Abstract
Fish diversity is generally high in drainage networks in perennial systems. On the other hand, little is known about the importance of drainage networks in semiarid regions. The scant attention given to rivers in semiarid regions so far may in part be explained by the high intermittency and low ramification of these drainage networks and their incorrect classification as systems of low diversity and productivity. In this study, we investigated the importance of the drainage system of an intermittent river system in Brazil with regard to taxonomic and functional β-diversity. To do so, we evaluated the importance of both tributaries (branches) (12 sampling points) and the main stem (7 sampling points).We initially modeled the spatial structure within the branches and the main stem using asymmetric eigenvector maps. We then partitioned β-diversity values between local (biotic and abiotic) and spatial factors (positive spatial autocorrelation). The higher β-diversity values observed for branches were explained mainly by predation and environmental selection. On the other hand, in the main stem, β-diversity was best explained by a combination of environmental conditions and spatial factors. In general, local factors were more important in the functional approach than in the taxonomic approach, supporting their joint use in metacommunity studies. Our results suggest that fish metacommunity structure in branches may be responsible for maintaining regional patterns of β-diversity in semiarid river systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The structuring of local communities is commonly assumed to be the result of the joint action of dispersal and niche-based processes (metacommunity dynamics) (Hubbell 2001; Leibold et al. 2004). Spatial distance and local environmental conditions determine which species will colonize and eventually settle in local communities (Heino et al. 2015). The importance of these processes depend on the aspect (taxonomic or functional) of the biodiversity investigated (Tolonen et al. 2018). Thus, while the taxonomic aspect is usually dependent on spatial distance and stochasticity, the functional aspect is determined by the biotic interactions between species and the environment (Spasojevic et al. 2014; Gianuca et al. 2017; Rodrigues-Filho et al. 2018a). This is the framework adopted in many studies contrasting spatial distance with environmental conditions (e.g., habitats) (Logue et al. 2011). Such studies assume that local processes are represented by environmental conditions only, although biotic interactions (e.g., competition and predation) may also have a direct influence on local fish assemblage and functional attributes (Jackson et al. 2001; Chase 2003; Spasojevic et al. 2014; Giam and Olden 2016). For example, predators may serve as strong biotic filters by consuming preferential prey (species with specific functional attributes), thereby directly impacting the local assemblage (Livingston et al. 2017). In other words, information on predation patterns at the metacommunity level can help understand the mechanisms responsible for local biodiversity and assemblage, from both the taxonomic and functional perspective.
The dynamics of metacommunities depends on taxonomic group (Schmera et al. 2017) and ecosystem type (Heino et al. 2015), but must also take into account the dynamics of adjacent ecosystems which directly influence in the species’ dispersal rates and interaction with the environment. Local processes involving environmental and biotic factors presuppose the dispersal of individuals and the existence of local communities. In other words, the spatial configuration of an ecosystem can directly affect community structure patterns by limiting or facilitating dispersal among communities (Heino et al. 2015). In intermittent aquatic ecosystems, for example, the frequent isolation of dendritic systems generates different levels of connectivity between the tributaries (branches) and the main channel (main stem), ranging from completely isolated to sporadically connected communities (Datry et al. 2016a). To understand how the complex dynamics of intermittent rivers affects the organization of local communities, changes in local biodiversity need to be broken down (β-diversity; Socolar et al. 2016). Defined as dissimilarity in species composition (Baselga et al. 2012) or functional attributes (Villéger et al. 2013) between pairs of local communities, β-diversity may be divided into turnover and nestedness. Identifying which of these two components is more important to β-diversity helps understand the mechanisms responsible for local biodiversity. For example, a high turnover rate is evidence of large differences in composition or functional attributes between local communities and suggests that more locations are required to preserve regional biodiversity. On the other hand, a high nestedness value indicates that communities with poor species diversity and a narrow range of functional attributes are subsets of richer communities; thus, few locations are required to preserve regional biodiversity (Socolar et al. 2016).
In branches of intermittent rivers, disconnection events compromise the dispersal of individuals, increasing the compositional dissimilarity between local communities (Datry et al. 2016b). Once species have become established, adverse environmental conditions and biotic interactions (e.g., competition and predation) further shape the local assemblage (Boulton 2014; Larned et al. 2010; Altermatt et al. 2011). This combination of factors tends to increase the complexity of spatial organization and goes a long way to explain the dissimilarity (high β-diversity) observed between assemblages in such environments (Datry et al. 2016b). β-diversity patterns are mainly explained by turnover associated with isolation and environmental and biotic filters (Brown and Swan 2010; Finn et al. 2011). Conversely, the unidirectional flow of the main stem favors dispersal among local communities (mass effect mechanism), allowing species to maintain local populations under adequate conditions (Logue et al. 2011; Vitorino-Júnior et al. 2016). This mass effect mechanism tends to homogenize neighboring assemblages, regardless of local conditions, resulting in lower β-diversity and turnover compared to branches (Strecker and Brittain 2017). Therefore, in intermittent drainage systems the branches and the main stem often display different β-diversity patterns.
Functional metacommunity organization is poorly understood and difficult to predict in systems with intermittent dynamics (Datry et al. 2015). However, some expectations can be made based on the hydrological dynamics peculiar to intermittent rivers. For example, the isolation of branches tends to favor small species with high fertility rates (Winemiller and Rose 1992), a functional attribute which confers greater resilience to ephemeral water bodies (Townsend and Hildrew 1994). Nevertheless, a variety of morphotypes may be expected in branches due to differential habitat exploitation. Isolation can also lead to more intense biotic interactions, favoring species with efficient physical protection (e.g., barbs) (Villéger et al. 2017). Based on this set of factors, one would expect considerable functional dissimilarities between local communities in different branches. However, while environmental selection of species-specific attributes associated with ephemerality and habitat exploitation tends to intensify the functional dissimilarity between communities (turnover), local extirpation associated with predation or competition tends to generate nestedness (McAbendroth et al. 2005). Thus, functional β-diversity in branches may also be explained by turnover and nestedness. On the other hand, the unidirectional flow of the main stem is selective of species with high dispersal capacity (greater size and absence of parental care) (Comte and Olden 2018) which then tend to become widely distributed within local communities (low functional β-diversity). As a result, despite the high turnover observed for β-diversity with the taxonomic approach, small turnover rates and nestedness may be expected in the functional approach (Virorino-Júnior et al. 2016).
In view of the unique nature of intermittent systems and the scarcity of studies on the factors determining metacommunity structure in such systems, we evaluated the metacommunity organization of an intermittent riverine network in a semiarid region of Brazil. To do so, two hypotheses were formulated: (1) greater hydrological complexity and isolation in branches lead to greater taxonomic and functional diversity in relation to the main stem. Taxonomic and functional β-diversity may be expected to be higher in branches than in the main stem due to turnover (taxonomic approach) or the combination of turnover and nestedness (functional approach). (2) Dissimilarities between isolated locations are usually due to local processes, while spatial processes are more important in connected locations. Because of the adverse conditions (e.g., high temperatures) and intense biotic interactions (e.g., predation) (Larned et al. 2010) prevalent in isolated communities in intermittent systems, functional and compositional differences between branches are best explained by predation and local environmental conditions, whereas spatial factors are more important in the analysis of main stem dynamics.
Methods
Study site and data collection
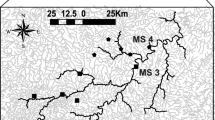
The Jaguaribe River displays unusual dynamics for rivers in semiarid regions (Cavalcante 2018) due to the intensity and frequency of precipitation upon the scant Caatinga vegetation during the 4-month long rainy season (February to May). In addition, an abundance of barrages have been built locally to store rainwater and control flow. Rivers in semiarid Northeastern Brazil tend to be shallow and slow-flowing, with predominantly sandy bottoms (Cavalcante 2018). Discharge rates vary greatly because of unevenly distributed rainfalls and/or dam control, producing a large number of sandy banks along the main river courses. It is very common for the main stem to dry out totally or form a small and very slow-flowing (10 m3 s−1) permanent water course (50–90% of the time), leaving most of the bed exposed (Cavalcante 2018; van Oel et al. 2018). This combination of events promotes intense evapotranspiration, which in turn increases the intermittency of the branches, disconnecting them from the main stem (Maltchik and Medeiros 2012). In addition, the Jaguaribe River is currently regulated by two very large reservoirs, Orós and Castanhão (Fig. 1), both of which were built within the last 50 years.
Sampling was conducted during the rainy season (May–April 2014 and 2015). The amount of rain registered in these two years was comparatively small (Fig. 2), making it possible to observe numerous disconnection events between the branches and the main stem. The study area included 19 sampling points (branches n = 12; main stem n = 7) (Fig. 1), each consisting of 50-m river stretch. After isolating the stretch with nets, physicochemical, morphometric and vegetation data were collected to draw an environmental profile of the sampling location (q.v. Mendonça et al. 2005). Temperature (°C), dissolved oxygen (mg/L) and pH were measured once at the downstream extremity with a Hanna HI9146 device and a PHscan 30 device. Morphometric and vegetation data were collected for four transects along the 50-m stretch. Depth was measured at nine equidistant points along each transect, starting 10 cm from the margin (totaling 36 measurements per sampling point). Based on black-and-white photographs taken in four directions (north, south, east and west) at each transect, the canopy cover (%) was calculated by dividing the dark area by the total area (light + dark area). Using a flow meter, the flow rate was measured once at each transect. The readings obtained for each set of 4 transects were averaged for statistical analysis.
Immediately following the environmental profiling, fish were sampled with a 5.3 m2 trawl net (mesh size: 14 mm between opposing knots), a 0.7 m2 sieve net (mesh size: 1 mm between opposing knots) and a 1.3 m2 seine (mesh size: 2 mm between opposing knots), employing a fishing effort of two man-hours. All the gear was used at all the sampling points (branches and main stem). The 50-m delimitation per sampling point was representative of the distribution of the habitats present within and along the branches and the main stem (backwaters, pools, macrophyte beds and rapids) (Uieda and Castro 1999). Captured specimens were anesthetized with a eugenol solution, followed by fixation in 10% formaldehyde. Voucher specimens were deposited at the Federal University of Rio Grande do Norte (Natal, Rio Grande do Norte, Brazil) (see Table S1).
Functional data
The functional characterization of fish community was based on attributes related to dispersal capacity, habitat use and defense (Table 1; see Online Appendix 2 for more details). These functional traits were chosen due to their well-documented relationship with abiotic, biotic and spatial factors of freshwater fish populations (Villéger et al. 2017). For example, life history attributes are good indicators of a species’ dispersion capacity and resistance to hydrological changes (Winemiller and Rose 1992). This is illustrated by the conflicting demands of body size (the larger the individual, the greater the dispersal capacity) and parental care (the greater the energy expenditure, the smaller the dispersal capacity). Moreover, morphological attributes are related to habitat preference and locomotion in the water column, including the position of the pectoral fin on the body (e.g., centrally positioned fins increase maneuverability; Gatz 1987) and the width of the caudal peduncle (slim peduncles facilitate permanence in locations with constant flow) (Webb 1984). Also, agonistic interactions are minimized by the presence of defense mechanisms such as spines. Taken together, these attributes are key to the processes considered in metacommunity theory: dispersal, niche selection and predation (Leibold et al. 2004).
Since some of the variables were non-continuous, we used Gower’s distance to estimate the functional distance between pairs of species (see Table S2). To do so, we conducted a principal coordinates analysis (PCoA). The position of each species on the PCoA axis (multifunctional space) indicated the degree of functional similarity and was used to quantify the functional amplitude in each local community (Villéger et al. 2008). The amplitude value was then used as a proxy for functional richness (FRic) in the estimations of functional β-diversity (Cornwell et al. 2006; Villéger et al. 2013). Using the protocol of Maire et al. (2015), a number of PCoA axes were selected to construct the functional space. The protocol defines the minimum number of axes representative of the functional community structure by quantifying the mean square deviation (mSD) index. The first two axes were maintained because they represented the minimum required to preserve the quality of the functional space (i.e., mSD < 0.01).
Taxonomic and functional diversity
Regional and local diversity was quantified based on the number of species and the amplitude of functional space occupation. The total number of the species observed in the branches and the main stem was used to define regional taxonomic diversity, while functional diversity was estimated by the volume occupied by all the species. Locally, taxonomic and functional diversity was defined by the number of species registered in each community and by the volume occupied. In the functional approach, regional diversity was used to standardize local diversity values so as to make comparisons possible between communities with different numbers of species (Villéger et al. 2008). Finally, we quantified the spatial variation in local diversity for each region (β-diversity).
Taxonomic β-diversity and its components were calculated from the incidence matrix using Sørensen’s dissimilarity index. The latter is widely used in ecological studies and is considered robust in β-diversity calculations (Koleff et al. 2003). Taxonomic β-diversity may be described thus:
where a is the number of species common to both communities, b is the number of species occurring in the first community but not in the second and c is the number of species occurring in the second community but not in the first. The taxonomic β-diversity was unpacked into turnover and nestedness using the following equation (Baselga 2010):
Taxonomic β-diversity and its components range from 0 to 1 (Baselga et al. 2012). If two assemblages have the same taxonomic composition, taxonomic β-diversity equals 0 (b = c = 0). Taxonomic turnover equals 0 if two assemblages have identical taxonomic subsets (b = c = 0) and 1 if no species are shared (b = 0, or c = 0). Nestedness equals 0 if two assemblages have the same set of species (a = 0) and tends toward 1 when one assemblage is a total subset of another. Following a similar analytical protocol, Villéger et al. (2013) applied the concept of taxonomic β-diversity to functional β-diversity using convex hull volume (FRic). As an analogy, a (species present at both sampling points) represents the functional intersection between two assemblages in the multidimensional space, while b (species present only at the first sampling point) and c (species present only at the second sampling point) represent the unique volumes of each assemblage. Like taxonomic β-diversity and its components, functional β-diversity and its components range from 0 (identical functional volumes) to 1 (no overlap in the multidimensional space).
Predictor variables
Two sets of predictor variables for taxonomic and functional β-diversity patterns were defined: local (biotic and abiotic) and spatial. Local factors included environmental conditions characterizing the morphometric and physicochemical structure at each sampling point and predation (Table 2). As shown by Howeth and Leibold (2010), within metacommunities predators are able to affect the distribution of their prey. Despite their undeniable importance to metacommunity studies, biotic factors can be difficult to evaluate (but see Livingston et al. 2017). To consider the effect of predation, we used the relative abundance of piscivorous species per community. Of all the species sampled, only three (Hoplias malabaricus, Serrasalmus rhombeus and Synbranchus marmoratus) are described as piscivorous in the literature. However, in view of the phenomenon of ontogenetic diet shift observed for many fish species, we performed stomach content analyses to ascertain whether individuals (including juveniles) of these three species were in fact piscivorous. This made it possible to establish the presence of predation in local communities with greater accuracy. However, it should be kept in mind that only 30% of individuals of H. malabaricus, S. rhombeus and S. marmoratus are likely to present any stomach content upon dissection (for more details about stomach content analysis, see Online Appendix 3).
The spatial distance between the communities was evaluated with most accuracy by following the watercourse, i.e., the dispersal routes (Vogt et al. 2013; Heino et al. 2015). Flow unidirectionality should be considered in any aquatic system when studying the dispersal of species among communities. This can be achieved with spatial vectors from asymmetric eigenvectors maps (AEM; Blanchet et al. 2008). AEM analysis produces eigenvectors related to dispersal and mass effect processes. For example, eigenvectors with high eigenvalues are associated with large spatial scales and suggest dispersal limitation is the best explanation for the observed spatial configuration of communities. Conversely, eigenvectors with low eigenvalues represent fine scales and point to mass effects as the best explanation for spatial configuration (Griffith and Peres-Neto 2006). In addition, one of the advantages of using spatial eigenvectors is that Moran’s I coefficients can be used to select eigenvectors with positive spatial autocorrelation (p < 0.05) (Blanchet et al. 2011).
Statistical analysis
We calculated the taxonomic and functional richness for each local community in order to investigate the dependence of spatial patterns of α-diversity on the position in the intermittent drainage network, using the general least squares (GLS) method to control for heteroskedasticity. Taxonomic richness was defined as the number of species in each local community, while functional richness was based on the percentage of convex hull volume occupied by all species of a local community (FRic) (Cornwell et al. 2006; Villéger et al. 2008). Patterns of taxonomic and functional β-diversity (and their components) in the branches and the main stem were determined with permutational multivariate analysis of variance using 999 permutations (PERMANOVA) (Anderson et al. 2006).
The relative importance of local (biotic and abiotic) and spatial factors in metacommunity research has mainly been demonstrated with multivariate partitioning of variance (Cottenie 2005; Leibold and Chase 2017; Tolonen et al. 2018), although the technique has been criticized for producing spurious correlations (Smith and Lundholm 2010) due to the incorporation of neutral dynamics (random events, Hubbell 2001) into the component shared by the environmental and spatial fractions (fraction [b] of Peres-Neto et al. 2006). Moreover, in nature species are distributed in an autocorrelated manner which violates the basic assumptions of independence in multivariate analysis (Legendre 1993). To accommodate this unique distribution of species, Clappe et al. (2018) proposed a new statistical method of assessing the importance of local and spatial factors in β-diversity patterns. Though developed for the taxonomic approach, the framework may also be used in the functional approach (Clappe et al. 2018).
Thus, we used the method of Clappe et al. (2018) to evaluate the explanatory power of local (biotic and abiotic) factors only [a], spatial factors only [c], local and spatial factors combined [b] and residual factors [d]. These fractions are easily obtained from multiple regressions of the β-diversity matrices using local factors to obtain the fraction [ab], spatial factors to obtain the fraction [bc], and local and spatial factors combined (all predictors) to obtain the fraction [abc] (Peres-Neto et al. 2006). By using association matrices (e.g., β-diversity), the whole procedure can be summarized with a distance-based redundancy analysis (db-RDA). The explanatory power of each fraction and its statistical significance were determined with the R2adj value and 999 randomizations, respectively (Peres-Neto et al. 2006). The final model of each multiple regression was composed only of forward-selected local variables (p < 0.05; Blanchet et al. 2008). We chose to use all positive spatial eigenvectors in the model because the previous selection did not decrease the Type I error rate for fraction [a] (no association between β-diversity patterns and local factors was detected as false when it should be true; Peres-Neto and Legendre 2010).
Complementary analyses were performed to determine whether the difference between the branches and the main stem with regard to the number of samples had influenced the observed β-diversity patterns (and their components) (Online Appendix 1). All analyses were performed with the software R (R Core Team 2017). Functional richness and β-diversity were calculated with Villéger’s function (multidimFD and multidimFbetaD; http://villeger.sebastien.free.fr/Rscripts.html), whereas PCoA and PERMANOVA were performed with the vegan package (Oksanen et al. 2015). The spatially constrained null model was estimated with the script provided by Clappe et al. (2018).
Results
Taxonomic and functional structure
Altogether, 5660 individuals were captured in this study (main stem n = 3144; branches n = 2516), corresponding to 34 species (branches n = 29; main stem n = 25; shared n = 20). Piscivorous species accounted for 3% (main stem) and 1.5% (branches) of the captured individuals. Only four of the 19 sampling points yielded no piscivorous species. The analyzed stomach contents confirmed the piscivorous feeding habits of H. malabaricus and S. rhombeus (even in individuals < 5 cm), but not of S. marmoratus, contradicting the literature. Despite the differences in abundance, no significant difference in taxonomic and functional richness was observed between the branches and the main stem (Fig. 3).
The first two axes of the PCoA explained 56.7% of the observed variation in the functional structure of the local communities (Figure S1). In the functional space defined by these two axes, the area occupied by species was similar for the main stem and the branches (Fig. 4). In general, the species comprising local communities were characterized by a lack of defense-related morphologies, low investment in parental care and centrally located pectoral fins (negative scores in PCoA 1; Fig. 4), all of which are associated with high dispersal capacity.
Functional space occupation by species observed only in the main stem (a) or only in the branches (b). Density curves were defined from Kernel density calculations, in which darker areas indicate regions of the functional space with a higher concentration of species. The direction of the arrows indicates the gradients of the functional attributes within the multifunctional space. Only attributes with loadings greater than 0.5 were used (for more information, see Table S2)
β-diversity patterns
Regardless of the approach (taxonomic or functional), β-diversity was greater in branches than in the main stem or the entire drainage network (p = 0.01; Table 3). The difference remained significant even after controlling for the number of communities in the branches and the main stem, suggesting that our results were not biased by differences in the number of samples (Figure S1.2, Online Appendix 1). The high turnover values explained the greater β-diversity in branches in the taxonomic approach, while nestedness was more important in the functional approach (p = 0.01; Table 3).
Metacommunity structure
Based on the forward selection criterion, three spatial eigenvectors were selected to explain main stem β-diversity, while four eigenvectors (AEM-1, AEM-2, AEM-3 and AEM-4) were used to explain the β-diversity of the entire drainage network (Table 4). Taken together, these eigenvectors had little explanatory power (Fig. 5), but the combination of unique local and spatial factors explained a considerable part of the observed patterns of taxonomic and functional β-diversity.
Six local factors were significantly associated with β-diversity (three in the taxonomic approach and four in the functional approach; Table 4). For example, relative predator abundance was an important determinant of community structure, but only in branches. Regardless of the approach, local factors alone were more explanatory of branch diversity, whereas the combination of local and spatial factors was more explanatory of main stem diversity (Fig. 5).
Discussion
Using an analytical framework which excludes spurious correlations between local and spatial factors (Clappe et al. 2018), we found a clear difference in the importance of local and spatial processes for taxonomic and functional β-diversity. For instance, species distribution was strongly influenced by spatially structured local processes [b], whereas local processes were predictive of functional traits [a]. This may in part be explained by environmental tolerance to semiarid conditions (species with high fecundity) and ability to escape from predators (Larned et al. 2010; Pelaez and Pavanelli 2019). In addition, our findings match the predictions of Brown and Swan (2010), according to which local factors are more important in explaining diversity patterns in branches, whereas the combination of local and spatial factors is better at explaining the structure of the main stem. However, since these two factors depended on the position in the drainage network, the β-diversity patterns observed in the branches and the main stem were different.
The object of this study was a dendritic system in a semiarid region of Northeastern Brazil, with high rates of discontinuity and low taxonomic and functional richness of fish communities. In general, the ichthyofauna of this ecoregion displays lower taxonomic richness than that of other Brazilian regions. In fact, the species sampled in a recent survey of several aquatic ecosystems in the ecoregion (Mid-Northeastern Caatinga) (Rodrigues-Filho et al. 2016) represented 60% of the taxonomic diversity of fishes reported from the Jaguaribe River. The small diversity is due mainly to an extensive history of adverse events (i.e., past climate change and marine incursions; Rodrigues-Filho et al. 2018b) compromising the aquatic biota. These events led to functional redundancy, since only the most resilient species have managed to survive. This may explain the absence of a significant difference in functional richness between the branches and the main stem.
Branches constrain regional β-diversity patterns
The β-diversity values were higher for the branches than for the main stem (Table 3). Local communities displayed different taxonomic and functional compositions, explained primarily by turnover and nestedness, respectively. This scenario diverges from the functional homogenization commonly observed in perennial systems (Vitorino-Júnior et al. 2016), which may be explained by high functional similarity between local communities (Logez et al. 2010) or by allopatric speciation (Baselga et al. 2012). In our study, we found evidence of substitution of species with similar functions (redundancy) from one sample to another. Thus, the observed functional β-diversity is explained by selective loss of species, producing a nested pattern. Nestedness indicates high levels of functional redundancy (i.e., different species with the same function) and is therefore important in the analysis of metacommunities of fishes and other taxa in semiarid regions. In intermittent systems under harsh environmental conditions, this redundancy could act as a mechanism of resistance to hydrological variability (Cadotte et al. 2011; Mouillot et al. 2011). Similar results have been reported for temporary ponds, in association with environmental severity and reduced habitat connectivity (Brendonck et al. 2015; Hill et al. 2017; Gianuca et al. 2017). Such biodiversity patterns are considered important from the environmental perspective because they identify the highest concentrations of biodiversity and the areas with the greatest potential for conservation (Vodǎ et al. 2016). However, patterns should be interpreted with caution when dealing with systems in semiarid regions, especially in view of the role branches play in regional β-diversity patterns and the current scarcity of research in this field.
Importance of local and spatial factors
In a seminal paper, Brown and Swan (2010) predicted that local factors would be the most important determinants of composition of communities isolated in branches. When Schmera et al. (2017) tested this claim, they found patterns to depend on taxonomic groups. According to the authors, in fish communities spatial factors are as important as environmental factors, contradicting the claim of Brown and Swan (2010). Other authors have reported similar results (Mykra et al. 2007; Cetra et al. 2017; Rodrigues-Filho et al. 2018a), suggesting that dispersal processes are also important in branches. These discrepancies should be analyzed in light of the dispersal capacity of the studied taxonomic group (as suggested by Schmera et al. 2017). Moreover, branches are not necessarily physically isolated from the main stem. In the present study, which evaluated a drainage network with highly disconnected branches, as predicted by Brown and Swan (2010) local factors were very important from both the taxonomic and functional perspective due to the intensification of biotic interactions and/or environmental filters in partially or intermittently isolated communities. In fact, numerous disconnection events were observed throughout the dendritic system (Figure S2, for example). In other words, in intermittent systems the relative importance of local and spatial processes for the structuring of isolated communities depends on the level of connectivity with the main stem: high levels of connection favor the colonization of communities farther away by species with greater dispersal capacity, while low levels of connection intensify local processes (e.g., high temperatures and high predation rates), favoring hardy species. In the present study, the intensification of local processes associated with isolation (Larned et al. 2010) explained most of the observed patterns of taxonomic turnover and functional nestedness.
Predation (the only local branch variable forward-selected in both the taxonomic and the functional approach) explained more than 10% of the taxonomic β-diversity and 14% of the functional β-diversity, suggesting that the isolation of communities can intensify biotic interactions. In fact, the combination of predator preference and adverse environmental conditions in branches explained most of the observed β-diversity values. In the taxonomic approach, β-diversity was mainly explained by turnover (0.65), which may be due to differences in predation rates between local communities or to the fact that sampling was performed early in the dry season, shortly after the system had become disconnected (Chaves et al. 2008). Contrasting with the taxonomic approach, nestedness explained most of the observed functional β-diversity patterns in branches. These results suggest that different connectivity rates can generate different levels of mortality and colonization (Lake 2003), favoring species with resistance traits such as high fecundity (Larned et al. 2010). For example, local extirpations in isolated communities promoted by predation or environmental filters contribute to the divergence of functional structures. Thus, width and depth gradients may have an influence on extinction rates because the permanence of a water body during the dry season depends on the surface area (White et al. 2016). On the other hand, in communities connected with the main stem, species with functional attributes associated with high dispersal capacity (less parental care) are favored (Soininen et al. 2016). As shown by the literature, environmental conditions are strongly related to the functional characteristics (e.g., life history, habitat-type exploitation, defense-related morphologies) of the species surveyed (Rodrigues-Filho et al. 2017; Villéger et al. 2017).
In the main stem, a combination of local and spatial factors explained the patterns of taxonomic and functional β-diversity. In our analysis of the branches, the first spatial eigenvectors were selected. This runs counter to our initial hypothesis that mass effect (selection of the last spatial eigenvectors) would be the main determinant of main stem community structure. The greater explanatory power of the combination of local and spatial factors is compatible with the concept of environment-space continuum posited by metacommunity theory and suggests localized spatial processes are responsible for the observed metacommunity structure (Gravel et al. 2006). Thus, species’ differential dispersal capacity and environmental affinities are likely responsible for the main stem β-diversity patterns observed in intermittent rivers. Due to the combined action of local spatial factors, taxonomic and functional β-diversity values were higher in our drainage network than in most perennial systems, in which the mass effect is a predominant limitation of over-dispersal (Araújo et al. 2013; Vitorino-Júnior et al. 2016; Pelaez and Pavanelli 2019).
Analytical limitations
The role of environmental and spatial factors in the organization of aquatic metacommunities depends on taxonomic (Schmera et al. 2017) and functional groups (Rodrigues-Filho et al. 2018a), spatial scale (Brown and Swan 2010; Heino et al. 2015) and ecosystem type (Larned et al. 2010). The complexity of these factors (and their inter-relationships) is the main reason for the small extrapolative power of metacommunity patterns observed in ecological studies (low R2adj values; Siqueira et al. 2012; Schmera et al. 2017). Another possible explanation is the omission of variables important for metacommunity structure (e.g., biotic interactions) (Legendre 2008). However, when this important factor of community assemblage was included, the residual variation in our study remained high ([d] > 75%), most likely because of the unpredictability of aquatic systems (Schmera et al. 2017). Another possible explanation for the high values of stochastic events ([d]) is the underestimation of local components ([a]) and the overestimation of spatial factors ([c]) due to the difficulty of capturing the influence of local processes across extended spatial scales (Smith and Lundholm 2010; Vellend et al. 2014). This problem of partitioning analysis has been reported by several authors (Cetra et al. 2017; Rodrigues-Filho et al. 2018a; Zbinden and Matthews 2017).
Implications for conservation
The conservation of local biodiversity is extremely important for the maintenance of regional patterns of β-diversity (Teshima et al. 2016). In this study, we observed high functional and taxonomic β-diversity in branches, suggesting that the conservation of regional patterns depends on the preservation of these local communities, despite the high levels of nestedness. We also recorded a variety of anthropic impacts, such as flow regulation (Figure S3), agriculture and pollution (Figure S4). The latter is known to influence longitudinal gradients of β-diversity directly (Agostinho et al. 2008; Araújo et al. 2013). Such disturbances affect the two main forces found to regulate biodiversity in this study: dispersal limitation and local processes (abiotic and biotic). Changes in environmental conditions can directly affect the selection of functional traits (Mims and Olden 2013). For example, agricultural activities are known to promote functional and taxonomic simplification of the fish fauna by reducing the number of available habitats (Casatti et al. 2015) and homogenizing the underlying substrate via siltation (Dala-Corte et al. 2016). In addition, reservoirs can promote artificial patterns of nested subsets because different zones select for different species traits, which change as distance increases (Araújo et al. 2013). These considerations, along with the importance of small branches to the main stem (Biggs et al. 2017), show how potential local disturbances can directly affect regional patterns of fish community diversity in riverine networks in semiarid regions. Despite claims to the contrary in much of the literature on ecology, such environments have crucial ecosystem functions for both aquatic and terrestrial communities (Datry et al. 2014), including human populations (Kingsford et al. 2016). Hence, a proper understanding of the dynamics of intermittent rivers and their drainage networks is essential when developing conservation strategies for specific ecosystems (Boulton 2014).
Conclusions
Branches were found to be responsible for the observed high levels of regional taxonomic and functional β-diversity, mainly by way of predation and environmental selection. Although many studies have documented the importance of adjacent water bodies to regional biodiversity (Curry et al. 2012; Espírito-Santo et al. 2013; Espírito-Santo and Zuanon 2016; Vitorino-Júnior et al. 2016), little attention has been given to the mechanisms regulating their diversity. The complex dynamics of connections and disconnections of adjacent water bodies (branches) in semiarid regions have made some researchers regard them as ecosystems of low taxonomic richness (Datry et al. 2014). In the present study, this notion is shown to be incorrect since functional and taxonomic β-diversity was greater in the branches than in the main stem. The observed pattern was explained primarily by the combination of local and spatial processes, making it possible to predict how future anthropic interventions (such as agriculture, pollution and flow regulation) might affect fish assemblages which, despite their natural resistance to seasonal fluctuations, are sensitive to such abrupt interventions (Larned et al. 2010).
References
Agostinho AA, Pelicice FM, Gomes LC (2008) Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz J Biol 68:1119–1132. https://doi.org/10.1590/S1519-69842008000500019
Altermatt F, Schreiber S, Holyoak M (2011) Interactive effects of disturbance and dispersal directionality on species richness and composition in metacommunities. Ecology 92:859–870. https://doi.org/10.1890/10-1095.1
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693. https://doi.org/10.1111/j.1461-0248.2006.00926.x
Araújo ES, Marques EE, Freitas IS, Neuberger AL, Fernandes R, Pelicice FM (2013) Changes in distance decay relationships after river regulation: similarity among fish assemblages in a large Amazonian river. Ecol Freshw Fish 22:543–552. https://doi.org/10.1111/eff.12054
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Global Ecol Biogeogr 19:134–143. https://doi.org/10.1111/j.1466-8238.2009.00490.x
Baselga A, Gómez-Rodríguez C, Lobo JM (2012) Historical legacies in world amphibian diversity revealed by the turnover and nestedness components of beta diversity. PLoS ONE 7:1–10. https://doi.org/10.1371/journal.pone.0032341
Biggs J, Fumetti S, Kelly-Quinn M (2017) The importance of small waterbodies for biodiversity and ecosystem services: implications for policy makers. Hydrobiologia 793:3–39. https://doi.org/10.1007/s10750-016-3007-0
Blanchet FG, Legendre P, Borcard D (2008) Modelling directional spatial processes in ecological data. Ecol Model 215:325–336. https://doi.org/10.1016/j.ecolmodel.2008.04.001
Blanchet FG, Legendre P, Maranger R, Monti D, Pepin P (2011) Modelling the effect of directional spatial ecological processes at different scales. Oecologia 166:357–368. https://doi.org/10.1007/s00442-010-1867-y
Boulton AJ (2014) Conservation of ephemeral streams and their ecosystem services: what are we missing? Aquat Conserv 24:733–738. https://doi.org/10.1002/aqc.2537
Brendonck L, Jocqué M, Tuytens K, Timms BV, Vanschoenwinkel B (2015) Hydrological stability drives both local and regional diversity patterns in rock pool metacommunities. Oikos 124:741–749. https://doi.org/10.1111/oik.01710
Brown BL, Swan CM (2010) Dendritic network structure constrains metacommunity properties in riverine ecosystems. J Anim Ecol 79:571–580. https://doi.org/10.1111/j.1365-2656.2010.01668.x
Cadotte MW, Carscadden K, Mirotchnik N (2011) Beyond species: functional diversity and the maintenance of ecological processes and services. J Appl Ecol 48:1079–1087. https://doi.org/10.1111/j.1365-2664.2011.02048.x
Casatti L, Teresa FB, Zeni JO, Ribeiro MD, Brejão GL, Ceneviva-Bastos M (2015) More of the same: high functional redundancy in stream fish assemblages from tropical agroecosystems. Environ Manag 55:1300–1314. https://doi.org/10.1007/s00267-015-0461-9
Cavalcante AA (2018) Temporary distribution of discharges and morphological changes at semiarid rivers: the Jaguaribe River in Ceará State, Brazil. Revista do Departamento de Geografia, vol 35, pp 28–36. https://doi.org/10.11606/rdg.v35i0.133598
Cetra M, Petrere M Jr, Barrella W (2017) Relative influences of environmental and spatial factors on stream fish assemblages in Brazilian Atlantic rainforest. Fisheries Manag Ecol 24:139–145. https://doi.org/10.1111/fme.12207
Chase JM (2003) Community assembly: when should history matter? Oecologia 136:489–498. https://doi.org/10.1007/s00442-003-1311-7
Chaves ML, Rieradevall M, Chainho P, Costa JL, Costa MJ, Prat N (2008) Macroinvertebrate communities of non-glacial high altitude intermittent streams. Freshw Biol 53:55–76. https://doi.org/10.1111/j.1365-2427.2007.01867.x
Clappe S, Dray S, Peres-Neto PR (2018) Beyond neutrality: disentangling the effects of species sorting and spurious correlations in community analysis. Ecology 99:1737–1747. https://doi.org/10.1002/ecy.2376
Comte L, Olden DJ (2018) Evidence for dispersal syndromes in freshwater fishes. P Roy Soc B Biol Sci 285:20172214. https://doi.org/10.1098/rspb.2017.2214
Cornwell WK, Schwilk DW, Ackerly DD (2006) A trait-based test for habitat filtering: convex hull volume. Ecology 87:1465–1471. https://doi.org/10.1890/0012-9658(2006)87%5b1465:ATTFHF%5d2.0.CO;2
Cottenie K (2005) Integrating environmental and spatial processes in ecological community dynamics. Ecol Lett 8:1175–1182. https://doi.org/10.1111/j.1461-0248.2005.00820.x
Curry CJ, Curry RA, Baird DJ (2012) The contribution of riffles and riverine wetlands to benthic macroinvertebrate biodiversity. Biodiversity Conservation 21:895–913. https://doi.org/10.1007/s10531-011-0219-5
Dala-Corte RB, Giam X, Olden JD, Becker FG, Guimarães TF, Melo AS (2016) Revealing the pathways by which agricultural land-use affects stream fish communities in South Brazilian grasslands. Freshw Biol 61:1921–1934. https://doi.org/10.1111/fwb.12825
Datry T, Larned ST, Tockner K (2014) Intermittent rivers: a challenge for freshwater ecology. Bioscience 64:229–235. https://doi.org/10.1093/biosci/bit027
Datry T, Bonada N, Heino J (2015) Towards understanding the organisation of metacommunities in highly dynamic ecological systems. Ecography 125:149–159. https://doi.org/10.1111/oik.02922
Datry T, Bonada N, Heino J (2016a) Towards understanding the organisation of metacommunities in highly dynamic ecological systems. Oikos 125:149–159. https://doi.org/10.1111/oik.02922
Datry T, Moya N, Zubieta J, Oberdorff T (2016b) Determinants of local and regional communities in intermittent and perennial headwaters of the Bolivian Amazon. Freshw Biol 61:1335–1349. https://doi.org/10.1111/fwb.12706
Dumay O, Tari PS, Tomasini JA, Mouillot D (2004) Functional groups of lagoon fish species in Languedoc Roussillon, southern France. J Fish Biol 64:970–983. https://doi.org/10.1111/j.1095-8649.2004.00365.x
Espírito-Santo HMV, Zuanon J (2016) Temporary pools provide stability to fish assemblages in Amazon headwater streams. Ecol Freshw Fish 26:475–483. https://doi.org/10.1111/eff.12292
Espírito-Santo HMV, Rodríguez MA, Zuanon J (2013) Reproductive strategies of Amazonian stream fishes and their fine-scale use of habitat are ordered along a hydrological gradient. Freshw Biol 58:2494–2504. https://doi.org/10.1111/fwb.12225
Finn DS, Bonada N, Murria C, Hughes JM (2011) Small but mighty: headwaters are vital to stream network biodiversity at two levels of organization. J N Am Benthol Soc 30:963–980. https://doi.org/10.1899/11-012.1
Gatz AJ Jr (1987) Morphologically inferred niche differentiation in stream fishes. Am Midl Nat 106:10–21. https://doi.org/10.2307/2425131
Giam X, Olden JD (2016) Environment and predation govern fish community assembly in temperate streams. Global Ecol Biogeogr 25:1194–1205. https://doi.org/10.1111/geb.12475
Gianuca AT, Declerk SAJ, Lemmens P, Meester L (2017) Effects of dispersal and environmental heterogeneity on the replacement and nestedness components of β-diversity. Ecology 98:525–533. https://doi.org/10.1002/ecy.1666
Gravel D, Canham CD, Beaudet M, Messeier C (2006) Reconciling niche and neutrality: the continuum hypothesis. Ecol Lett 9:399–409. https://doi.org/10.1111/j.1461-0248.2006.00884.x
Griffith DA, Peres-Neto PR (2006) Spatial modeling in ecology: the flexibility of eigenfunction spatial analyses. Ecology 87:2603–2613. https://doi.org/10.1890/0012-9658(2006)87%5b2603:SMIETF%5d2.0.CO;2
Heino J, Melo AS, Siqueira T, Soininen J, Valanko S, Bini LM (2015) Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshw Biol 60:845–869. https://doi.org/10.1111/fwb.12533
Hill MJ, Heino J, Thornhill I, Ryves DB, Wood PJ (2017) Effects of dispersal mode on the environmental and spatial correlates of nestedness and species turnover in pond communities. Oikos 126:1575–1585. https://doi.org/10.1111/oik.04266
Howeth JG, Leibold MA (2010) Prey dispersal rate affects prey species composition and trait diversity in response to multiple predators in metacommunities. J Anim Ecol 79:1000–1011. https://doi.org/10.1111/j.1365-2656.2010.01715.x
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton
Jackson DA, Peres-Neto PR, Olden JD (2001) What controls who is where in freshwater fish communities - the roles of biotic, abiotic, and spatial factors. Can J Fish Aquat Sci 58:157–170. https://doi.org/10.1139/f00-239
Kingsford RT, Basset A, Jackson L (2016) Wetlands: conservation’s poor cousins. Aquat Conserv 26:892–916. https://doi.org/10.1002/aqc.2709
Koleff P, Gaston KJ, Lennon JJ (2003) Measuring beta diversity for presence–absence data. J Anim Ecol 72:367–382. https://doi.org/10.2307/2259551
Lake PS (2003) Ecological effects of perturbation by drought in flowing waters. Freshw Biol 48:1161–1172. https://doi.org/10.1046/j.1365-2427.2003.01086.x
Larned ST, Datry T, Arscott DB, Tockner K (2010) Emerging concepts in temporary-river ecology. Freshw Biol 55:717–738. https://doi.org/10.1111/j.1365-2427.2009.02322.x
Legendre P (1993) Spatial autocorrelation: trouble or new paradigm? Ecology 74:1659–1673. https://doi.org/10.2307/1939924
Legendre P (2008) Studying beta diversity: ecological variation partitioning by multiple regression and canonical analysis. J Plant Ecol 1:3–8. https://doi.org/10.1093/jpe/rtm001
Leibold MA, Chase JM (2017) Metacommunity Ecology. Princeton University Press, Princeton
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7:601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x
Livingston G, Fukumori K, Provete DB, Kawachi M, Takamura N, Leibold MA (2017) Predators regulate prey species sorting and spatial distribution in microbial landscapes. J Anim Ecol 86:501–510. https://doi.org/10.1111/1365-2656.12639
Logez M, Pont D, Ferreira MT (2010) Do Iberian and European fish faunas exhibit convergent functional structure along environmental gradients? J N Am Benthol Soc 29:1310–1323. https://doi.org/10.1899/09-125.1
Logue JB, Mouquet N, Peter N, Hillebrand H, Metacommunity Working Group (2011) Empirical approaches to metacommunities: a review and comparison with theory. Trends Ecol Evol 26:482–491. https://doi.org/10.1016/j.tree.2011.04.009
Maire E, Grenouillet G, Brosse S, Villéger S (2015) How many dimensions are needed to accurately assess functional diversity? A pragmatic approach for assessing the quality of functional spaces. Global Ecol Biogeogr 24:728–740
Maltchik L, Medeiros ESF (2012) Conservation importance of semiarid streams in north-eastern Brazil: implications of hydrological disturbance and species diversity. Aquat Conserv 16:665–677. https://doi.org/10.1002/aqc.805
McAbendroth L, Foggo A, Rundle SD, Bilton DT (2005) Unravelling nestedness and spatial pattern in pond assemblages. J Anim Ecol 74:41–49. https://doi.org/10.1111/j.1365-2656.2004.00895.x
Mendonça FP, Magnusson WE, Zuanon J (2005) Relationships between habitat characteristics and fish assemblages in small streams of central Amazonia. Copeia 4:751–764. https://doi.org/10.1643/0045-511(2005)005%5b0751:RBHCAF%5d2.0.CO;2
Mims MC, Olden J (2013) Fish assemblages respond to altered flow regimes via ecological filtering of life history strategies. Freshw Biol 58:50–62. https://doi.org/10.1111/fwb.12037
Mouillot D, Villéger S, Scherer-Lorenzen M, Mason NWH (2011) Functional structure of biological communities predicts ecosystem multifunctionality. PLoS ONE 6:1–9. https://doi.org/10.1371/journal.pone.0017476
Mykra H, Heino J, Muotka T (2007) Scale-related patterns in the spatial and environmental components of stream macroinvertebrate assemblage variation. Global Ecol Biogeogr 16:149–159. https://doi.org/10.1111/j.1466-8238.2006.00272.x
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2015) vegan: Community Ecology Package. R package version 2.2-1. https://cran.r-project.org/web/packages/vegan/vegan.pdf. Accessed 27 June 2017
Pelaez O, Pavanelli CS (2019) Environmental heterogeneity and dispersal limitation explain different aspects of β-diversity- in -Neotropical -fish -assemblages. Fresh Biol 64:497–505. https://doi.org/10.1111/fwb.13237
Peres-Neto PR, Legendre P (2010) Estimating and controlling for spatial structure in the study of ecological communities. Global Ecol Biogeogr 19:174–184. https://doi.org/10.1111/j.1466-8238.2009.00506.x
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625. https://doi.org/10.1890/0012-9658(2006)87%5b2614:VPOSDM%5d2.0.CO;2
R Foundation for Statistical Computing (2017). R version 3.4.2. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 27 June 2017
Rodrigues-Filho CAS, Gurgel-Lourenço RC, Bezerra LAV, Souza WAD, Garcez DS, Lima SMQ, Ramos TPA, Sánchez-Botero JI (2016) Ichthyofauna of the humid forest enclaves in the tablelands of Ibiapaba and Araripe,Northeastern Brazil. Biota Neotrop 16:e20160273. https://doi.org/10.1590/1676-0611-BN-2016-0273
Rodrigues-Filho CAS, Gurgel-Lourenço RC, Lima SMQ, Oliveira EF, Sánchez-Botero JI (2017) What governs the functional diversity patterns of fishes in the headwater streams of the humid forest enclaves: environmental conditions, taxonomic diversity or biotic interactions? Environ Biol Fish 100:1023–1032. https://doi.org/10.1007/s10641-017-0603-4
Rodrigues-Filho CAS, Gurgel-Lourenço RC, Bezerra LAV, Sousa WA, Oliveira EF, Leitão RP, Garcez DS, Sánchez-Botero JI (2018a) How are local fish communities structured in Brazilian semiarid headwater streams? Hydrobiologia 819:93–108. https://doi.org/10.1007/s10750-018-3650-8
Rodrigues-Filho CAS, Leitão RP, Zuanon J, Sánchez-Botero JI, Baccaro FB (2018b) Historical stability promoted higher functional specialization and originality in Neotropical stream fish assemblages. J Biogeogr 45:1345–1354. https://doi.org/10.1111/jbi.13205
Schmera D, Árva D, Boda P, Bódis E, Bolgovics A, Borics G, Csercsa A, Deák C, Krasznai EA, Lukács BA, Mauchart P, Móra A, Sály P, Specziár A, Suveges K, Szivák I, Takács P, Tóth M, Várbíró G, Votjkó A, Eros T (2017) Does isolation influence the relative role of environmental and dispersal-related processes in stream networks? An empirical test of the network position hypothesis using multiple taxa. Freshw Biol 63:74–85. https://doi.org/10.1111/fwb.12973
Siqueira T, Bini LM, Roque FO, Cottenie K (2012) A Metacommunity Framework for Enhancing the Effectiveness of Biological Monitoring Strategies. PLoS ONE 7:e43626. https://doi.org/10.1371/journal.pone.0043626
Smith TW, Lundholm JT (2010) Variation partitioning as a tool to distinguish between niche and neutral processes. Ecography 33:648–655. https://doi.org/10.1111/j.1600-0587.2009.06105.x
Socolar JB, Gilroy JJ, Kunin WE, Edwards DP (2016) How should beta-diversity inform biodiversity conservation? Trends Ecol Evol 31:67–80. https://doi.org/10.1016/j.tree.2015.11.005
Soininen J, Jamoneau A, Rosebery J, Passy SI (2016) Global patterns of species and trait composition in diatoms. Global Ecol Biogeogr 25:940–950. https://doi.org/10.1111/geb.12452
Spasojevic MJ, Copeland S, Suding KN (2014) Using functional diversity patterns to explore metacommunity dynamics: a framework for understanding local and regional influences on community structure. Ecography 37:939–949. https://doi.org/10.1111/ecog.00711
Strecker AL, Brittain JT (2017) Increased habitat connectivity homogenizes freshwater communities: historical and landscape perspectives. J Appl Ecol 54:1343–1352. https://doi.org/10.1111/1365-2664.12882
Teshima FA, Mello BJG, Ferreira FC, Cetra M (2016) High β-diversity maintains regional diversity in Brazilian tropical coastal stream fish assemblages. Fisheries Manag Ecol 6:531–539. https://doi.org/10.1111/fme.12194
Tolonen KT, Cai Y, Vilmi A, Karjalainen SM, Sutela T, Heino J (2018) Environmental filtering and spatial effects on metacommunity organisation differ among littoral macroinvertebrate groups deconstructed by biological traits. Aquat Ecol 52:119–131. https://doi.org/10.1007/s10452-018-9649-4
Townsend CR, Hildrew AG (1994) Species traits in relation to a habitat templet for river systems. Freshw Biol 31:265–275
Uieda VS, Castro RMC (1999) Coleta e fixação de peixes de riachos. In: Caramaschi EP, Mazzoni R., Bizerril CRSF, Peres-Neto PR (eds.), Ecologia de peixes de riachos. Série Oecologia Brasiliensis, Rio de Janeiro, pp 1–22
van Oel RR, Martins ESPR, Costa AC, Wanders N, van Lanen HAJ (2018) Diagnosing drought using the down streamness concept: the effect of reservoir networks on drought evolution. Hydrolog Sci J 63:979–990. https://doi.org/10.1080/02626667.2018.1470632
Vellend M, Srivastava DS, Anderson KM, Brown CD, Jankowski JE, Kleynhans EJ, Kraft NJB, Letaw AD, Macdonald AAM, Maclean JEI, Myers-Smith H, Norris AR, Xue X (2014) Assessing the relative importance of neutral stochasticity in ecological communities. Oikos 123:1420–1430. https://doi.org/10.1111/oik.01493
Villéger S, Mason NWH, Mouillot D (2008) New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89:2290–2301. https://doi.org/10.1890/07-1206.1
Villéger S, Grenouillot G, Brosse S (2013) Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in European fish assemblages. Global Ecol Biogeogr 22:671–681. https://doi.org/10.1111/geb.12021
Villéger S, Brosse S, Mouchet M, Mouillot D, Vanni MJ (2017) Functional ecology of fsh: current approaches and future challenges. Aquat Sci 79:783–801. https://doi.org/10.1007/s00027-017-0546-z
Vitorino-Júnior OB, Fernandes R, Agostinho CS, Pelicice FM (2016) Riverine networks constrain β-diversity patterns among fish assemblages in a large Neotropical river. Freshw Biol 61:1733–1745. https://doi.org/10.1111/fwb.12813
Vodǎ R, Dapporto L, Dincǎ V, Shreeve TG, Khaldi M, Barech G, Rebbas K, Sammut P, Scalercio S, Hebert PDN, Vila R (2016) Historical and contemporary factors generate unique butterfly communities on islands. Sci Rep-UK 6:1–11. https://doi.org/10.1038/srep28828
Vogt RJ, Peres-Neto PR, Beisner BE (2013) Using functional traits to investigate the determinants of crustacean zooplankton community structure. Oikos 122:1700–1709. https://doi.org/10.1111/j.1600-0706.2013.00039.x
Webb PW (1984) Form and function in fish swimming. Sci Am 251:72–82
White RSA, McHugh PA, McIntosh AR (2016) Drought survival is a threshold function of habitat size and population density in a fish metapopulation. Glob Change Biol 22:3341–3348. https://doi.org/10.1111/gcb.13265
Winemiller KO, Rose KA (1992) Patterns of life-history diversification in North American fishes: implications for population regulation. Can J Fish Aquat Sci 49:2196–2218. https://doi.org/10.1139/f92-242
Zbinden ZD, Matthews WJ (2017) Beta diversity of stream fish assemblages: partitioning variation between spatial and environmental factors. Freshw Biol 62:1460–1471. https://doi.org/10.1111/fwb.12960
Acknowledgements
The study was funded by CNPq/PPBIO (National Council for Scientific and Technological Development/Biodiversity Research Program) through Grant #457463/2012-0 and by CNPq/ICMBio through Grant #552009/2011-3. We would also like to thank the Ministry of the Environment (MMA; 26174-2) and the Chico Mendes Institute for Biodiversity Conservation (ICMBio/SISBIO; 32921-4) for granting licenses to collect fish samples and Universidade Federal Rural do Semi-Árido for logistic support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CASRF designed the study. CASRF, RCGL, EAR, JLCN, RSC and JISB conducted the field work and collected the data, with additional material from collaborators. CASRF analyzed the data. CASRF drafted the manuscript, assisted by RCGL, EAR, JLCN, DSG and JISB.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Télesphore Sime-Ngando.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rodrigues-Filho, C.A.S., Gurgel-Lourenço, R.C., Ramos, E.A. et al. Metacommunity organization in an intermittent river in Brazil: the importance of riverine networks for regional biodiversity. Aquat Ecol 54, 145–161 (2020). https://doi.org/10.1007/s10452-019-09732-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-019-09732-1