Abstract
We investigated how the riverine network influences taxonomic and functional beta diversity patterns of fish assemblages in the mainstem/headwater (lateral) and upstream/downstream (longitudinal) gradients in a Neotropical river system. We investigated the following questions: which component (turnover or nestedness) explains taxonomic and functional beta diversity in both gradients? Is this component a consistent pattern for different lateral sections of the river basin? Is this component influenced by the spatial extent? Finally, how are taxonomic and functional beta diversity structured by space and environment along the longitudinal gradient? Taxonomic and functional turnover were the main patterns found for the lateral gradient and they were consistent for all lateral sections considered. Taxonomic and functional turnover were also the main patterns for the longitudinal gradient, increasing with the spatial extent increase and being structured by space and spatially structured environments. Our study demonstrates that the dendritic nature of riverine systems constrains species and traits occurrence along lateral and longitudinal gradients in a Neotropical region, generating taxonomic and functional turnover patterns due to the influence of space and spatially structured environments on niche- and dispersal-based processes. These results show that Neotropical riverine systems conservation must go beyond traditional approaches and consider the metacommunity perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adoption of a metacommunity perspective in understanding riverine fish community organization requires the recognition of suitable spatial scales in which ecological mechanisms operate (Heino et al., 2015). Riverine systems have a hierarchical branching structure where multiple isolated branches (headwaters) are connected to the mainstem channel forming a riverine network (Grant et al., 2007; Muneepeerakul et al., 2008; Lynch et al., 2011; Altermatt, 2013; Altermatt et al., 2013; Vitorino Jr. et al., 2016; Tonkin et al., 2018). The notion that freshwater fish communities are linked by species movement through the river network has stimulated aquatic ecologists to consider the dendritic nature of riverine metacommunities (Altermatt, 2013; Tonkin et al., 2018). In this context, headwater streams and mainstem channel sites are potential patches to be occupied by species but this occupation depends on the characteristics and dispersal constraints of each species (Erős, 2017), and the relative influence of dispersal and niche-based processes on the structure of metacommunities may vary spatially (Brown & Swan, 2010; Henriques-Silva et al., 2019). Therefore, studies encompassing the broader range of habitats present in riverine networks, such as headwater streams and mainstem channel sites, may provide valuable information about the context dependency of processes underlying the metacommunity organization in freshwater systems (Carvalho & Tejerina-Garro, 2015).
Headwater streams are located at the most upstream section of the river network and their environmental variability and unpredictability act as strong filters to fish species and their ecological traits (Poff, 1997; Jackson et al., 2001; Grenouillet et al., 2004; Carvalho & Tejerina-Garro, 2015). Therefore, fish assemblages of headwater streams are often composed of only a few species with some level of endemism (Meyer et al., 2007; Altermatt, 2013; Borges et al., 2020) and with similar functional traits (Carvalho & Tejerina-Garro, 2015). Nevertheless, in headwater habitats, environment and physiography may differ among streams (Tejerina-Garro et al., 2005; Súarez & Petrere Jr., 2006; Vitorino Jr. et al., 2016) suggesting that each fish assemblage will be primarily structured by its own set of local factors (species sorting mechanism, sensu Leibold et al., 2004) with species and trait selection leading to taxonomic and functional turnover across headwater streams. In fact, recent studies conducted in the Neotropical region reveal that taxonomic turnover and functional nestedness explain beta diversity patterns in tributaries (Vitorino Jr. et al., 2016; Peláez et al., 2017; Peláez & Pavanelli, 2019). On the other hand, mainstems are recognized to be environmentally stable, presenting higher habitat heterogeneity than headwater streams and providing connection among fish assemblages along the upstream/downstream gradient (Meffe & Minkley, 1987; Jackson et al., 2001). These characteristics are expected to reduce the environmental effects on fish assemblages and favor regional processes, such as species dispersal and mass effects (Heino et al., 2015). In this context, one may expect the occurrence of species turnover to be related to species dispersal abilities and a decrease in functional dissimilarities among assemblages along this longitudinal gradient, which represents an intermediate spatial scale. At this scale, evidence has been found for fish taxonomic turnover (Vitorino Jr. et al., 2016; Peláez et al., 2017; Zbinden & Matthews, 2017; Peláez & Pavanelli, 2019), functional turnover (Vitorino Jr. et al., 2016), and functional nestedness patterns (Peláez & Pavanelli, 2019). Despite the increasing number of studies regarding fish beta diversity patterns (e.g., Brown & Swan, 2010; Logue et al., 2011; Vitorino Jr. et al., 2016; Medina Torres & Higgins, 2016; Peláez et al., 2017; Zbinden & Matthews, 2017; Huang et al., 2019; Peláez & Pavanelli, 2019), few have addressed the role of the riverine network in structuring fish β-diversity within the Neotropical region. Furthermore, there is a paucity of knowledge regarding the spatial distribution of β-diversity components (turnover and nestedness; Baselga, 2010) in different ecological gradients; the consistency of these patterns among different regions/sections within a river basin; and the effect of the overall area encompassed by a study (spatial extent; Wiens, 1989) on beta diversity patterns along a longitudinal gradient (upstream to downstream habitats).
Although several ecological studies consider the environment as a key driver of local community structure, freshwater ecologists have found evidence that space and spatially structured environments play an important role in structuring taxonomic and functional diversity of fish assemblages even in small spatial scales (Azevedo et al., 2007; Sharma et al., 2011; Nakagawa, 2014; Carvalho & Tejerina-Garro, 2015; Borges et al., 2020). While space reflects how species composition and traits vary among sites due to spatial distance and specific species dispersal constraints, the spatially structured environment expresses the variation in species composition or traits linked to dispersal limitations. This is because nearby sites are similar to each other due to structured environmental variation (Münkemüller et al., 2012; Soininen, 2016). In this context, procedures that allow us to access these components in order to model spatial structures representing possible patterns of dispersal among sampling sites (Blanchet et al., 2008, 2011), including spatial trends of fish assemblages (Bertolo et al., 2012; Carvalho & Tejerina-Garro, 2015; Massicotte et al., 2015; Cetra et al., 2017; Rodrigues-Filho et al., 2018), are important because they reflect processes based on species dispersal (Gilbert & Lechowicz, 2004; Diniz-Filho et al., 2012).
In this paper, we investigated how the riverine dendritic network constrains taxonomic and functional beta diversity patterns of fish assemblages in two ecological gradients: mainstem/headwater (hereafter lateral gradient) and upstream/downstream (hereafter longitudinal gradient). For the lateral gradient, we aimed to answer the following questions: i) which component (turnover or nestedness) explains taxonomic and functional beta diversity patterns along the mainstem/headwater gradient?; ii) is the main component explaining beta diversity a consistent pattern for different lateral sections of the river basin? Based on recent findings for the Neotropical region (Vitorino Jr. et al., 2016; Peláez et al., 2017; Peláez & Pavanelli, 2019), we expect to find turnover and nestedness components explaining taxonomic and functional beta diversity at the lateral gradient, respectively, in different sections of the river basin given that similar ecological processes are expected to structure fish assemblages towards headwaters independently of its localization. For the longitudinal gradient, we sought to answer the following questions: (i) which component (turnover or nestedness) explains taxonomic and functional beta diversity patterns along the upstream/downstream gradient?; (ii) how does spatial extent affect beta diversity patterns?; iii) how do spatial and environmental factors structure taxonomic and functional fish beta diversity? While recent studies agree that taxonomic turnover related to fish species dispersal explains beta diversity when both mainstem and tributaries are considered (Vitorino Jr. et al., 2016; Peláez et al., 2017; Zbinden & Matthews, 2017; Peláez & Pavanelli, 2019), the same is not observed for functional beta diversity and divergent patterns are found (Vitorino Jr. et al., 2016; Peláez & Pavanelli, 2019). Here, we assumed that the connection among mainstem sites and headwater streams along the longitudinal gradient will reduce the environmental signal on fish assemblages and favor species dispersal (regional process; Heino et al., 2015), increasing species turnover but decreasing functional dissimilarities among patches. At this intermediate spatial scale (longitudinal gradient), we expect that beta diversity patterns will be primarily structured by spatial components. We also expect that increasing the spatial extent of the longitudinal gradient will potentialize dispersal constraints and lead to increased beta diversity.
To answer these questions, we used samplings conducted at the Almas River basin (Tocantins River, Amazon) that exhibits an expressive altitudinal gradient, waterfalls, steep streams, and considerable environmental variability among streams (Oliveira & Bispo, 2001). These features may affect fish species and traits selection and dispersal in both lateral and longitudinal gradients; therefore, the study area is appropriate to investigate and answer our questions about fish metacommunities and beta diversity patterns in the riverine dendritic network.
Materials and methods
Study area and habitat description
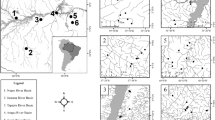
The study was conducted in the Almas River basin, upper section of the Tocantins-Araguaia River basin, Central Brazil (Fig. 1). Its climate is Aw according to Köppen–Geiger classification (Cardoso et al., 2014), characterized by a well-defined dry (April to September) and rainy (December to February) seasons (Nimer, 1989). The headwaters of the Almas River basin are located at the Serra dos Pirineus with an altitudinal gradient varying between 450 m and 1200 m above sea level (Barbalho et al., 2018); its streams are usually steep, presenting rock, sand, and foliage substrates and are often associated with waterfalls (Oliveira & Bispo, 2001). The region is historically threatened by human actions with direct impacts on freshwater habitats (Tejerina-Garro, 2008; Tejerina-Garro et al., 2017).
Spatial distribution of mainstem river (MS) and headwater stream sites sampled in the Almas River, Upper Tocantins-Araguaia River basin in Goiás State (gray area), Central Brazil. Each basin section is composed of one mainstem river site and a set of headwater streams. Watercourses of each basin section are identified by a code and different symbols (MS1, triangles; MS2, circles; MS3, squares; MS4, pentagons). MS1 the most upstream section sampled, MS4 the most downstream section
Headwater streams sampled had an average channel width and depth of 5.35 m (min. 1.05 m, max. 16.54 m) and 0.30 m (min. 0.09 m, max. 0.59 m), respectively; mainstem river sites had an average channel width and depth of 108 m (min. 36 m, max. 173 m) and 1.67 m (min. 1.21 m, max. 2.64 m), respectively. Headwater streams display ponds, pools, and rapids with a substrate predominantly composed of sand, gravel and rocks or a mix of clay, leaves, and branches; the banks displayed three vertical vegetal layers (herbs, shrubs, trees), but it was sometimes absent in some tributaries. In the mainstem, the presence of pools and rapids with a substrate composed mainly of rocks was observed; the riverbank was visually distinguished by two vertical layers (shrubs and trees) with the absence of these at the fourth site.
Sampling design and fish species sampling
Fish species were sampled in four mainstem river sites distributed along the river channel and in 29 headwater streams (Fig. 1). For each mainstem site, we sampled a set of headwater streams located upstream to it, describing a lateral gradient along four distinct sections of the river (Fig. 1). Given the connection among all headwater streams through the mainstem, the set formed by all mainstem sites and headwater streams also described a longitudinal gradient along the river basin. Therefore, this sampling design allowed us to evaluate beta diversity patterns in both lateral and longitudinal gradients and to compare these patterns among different lateral sections of the river basin. In this case, each lateral section was represented by one mainstem river site and some headwater streams sampled upstream to it.
Watercourses were sampled once between April and September 2016, during the dry season when fish sampling is more efficient (Pease et al., 2012). Sampling sites were defined according to accessibility and in the case of the mainstem river sites at least 30 km of hydrological distance was preserved between them so as to avoid spatial autocorrelation. At mainstem sites, species were sampled along a 1000 m stretch using four sets of gillnets with different mesh sizes (10 m × 1.5 m × 12–70 mm) and placed in pools at 5:00 PM and retrieved at 7:00 AM. In headwater streams, species were sampled using the electrofishing method (Mazzoni et al., 2000), with four persons traversing a 50 m stretch three times for two hours, in a downstream to upstream direction. Despite the use of different sampling methods in mainstem sites and headwater streams, both techniques are known to return a reliable estimation of fish species composition (Tejerina-Garro & Mérona, 2000; Medeiros et al., 2010). Therefore, to avoid any bias related to abundance data, we only used presence-absence data.
All fishes were fixed in formalin (10%), placed in identified plastic bags and stored in barrels containing formaldehyde. In the laboratory of the Centro de Biologia Aquática (CBA, Pontifícia Universidade Católica de Goiás), fishes were identified using taxonomical keys (Santos et al., 2004; Rocha, 2012; Costa e Silva, 2015; Fernando R. de Carvalho, personal communication) and stored in containers containing alcohol 70%.
Environmental data
Concomitantly to fish sampling, we measured nine environmental variables in the mainstem and headwater streams: channel depth (m) and width (m), water velocity (cm s−1), water turbidity (NTU), dissolved oxygen (mg l−1), pH, water conductivity (µS cm−1), water temperature (°C), and altitude (m). These parameters were measured in 10 transversal transects along the mainstem river stretch (1000 m) and five transversal transects along headwater stream (50 m stretch). All transects were equidistant from each other. For analyses, we used the mean value of each environmental variable considering all transects sampled.
Functional traits
We collected data for seven ecological and life-story functional traits: body mass, trophic guild, parental care, foraging method, water column position, foraging locality, and migratory habit (see traits categories and functional data in the supplementary file, respectively, Online Resources S1 and S2). Body mass was calculated as the average body mass for all specimens found of a given species. All other traits were obtained by consulting specialized literature (scientific papers, books, and doctoral thesis) and the world database for fishes (FishBase; Froese & Pauly, 2019; to access literature consulted see the Online Resource S4). Functional traits collected are commonly used in studies of fish functional diversity (Logez et al., 2013; Stuart-Smith et al., 2013; Keck et al., 2014; Carvalho & Tejerina-Garro, 2015; Gravel et al., 2016), reflecting habitat alteration (Frimpong & Angermeier, 2010), abiotic and biotic interactions (Winemiller, 2005), and ecosystem processes (Holmlund & Hammer, 1999). Fish body mass or size are related to food acquisition, mobility, and nutrient budget (Villéger et al., 2017); trophic level is associated to bioaccumulation, food web stability, resilience and dynamics (see Holmlund & Hammer, 1999); parental care is a response to species interactions such as predation (Winemiller, 2005) or represents different reproductive modes (Winemiller, 1989); benthic species redistribute sediments/nutrients; and migratory species transport and distribute nutrients (Holmlund & Hammer, 1999). Therefore, chosen traits are relevant to describe functional patterns of assemblages and ecosystem processes.
Whenever we could not define a given trait (absence of data or species identified only at the morphospecies level), we extrapolated information found for the genus or family level. This kind of extrapolation is possible because recent studies showed that aspects of the ecological niche of fishes are conserved, and species from the same clade maintain similar traits and responses (Roa-Fuentes et al., 2015; Pease et al., 2018; Rodrigues-Filho et al., 2018; Carvalho et al., 2020). If the extrapolation was not possible, functional trait was considered as not available (NA).
Data analyses
To test whether taxonomic turnover and functional nestedness are the main patterns of beta diversity along the lateral gradient, we calculated the turnover (βSIM) and nestedness (βNES) values for each lateral section of the river basin. For the calculation of the taxonomic turnover (βTSIM) and nestedness (βTNES), we decomposed a taxonomic matrix (species vs. sites) according to each lateral section and computed fish composition dissimilarities among sites via Sorensen dissimilarity index (Baselga et al., 2018). For the functional turnover (βFSIM) and nestedness (βFNES) calculation, we used a functional matrix (species vs. traits) to obtain a functional dendrogram for each lateral section of the river basin and we computed fish trait dissimilarities among sites via Sorensen dissimilarity index (Baselga et al., 2018). The functional dendrogram was obtained converting the functional matrix into a distance matrix using the Gower distance (Pavoine et al., 2009). Then, the functional distance matrix was converted into a functional dendrogram using the unweighted pair group method with arithmetic averages (UPGMA) as the clustering method. Both protocols allowed us to access the components of taxonomic and functional beta diversity and compare their trends among different sections of the lateral gradient. We used the function beta.multi to calculate βTSIM and βTNES (Baselga 2012) and phylo.beta.multi to calculate βFSIM and βFNES, both available in the betapart package (Baselga 2012) of the R Software (R Core Team, 2019). In the case of the phylo.beta.multii function, we substituted the phylogenetic tree required for calculation by the functional dendrogram since both structures have similar properties (Kembel et al., 2010; Pavoine & Bonsall, 2010). To obtain the dendrogram we used vegan (Oksanen et al., 2019), ade4 (Dray & Dufour, 2007) and picante (Kembel et al., 2010) packages of the R Software.
The same set of procedures mentioned above was used to evaluate whether taxonomic turnover and functional nestedness are the main patterns explaining fish beta diversity along the longitudinal gradient. However, the taxonomic matrix included all watercourses sampled (mainstem sites and headwater streams) within the river basin, and the functional matrix included all species found and their traits in order to represent the variation of all species and traits along the longitudinal gradient.
To evaluate how the increase of the spatial extent influences on taxonomic and functional beta diversity patterns along the longitudinal gradient, we constructed four different scenarios. In the first one, we calculated values of taxonomic and functional beta diversity (including turnover and nestedness components) for the most upstream lateral section of the river basin (MS1, Fig. 1). In the second scenario, we added to the design the section MS2 (lateral section located downstream to MS1) and we recalculated taxonomic and functional beta diversity values. The third and fourth scenarios included the addition of sections MS3 and MS4 to the design, respectively, followed by the calculation of taxonomic and functional diversity values. Then, we compared the variation of beta diversity when the spatial extent was sequentially increased.
To disentangle environmental and spatial effects on taxonomic beta diversity and its components, we used the taxonomic matrix with all sites and Sorensen dissimilarity index to extract three distance matrices describing: the overall beta diversity (βTSOR), beta turnover (βTSIM) and beta nestedness (βTNES). Each matrix was submitted to a Principal Coordinate Analysis (PCoA) to retain the two first axes (eigenvectors) representing most of the covariability observed among data. Then, PCoA axes were tested against standardized values of environmental variables to find those correlated to beta diversity, and the significance of the squared correlation coefficient (R2) was tested with a randomized procedure (9,999 permutations) using the envfit analysis available in the R software. The environmental variables with significant values (P < 0.05) were retained and tested for multicollinearity with the variance inflation factor (VIF). If an environmental variable had a VIF value > 3, it was removed from the analysis (Zuur et al., 2010; Dorman et al., 2012). After that, PCoA axes were tested against spatial predictors to find those correlated to beta diversity. The correlation was tested through a redundancy analysis (RDA) and randomized procedures (9,999 permutations; Legendre & Legendre, 2012; Oksanen et al., 2019), and only significant spatial axes were retained for analyses (P < 0.05). To obtain the spatial predictors, we used the Asymmetric Eigenvectors Maps (AEM) considering flow direction to create a sites-by-edges matrix and represent possible patterns of dispersal among sampling sites (for more details see Blanchet et al., 2008). A sites-by-edges matrix and the spatial coordinates of watercourses were used to construct a connection diagram, where sites are linked according to asymmetric processes. We used the adespatial package for multivariate spatial analysis (Dray et al., 2019) available in the R software (R Core Team, 2019) to construct AEMs. Finally, the environmental and spatial predictors retained were used in a RDA and variation partitioning analysis against PCoA axes (Borcard et al., 1992; Peres-Neto & Legendre 2010) with rda and varpart functions available in the vegan package of the R software (R Core Team, 2019). The distance matrices describing beta diversity were obtained using the beta.pair function available in the betapart package of the R Software.
To disentangle environmental and spatial influence on functional beta diversity and its components, we used the taxonomic matrix with all sites and the functional matrix with all traits to extract distance matrices describing: the overall functional beta diversity (βFSOR), functional beta turnover (βFSIM), and functional beta nestedness (βFNES). After that, the same steps and procedures used for taxonomic beta diversity were performed, excepting for the use of the function phylo.beta.pair (betapart package of the R Software) to extract functional distance matrices. Additionally, we compared environmental variables values (log10) among basin sections (MS1 to MS4) using an ANOVA for unequal variances.
Results
We sampled 82 fish species belonging to six orders and 26 families (Table 1). In the mainstem, we found 25 fish species from three orders and 13 families, whereas in headwater streams we found 67 species from six orders and 21 families (Table 1). Characiformes (36) and Siluriformes (32) were the most speciose orders and Characidae (17), Loricariidae (16), and Cichlidae (7) were the most speciose families (Table 1).
Taxonomic and functional beta turnover were the main patterns observed along lateral gradients of the river basin (Table 2a). These patterns were consistent for all four sections despite a functional nestedness increase (Table 2a), indicating the occurrence of species and functional trait replacement among fish assemblages along the mainstem/headwater gradient. Taxonomic and functional beta turnover were also the main patterns along the longitudinal gradient of the river basin (Table 2b), suggesting that species and functional trait replacement occur in the upstream/downstream gradient at this intermediate spatial scale. The increase in spatial extent led to an increase in turnover component along the longitudinal gradient for both taxonomic and functional diversity, despite the low increment when the last sections of the river basin (MS3 and MS4) were added (Table 2b).
The ANOVA indicated that environmental variables values differed among basin sections (P < 0.05 in all cases; Online Resource S3). The evaluation of fish species distribution and environmental variables relationship via envifit analysis and permutation procedures for the taxonomic and functional beta fish diversity revealed, respectively, that the altitude, channel depth and width, water velocity, and water temperature were significantly correlated to βTSOR and βTSIM (P < 0.006 for all cases) and only conductivity to βTNES (P = 0.03), whereas the altitude, channel width, dissolved oxygen, water velocity, and water temperature were significantly correlated to βFSOR and βFSIM (P < 0.006 for all cases) and only conductivity to βFNES (P = 0.03). In all cases, the multicollinearity tests among environmental variables selected by envifit analysis indicated a VIF < 2 for all variables, and all of them were retained for RDA and variation partitioning models. AEM models generated 14 spatial predictors and, in all cases, RDA models indicated spatial predictors 1 and 12 as important for both taxonomic and functional beta fish diversity and its components (P < 0.05 for all cases). Therefore, both axes were used in RDA and variation partitioning models.
The RDA models showed that space and spatially structured environments affected taxonomic (βTSOR and βTSIM) and functional (βFSOR and βFSIM) beta diversity patterns (Table 3; Variation partitioning). Even combined, their contribution in structuring beta diversity patterns was low and most variation was explained by residuals (Table 3).
Discussion
Studies addressing the influence of the riverine dendritic network on metacommunities’ organization form a baseline for fish ecology and conservation (Altermatt, 2013), but their number in tropical regions remains low (Vitorino Jr. et al., 2016). Our findings revealed that taxonomic and functional turnover are the main components explaining beta diversity patterns of fish metacommunities along the lateral gradient (mainstem/headwater) as it appears to be related to species sorting by environmental filtering and species dispersal limitation in headwater habitats. Species sorting assumes that environmental differences among patches will drive the structure of local communities in the river network (Altermatt, 2012; Brown et al., 2016), a condition that can be observed among headwater streams (Tejerina-Garro et al., 2005; Súarez & Petrere Jr., 2006) and it is frequently associated with species and functional trait filtering in headwaters (Poff, 1997; Altermatt, 2013; Carvalho & Tejerina-Garro, 2015; Borges et al., 2020). Environmental influence was already reported as an important driver of species turnover and functional dissimilarities in fish assemblages of tributaries (Medina Torres & Higgins, 2016; Vitorino Jr. et al., 2016; Peláez et al., 2017; Zbinden & Matthews, 2017; Huang et al., 2019; Peláez & Pavanelli, 2019). Our results reinforce these findings, showing that taxonomic and functional turnover towards headwater habitats is a consistent trend for different lateral sections of a river basin. However, it is important to point out that the study region is characterized by the presence of waterfalls, steep slopes, and an expressive altitudinal gradient (Oliveira & Bispo, 2001), factors that usually constrain species dispersal. Although dispersal limitation is expected to generate a nestedness pattern (Gianuca et al., 2017; Peláez et al., 2017; Peláez & Pavanelli, 2019), if the environment leads to a unique fish assemblages formation because different filters act on headwater streams, then species dispersal limitation generated by natural barriers along the dendritic network may help to maintain species and traits replacement among streams (Finn et al., 2011; Carrara et al., 2012). In this case, both environmental filtering and species dispersal limitation would be responsible for generating taxonomic and functional turnover trends in the lateral gradient of the Almas River basin network.
The spatial congruence between taxonomic and functional beta diversity patterns considering the lateral gradient suggests that basin’s regions displaying high taxonomic turnover will present high functional turnover. Although the spatial congruence among different diversity facets was already observed in fish assemblage’s studies (Strecker et al., 2011; Pool et al., 2014), we consider our result carefully. We found a higher contribution of the nestedness pattern in structuring functional beta diversity than taxonomic beta diversity; other studies conducted in the Neotropical region have already found the turnover component associated with taxonomic diversity and nestedness to functional diversity in tributaries (Vitorino Jr. et al., 2016; Peláez & Pavanelli, 2019). Thus, if environmental filtering selects different species with similar fish functional traits in headwaters as observed by Carvalho & Tejerina-Garro (2015), the congruence between taxonomic and functional beta diversity may change from place to place according to the strength of filters acting on headwater habitats. As stated before, in the case of the study region the specific combination of environmental filtering and species dispersal limitation mediated by natural barriers seems to generate taxonomic and functional turnover among headwater streams.
We found that taxonomic and functional turnover are the main patterns explaining the variation in fish beta diversity along the longitudinal gradient, and both patterns gradually increased with increasing spatial extent. Increasing the extension of the overall area studied possibly implies in higher levels of connectivity and environmental heterogeneity between mainstem sites and headwater streams (Altermatt, 2013; Tonkin et al., 2018), favoring species dispersal through the river network and leading to a high species turnover along this gradient (Heino et al., 2015). Additionally, given that species may move between different habitats (mainstem and headwaters) with different local environmental conditions, it is possible that filters select species with different functional traits, explaining the functional turnover pattern. The results of the variation partitioning analyses seem to reinforce such perspective because the space and spatially structured environments were important drivers of taxonomic and functional beta diversity. In our models, space represents the spatial distance among assemblages considering the river flow, and its influence possibly indicates the occurrence of dispersal-based process in structuring fish assemblages, as already reported by Carvalho & Tejerina-Garro (2015) for fish dispersal between main channel sites and headwater streams. The spatially structured environment indicates that spatial distance is important since nearby sites are more similar to each other due to structured environmental conditions. This situation is responsible for driving composition and functional dissimilarities of fish assemblages between main channel and headwater habitats in Neotropical regions (Carvalho & Tejerina-Garro, 2015). Such results suggest that both niche- and dispersal-based processes are important in structuring taxonomic and functional beta fish diversity along the longitudinal gradient considering an intermediate spatial scale. However, it is important to notice that the observed contribution of space and spatially structured environments in explaining beta diversity patterns was low and most of the variation was related to unknown factors. One explanation is associated with the spatial scale dependency, because the strength of spatial components in structuring diversity patterns can be higher at broader spatial scales (Gilbert & Lechowicz, 2004; Diniz-Filho et al., 2012; Heino et al., 2015), including beta diversity patterns of fish assemblages (Leprieur et al., 2011). Another explanation is related to the number of environmental variables considered in this study, as they represent only a fraction of the variables that can structure fish assemblages in mainstem and headwater streams (Poff, 1997). Therefore, increasing the number of environmental variables may reveal stronger associations between beta diversity patterns and spatially structured environments at this spatial scale.
The degree of connectivity and dispersal among sites regulates how diversity varies among assemblages in riverine systems (Altermatt, 2013; Tonkin et al., 2018), and conservation strategies must go beyond traditional approaches that focus on local scales to incorporate the metacommunity perspective. The predominance of the turnover component in explaining taxonomic and functional diversity along lateral and longitudinal gradients, as we observed, has important implications for the conservation of Neotropical riverine systems. It indicates that (1) a successful conservation strategy should consider the protection of multiple and contiguous regions or sites to preserve the regional variation of diversity (Vitorino Jr. et al., 2016; Gianuca et al., 2017); (2) anthropogenic impacts that affect mainstem and headwater stream sites locally, such as river impoundments along the Tocantins River (Lima et al. 2016), may jeopardize the preservation of diversity in a regional scale; and (3) similar conservation strategies may be applied to preserve both taxonomic and functional beta diversity in a region. Therefore, our study not only shows how taxonomic and functional diversity are structured along mainstem/headwater (lateral) and upstream/downstream (longitudinal) gradients in Neotropical systems, but it also provides an initial baseline for future conservation approaches in these regions addressing the dendritic nature of riverine networks.
References
Altermatt, F., 2012. Metacommunity dynamics. In Gibson, D. (ed.), Oxford Bibliographies Online: Ecology. Oxford University Press, New York.
Altermatt, F., 2013. Diversity in riverine metacommunities: a network perspective. Aquatic Ecology 47: 365–377.
Altermatt, F., M. Seymour & N. Martinez, 2013. River network properties shape a-diversity and community similarity patterns of aquatic insect communities across major drainage basins. Journal of Biogeography 40: 2249–2260.
Azevedo, M. C. C., F. G. Araújo, A. G. Cruz-Filho, A. L. M. Pessanha, M. A. Silva & A. P. P. Guedes, 2007. Demersal fishes in a tropical bay in southeastern Brazil: partitioning the spatial, temporal and environmental components of ecological variation. Estuarine, Coastal and Shelf Science 75: 468–480.
Barbalho, M. G. S., A. C. Leal, J. O. R. Nunes, C. G. Moraes & J. C. Peixoto, 2018. Unidades da paisagem da bacia do Rio das Almas, microrregião de Ceres/GO. Planeta Amazônia 10: 153–166.
Baselga, A., 2010. Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography 19: 134–143.
Baselga, A., 2012. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Global Ecology and Biogeography 21: 1223.
Baselga, A., D. Orme, S. Villéger, J. De Bortoli, & F. Leprieur, 2018. Betapart: Partitioning beta diversity into turnover and nestedness components. R package version 1.3. Available at: https://cran.r-project.org/web/packages/betapart/.
Bertolo, A., F. G. Blanchet, P. Magnan, P. Brodeur, M. Mingelbier & P. Legendre, 2012. Inferring processes from spatial patterns: the role of directional and non-directional forces in shaping fish larvae distribution in a freshwater lake system. PLoS ONE 7: e50239.
Blanchet, F. G., P. Legendre & D. Borcard, 2008. Modelling directional spatial processes in ecological data. Ecological Modelling 215: 325–336.
Blanchet, F. G., R. Maranger, D. Monti & P. Pepin, 2011. Modelling the effect of directional spatial ecological processes at different scales. Oecologia 166: 357–368.
Borcard, D., P. Legendre & P. Drapeau, 1992. Partialling out the spatial component of ecological variation. Ecology 73: 1045–1055. https://doi.org/10.2307/1940179.
Borges, P. P., M. S. Dias, F. R. Carvalho, L. Casatti, P. S. Pompeu, M. Cetra, F. L. Tejerina-Garro, Y. R. Súarez, J. C. Nabout & F. B. Teresa, 2020. Stream fish metacommunity organisation across a Neotropical ecoregion: the role of environment, anthropogenic impact and dispersal-based processes. PLoS ONE 15: e0233733.
Brown, B. L. & C. M. Swan, 2010. Dendritic network structure constrains metacommunity properties in riverine ecosystems. Journal of Animal Ecology 79: 571–580.
Brown, B. L., E. R. Sokol, J. Skelton & B. Tornwall, 2016. Making sense of metacommunities: dispelling the mythology of a metacommunity typology. Oecologia 183: 643–652.
Cardoso, M. R. D., F. F. N. Marcuzzo & J. R. Barros, 2014. Classificação Climática de Köppen-Geiger para o Estado de Goiás e o Distrito Federal. Acta Geographica 8(16): 40–55.
Carrara, F., F. Altermatt, I. Rodriguez-Iturbe & A. Rinaldo, 2012. Dendritic connectivity controls biodiversity patterns in experimental metacommunities. Proceedings of the National Academy of Sciences of the United States of America 109: 5761–5766.
Carvalho, R. A. & F. L. Tejerina-Garro, 2015. Environmental and spatial processes: what controls the functional structure of fish assemblages in tropical rivers and headwater streams? Ecology of Freshwater Fish 24: 317–328.
Carvalho, R. A., H. T. Santana, & F. L. Tejerina-Garro, 2020. Environmental influence on higher fish taxonomic levels: relationships in tropical headwater streams. Studies on Neotropical Fauna and Environment.
Cetra, M., M. Petrere Jr. & W. Barrela, 2017. Relative influences of environmental and spatial factors on stream fish assemblages in Brazilian Atlantic rainforest. Fisheries Management and Ecology 24: 139–145.
Costa e Silva, T., 2015. Revisão taxonômica das espécies do gênero Pimelodus Lacépède, 1803 (Siluriformes: Pimelodidae) na drenagem do rio Tocantins, Brasil. Programa de Pós-Graduação em Ecologia de Ecótonos. Universidade Federal do Tocantins.
Diniz-Filho, J. A. F., T. Siqueira, A. A. Padial, T. F. Rangel, V. L. Landeiro & L. M. Bini, 2012. Spatial autocorrelation analysis allows disentangling the balance between neutral and niche processes in metacommunities. Oikos 121: 201–210.
Dorman, D. F., J. Elith, S. Bacher, C. Buchmann, G. Carl, G. Carré, et al., 2012. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36: 27–46.
Dray, S. & A. Dufour, 2007. The ade4 Package: implementing the Duality Diagram for Ecologists. Journal of Statistical Software 22(4): 1–20.
Dray, S., D. Bauman, G. Blanchet, D. Borcard, S. Clappe, G. Guenard, et al., 2019. adespatial: Multivariate Multiscale Spatial Analysis. R package version 0.3-7. https://CRAN.R-project.org/package=adespatial.
Erős, T., 2017. Scaling fish metacommunities in stream networks: synthesis and future research avenues. Community Ecology 18: 72–86.
Finn, D. S., N. Bonada, C. Múrria & J. M. Hughes, 2011. Small but mighty: headwaters are vital to stream network biodiversity at two levels of organization. Journal of the North American Benthological Society 30: 963–980.
Frimpong, E. A. & P. L. Angermeier, 2010. Trait-based approaches in the analysis of stream fish communities. American Fisheries Society Symposium 73(109–136): 2010.
Froese, R., & D. Pauly, 2019. FishBase. World Wide Web electronic publication. Available in: www.fishbase.org, version (08/2019).
Gianuca, A. T., S. A. J. Declerck, P. Lemmens & L. Meester, 2017. Effects of dispersal and environmental heterogeneity on the replacement and nestedness components of β-diversity. Ecology 98(2): 525–533.
Gilbert, B. & M. J. Lechowicz, 2004. Neutrality, niches, and dispersal in a temperate forest understory. Proceedings of the National Academy of Sciences of the United States of America 101: 7651–7656.
Grant, E. H. C., W. H. Lowe & W. F. Fagan, 2007. Living in the branches: population dynamics and ecological processes in dendritic networks. Ecology Letters 10: 165–175.
Gravel, D., C. Albouy & W. Thuiller, 2016. The meaning of functional trait composition of food webs for ecosystem function. Philosophical Transactions of Royal Society B 371: 20150268.
Grenouillet, G., D. Pont & C. Herissé, 2004. Within-basin fish assemblage structure: the relative influence of habitat versus stream spatial position on local species richness. Canadian Journal of Fisheries and Aquatic Sciences 61: 93–102.
Heino, J., A. S. Melo, T. Siqueira, J. Soininen, S. Valanko & L. M. Bini, 2015. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwater Biology 60: 845–869.
Henriques-Silva, R., M. Logez, N. Reynaud, P. A. Tedesco, S. Brosse, S. R. Januchowski-Hartley, T. Oberdorff & C. Argillier, 2019. A comprehensive examination of the network position hypothesis across multiple river metacommunities. Ecography 42: 284–294.
Holmlund, C. M. & M. Hammer, 1999. Ecosystem services generated by fish populations. Ecological Economics 29: 253–268.
Huang, L., J. Huang, Z. Wu, Y. Mo, Q. Zou, E. Jeppesen & N. Wu, 2019. Beta diversity partitioning and drivers of variations in fish assemblages in a headwater stream: Lijiang River, China. Water 11: 1–16.
Jackson, D. A., P. R. Peres-Neto & J. D. Olden, 2001. What controls who is where in freshwater fish communities—the roles of biotic, abiotic, and spatial factors. Canadian Journal of Fisheries and Aquatic Sciences 58: 157–170.
Keck, B. P., Z. H. Marion, D. J. Martin, J. C. Kaufman, C. P. Harden, J. S. Schwartz & R. J. Strange, 2014. Fish functional traits correlated with environmental variables in a temperate biodiversity hotspot. PLoS ONE 9: e93237.
Kembel, S. W., P. D. Cowan, M. R. Helmus, W. K. Cornwell, H. Morlon, D. D. Ackerly, et al., 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26: 1463–1464.
Legendre, P., & L. Legendre, 2012. Numerical Ecology. 3rd English ed. Elsevier.
Leibold, M. A., M. Holyoak, N. Mouquet, P. Amarasekare, J. M. Chase, M. F. Hoopes, R. D. Holt, J. B. Shurin, R. Law, D. Tilman, M. Loreau & A. Gonzalez, 2004. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters 7: 601–613.
Leprieur, F., P. A. Tedesco, B. Hugueny, O. Beauchard, H. H. Dürr, S. Brosse & T. Oberdorff, 2011. Partitioning global patterns of freshwater fish beta diversity reveals contrasting signatures of past climate changes. Ecology Letters 14: 325–334.
Lima, A. C., C. S. Agostinho, D. Sayanda, F. M. Pelicice, A. M. V. M. Soares & K. A. Monaghan, 2016. The rise and fall of fish diversity in a neotropical river after impoundment. Hydrobiologia 763: 207–221.
Logez, M., P. Bady, A. Melcher & D. Pont, 2013. A continental-scale analysis of fish assemblage functional structure in European rivers. Ecography 36: 080–091.
Logue, J. B., N. Mouquet, H. Peter, H. Hillebrand, P. Declerck, A. Flohre, S. Gantner, N. Gülzow, P. Hörtnagl, S. Meier & B. Pecceu, 2011. Empirical approaches to metacommunities: a review and comparison with theory. Trends in Ecology and Evolution 26: 482–491.
Lynch, H. J., E. H. C. Grant, R. Muneepeerakul, M. Arunachalam, I. Rodriguez-Iturbe & W. F. Fagan, 2011. How restructuring river connectivity changes freshwater fish biodiversity and biogeography. Water Resources Research 47: W05531.
Mazzoni, R., N. Fenerich-Verani & E. P. Caramaschi, 2000. Electrofishing a sampling technique for coastal stream fish populations and communities in the southeast of Brazil. Revista Brasileira de Biologia 60: 205–216.
Medeiros, E. S. F., M. J. Silva, B. R. S. Figueiredo, T. P. A. Ramos & R. T. C. Ramos, 2010. Effects of fishing technique on assessing species composition in aquatic systems in semi-arid Brazil. Brazilian Journal of Biology 70: 255–262.
Medina Torres, K. M. M. & C. L. Higgins, 2016. Taxonomic and functional organization in metacommunity structure of stream-fish assemblages among and within river basin in Texas. Aquatic Ecology 50: 247–259.
Meyer, J. L., D. L. Strayer, J. B. Wallace, S. L. Eggert, G. S. Helfman & N. E. Leonard, 2007. The contribution of headwater streams to biodiversity in river networks. Journal of the American Water Resources Association 43: 86–103.
Nimer, E., 1989. Climatologia do Brasil. IBGE, Rio de Janeiro: 421.
Massicotte, P., A. Bertolo, P. Brodeur, C. Hudon, M. Mingelbier & P. Magnan, 2015. Influence of the aquatic vegetation landscape on larval fish abundance. Journal of Great Lakes Research 41: 873–880.
Meffe, G. K. & W. L. Minkley, 1987. Persistence and stability of fish and invertebrate assemblages in a repeatedly disturbed Sonoran Desert stream. American Midland Naturalist 117: 177–191.
Muneepeerakul, R., E. Bertuzzo, H. J. Lynch, W. F. Fagan, A. Rinaldo & I. Rodriguez-Iturbe, 2008. Neutral metacommunity models predict fish diversity patterns in Mississippi-Missouri basin. Nature 453: 220–222.
Münkemüller, T., F. Bello, C. N. Meynard, D. Gravel, S. Lavergne, D. Mouillot, N. Mouquet & W. Thuiller, 2012. From diversity indices to community assembly processes: a test with simulated data. Ecography 35: 468–480.
Nakagawa, H., 2014. Contribution of environmental and spatial factors to the structure of stream fish assemblages at different spatial scales. Ecology of Freshwater Fish 23: 208–223.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, et al., 2019. Package “vegan”. R package version 2.5-6. Available at: https://cran.r-project.org/web/packages/vegan/.
Oliveira, L. G. & P. C. Bispo, 2001. Ecologia de comunidades das larvas de Trichoptera Kirby (Insecta) em dois córregos de primeira ordem da Serra dos Pirineus, Pirenópolis, Goiás, Brasil. Revista Brasileira de Zoologia 18: 1245–1252.
Pavoine, S. & M. B. Bonsall, 2010. Measuring biodiversity to explain community assembly: a unified approach. Biological Reviews 86: 792–812.
Pavoine, S., J. Vallet, A.-B. Dufour, S. Gachet & H. Daniel, 2009. On the challenge of treating various types of variables: application for improving the measurement of functional diversity. Oikos 118: 391–402.
Pease, A. A., A. A. González-Díaz, R. Rodiles-Hernández & K. O. Winemiller, 2012. Functional diversity and trait–environment relationships of stream fish assemblages in a large tropical catchment. Freshwater Biology 5: 1060–1075.
Pease, A. A., M. Mendoza-Carranza & K. O. Winemiller, 2018. Feeding ecology and ecomorphology of cichlid assemblages in a large Mesoamerican river delta. Environmental Biology of Fishes 101: 867–879.
Peláez, O. E., F. M. Azevedo & C. S. Pavanelli, 2017. Environmental heterogeneity explains species turnover but not nestedness in fish assemblages of a Neotropical basin. Acta Limnologica Brasiliensia 29: e117.
Peláez, O. E. & C. S. Pavanelli, 2019. Environmental heterogeneity and dispersal limitation explain different aspects of β-diversity in Neotropical fish assemblages. Freshwater Biology 64: 497–505.
Peres-Neto, P. R. & P. Legendre, 2010. Estimating and controlling for spatial structure in the study of ecological communities. Global Ecology and Biogeography 19: 174–184.
Poff, N. L., 1997. Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. Journal of the North American Benthological Society 16: 391–409.
Pool, T. K., G. Grenouillet & S. Villéger, 2014. Species contribute differently to the taxonomic, functional, and phylogenetic alpha and beta diversity of freshwater fish communities. Diversity and distributions 20: 1235–1244.
R Core Team. 2019. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. URL https://www.R-project.org/.
Roa-Fuentes, C. A., L. Casatti & R. M. Romero, 2015. Phylogenetic signal and major ecological shifts in the ecomorphological structure of stream fish in two river basins in Brazil. Neotropical Ichthyology 13: 165–178.
Rocha, M. S., 2012. Sistemática da família Pimelodidae Swainson, 1838 (Teleostei: Siluriformes). Instituto Nacional de Pesquisas da Amazônia, INPA.
Rodrigues-Filho, C. A. S., R. C. Gurgel-Lourenço, L. A. V. Bezerra, E. F. Oliveira, R. P. Leitão, D. S. Garcez & J. L. Sánchez-Botero, 2018. How are local fish communities structured in Brazilian semiarid headwater streams? Hydrobiologia 819: 93–108.
dos Santos, G. M., B. de Mérona, A. A. Juras & M. Jégu, 2004. Peixes do baixo rio Tocantins: 20 anos depois da usina hidrelétrica Tucuruí. Eletronorte, Brasília, DF.
Soininen, J., 2016. Spatial structure in ecological communities—a quantitative analysis. Oikos 125: 160–166.
Sharma, S., P. Legendre, M. Cáceres & D. Boisclair, 2011. The role of environmental and spatial processes in structuring native and non-native fish communities across thousands of lakes. Ecography 34: 762–771.
Strecker, A. L., J. D. Olden, J. B. Whittier & C. P. Paukert, 2011. Defining conservation priorities for freshwater fishes according to taxonomic, functional, and phylogenetic diversity. Ecological Applications 21: 3002–3013.
Stuart-Smith, R., A. Bates, J. Lefcheck, et al., 2013. Integrating abundance and functional traits reveals new global hotspots of fish diversity. Nature 501: 539–542.
Súarez, Y. R. & M. Petrere Jr., 2006. Gradientes de diversidade nas comunidades de peixes da bacia do rio Iguatemi, Mato Grosso do Sul, Brasil. Iheringia 96: 197–204.
Tejerina-Garro, F. L., 2008. Biodiversidade e impactos ambientais no estado de Goiás. In Rocha, C., F. L. Tejerina-Garro & J. P. Pietrafesa (eds), Cerrado, Sociedade e Meio Ambiente: Desenvolvimento Sustentável em Goiás. Editora da Universidade Católica de Goiás, Goiânia, GO: 15–47.
Tejerina-Garro, F. L. & B. Mérona, 2000. Gill net sampling standardisation in large rivers of French Guiana (South America). Bulletin Français de La Pêche et de La Pisciculture 357(358): 227–240.
Tejerina-Garro, F. L., M. Maldonado, C. Ibañez, D. Pont, N. Roset & T. Oberdorff, 2005. Effects of natural and anthropogenic environmental changes on riverine fish assemblages: a framework for ecological assessment of rivers. Brazilian Archives of Biology and Technology 48: 91–108.
Tejerina-Garro, F. L., R. A. Carvalho & F. B. Teresa, 2017. A biodiversidade e a conservação da ictiofauna do alto da bacia do rio Paraná no estado de Goiás, Brasil Central. In Hannibal, W., R. F. Rossi, I. L. Morais & L. H. M. Teixeira (eds), Biodiversidade, Manejo e Conservação do Sul de Goiás. Paco Editorial, Jundiaí, SP: 93–126.
Tonkin, J. D., F. Altermatt, D. S. Finn, J. Heino, J. D. Olden, S. U. Pauls & D. A. Lytle, 2018. The role of dispersal in river network metacommunities: patterns, processes, and pathways. Freshwater Biology 63: 141–163.
Villéger, S., S. Brosse, M. Mouchet, D. Mouillot & M. J. Vanni, 2017. Functional ecology of fish: current approaches and future challenges. Aquatic Sciences 79: 783–801.
Vitorino Jr., O. B., R. Fernandes, C. S. Agostinho & F. M. Pelicice, 2016. Riverine networks constrain β-diversity patterns among fish assemblages in a large Neotropical river. Freshwater Biology 61: 1733–1745.
Wiens, J. A., 1989. Spatial scaling in ecology. Functional Ecology 3: 385–397.
Winemiller, K. O., 1989. Patterns of variation in life history among South American fishes in seasonal environments. Oecologia 81: 225–241.
Winemiller, K. O., 2005. Life history strategies, population regulation, and implications for fisheries management. Canadian Journal of Fisheries and Aquatic Sciences 62: 872–885.
Zbinden, Z. D. & W. J. Matthews, 2017. Beta diversity of stream fish assemblages: partitioning variation between spatial and environmental factors. Freshwater Biology 62: 1460–1471.
Zuur, A. F., E. N. Ieno & C. S. Elphick, 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1: 3–14.
Acknowledgements
We thank FAPEG for the financial support to the Project #14597. We thank Waldeir Francisco de Menezes and Nicelly Braudes de Araújo for their help with data collection in field and the extensive work at the laboratory. We also thank reviewers for comments that helped to improve the manuscript. FBT is supported by CNPq (306912/2018-0).
Author information
Authors and Affiliations
Contributions
RAC and FLTG were responsible for study design and fish species sampling. RAC, FLTG, and FBT were responsible for statistical analyses and manuscript writing. All authors read the manuscript and gave their final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Fernando M. Pelicice
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carvalho, R.A., Teresa, F.B. & Tejerina-Garro, F.L. The effect of riverine networks on fish β-diversity patterns in a Neotropical system. Hydrobiologia 848, 515–529 (2021). https://doi.org/10.1007/s10750-020-04459-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04459-9