Abstract
Foraging characteristics of siscowet lake trout (Salvelinus namaycush siscowet) on deepwater sculpin (Myoxocephalus thompsonii) were studied under ecologically relevant downwelling light intensities (9.0 × 108 to 1.62 × 1011 photons m−2 s−1) and emission spectrum (500–510 nm) on varying substrates (gravel, sand, and black fabric). Siscowet reaction distance within our trials increased with light intensity up to 6.0 × 109 photons m−2 s−1, after which reaction distance remained constant with additional increases in light intensity following the Michaelis–Menten saturation function. Reaction distances were not affected by substrate type under any light intensity. The number of prey captures also increased with increasing light intensity, with most orientations toward prey occurring within the siscowet’s forward sector (± 0°–60°, where 0° represents the tip of the siscowet rostrum). Finally, the overall probability of prey capture was positively related to reaction distance at each light intensity. Results suggest that siscowet can visually forage on benthic prey at great depth in Lake Superior, and reaction distance (≤ 27 cm) to sculpin may not diminish until depths exceed 200 m (6.00 × 109 photons m−2 s−1).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predator–prey interactions influence the distribution and abundance of freshwater species (Kerfoot & Sih, 1987). Habitat characteristics and the physical–chemical environment (Crowder & Cooper, 1979; Crowder et al., 1981) affect the outcome of these interactions, which are ultimately a determining factor in the structure of aquatic communities (Carpenter et al., 1985; Beauchamp et al., 2007). Encounters between piscivorous fishes and their prey depend upon the degree to which environmental characteristics modify detection (Beauchamp et al., 1999). Changing light intensity, (Mazur & Beauchamp, 2003; Hansen et al., 2013; Keyler et al., 2015) and substrate type (Houtman & Dill, 1994; Ellis et al., 1997; Sowersby et al., 2015) can influence predator–prey interactions between fishes. Light can influence daily movements and success of visually foraging fishes (Aksnes & Utne, 1997; Boscarino et al., 2010), while different substrates can provide cryptic camouflage for prey fishes and simultaneously alter visual detection by predators (Ruxton et al., 2004; Stevens & Cuthill 2006). These environmental characteristics can affect both the visually mediated behavior and the distance at which a piscivore first responds to prey (Munz & McFarland, 1977; Ruxton et al., 2004).
Reaction distance is a critical measurement for evaluating prey detection (Howick & O’Brien, 1983; Miner & Stein, 1996; Vogel & Beauchamp, 1999) and is an important metric in visual foraging models used for predicting predation effects, foraging success, and fish distributions in response to environmental changes such as decreased water clarity (Vogel & Beauchamp, 1999). To estimate the volume of water searched during foraging (e.g., volume of a cylinder), foraging models typically use the square, or another exponent, of the reaction distance (Eggers, 1977). Therefore, minor errors in reaction distance will be exaggerated and thereby influence the accuracy of model predictions (Vogel & Beauchamp, 1999). For example, use of reaction distance for a piscivore detecting pelagic prey in a benthic foraging model may overestimate reaction distance, and therefore search volume, due to differences in prey behavior and the additional substrate component. Current model simulations of predator–prey interactions without accurate sensory input are inaccurate (Roth et al., 2008). By determining visual and spectral sensitivity, and conducting experiments under natural lighting conditions, our understanding of how predation is influenced by differences in light intensity, prey type, and associated foraging habitat (e.g., benthic vs. pelagic) can be improved.
A thorough examination of predator–prey interactions between species that occupy the offshore benthic environment is needed to determine how perception of prey can vary between benthic and pelagic environments. Within the Lake Superior offshore system, the siscowet (Salvelinus namaycush siscowet Walbaum), a deepwater morphotype of lake trout, is the most abundant piscivore (Gorman et al., 2012a, b). Siscowet migrate vertically daily (diel vertical migration, DVM), characterized by moving shallower in the water column at night to consume kiyi (Coregonus kiyi Koelz), which are in turn following migrating mysid shrimp (Mysis relicta Lovén; Hrabik et al., 2006; Jensen et al., 2006; Gorman et al., 2012a). During the day, siscowet move to deeper water and are found between 80 m (Harvey et al., 2003) and 400 m (Sitar et al., 2008), where they prey upon deepwater sculpin (Myoxocephalus thompsonii Girard). Deepwater sculpin (hereafter sculpin) are a benthic, profundal species that represent the bulk of demersal prey in the deepest areas of the lake, ~ 11% of the total fish biomass in Lake Superior (Gorman et al., 2012a, b; Sierszen et al., 2014), and comprise up to 70% of the siscowet diet by mass (Stockwell et al., 2010). Due to the greater depths where siscowet forage on sculpin, these predator–prey interactions take place within a light-limited environment.
Our objective was to determine how visual foraging by siscowet on deepwater sculpin was affected by light intensity and substrate. We determined siscowet reaction distance to prey, angle of attack, forage activity, and foraging success over a range of ecologically relevant light intensities (9.0 × 108 to 1.62 × 1011 photons m−2 s−1) and substrate types (gravel, sand, and black fabric). Finally, a predictive model of benthic prey detection was developed for siscowet using the association between light intensity and reaction distance. By studying both the effects of light and substrate, we sought to determine the relative importance of each factor and their interactions (Vogel & Beauchamp, 1999). This work examines rarely studied interactions between a benthic–pelagic predator and a benthic prey that occupy a low-light environment.
Materials and methods
Fish collection and husbandry
Siscowet lake trout (N = 5) and deepwater sculpin (N = 215) were collected in collaboration with the US Geological Survey (Ashland, WI) aboard the R/V Kiyi via bottom trawls (Gorman & Keyler, 2016). Sculpin were collected on May 19, 2015, June 24, 2015, and July 31, 2015 while siscowet were collected on November 18, 2015. Two trawls (12 m Yankee bottom trawl) were towed for 10 min on each date east of Stockton Island on Lake Superior (46°54.751′N 90°30.611′W) at depths between 110 and 120 m. Trawls were towed on contour between 1000 and 1300 h.
To reduce capture, handling, and transport stress (e.g., Carmichael et al., 1984), siscowet and sculpin were transferred to temporary tanks (100 l) for 5 min containing 4–6 °C oxygenated water with 0.5% NaCl, 24 mg/l Stresscoat® (Mars Fishcare North America Inc., Chalfont, PA), and 15 mg/l tricaine methanesulfonate (MS-222). Siscowet swim bladders were vented using a sterilized (70% ethanol emersion) 14-gage veterinary needle. Needle insertion sites were cleansed with povidone iodine 10% topical solution. Fish were then transferred to continuously oxygenated 284 l transport tanks containing 4-6 °C oxygenated lake water with 0.5% NaCl, 24 mg/l Stresscoat®, and 15 mg/l MS-222 for truck transport from Ashland, WI to laboratory facilities at the University of Minnesota Duluth (Duluth, MN).
In the laboratory, Siscowets and sculpins were housed separately in four 568 l polyethylene stock tanks holding oxygenated water treated with 0.5% NaCl, 24 mg/l Stresscoat®, and 5 mg/l MS-222. Tank water was mechanically filtered (700 l/h) by electric-powered canister filters with biological media. Oxygenation of water was ceased after 7 days and carbon filtration was initiated. All tanks were maintained in cold rooms at 5.5 °C with a diel photo period of 14 h light: 10 h dark and a light intensity of 3.05 × 109 photons m−2 s−1 for the diurnal segment. Water quality was maintained at < 1 ppm ammonia, < 10 ppm nitrate, and ~ 7.2 pH, and measured twice daily for the first 2 weeks and daily thereafter. All husbandry and tank maintenance was conducted under low intensity red light (Sunbeam 40 W, 630–700 nm, ~ 1.62 × 1013 photons m−2 s−1) to limit disturbance of fish. All fish husbandry and experimentation conformed to University of Minnesota animal care protocols that were approved by the Institutional Animal Care and Use Committee Protocol ID: 1504-32496A and to recommendations in the Guide for Care and Use of Laboratory Animals of the National Institutes of Health.
Foraging arena
One 1892 l rectangular (2.28 m × 1.0 m) fiberglass tank with water temperature maintained at 5.5 °C was used for the foraging arena. The inside of the tank was lined with black cloth and water depth maintained at 0.4 m. Tank water was filtered by two power filters which were turned off when foraging trials commenced. Siscowet and sculpin were separated by an opaque acrylic lift gate before commencing trials (Fig. 1), which was then manually raised by a technician positioned behind suspended black fabric to eliminate observer interference.
Substrates
Three different substrates were used independently for each round of trials (5 light intensities), including gravel, sand, and black fabric. Using the Wentworth scale (Wentworth, 1922), gravel and sand substrates were classified by diameter (mm) of grain. Diameters of gravel (8–16 mm) and sand (0.5–1.0 mm) were designated into substrates as medium gravel and coarse sand by Wentworth class, respectively. Sand and gravel were washed and applied to the tank bottom to create a level 5-cm deep substrate.
Lighting and recording
Experimental lighting, light intensities, and recording procedures followed Keyler et al. (2015). Briefly, 500–510 nm cyan LED lights replicating downwelling light in Lake Superior (Jerome et al., 1983) were mounted above the foraging arena. Light intensity was manipulated via a controller (outside of cold room) and application of neutral density filters. For upper light intensities (1.86 × 1011 and 1.62 × 1013 photons m−2 s−1), a 0.3 neutral density filter was placed over each light source. To reach the lowest light intensities tested (6 × 109 to 9 × 108 photons m−2 s−1), a combination of 0.6 and 0.9 neutral density filters was added in addition to the 0.3 filter.
Light intensity was measured in watts cm−2 s−1 at the water’s surface using a precision radiometer and a broadband silicon light detector. Infrared LED lights provided additional illumination for three wide-angle cameras suspended above the tank for a top-down perspective. Recordings were digitally saved to a DVR.
Five tested light intensities, 9.00 × 108, 3.05 × 109, 6.00 × 109, 1.86 × 1011, and 1.62 × 1013 photons m−2 s−1 were equivalent to 0.0001, 0.0005, 0.001, 0.01, and 1 lx, and 9.00 × 108 photons m−2 s−1 (0.0001 lx) represented total darkness (no light source, sealed, blackened room). Watts cm−2 s−1 were converted to photons m−2 s−1 using the method described by Harrington et al. (2015). To determine depth (x) in Lake Superior that correlated to each tested light intensity, the Beer–Lambert equation was used (Hutchinson, 1957):
where I0 is the average Lake Superior summer solar surface irradiance from Fahnenstiel et al. (1984), and k is the attenuation value for Lake Superior waters. For lunar surface irradiance, values from Arizona (Cramer et al., 2013) were used because Lake Superior surface irradiance is reported in lux, an inappropriate unit for fish visual studies (Johnsen, 2012).
Foraging trials
Foraging trials were conducted between February 2, 2016 and March 23, 2016 after the collected siscowet resumed actively foraging for prey. Each siscowet (N = 5) was tested twice at each light intensity (N = 5) on each substrate (N = 3) for a total of 150 trials. To minimize stress on test fish, individual siscowet were limited to a maximum of 3 trials on a testing day (30 min foraging time), followed by a 24-h rest period with no trials. Each siscowet was marked with a visible implant tag to identify individual fish for foraging trials. Tags were implanted in the transparent tissue posterior to the left eye and anterior to the operculum in dermal tissue as to not affect or interfere with vision. Siscowet were small sexually mature adults ranging 420–510 mm in total length (LT) and 12–17 years in age (O. T. G., unpublished data), while adult sculpin used in foraging trials (N = 150) averaged 79.0 ± 0.58 mm LT. Prior to each trial, a 20 × 20-cm acrylic square outlined in red striping tape was temporarily placed on the bottom of the tank (removed before start of trial) and a brief recording was made to aid in digitally analyzing the distance of fish movements. Sculpins were then introduced into the foraging arena while a siscowet was placed within the holding area of the tank (Fig. 1), and both were acclimated to testing light intensity for at least 30 min. After acclimation, the gate was lifted, recording was started, and the trial was commenced for 10 min or until a prey was captured.
Foraging parameters
Digital foraging images were analyzed using ImageJ software (NIH, v. 1.5p) to determine predator reaction distance to prey and vice versa, and siscowet angle of attack. Angle of attack was calculated as the angle of the sculpin off-axis from the longitudinal axis of the siscowet from midpoint of predator eyes to midpoint of prey body just prior to first detection (turning of head) and orienting toward prey. For siscowet, we determined reaction distance as the distance from the midpoint of the siscowet’s eyes to the midpoint of the sculpin body calculated at the moment siscowet first orient toward prey. Foraging success was calculated as the overall probability of a siscowet detecting, pursuing, attacking, and retaining a sculpin (Richmond et al., 2004; also see Keyler et al., 2015). Sculpin activity prior to attack was classified as either moving or stationary. To investigate whether light intensity affected siscowet foraging activity, we arbitrarily chose a cut off of 50%, where siscowet were defined as ‘active’’ if moving around the arena for ≥ 50% of the trial and ‘non-active’ if moving < 50% of the trial.
Statistical analysis
We tested the effects of light intensity and substrate on siscowet reaction distance using a Two-way ANOVA. We used Tukey HSD post hoc tests to examine pairwise differences in reaction distance among light intensities and substrates. Differences in both prey captures and siscowet activity between foraging arena substrates were determined using a Pearson’s Chi-squared test. The correlation between overall probability of prey capture and reaction distance at each light intensity was determined using a Spearman’s rank correlation coefficient.
A Michaelis–Menten saturation function (O’Neill et al., 1989) was used to quantify the relationship between reaction distance (Rd, cm) and light intensity (Li, photons m−2 s−1):
where Rmax is the maximum reaction distance (cm) and α is the half saturation constant (photons m−2 s−1). Data were pooled across substrates because reaction distance was not significantly related to substrate type (see below). The relationship between reaction distance and light intensity for siscowet feeding on pelagic golden shiners (Notemigonus crysoleucas Mitchill) was accurately described by this model (Keyler et al., 2015).
Siscowet orientation to prey was tracked with overhead cameras over a 360° radius, with 0° located at the siscowet rostrum. Three equal segments of 60°, independent of the side of the fish, represented the forward sector (± 0°–60°), lateral sector (± 60°–120°), and rear sector (± 120°–180°). Differences in proportions of siscowet orientations among 60° increments between foraging arena substrates were tested in a 3 × 3 contingency table (3 substrates × 3 60° increments) using Fisher’s exact test. To reduce the chance of Type-I error with multiple comparisons, a Bonferroni correction was used with a significance level of 0.017. Increments of 60° were used to ensure sufficient activity within each segment for statistical analysis. The effects of light intensity and substrate on siscowet reaction distance within 60° sectors were tested for using a Two-way ANOVA.
Statistical analysis was performed using JMP software (JMP® Pro v.12.0.1, Statistical Analysis System Institute Inc., Cary, NC). Normality was tested using a Shapiro–Wilk test and equal variances were tested using a Brown–Forsythe test (α = 0.05). Pooled results were for 150 trials and those by substrate were for 50 trials.
Results
Siscowet reaction distance increased with light intensity, but was not affected by substrate type (Fig. 2). Reaction distance increased asymptotically with light intensity for all substrates from 9.00 × 108 to 6.0 × 109 photons m−2 s−1 (Two-way ANOVA, F11,180 = 3.22, p = 0.0005; light intensity main effect, F3,180 = 9.12, p < 0.0001) and then remained relatively constant at higher light intensities (Tukey’s HSD, p = 0.0146). Reaction distance was not significantly affected by substrate (substrate main effect, F2,180 = 2.79, p = 0.064). For combined substrate data, sensory detection at the lowest tested light intensity was sufficient to locate prey at 11 ± 2 cm, while increasing light intensities increased reaction distance up to 43 ± 3 cm at 1.62 × 1013 photons m−2 s−1. The effect of light intensity on reaction distance was similar among substrate types (light intensity × substrate interaction, F6,180 = 0.01, p = 0.996). Additionally, average reaction distances between individual siscowet were not significantly different (T. D. K. unpublished data).
A Michaelis–Menten function described 97% of the variation between light intensity (Li, photons m−2 s−1) and reaction distance (Rd, cm) to benthic prey (Fig. 3; R2 = 0.97, t3 = 24.01, p = 0.0016). The maximum reaction distance was Rmax= 44.02 cm and the light intensity at 50% of Rmax was α = 1.83 × 109 photons m−2 s−1.
Siscowet orientations to sculpins were unequal among 60° sectors for black fabric (Fig. 4; χ2 = 54.74, d.f. = 2, p < 0.0001), gravel (χ2 = 39.97, d.f. = 2, p < 0.0001), and sand (χ2 = 27.11, d.f. = 2, p < 0.0001), while reaction distances to sculpin were similar among 60° sectors and substrates (Two-way ANOVA, F7,196 = 1.96, p = 0.0617). For fabric and gravel, siscowet orientated toward prey more often within the forward sector (± 0°–60°, fabric N = 56, gravel N = 46) than the lateral sector (± 60°–120°, fabric N = 20, gravel N = 21) or the rear sector (± 120°–180°, fabric N = 4, gravel N = 3). For sand, siscowet never orientated toward prey in the rear sector, but oriented nearly equally to prey in front (N = 26) and lateral (N = 28) sectors.
Average reaction distance (x) and total number of orientations (n) plotted within 60° increments for combined light intensities on (A) gravel, (B) sand, and (C) black fabric substrates. The solid fish figure in the middle (left panel) represents the orientation of the fish relative to degrees off axis of prey. The degree of shading for each 60° increment indicates a higher proportion of orientations
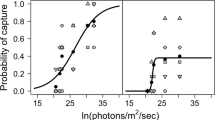
Prey capture did not differ significantly among substrates (Fig. 5; χ2 = 3.167, d.f. = 2, p = 0.21). Siscowet had 24% overall foraging success (36 prey captures) across all light intensities and substrates. Additionally, the time of day the foraging trial was conducted did not influence siscowet foraging activity or prey capture. Foraging success increased with light intensity from 0% (0 captures) at 9.00 × 108 photons m−2 s−1 to 43% success (13 captures) at 1.62 × 1013 photons m−2 s−1. Overall probability of prey capture was positively related to reaction distance at each light intensity (Fig. 6; Spearman’s rank correlation coefficient, ρ(rho) = 0.9, p = 0.037). Below 6.0 × 109 photons m−2 s−1, reaction distance and probability of capture began to decline for all 3 substrates and in dark trials, reactions to prey were all ≤ 20 cm with no captures (Fig. 7). Finally, foraging success was independent of siscowet activity at all non-zero light levels. Average siscowet activity exceeded 50% for 80% of trials on all substrates at non-zero light levels, but declined to 53% in dark trials. Activity did not differ significantly among the 3 substrates (Table 1; χ2 = 1.632, d.f. = 2, p = 0.44).
Correlation of prey capture probability (dashed line) to siscowet reaction distance (solid line) at corresponding light intensities for pooled substrates. Spearman’s rank correlation coefficient, ρ = 0.9. Gray, dotted vertical lines indicate light intensity range where both capture probability and reaction distance decline
Polar plot diagram of reaction distance (cm) and angle of attack for siscowet lake trout in response to benthic prey for pooled substrates at three light intensities ranging from high to low (A) 1.86 × 1011 (B) 3.05 × 109 and (C) 9.00 × 108 photons m−2 s−1. Solid shapes (unfilled square) represent orientation toward prey and open shapes (unfilled square) represent a capture. Each concentric circle represents 20 cm distance
Discussion
Light can determine the timing and success of visually foraging fishes (Aksnes & Utne, 1997; Boscarino et al., 2010). Due to diurnal migration behavior and depths of water inhabited, siscowet consistently occupy a low-light environment. Our study revealed that siscowet were able to forage in extremely low light, especially when compared to other lake trout morphotypes (see Vogel & Beauchamp, 1999; Mazur & Beauchamp, 2003). Siscowet reaction distance in our trials increased with light intensity up to 6.0 × 109 photons m−2 s−1, after which reaction distance was constant. The number of prey captures also increased with light intensity, with most orientations toward prey occurring within the siscowet’s forward sector (± 0°–60°). Finally, overall probability of prey capture was strongly related to reaction distance at each light intensity. While substrate is known to influence prey behavior and crypsis, (Ellis et al., 1997; Ruxton et al., 2004; Stevens & Cuthill, 2006), neither siscowet reaction distance nor prey capture were affected by substrate type in our trials, a possible product of decreased contrast perception at lower light intensities (Virostek & Franckowiak, 2014).
The effect of light intensity on prey detection and foraging success is critical when defining spatial and temporal limits of a species’ niche (Mazur & Beauchamp, 2003). On a clear summer day, our highest light intensity in our study correlates to a depth of ~ 130 m in Lake Superior, although siscowet are typically found below 100 m during the day (Selgeby & Hoff, 1996; Hrabik et al., 2006; Gorman, et al., 2012a). Our results suggest that siscowet can visually forage well below 150 m and reaction distance to prey would not be reduced until depths > 200 m (6.00 × 109 photons m−2 s−1) in Lake Superior (using solar light intensities predicted at depth from Keyler et al., 2015). At the second lowest light intensity (3.05 × 109 photons m−2 s−1, equivalent to ~ 205 m), siscowet may visually forage at reduced reaction distances (~ 27 cm; Fig. 8) and prey capture success (10%). Reaction distances of Siscowet in dark trials (≤ 20 cm) were consistent with other dark-tested species, thereby suggesting mechanosensory detection of prey that is limited to half the body length of a predator (Price & Mensinger, 1999; Palmer et al., 2005; Keyler et al., 2015). Siscowet in the dark profundal zone of Lake Superior (below 3.05 × 109 photons m−2 s−1) likely discontinue visual foraging in favor of other sensory modalities such as mechanosensory or chemosensory detection of prey.
Despite siscowet foraging efficiency being reduced at light intensities of 3.05 × 109 photons m−2 s−1 and lower, successful capture of prey within dark profundal zone of Lake Superior is likely a product of greater density and biomass of sculpins at depths > 100 m (Stockwell et al., 2010; Gorman et al., 2012a, b; Pratt et al., 2016). Trawl data and stomach analysis confirm consumption of sculpin by siscowet during the day when deeper in the water column (O. T. G., unpublished data). Some predators forage in suboptimal conditions, if prey densities are high (Crowder & Cooper, 1982), which compensates for increased search time (Ware, 1973). To optimize foraging, siscowet maximize energy (prey) per unit effort, including time and energy spent foraging (Werner & Hall, 1974). Due to diel vertical migration behavior, siscowet effectively change foraging habitats throughout a 24-h period, a strategy many animals use to optimize their foraging (Werner & Hall, 1979; Mittelbach, 1981). Lack of prey higher in the water column during the day likely drives siscowet into deeper and darker waters where prey density and foraging success are higher (Ahrenstorff et al., 2011).
Siscowet have physiological and morphological visual adaptations for foraging in dark, deep, offshore waters of Lake Superior. Siscowet in our trials generally oriented to prey head-on upon detection, while maneuvering to employ binocular vision within the forward sector (± 0°–60°; Vogel & Beauchamp, 1999). Morphologically, siscowet vision may be improved by their large eyes that are positioned higher on the head (when compared to the other morphotypes), thereby improving light capture (van der Meer & Anker, 1984), and binocular vision (Bond, 1996). Individually, a single fish eye has a wide field of view, approaching up to 360° (Land & Nilsson, 2012), although a binocular field of vision (binocular overlap) is small for fishes with laterally situated eyes. Increased forward orientations (± 0°–60°) may maximize binocular vision, which provides stereopsis, or depth perception, to aid in prey capture (Duke-Elder, 1958).
Physiologically, fishes are generally sensitive to the spectrum of light that is available within their environment. Accordingly, lake trout have a peak visual pigment sensitivity at 512 nm (rhodopsin) and 539 nm (porphyropsin) (Munz & McFarland, 1965), which matches the seasonally varying downwelling spectra in Lake Superior (500–550 nm) (Jerome et al., 1983). Similarly, Harrington et al. (2015) determined siscowet peak retinal sensitivity via electroretinography, which accounts for the in vivo neural processing associated with vision, and determined maximum siscowet sensitivity at 525 nm with relatively broad sensitivity from 500 to 550 nm, again optimally aligning with the seasonal downwelling spectra. Additionally, siscowet show aversion to light. Since they are found at depths > 80 m and are common at depths > 100 m, the light intensity from 80 to 100 m may represent the upper limit of light tolerance. Siscowet may move shallower in the water column at night when light levels are sufficiently low for visual foraging (M. Seider, U.S. Fish and Wildlife Service, Unpublished data).
Siscowets captured the fewest sculpins on gravel substrate, which may have provided the greatest camouflage for prey. The narrow band of wavelengths provided by LED lighting (500–510 nm) in our trials decreased contrast, likely obscuring camouflage effects at lower tested light levels. For higher tested light intensities, gravel likely offered disruptive patterning that complemented the sculpin’s coloration pattern to aid in crypsis. The disruptive pattern of the sculpin, including a brown body with dark dorsal saddles, banded pectoral fins, spotted sides and pelvic fins, and blotchy dorsal and anal fins (Scott & Crossman, 1973), may have hindered detection by siscowet (Muntz, 1990; De Robertis et al., 2003). Prey species employ a variety of strategies to avoid predator detection including crypsis (Ellis et al., 1997; Ruxton et al., 2004; Stevens & Cuthill, 2006), use of cover (Mittelbach, 1981; Cerri & Fraser, 1983; Sass et al., 2006), and reducing activity when predators are present (Dill, 1983; Dill & Fraser, 1984; Sih, 1986; Prejs, 1987). Crypsis is an especially effective tactic for benthic species, and substrates that offer sufficient camouflage can alter a prey’s response to predators (Houtman & Dill, 1994; Sowersby et al., 2015). Foraging trials with benthic species like sculpin must therefore consider the foraging arena substrate to encourage normal predator–prey response behavior, and ultimately ensure accuracy of foraging parameters.
Our tested substrates may not have facilitated natural sculpin behavior, which may have hindered their detection by siscowets. The natural lakebed of Lake Superior consists of soft gray clay in the eastern basin and red clay in the western basin (O. T. G., unpublished data). The use of clay substrate in our trials proved impractical due to high turbidity that obscured foraging recordings. Sand offered similar uniformly colored habitat, although clay would have allowed sculpin to burrow and better hide, which was not observed on sand substrate. For example, slimy sculpin (Cottus cognatus J. Richardson) have been observed burrowing into looser sediments when approached by divers (Brandt & Madon, 1986); however, this species occupies shallower depths where they are more readily detected. Burrowing behavior in sculpin would likely reduce siscowet reaction distance and foraging success, although observational data suggest slimy sculpins are more adept at burrowing into soft sediments than deepwater sculpins (C. P. Madenjian, U.S. Geological Survey 2019, unpublished data).
Future behavioral studies examining the response of prey fishes to piscivorous species, not just predators to prey, would help to provide a more comprehensive understanding of predator–prey interactions within offshore systems. By simultaneously determining prey response through metrics such as reaction distance, we may be able to determine which species has a first detection advantage. Sculpin, for example, may detect siscowet first because their eyes are oriented upward, which allows them to silhouette potential predators from a benthic position. Additionally, foraging mechanisms of siscowet on benthic and pelagic prey should be compared to examine how piscivory may vary among systems (Beauchamp et al., 1999). Reaction distances may be less for benthic species that lie below the siscowet’s plane of vision, compared to pelagic species. These considerations would allow for more accurate foraging models (Jensen et al., 2006; Hansen et al., 2013), which can provide better estimates of foraging success, effects on habitat use by benthic and pelagic fishes, and information on how apex predators like siscowet influence community structure.
Change history
11 May 2020
Due to a mistake during the production process, the online/HTML version of the original article was published under the terms of the Creative Commons Attribution 4.0 International License. The article is not open access and no special license applies. The original article has been corrected.
References
Ahrenstorff, T. D., T. R. Hrabik, J. D. Stockwell, D. L. Yule & G. G. Sass, 2011. Seasonally dynamic diel vertical migrations of Mysis diluviana, coregonine fishes, and siscowet lake trout in the pelagia of western Lake Superior. Transactions of the American Fisheries Society 140: 1504–1520.
Aksnes, D. L. & A. C. W. Utne, 1997. A revised model of visual range in fish. Sarsia 82: 137–147.
Beauchamp, D. A., C. M. Baldwin, J. L. Vogel & C. P. Gubala, 1999. Estimating diel, depth-specific foraging opportunities with a visual encounter rate model for pelagic piscivores. Canadian Journal of Fisheries and Aquatic Sciences 56: 128–139.
Beauchamp, D. A., A. D. Cross, J. L. Armstrong, K. W. Myers, J. H. Moss, J. L. Boldt & L. J. Haldorson, 2007. Bioenergetic responses by Pacific salmon to climate and ecosystem variation. North Pacific Anadromous Fish Commission Bulletin 4: 257–269.
Bond, C. E., 1996. Biology of Fishes. Saunders College Publishing, Fort Worth.
Boscarino, B. T., L. G. Rudstam, J. Tirabassi, J. Janssen & E. R. Loew, 2010. Light effects on alewife-mysid interactions in Lake Ontario: a combined sensory physiology, behavioral, and spatial approach. Limnology and Oceanography 55: 2061–2072.
Brandt, S. B. & S. P. Madon, 1986. Rainbow smelt (Osmerus mordax) predation on slimy sculpin (Cottus cognatus) in Lake Ontario. Journal of Great Lakes Research 12: 322–325.
Carmichael, G. J., J. R. Tomasso, B. A. Simco & K. B. Davis, 1984. Characterization and alleviation of stress associated with hauling largemouth bass. Transactions of the American Fisheries Society 113: 778–785.
Carpenter, S. R., J. F. Kitchell & J. R. Hodgson, 1985. Cascading trophic interactions and lake productivity. BioScience 35: 634–639.
Cerri, R. D. & D. F. Fraser, 1983. Predation and risk in foraging minnows: balancing conflicting demands. The American Naturalist 121: 552–561.
Crowder, L. B., W. E. Cooper, 1979. Structural complexity and fish–prey interactions in ponds: a point of view. In: Response of Fish to Habitat Structure in Standing Water. American Fisheries Society, North Central Division, Special Publication 6: 2–10.
Crowder, L. B. & W. E. Cooper, 1982. Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63: 1802–1813.
Crowder, L. B., J. J. Magnuson & S. B. Brandt, 1981. Complementarity in the use of food and thermal habitat by Lake Michigan fishes. Canadian Journal of Fisheries and Aquatic Sciences 38: 662–668.
Cramer, C. E., K. R. Lykke, J. T. Woodward & A. W. Smith, 2013. Precise measurement of lunar spectral irradiance at visible wavelengths. Journal of Research of the National Institute of Standards and Technology 118: 396.
De Robertis, A., C. H. Ryer, A. Veloza & R. D. Brodeur, 2003. Differential effects of turbidity on prey consumption of piscivorous and planktivorous fish. Canadian Journal of Fisheries and Aquatic Sciences 60: 1517–1526.
Dill, L. M., 1983. Adaptive flexibility in the foraging behavior of fishes. Canadian Journal of Fisheries and Aquatic Sciences 40: 398–408.
Dill, L. M. & A. H. Fraser, 1984. Risk of predation and the feeding behavior of juvenile coho salmon (Oncorhynchus kisutch). Behavioral Ecology and Sociobiology 16: 65–71.
Duke-Elder, S., 1958. A Century of International Ophthalmology, 1857–1957. Kimpton.
Eggers, D. M., 1977. The nature of prey selection by planktivorous fish. Ecology 58: 46–59.
Ellis, T., B. R. Howell & R. N. Hughes, 1997. The cryptic responses of hatchery-reared sole to a natural sand substratum. Journal of Fish Biology 51: 389–401.
Fahnenstiel, G. L., C. L. Schelske & R. A. Moll, 1984. In situ quantum efficiency of Lake Superior phytoplankton. Journal of Great Lakes Research 10: 399–406.
Gorman, O.T., & T. D. Keyler, 2016. A Hyperbaric Holding & Transport Vessel for Collection of Deepwater Fishes for Research and Broodstock Development. U.S. Geological Survey.
Gorman, O. T., D. L. Yule & J. D. Stockwell, 2012a. Habitat use by fishes of Lake Superior. I. Diel patterns of habitat use in nearshore and offshore waters of the Apostle Islands region. Aquatic Ecosystem Health & Management 15: 333–354.
Gorman, O. T., D. L. Yule & J. D. Stockwell, 2012b. Habitat use by fishes of Lake Superior. II. Consequences of diel habitat use for habitat linkages and habitat coupling in nearshore and offshore waters. Aquatic Ecosystem Health & Management 15: 355–368.
Hansen, A. G., D. A. Beauchamp & E. R. Schoen, 2013. Visual prey detection responses of piscivorous trout and salmon: effects of light, turbidity, and prey size. Transactions of the American Fisheries Society 142: 854–867.
Harrington, K. A., T. R. Hrabik & A. F. Mensinger, 2015. Visual sensitivity of deepwater fishes in Lake Superior. PLoS ONE 10: e0116173.
Harvey, C. J., S. T. Schram & J. F. Kitchell, 2003. Trophic relationships among lean and siscowet lake trout in Lake Superior. Transactions of the American Fisheries Society 132: 219–228.
Houtman, R. & L. M. Dill, 1994. The influence of substrate color on the alarm response of tidepool sculpins (Oligocottus maculosus; Pisces, Cottidae). Ethology 96: 147–154.
Howick, G. L. & W. J. O’Brien, 1983. Piscivorous feeding behavior of largemouth bass: an experimental analysis. Transactions of the American Fisheries Society 112: 508–516.
Hrabik, T. R., O. P. Jensen, S. J. D. Martell, C. J. Walters & J. F. Kitchell, 2006. Diel vertical migration in the Lake Superior pelagic community. I. Changes in vertical migration of coregonids in response to varying predation risk. Canadian Journal of Fisheries and Aquatic Sciences 63: 2286–2295.
Hutchinson, G. E., 1957. A Treatise on Limnology. Geology, Physics, and Chemistry, Vol. 1. Wiley, New York.
Jensen, O. P., T. R. Hrabik, S. J. Martell, C. J. Walters & J. F. Kitchell, 2006. Diel vertical migration in the Lake Superior pelagic community. II. Modeling trade-offs at an intermediate trophic level. Canadian Journal of Fisheries and Aquatic Sciences 63: 2296–2307.
Jerome, J. H., R. P. Bukata & J. E. Bruton, 1983. Spectral Attenuation and Irradiance in the Laurentian Great Lakes. Journal of Great Lakes Research 9: 60–68.
Johnsen, S., 2012. The Optics of Life: A Biologist’s Guide to Light. Princeton University Press, New Jersey.
Keyler, T. D., T. R. Hrabik, C. L. Austin, O. T. Gorman & A. F. Mensinger, 2015. Foraging mechanisms of siscowet lake trout (Salvelinus namaycush siscowet) on pelagic prey. Journal of Great Lakes Research 41: 1162–1171.
Kerfoot, W. C. & A. Sih, 1987. Predation: Direct and Indirect Impacts on Aquatic Communities. University Press of New England, Hanover.
Land, M. F. & D. E. Nilsson, 2012. Animal Eyes. Oxford University Press, Oxford.
Mazur, M. M. & D. A. Beauchamp, 2003. A comparison of visual prey detection among species of piscivorous salmonids: effects of light and low turbidities. Environmental Biology of Fishes 67: 397–405.
Miner, J. G. & R. A. Stein, 1996. Detection of predators and habitat choice by small bluegills: effects of turbidity and alternative prey. Transactions of the American Fisheries Society 125: 97–103.
Mittelbach, G. G., 1981. Foraging efficiency and body size: a study of optimal diet and habitat use by bluegills. Ecology 62: 1370–1386.
Muntz, W. R., 1990. Stimulus, environment and vision in fishes. The visual System of Fish. Springer, Dordrecht.
Munz, F. W. & W. N. McFarland, 1965. A suggested hereditary mechanism for visual pigments of chars (Salvelinus spp.). Nature 206: 955.
Munz, F. W. & W. N. McFarland, 1977. Evolutionary adaptations of fishes to the photic environment. The Visual System in Vertebrates. Springer, Berlin.
O’Neill, R. V., D. L. DeAngelis, J. J. Pastor, B. J. Jackson & W. M. Post, 1989. Multiple nutrient limitations in ecological models. Ecological Modelling 46: 147–163.
Palmer, L. M., M. Deffenbaugh & A. F. Mensinger, 2005. Sensitivity of the anterior lateral line to natural stimuli in the oyster toadfish, Opsanus tau (Linnaeus). Journal of Experimental Biology 208: 3441–3450.
Pratt, T. C., O. T. Gorman, W. P. Mattes, J. T. Myers, H. R. Quinlan, D. R. Schreiner, M. J. Seider, S. P. Sitar, D. L. Yule, & P. M. Yurista, 2016. The state of Lake Superior in 2011 [online]. Available from: http://www.glfc.org/pubs/SpecialPubs/Sp16_01.pdf [accessed 3 November 2017].
Price, N. N. & A. F. Mensinger, 1999. Predator-prey interactions of juvenile toadfish, Opsanus tau. The Biological Bulletin 197: 246–247.
Prejs, A., 1987. Risk of predation and feeding rate in tropical freshwater fishes: field evidence. Oecologia 72: 259–262.
Richmond, H. E., T. R. Hrabik & A. F. Mensinger, 2004. Light intensity, prey detection and foraging mechanisms of age-0 year yellow perch. Journal of Fish Biology 65: 195–205.
Roth, B. M., K. A. Rose, L. P. Rozas & T. J. Minello, 2008. Relative influence of habitat fragmentation and inundation on brown shrimp Farfantepenaeus aztecus production in northern Gulf of Mexico salt marshes. Marine Ecology Progress Series 359: 185–202.
Ruxton, G. D., T. N. Sherratt & M. P. Speed, 2004. Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry. Oxford University Press, Oxford.
Sass, G. G., C. M. Gille, J. T. Hinke & J. F. Kitchell, 2006. Whole-lake influences of littoral structural complexity and prey body morphology on fish predator–prey interactions. Ecology of Freshwater Fish 15: 301–308.
Scott, W. B. & E. J. Crossman, 1973. Freshwater fishes of Canada. Journal of the Fisheries Research Board of Canada 184: 966.
Selegby, J. H., & M. H. Hoff, 1996. Seasonal Bathymetric Distribution of 16 Fishes in Lake Superior 1958-75 (No. BSR-7). National Biological Service, Ashland WI, Great Lakes Science Center.
Sierszen, M. E., T. R. Hrabik, J. D. Stockwell, A. M. Cotter, J. C. Hoffman & D. L. Yule, 2014. Depth gradients in food-web processes linking habitats in large lakes: Lake Superior as an exemplar ecosystem. Freshwater Biology 59: 2122–2136.
Sih, A., 1986. Antipredator responses and the perception of danger by mosquito larvae. Ecology 67: 434–441.
Sitar, S. P., H. M. Morales, M. T. Mata, B. B. Bastar, D. M. Dupras, G. D. Kleaver & K. D. Rathbun, 2008. Survey of Siscowet Lake Trout at Their Maximum Depth in Lake Superior. Journal of Great Lakes Research 34: 276–286.
Sowersby, W., R. M. Thompson & B. B. M. Wong, 2015. Invasive predator influences habitat preferences in a freshwater fish. Environmental Biology of Fishes 99: 187–193.
Stevens, M. & I. C. Cuthill, 2006. Disruptive coloration, crypsis and edge detection in early visual processing. Proceedings of the Royal Society B 273: 2141–2147.
Stockwell, J. D., T. R. Hrabik, O. P. Jensen, D. L. Yule & M. Balge, 2010. Empirical evaluation of predator-driven diel vertical migration in Lake Superior. Canadian Journal of Fisheries and Aquatic Sciences 67: 473–485.
van der Meer, H. J. & G. C. Anker, 1984. Retinal resolving power and sensitivity of the photopic system in seven haplochromine species (Cichlidae, Teleostei). Netherlands Journal of Zoology 34: 197–209.
Virostek, K., & B. Franckowiak, 2014. What can you see in the dark? The effects of contrast, light, and age on contrast sensitivity in low light. Journal of Emerging Investigators 1–4.
Vogel, J. L. & D. A. Beauchamp, 1999. Effects of light, prey size, and turbidity on reaction distances of lake trout (Salvelinus namaycush) to salmonid prey. Canadian Journal of Fisheries and Aquatic Sciences 56: 1293–1297.
Ware, D. M., 1973. Risk of epibenthic prey to predation by rainbow trout (Salmo gairdneri). Journal of the Fisheries Research Board of Canada 30: 787–797.
Wentworth, C. K., 1922. A scale of grade and class terms for clastic sediments. The Journal of Geology 30 (5):377–392.
Werner, E. E. & D. J. Hall, 1974. Optimal foraging and the size selection of prey by the bluegill sunfish (Lepomis macrochirus). Ecology 55: 1042–1052.
Werner, E. E. & D. J. Hall, 1979. Foraging efficiency and habitat switching in competing sunfishes. Ecology 60: 256–264.
Acknowledgements
For assistance with collection of deepwater fishes, the authors thank the crew of the RV Kiyi. Funding for this project was provided by the US Geological Survey, Lake Superior Biological Station, University of Minnesota Duluth Biology Department, and National Science Foundation Grants IOS 1354745 and DOB 1359230 (AFM). The authors are grateful for the help and support from I. Harding, M. Pawlowski, J. Dobosenski, Minnesota Sea Grant, and the McNair Scholars Program at the University of Wisconsin-Superior. A special thanks to M. Joyce for help with statistical aspects of the paper, B. Matthias for his helpful editorial comments, and R. Mahling for his modeling expertise. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: C. E. Adams, C. R. Bronte, M. J. Hansen, R. Knudsen, & M. Power/Charr Biology, Ecology and Management
Rights and permissions
About this article
Cite this article
Keyler, T.D., Hrabik, T.R., Mensinger, A.F. et al. Effect of light intensity and substrate type on siscowet lake trout (Salvelinus namaycush siscowet) predation on deepwater sculpin (Myoxocephalus thompsonii). Hydrobiologia 840, 77–88 (2019). https://doi.org/10.1007/s10750-019-3944-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-3944-5