Abstract
Pseudomonas aeruginosa is considered an opportunistic pathogen of great clinical importance. The clearance of this bacterium occurs through recognition of the pathogen by innate immune system receptors, leading to a lung inflammatory response. However, this response must be controlled via immunoregulatory pathways. In this study, we evaluate the role of endogenous murine IL-10 after acute infection with the virulent strain P. aeruginosa PA14. To assess the role of IL-10, intratracheal infection with the PA14 strain was performed in C57BL/6 or IL-10 KO mice. The PA14 strain was recovered in both types of animals, although IL-10 KO mice presented a higher number of viable bacteria in the lung when compared to the C57BL/6 group. Histopathological and stereological analyses showed that IL-10 KO mice had higher tissue damage and inflammatory infiltrate when compared to control animals. The activity of MMP-9 but not MMP-2, as well as IL-6 and TNF-α expression, were augmented in the lungs of infected animals and was much more evident in IL-10 KO animals when compared to the other analyzed groups. This work indicates that endogenous IL-10 control P. aeruginosa infection, the expression of pro-inflammatory genes, MMP-9 activity and histopathological processes of the infectious process in question.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen that affects immunocompromised patients. Due to a variety of mechanisms of adaptation and resistance to multiple classes of antibiotics, infections by strains of P. aeruginosa can cause morbidity and mortality and represent a threat to public health (Moradali et al. 2017). To trigger an effective response against infection by P. aeruginosa, several pathogen-associated molecular patterns are recognized by innate immune receptors linked to specific acquired immunity. The innate immune response involves the production of inflammatory cytokines and cell recruitment. In addition, phagocytic clearance of this bacterium occurs by neutrophils and macrophages after bacterial components, including lipopolysaccharide and flagellar proteins, are recognized by innate immune system receptors such as TLR4 and TLR5, respectively (Raoust et al. 2009). This recognition and downstream signaling culminates in the secretion of proinflammatory cytokines such as IL-1β, IL-6 and TNF-α. Despite the onset of the immune response being beneficial in controlling infection by P. aeruginosa, this response must be controlled. IL-10 is an immunoregulatory cytokine that inhibits the overproduction of proinflammatory cytokines (Christofi and Apidianakis 2013; Hazlett et al. 2014; Lovewell et al. 2014). IL-10 modulates immune response intensity and allows for successful bacterial clearance, without excessive host tissue damage. Although in some cases the absence of IL-10 makes the immune response more effective in clearing the pathogen, the damage induced to host tissues is severe and compromises host survival (Peñaloza et al. 2018). Previously, Chmiel and colleagues (1999) demonstrated in a chronic model of P. aeruginosa infection embedded in agar beads, mimetizing the bacterium infection in cystic fibrosis disease, in IL-10 KO mice leading to severe lung inflammation but without differences in bacterial burden even with a certain knockout mice mortality. However, there is no scientific evidence between the acute infection by P. aeruginosa and IL-10 cytokine in the first hour’s post-infection. Recent studies have begun to elucidate how immunoregulators can influence bacterial infection and inflammation. Given the importance of the human pathogen P. aeruginosa and the need to understand the regulatory mechanisms of the immune response against this bacterium, we hypothesize that the lack of IL-10 influences acute P. aeruginosa lung infection. This study evaluates the role of IL-10 by examining acute infection with the virulent P. aeruginosa strain PA14 in IL-10-deficient mice. Our results demonstrate that the absence of IL-10 increased susceptibility to P. aeruginosa infection and exacerbated inflammatory patterns in the lungs, with higher expression of pro-inflammatory cytokines and MMP-9 activity.
Material and methods
Ethics statement
This study was carried out in strict accordance with the Brazilian laws on animal experimentation. The protocol was approved by the Ethics Committee on the Use of Animals at the Federal University of Alfenas, Minas Gerais, Brazil (Permit Number: CEUA no. 42/2017). The experimentation was carried out in collaboration with the Laboratory of Molecular Biology at the Federal University of São João Del-Rei, Dona Lindu Center West Campus, Minas Gerais, Brazil, which had certification CQB 301/10.
Mice and bacteria
C57BL/6 mice, 6–8 weeks of age, from the Central Vivarium of the Federal University of Minas Gerais (UFMG), Brazil and IL-10 KO mice (C57BL/6 background) from the Medical School of Ribeirão Preto, University of Sao Paulo, Brazil, were randomly separated into 2 groups, non-infected and infected. The virulent strain of P. aeruginosa PA14 was obtained from the bacterial culture collection of the Vaccine Laboratory of the Department of the Microbiology and Immunology at the Federal University of Alfenas, Minas Gerais, Brazil. The bacteria were cultivated in Luria Broth medium (LB), at 37 °C and at 180 rpm. For initial culture of the bacterial strain, 1 mL frozen culture (− 80 °C) of P. aeruginosa was inoculated in 50 mL LB and incubated at 37 °C under constant agitation until it had reached an exponential growth phase.
Infection, Pseudomonas aeruginosa counts and organ collection
C57BL/6 and IL-10 KO female mice were anesthetized (ketamine/xylazine (80/10 mg/kg) i.p.) and were intratracheally inoculated with 1 × 105 colony-forming units (CFUs) of P. aeruginosa PA14. Briefly, the trachea was exposed by a small incision of the neck skin and blunt dissection and the infection was performed by instilling 50 µL of bacterial suspension by intratracheal administration using a blunted 7-gauge needle. After instillation, the neck skin was sutured. Mice were maintained in a vertical position for 5 min and transferred to a warming pad until full recovery from anesthesia. After 10 h of infection, the mice were euthanized by cardiac puncture under anesthesia (ketamine/xylazine (150/16 mg/kg) i.p.) and the left lung lobe, spleen and left liver lobe were collected. After anesthesia, the trachea of all animals was exposed and occluded with a suture line after the expiration. After thoracotomy, the left main bronchus was occluded with a suture line, and the left lung was removed en bloc (Novaes et al. 2012a). The lung edges were attached with microdissecting needles to a paraffin-coated Petri dish without applying tension and the organ was longitudinally cleaved into three fragments about 3 mm thick. The lateral and medial fragments were used in biochemical and molecular analyses and the intermedium fragment was used in the morphological analysis. To determine P. aeruginosa CFU in the lungs, spleens and liver, fragments of the organs were macerated in 9 mL sterile saline and serially diluted. Dilutions were plated in duplicate on LB agar. Petri dishes were incubated at 37 °C for 24 h and CFUs were determined.
Zymography
Fragments of the left lung lobe of C57BL/6 and IL-10 KO mice were triturated and centrifuged at 3000 rpm for 15 min. The supernatant was collected for protein dosing and to determine matrix metalloproteinase (MMP)-2 and MMP-9 activities. The supernatant was subjected to electrophoresis on 7% polyacrylamide copolymerized with 1% gelatin (Himedia). The gel was incubated for 1 h at room temperature in a 2% Triton X-100 (Synth) solution after electrophoresis was completed. Then, it was incubated at 37 °C for 16 h in Tris–HCl buffer, pH 7.4 (Tris 50 mmol l−1, ZnCl2 1 μmol l−1; both from Sigma) and 10 mmol l−1 CaCl2. The gels were then stained with 0.05% Coomassie brilliant blue G-250 (Sigma-Aldrich, São Paulo, SP, Brazil) and distained with 30% methanol and 10% acetic acid. Images of the gels were digitized and the enzymatic activities of the MMPs were determined according to their molecular weights using the ImageJ image program.
Histological processing
To reduce organ retraction, the intermedium fragment of the left lung lobe was attached to a paraffin-coated Petri dish and fixed by immersion in 10% buffered formaldehyde solution (pH 7.4, 0.1 M) for 24 h. Then, fragments were dehydrated in ethanol, diaphanized in xylene and embedded in paraffin (Rocha Pereira et al. 2017). Ten 4-μm-thick lung vertical sections were obtained for each animal and morphological method. Five sections were stained with hematoxylin and eosin (H&E) and the remaining 5 sections were used for immunohistochemistry. One to every 20 sections were collected to avoid analysis of the same histological area (Novaes et al. 2012a). All sections were observed in a bright-field microscope (Axioscope A1; Carl Zeiss, Oberkochen, Germany) coupled with a × 40 objective lens. Digital images were obtained using Axion Vision LE image analysis software (Carl Zeiss, Oberkochen, Germany) (Rocha Pereira et al. 2017). For each group, fifty random histological fields equally distributed in the cranial, median and caudal lung areas were sampled and a total lung area of 2.19 × 106 µm2 was analyzed from each group.
Immunohistochemistry
Histological sections were dewaxed with xylene and rehydrated with decreasing concentrations of ethanol (100 to 70%) until distilled water. Antigen recovery was performed with citrate buffer (pH 6.0) in a pressure cooker for 4 min. The sections were then incubated for 10 min in 3% hydrogen peroxide to block endogenous peroxidase, followed by 15 min in 5% non-fat milk prepared in pH 7.6 TBST (1× Tris-buffered saline with 0.05% Tween 20). Then, sections were incubated for 12 h at 4 °C with a primary rabbit anti-F4/80 antibody (1:1000 dilution) for macrophages (ab15191, Abcam, Cambridge, UK) and anti-myeloperoxidase antibody (1:200 dilution) for neutrophils (PA5-16672, ThermoFisher Scientific, Waltham, MA, USA). Control slides were obtained by omitting the primary antibody, incubating the slides only with antibody diluent. The slides were washed with TBST and incubated for 2 h at room temperature with a ready-to-use secondary goat anti-rabbit IgG antibody conjugated with horseradish peroxidase (Dako EnVision™ + Dual Link System-HRP, Agilent, Santa Clara, CA, USA). The slides were washed with TBST and the immunohistochemical reactions were revealed with 0.5% 3,3′-diaminobenzidine for 5 min. Finely, the slides were dehydrated in ethanol, treated with xylene and mounted with coverslips and analyzed from stereological principles.
Stereological analysis
Lung microstructure was analyzed from classical stereological principles previously reported by Weibel (1989), Mandarim-de-Lacerda (2003) and Weibel et al. (2007). Considering a relative anisotropy in lung structure (Mitzner et al. 2008), vertical sections were used and different lung areas (cranial, median and caudal) were analyzed to reduce measure bias (Baddeley et al. 1986; Mitzner et al. 2008). The following parameters were estimated: (i) volume density (Vv, %) of alveolar septum, alveolar space, macrophages and neutrophils; (ii) number density (NA) of alveoli (alveoli/mm2); and (iii) surface density (Sv) of alveolar septum (μm2/μm3). The representative number of histological images analyzed for each animal was determined considering the stabilization of the coefficient of variation of the alveoli number in ascending random samples (3, 6, 9, 12, 15, 18 and 21 histological fields), according to principles previously reported (Novaes et al. 2012b). Briefly, when the increase in image analysis resulted in a similar (p > 0.05) coefficient of variation (CV = mean/standard deviation) between 3 consecutive samples, the smallest sample size (n = 15) was admitted as the minimal representative histological fields. Volume density was estimated as Vv = PP/PT, where PP is the number of test points hitting the interest structures (septum or alveolar space) and PT is the total number of points in the test system. In this analysis, a quadratic test system with 300 test points delimited by a test area (AT) with an area of 35 × 103 μm2 was used. Number density was estimated as NA = ∑QA/AT, where ∑QA is the number of alveoli counted in the test area (AT = 35 × 103 μm2). Surface density was estimated as Sv = 2 × ΣI/LT, where ΣI is the total number of intersections between cycloid arcs and the alveolar surface and LT is the total length of the cycloid arcs. For this analysis, a test system with 24 cycloid arcs with 31.54 µm each was used (LT = 757.02 μm). All stereological measures were performed using Image-Pro Plus 4.5 image analysis software (Media Cybernetics, Rockville, MD, USA) (Novaes et al. 2012a,b).
Real-time RT-PCR
For analysis of differential gene expression, total RNA from the left lung lobe of uninfected and P. aeruginosa infected C57BL/6 or IL-10 KO mice were extracted using TRIzol® LS reagent (Life Technologies, Carlsbad, CA, USA). Reverse transcription of 1 µg total RNA was performed using illustra™ Ready-To-Go RT-PCR Beads (GE Healthcare, Little Chalfont, UK). Real-time RT-PCR was performed with an ABI 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) containing SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), cDNA as the PCR template and primers to amplify specific fragments corresponding to specific gene targets. PCR reactions followed the cycling parameters: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The data were analyzed according to the comparative method of the threshold cycle (Ct), using the equation 2−ΔΔCt. Primers were used to amplify a specific 100–120 bp fragment corresponding to specific gene targets as follows: IL-10 F 5′-GGTTGCCAAGCCTTATCGGA-3′, IL-10 R 5′-ACCTGCTCCACTGCCTTGCT-3′, IL-6 F 5′-CCAGGTAGCTATGGTACTCCAGAA-3′, IL-6 R 5′-GATGGATGCTACCAAACTGGA-3′, TNF-α F 5′-CATCTTCTCAAAATTCGAGTGACA-3′, TNF-α R 5′-TGGGAGTAGACAAGGTACAACCC-3′, β-actin F 5′AGGTGTGCACTTTTTATTGGTCTCAA-3′, β-actin R 5′-TGTATGAAGGTTTGGTCTCCCT-3′. All data were presented as relative expression units after normalization to the β-actin gene. PCR measurements were conducted in triplicate.
Statistical analysis
All experiments were repeated at least 3 times. As similar results were obtained between repetitions, figures show data from one representative experiment. Graphs and data analysis were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Error bars represent the standard error of the mean. Normality in data distribution was verified by the D′Agostino-Pearson test. Nonparametric variables were compared by one-way ANOVA followed by the Tukey’s post-test. The confidence level of all tests was set at 95% (p ≤ 0.05).
Results
IL-10 KO mice were more susceptible to pulmonary infection by P. aeruginosa, but bacteremia did not occur
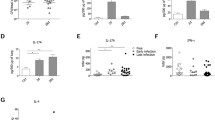
The number of bacteria was evaluated in the lungs, spleen and liver of these animals 10 h post-infection. A CFU count was performed, and significant results were observed only in the lung, showing that, during this period, there was no bacterial spread to other organs. In vivo analyses demonstrated that the PA14 strain was recovered from the lungs of both types of animals; IL-10 KO mice were more susceptible, indicated by the higher number of viable bacteria in the organ (mean 4.8 log CFU), when compared to the C57BL/6 mice (mean 3.3 log CFU) (Fig. 1). The difference between the groups was on average 1.5 log CFU recovered from the lungs of these animals. Although none of the mice died prior to being euthanized, the IL-10 KO infected group presented prostrated with more signs of fever including chills and lethargy when compared to C57BL/6 infected mice.
IL-10 KO mice are more susceptible to P. aeruginosa lung infection. The lungs of animals infected with 1 × 105 CFU of the P. aeruginosa PA14 strain were recovered 10 h after infection and viable CFUs were counted. The data were transformed into log values of CFU per largest lung lobe (left lung). The result presented is representative of 3 independent experiments (n = 5 mice/group). *p < 0.05
Endogenous IL-10 deletion led to an increase in lung damage during P. aeruginosa infection and a higher influx of macrophages and neutrophils but did not alter the hepatic architecture
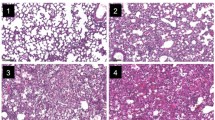
After the CFU evaluation, histopathological parameters were assessed in the lung and liver of mice infected with P. aeruginosa or not; a stereological analysis was performed. IL-10 KO mice had thickened alveolar septa, intense diffuse inflammation with a higher degree of inflammatory cell infiltration and collapsed alveoli when compared to the other groups (Fig. 2). Histopathological analysis of the liver of infected C57BL/6 and IL-10 KO mice showed no alterations in their architecture compared to the liver of the control animals (data not shown), corroborating with the results observed in the CFU count, demonstrating that in 10 h of infection, the P. aeruginosa PA14 strain was not able to spread and cause damage to other tissues. Based on the stereological analysis, alveoli counts were not statistically different between groups (Fig. 3a). There was a decrease in the alveolar space of infected IL-10 KO animals when compared to uninfected C57BL/6 and uninfected IL-10 KO mice (Fig. 3b) and a decrease in lung surface density of IL-10 KO infected when compared to uninfected IL-10 KO mice (Fig. 3c). Immunohistochemistry revealed the influx of both macrophages and neutrophils in the lung of infected mice compared to non-infected mice (Figs. 4 and 5, respectively). Regarding the observed influx of those cells, the differential evaluation of the quantitate of macrophages or neutrophils demonstrated an increase in both cell types in the lung of infected IL-10 KO mice when compared to wild-type mice (Figs. 4e and 5e, respectively).
Histopathological analysis of the lung of mice infected with the P. aeruginosa PA14 strain demonstrated a greater inflammatory pattern in IL-10 KO mice when compared to the controls. Fragments of the largest lung lobe (left lung) from C57BL/6 or IL-10 KO infected or non-infected animals were fixed in 10% formaldehyde solution, dehydrated, diaphanized and embedded in paraffin. Sections of tissue were stained with hematoxylin and eosin. a Normal histological architecture of uninfected C57BL/6. b Septum thickening and presence of inflammatory infiltrate with moderate cellularity in the infected C57BL/6 mice. c Normal histological architecture, presenting larger alveoli compared to uninfected C57BL/6 in uninfected IL-10 KO mice. d Septum thickening, diffuse inflammatory infiltrate, high cellularity and collapsed alveoli in infected IL-10 KO mice. Ampersand: alveolar sac; arrowhead: alveolar septum; asterisk: alveoli. Increase by 40×. Bars (50 μm). The result presented is representative of 3 independent experiments (n = 5 mice/group)
Stereological analysis of the lung of mice infected with the P. aeruginosa PA14 strain demonstrated greater damage in IL-10 KO mice when compared to the controls. Stereological analysis from the histopathological results of the largest lung lobe (left lung) from C57BL/6 or IL-10 KO infected or non-infected animals shows no significant differences in the number of alveoli (a). Infected IL-10 KO mice had less alveolar space compared to uninfected C57BL/6 mice and uninfected IL-10 KO, a decrease in alveolar space in lung tissues of infected C57BL/6 mice was also observed compared to the infected C57BL/6 mice uninfected IL-10 KO (b). A decrease in alveolar surface density was shown in infected IL-10 KO mice compared to uninfected IL-10 KO (c). *p < 0.05 compared to non-infected C57BL/6 mice; #p < 0.05 compared to non-infected IL-10 KO mice; &p < 0.05 compared to C57BL/6 infected with P. aeruginosa. The result presented is representative of 3 independent experiments (n = 5 mice/group)
IL-10 KO mice infected with P. aeruginosa present a higher influx of macrophages in the lung when compared to the control animals. Immunochemistry of the largest lung lobe (left lung) histological sections from the C57BL/6 or IL-10 KO mice non-infected (a and c, respectively) or infected with P. aeruginosa intratracheally (b and d, respectively) incubated with a primary rabbit anti-F4/80 antibody for macrophage identification. Macrophages are marked in brown color. Ampersand: alveolar sac; arrowhead: alveolar septum; asterisk: alveoli. Increase by 40×. Bars (50 μm). Stereological analysis from the immunohistochemistry results of the largest lung lobe (left lung) from C57BL/6 or IL-10 KO infected or non-infected animals. The results show significant differences in the macrophages influx in infected mice with the higher influx in IL-10 KO infected mice when compared to C57BL/6 infected mice (e). *p < 0.05 compared to non-infected C57BL/6 mice; #p < 0.05 compared to non-infected IL-10 KO mice; &p < 0.05 compared to C57BL/6 infected with P. aeruginosa. The result presented is representative of 3 independent experiments (n = 5 mice/group)
IL-10 KO mice infected with P. aeruginosa present a higher influx of neutrophils in the lung when compared to the control animals. Immunochemistry of the largest lung lobe (left lung) histological sections from the C57BL/6 or IL-10 KO mice non-infected (a and c, respectively) or infected with P. aeruginosa intratracheally (b and d, respectively) incubated with a primary rabbit anti-myeloperoxidase antibody for neutrophil identification. Neutrophils are marked in brown color. Ampersand: alveolar sac; arrowhead: alveolar septum; asterisk: alveoli. Increase by 40×. Bars (50 μm). Stereological analysis from the immunohistochemistry results of the largest lung lobe (left lung) from C57BL/6 or IL-10 KO infected or non-infected animals. The results show significant differences in the neutrophils influx in infected mice with the higher influx in IL-10 KO infected mice when compared to C57BL/6 infected mice (e). *p < 0.05 compared to non-infected C57BL/6 mice; #p < 0.05 compared to non-infected IL-10 KO mice; &p < 0.05 compared to C57BL/6 infected with P. aeruginosa. The result presented is representative of 3 independent experiments (n = 5 mice/group)
Infected lungs of IL-10 KO mice showed higher expression of IL-6 and TNF-α when compared to wild-type infected mice
Lung digests of infected C57BL/6 and IL-10 KO mice were collected for an evaluation of IL-10, IL-6 and TNF-α gene expression. In the differential expression analysis of IL-10, an increase in this cytokine was observed in infected C57BL/6 mice relative to uninfected C57BL/6 mice. IL-10 expression was not observed in the IL-10 KO mice, as expected (Fig. 6a). In the analysis of the differential expression of the IL-6 gene, an increase in this cytokine was demonstrated in the lung digests of IL-10 KO mice when compared to the other groups. In addition, an increase in IL-6 expression was seen in infected C57BL/6 mice compared to uninfected C57BL/6 mice; however, IL-6 gene expression was significantly higher in IL-10 KO animals (Fig. 6b). In the analysis of TNF-α expression, a significant increase in this cytokine was observed in infected IL-10 KO and C57BL/6 infected animals compared to control animals; this increase was relatively higher in infected IL-10 KO mice than in C57BL/6 mice infected with the P. aeruginosa PA14 strain (Fig. 6c).
IL-10 KO mice infected with P. aeruginosa present greater differential expression of the IL-6 and TNF-α genes in the lung when compared to the control animals. Expression analysis of the IL-6 (b) and TNF-α (c) proinflammatory genes or IL-10 anti-inflammatory gene (a), performed by RT-qPCR, in the lungs of C57BL/6 or IL-10 KO mice and intratracheally infected with P. aeruginosa PA14. *p < 0.05 compared to the uninfected C57BL/6 group; #p < 0.05 compared to the uninfected IL-10 KO group, &p < 0.05 compared to C57BL/6 infected with P. aeruginosa. The result presented is representative of 3 independent experiments (n = 5 mice/group)
MMP-9 was more activated in lungs of IL-10 KO mice infected with P. aeruginosa than the lungs of wild-type infected mice
Lung digests were used to analyze the activity of MMP-2 and MMP-9 by zymography. The zymography gels showed the presence of bands of approximately 64 kDa corresponding to the activity of the MMP-2 enzyme in lung samples from uninfected C57BL/6 and IL-10 KO animals (controls) and C57BL/6 animals and IL-10 KO 10 h after infection with P. aeruginosa PA14. No significant difference in MMP-2 activity was observed between control and IL-10 KO animals, as shown in Fig. 7(a). However, a significant increase in MMP-9 activity was observed in C57BL/6 animals as well as the presence of high levels of MMP-9 in P. aeruginosa-infected IL-10 KO animals compared to uninfected C57BL/6 animals, shown by the presence of approximately 89-kDa bands Fig. 7(b).
IL-10 KO mice infected with P. aeruginosa exhibit greater enzymatic activity of MMP-9 but not of MMP-2, in the lung when compared to the control animals. The enzymatic activity of MMP-2 and MMP-9 in the lung tissue of C57BL/6 or depleted IL-10 KO mice infected or uninfected with the P. aeruginosa PA14 strain was evaluated by zymography. Evaluation of MMP-2 (a and a′) or MMP-9 (b and b′) activity in the lung tissue of uninfected C57BL/6 (C57) mice, uninfected IL-10 KO (IL-10) mice, infected C57BL/6 (C57I), or IL-10 KO infected (IL-10I) mice. The zymography gel representation is demonstrated by the intensity of the bands using the image editor program ImageJ in relation to the group of less enzyme activity. *p < 0.05 compared to the uninfected C57BL/6 group; #p < 0.05 compared to the uninfected IL-10 KO group, &p < 0.05 compared to C57BL/6 infected with P. aeruginosa. The result presented is representative of 3 independent experiments (n = 5 mice/group)
Discussion
IL-10 is an important anti-inflammatory cytokine responsible for controlling the immune response, thereby avoiding an exacerbated response and preventing tissue damage. In this study, it was shown that the absence of endogenous IL-10 culminates in greater recovery of viable bacteria from the lungs of animals infected with the virulent P. aeruginosa PA14 strain when compared to wild-type mice. However, no bacteria were recovered in the spleen or liver of these animals, showing that after 10 h of infection, P. aeruginosa was not able to spread to other organs differing from what occurred in the gut-dysbiotic mice infected with tenfold more P. aeruginosa leading to higher lung inflammatory damage (Rosa et al. 2020). According to Peñaloza et al. (2018), IL-10 production is required for host survival during infections caused by extracellular and/or highly proinflammatory bacteria, including Streptococcus pneumoniae, Pseudomonas aeruginosa, Francisella tularensis, Escherichia coli and Mycobacterium tuberculosis, since the cytokine modulates the intensity of the immune response and allows for successful bacterial clearance without excessive tissue damage to the host (Peñaloza et al. 2016). However, IL-10 production impairs host survival during infections caused by intracellular bacteria or bacterial pathogens that modulate the inflammatory response, such as Klebsiella pneumoniae, Bordetella pertussis, Listeria monocytogenes, Brucella abortus and Salmonella enterica serovar Typhimurium, since production of IL-10 during the infectious cycle of these bacteria helps to evade the immune response and to spread within the host, seriously impairing host survival (Peñaloza et al. 2016; Corsetti et al. 2013). Chen et al. (2017) demonstrated that mice with secondary infection by P. aeruginosa after sepsis showed decreased bacterial clearance in the lung, increased mortality of animals and increased IL-10 expression. In the present study, robust infiltration of inflammatory cells in the lung tissue was observed in the histopathological analysis and through the stereological analysis, a decrease in the alveolar space and alveolar surface density were observed in infected IL-10 KO mice. In spite of a greater influx of inflammatory cells to the site of infection, IL-10 KO animals failed to maintain efficient infection control, as seen in C57BL/6 animals. IL-10 has been demonstrated to be critical in restricting excessive inflammatory responses during infection, as in the absence of IL-10, proinflammatory cytokine concentrations are significantly elevated in comparison to wild-type mice (Leech et al. 2017). These results corroborate with the histopathological and immunohistochemistry analyses observed in this study and by Buff and colleagues (2010) that used a recombinant adeno-associated virus type 5 (AAV5) vector expressing murine IL-10 that was able to decrease airway inflammation in IL-10 KO mice chronically infected with mucoid P. aeruginosa (Buff et al. 2010). Our data suggest that the cytokine IL-10 plays an important role in P. aeruginosa clearance and in controlling the inflammatory response. Conversely, Chmiel and colleagues (2002) showed no difference between IL-10 KO and C57Bl/10J mice in the number of bacteria isolated 2, 4, 6 and 8 days post-infection with a mucoid clinical isolate of P. aeruginosa. However, the IL-10 KO mice presented prolonged NF-κB activation with greater proinflammatory cytokine levels and neutrophil numbers in the bronchoalveolar lavage fluid (BALF) (Chmiel et al. 2002). We also observed an increase in the expression of both the IL-6 and TNF-α encoding genes in the lung of P. aeruginosa-infected mice with a higher influx of neutrophils and macrophages; this increase was more evident and differentially increased in the lungs of the IL-10 KO animals compared to the other analyzed groups, corroborating the histopathological and stereological analyses in which inflammation was more evident in knockout mice. Chmiel and colleagues (1999) also identified marked differences in lung extent inflammation between wild-type and IL-10 KO mice infected with P. aeruginosa containing agar beads 10 days after infection and increased systematic knockout mice morbidity but without differences in lung bacterial burden (Chmiel et al. 1999). Guilbault and colleagues (2002), using a model of cystic fibrosis chronic infection by P. aeruginosa-impregnated in agar beads, demonstrated higher levels of TNF-α in the lungs from IL-10 KO mice compared to wild-type mice maintaining higher levels of polymorphonuclear cells and lower levels of macrophages for a longer period of time. Our results demonstrated a higher influx of both macrophage and neutrophils in an acute intratracheal infection by P. aeruginosa in IL-10 KO mice reinforcing the histopathological findings of an increase of pro-inflammatory cytokine expression compared to controls. Wonnenberg and colleagues (2016) reviewed the importance of the reduction of inflammasome activation and pro-inflammatory cytokine IL-1β release to avoid lung damage during P. aeruginosa infection (Wonnenberg et al. 2016). Inflammasome activation may be another important innate immunity response to be investigated in order to regulate the exacerbate lung inflammatory response to P. aeruginosa. Considering the effective increase in the expression of genes encoding the proinflammatory cytokines and the increase of the inflammatory influx in the lungs of IL-10 KO animals infected with P. aeruginosa, the present study evaluated the activity of MMP-2 and MMP-9 in the lungs of C57B/6 and IL-10 KO animals after P. aeruginosa infection. MMPs are considered to be important controllers of the immune response and it is therefore of paramount importance to determine what other immunoregulatory factors may act in the absence of IL-10 during the infectious process under study. Our results demonstrated that there was an increase in the activity of MMP-9 but not of MMP-2, in the lungs of infected animals, to a greater extent in IL-10 KO animals. MMP-9 plays an important role in protection against shock-mediating endotoxins, in reducing dendritic cell migration, angiogenesis and bronchiolysis after acute lung injury and in altering chemokine and leukocyte gradients inducing inflammation (Parks et al. 2004; Li et al. 2015; Zhang et al. 2017). Despite increased inflammation and MMP-9 activity, more bacteria were recovered from the lungs of IL-10 KO mice compared to wild-type mice. This suggests that IL-10 plays a role in the modulation of inflammation, in the expression of MMP-9 activity and in P. aeruginosa clearance. Kothari et al. (2014) reported a blockade of MMP-9 expression in macrophages exposed to exogenous IL-10 and an increase in MMP-9 expression in macrophages incubated in the presence of anti-IL-10 IgG. These data suggest that IL-10 plays a role in modulating MMP-9 levels, corroborating the results of the present study. Zhang et al. (2017) showed that elevated expression of MMP-9 may lead to an exacerbated inflammatory response of the pulmonary artery, the induction of smooth muscle cell proliferation, and degradation of the extracellular matrix. The authors suggested that inhibition of MMP-9 may have satisfactory results in the prevention and treatment of chronic obstructive pulmonary disease (COPD). More studies must be carried out to deeply understand if the inhibition of MMP-9 is also a promising target for P. aeruginosa infection treatment. In a cognate manner, Li et al. (2016) demonstrated that silencing of the MMP extracellular inducer gene (EMMPRIN) results in host resistance to P. aeruginosa infection due to increased Th1 cytokine expression, i.e., IFN-γ, IL-12 and IL-18 and decreased expression of Th2 cytokines, i.e., IL-4, IL-5, as well as a decrease in IL-10 expression. In conclusion, this work describes the close relationship between the control of acute Pseudomonas aeruginosa infection by endogenous IL-10 and its control over the expression of pro-inflammatory genes such as IL-6 and TNF-α, MMP-9 activity and histopathological processes of the infectious process in question. This work supports the possibility of new research aimed at the search for therapies based on immune response mechanisms, aiming for a higher quality of life for individuals who are at risk of being infected with P. aeruginosa.
References
Baddeley AJ, Gundersen HJ, Cruz-Orive LM (1986) Estimation of surface area from vertical sections. J Microsc 142:259–276
Chen W, Lian J, Ye J, Mo Q, Qin J, Hong G, Long-Wang C, Shao-Ce Z, Guang-Ju Z, Zhong-Qiu L (2017) Ethyl pyruvate reverses development of Pseudomonas aeruginosa pneumonia during sepsis-induced immunosuppression. Int Immunopharmacol 52:61–69
Chmiel JF, Konstan MW, Knesebeck JE, Hilliard TL, Dawson DV, Berger M (1999) IL-10 attenuates excessive inflammation in chronic pseudomonas infection in mice. Am J Respir Crit Care Med 160:2040–2047
Chmiel JF, Konstan MW, Saadane A, Krenicky JE, Kirchner HL, Berger M (2002) Prolonged inflammatory response to acute pseudomonas challenge in interleukin-10 knockout mice. Am J Respir Crit Care Med 165:1176–1181
Corsetti PP, De Almeida LA, Carvalho NB, Azevedo V, Silva T, Teixeira HC, Faria AC, Oliveira S (2013) Enhances production of proinflammatory cytokines and leads to Brucella abortus clearance in mice. PLoS ONE 9:0074729
Guilbault C, Stotland P, Lachance C, Tam M, Keller A, Thompson-Snipes L, Cowley E, Hamilton T, Eidelman DH, Stevenson MM, Radiozioch D (2002) Influence of gender and interleukin-10 deficiency on the inflammatory response during lung infection with Pseudomonas aeruginosa in mice. Immunology 107:297–305
Kothari P, Pestana R, Mesraoua R, Elchaki R, Khan KM, Dannenberg AJ, Falcone D (2014) IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J Immunology 192:349–357
Leech JM, Lacey KA, Mulcahy ME, Medina E, Mcloughlin RM (2017) IL-10 plays opposing roles during Staphylococcus aureus systemic and localized infections. J Immunol 198:2352–2365
Li WL, Wu CH, Yang J, Tang M, Chen LJ, Zhao SL (2015) Local inflammation alters MMP-2 and MMP-9 gelatinase expression associated with theseverity of nifedipine-induced gingival overgrowth: a rat model study. Inflammation. 38(4):1517-28.
Li Y, Chen L, Wang C, Chen J, Zhang X, Hu Y, Niu X, Pei D, He Z, Bi Y (2016) Extracellular matrix metalloproteinase inducer enhances host resistanceagainst Pseudomonas aeruginosa infection through MAPK signaling pathway. Am J Transl Res 8(12):5619-5627.
Lovewell RR, Patankar YR, Berwin B (2014) Mechanisms of phagocytosis and host clearance of Pseudomonas aeruginosa. Am J Physiol Lung Cell MoL Physiol 306:591–603
Mandarim-de-Lacerda CA (2003) Stereological tools in biomedical research. An Acad Bras Cienc 75:469–486
Mitzner W, Fallica J, Bishai J (2008) Anisotropic nature of mouse lung parenchyma. Ann Biomed Eng 36:2111–2120
Moradali M, Ghods S, Rehm B (2017) Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol 7:39
Novaes RD, Gonçalves RV, Cupertino MC, Marques DCS, Rosa DD, Peluzio MCG, Neves CA, Leite JPV (2012) Bark extract of Bathysa cuspidata attenuates extra-pulmonary acute lung injury induced by paraquat and reduces mortality in rats. Int J Exp Pathol 93:225–233
Novaes RD, Penitente AR, Talvani A, Natali AJ, Neves CA, Maldonado IR (2012) Use of fluorescence in a modified disector method to estimate the number of myocytes in cardiac tissue. Arq Bras Cardiol 98:252–258
Parks WC, Wilson CL, López-Boado Y (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 8:617–629
Peñaloza HF, Schultz BM, Nieto PA, Salazar GA, Suazo I, Gonzalez PA et al (2016) Opposing roles of IL-10 in acute bacterial infection. Cytokine Growth Factor Rev 32:17–30
Peñaloza HF, Noguera LP, Riedel CA, Bueno SM (2018) IL-10 plays opposing roles during Staphylococcus aureus systemic and localized infections. Frontiers in Microbiology 198:2352–2365
Rocha Pereira AE, Rodrigues MÂ, Novaes RD, Caldas IS, Martins Souza RL, Costa Pereira AA (2017) Lipopolysaccharide-induced acute lung injury in mice chronically infected by Schistosoma mansoni. Exp Parasitol 178:21–29
Rosa CP, Pereira JA, de Melo C, Santos N, Brancaglion GA, Silva EM, Tagliati CA, Novaes RD, Corsetti PP, de Almeida LA (2020) Vancomycin-induced gut dysbiosis during Pseudomonas aeruginosa pulmonary infection in a mice model. J Leukoc Biol 107(1):95–104
Weibel ER (1989) Measuring through the microscope: development and evolution of stereological methods. J Microsc 155:393–403
Weibel ER, Hsia CC, Ochs M (2007) How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol 102:459–467
Wonnenberg B, Bischoff M, Beisswenger C, Dinh T, Bals R, Singh B, Tschering T (2016) The role of IL-1β in Pseudomonas aeruginosa in lung infection. Cell Tissue Res 364(2):225–229
Zhang R, Ni HJ, Zhou HS (2017) Study on the expression of Toll-like receptor 4 and matrix metalloproteinase-9 in patients with chronic obstructive pulmonary disease and their clinical significance. Eur Rev Medical Pharmacol Sci 21(9):2185–2191
Buff SM, Yu H, McCall JN, Caldwell SM, Ferkol TW, Flotte TR, Virella-Lowell IL (2010) IL-10 delivery by AAV5 vector attenuates inflammation in mice with Pseudomonas pneumonia. Gene Ther. 17(5):567‐576.
Christofi T, Apidianakis Y (2013) Drosophila immune priming against Pseudomonas aeruginosa short-lasting and depends on cellular and humoral immunity. F1000Research 1: 2–76
Raoust E, Balloy V, Garcia-Verdugo I, Touqui L, Ramphal R, Chignard, M (2009) Pseudomonas aeruginosa LPS or flagellin are sufficient to activate TLR-dependent signaling in murine alveolar macrophages and airway epithelial cells. PLoS ONE.
Funding
This study was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) (Finance Code 001).
Author information
Authors and Affiliations
Contributions
VAB, SFDS, FLT, DOL, CSC, RDN, PPC and LAA designed the project and experiments. VAB, JAP, SFDS, FLT and BPP carried out most of the experiments. VAB, JAP, RDN, PPC and LAA wrote the manuscript. VAB, SFDS, BPP and LAA carried out statistical analysis and prepared figures. LAA submitted this paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was carried out in strict accordance with the Brazilian laws on animal experimentation. The protocol was approved by the Ethics Committee on the Use of Animals at the Federal University of Alfenas, Minas Gerais, Brazil (Permit Number: CEUA no. 42/2017). The experimentation was carried out in collaboration with the Laboratory of Molecular Biology at the Federal University of São João Del-Rei, Dona Lindu Center West Campus, Minas Gerais, Brazil, which had certification CQB 301/10
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Belo, V.A., Pereira, J.A., Souza, S.F.D. et al. The role of IL-10 in immune responses against Pseudomonas aeruginosa during acute lung infection. Cell Tissue Res 383, 1123–1133 (2021). https://doi.org/10.1007/s00441-020-03308-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03308-4