Abstract
Two sand-dwelling cichlids from Lake Malawi (Aulonocara stuartgranti, Tramitichromis sp.) that feed on benthic invertebrates, but have different lateral line phenotypes, use lateral line and/or visual cues to detect prey under light versus dark conditions. The current study examined how ecologically relevant variation in light intensity [0–800 lux (lx)] influences detection of prey (mobile, immobile) in each species by analyzing six behavioral parameters. Both species fed at light intensities ≥1 lx and trends in behavior among light intensities were informative. However, prey type and/or time of day (but not light intensity) predicted all four parameters analyzed with generalized linear mixed models in A. stuartgranti, whereas the interaction of light intensity and time of day predicted three of these parameters in Tramitichromis sp. Data suggest that the critical light intensity is 1–12 lx for both species, that the integration of visual and lateral line input explains differences in detection of mobile and immobile prey and behavioral changes at the transition from 1 to 0 lx in A. stuartgranti, and that Tramitichromis sp. likely uses binocular vision to locate prey. Differences in the sensory biology of species that exploit similar prey will have important implications for the trophic ecology of African cichlid fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light in aquatic habitats varies in quality and quantity over time and space (Kirk 2011) and influences the ability of visual predators to detect and capture mobile prey (Vinyard and O’Brien 1976; Confer et al. 1978; Lythgoe 1979; Ryer and Olla 1999; Vogel and Beauchamp 1999; Rickel and Genin 2005). Fishes occupying similar habitats may demonstrate variation in visually mediated prey detection abilities as a result of different visual thresholds and absorption spectra of visual pigments, which may provide a competitive advantage under particular light conditions (Vogel and Beauchamp 1999; Hofmann et al. 2009). Many fishes are also able to detect prey at low light intensities (e.g., dawn, dusk, at depth, or with increased turbidity), but with reduced capabilities compared to that at higher light intensities. The distance at which free swimming prey are detected dramatically decreases below a certain light intensity (“critical light intensity,” Confer et al. 1978) in salmonids (Dunbrack and Dill 1984; Henderson and Northcote 1985) and some freshwater percomorphs (bluegill, Lepomis macrochirus, Vinyard and O’Brien 1976; largemouth bass, Micropterus salmoides, Howick and O’Brien 1983; yellow perch, Perca flavescens, Richmond et al. 2004).
Given the importance of multimodal sensory integration in the formulation of behavior, the contributions of the non-visual sensory systems to prey detection (e.g., mechanosensory lateral line, auditory, olfactory, gustatory, somatosensory/tactile, and in some cases, the electrosensory system; reviewed in Montgomery et al. 2014) must also be considered. Morphological and/or physiological specializations of non-visual sensory systems, including the olfactory system (Parzefall 1993; Montgomery et al. 1999), gustatory system (Atema 1971), and the lateral line system (Janssen 1997; Schwalbe et al. 2012; reviewed in Webb 2014), have been used to predict how these senses provide alternatives to vision for prey detection in light-limited environments. Futhermore, the integration of different combinations of sensory inputs may explain variation in behavior under different environmental conditions (Partridge and Pitcher 1980; Moller 2002; Montgomery et al. 2003; Gardiner and Motta 2012). For instance, several species of fishes have been shown to modulate feeding strategies using a combination of visual and non-visual cues that allow them to feed under a range of light conditions, including darkness (Townsend and Risebrow 1982; Batty et al. 1986; Diehl 1988; Schwalbe et al. 2012).
The mechanosensory lateral line system is known to play important roles in prey detection, as well as in predator avoidance, communication, and navigation around obstacles (Webb et al. 2008; Montgomery et al. 2014). The system demonstrates a great deal of variation, which is defined by the morphology of the cranial and trunk lateral line canals and neuromast receptor organs within them, and the distribution of superficial neuromasts on the skin of the head, trunk, and tail (reviewed in Webb 2014). Widened lateral line canals, one of five cranial lateral line canal phenotypes found among bony fishes, has evolved convergently in ~15 teleost families (including many deep sea taxa) and appears to be an adaptation for enhanced sensitivity to water flows including those generated by prey (Denton and Gray 1988, 1989; Montgomery and Coombs 1992; discussed in Schwalbe et al. 2012; reviewed in Webb 2014).
The speciose cichlid fishes of the African Rift Lakes are typically described as visual feeders (Fryer and Iles 1972) and most genera have narrow cranial lateral line canals, but all members of a few genera (e.g., Alticorpus, Aulonocara, Aulonocranus, Trematocara, Trematocranus, Konings 2007) have widened lateral line canals suggesting the capacity for lateral-line-mediated prey detection (Konings 1990). Two genera of non-mbuna (sand-dwelling, demersal), haplochromine cichlids in Lake Malawi, Aulonocara (widened canals) and Tramitichromis (narrow canals; Fig. 1), provide an interesting taxon pair for comparison of prey detection strategies since both feed on benthic invertebrates in the sand. Schwalbe et al. (2012) and Schwalbe and Webb (2014) analyzed the behavioral responses of Aulonocara stuartgranti and Tramitichromis sp. to tethered live and dead prey (=adult brine shrimp, Artemia sp.), in experiments carried out under light and dark conditions and in which the lateral line system was experimentally inactivated. These studies demonstrated that A. stuartgranti uses a combination of inputs to its visual and lateral line systems to detect prey in the light, but that it depends on its lateral line system to detect prey in the dark. Furthermore, these studies showed that, in the dark, deactivation of the lateral line system of A. stuartgranti significantly affected prey detection behavior and revealed that other senses (olfaction, gustation, and somatosensory/tactile) were insufficient to initiate prey detection behavior. In contrast, Tramitichromis sp. did not feed in the dark, and the inactivation of the lateral line system had little effect on prey detection behavior in the presence of light, demonstrating its dependence on vision for prey detection.
Lateral line canals and canal neuromasts in Aulonocara stuartgranti (widened canals) and Tramitichromis (narrow canals) visualized using fluorescent vital staining (4-di-2-ASP, 63 µM, 5 min; a, c), µCT imaging (reconstructed from 14 µm slices; b, d, e, g, h), and scanning electron microscopy (f). a Lateral view of A. stuartgranti revealing series of larger infraorbital (IO), mandibular (MD), and preopercular (PO) canal neuromasts and very small superficial neuromasts on the skin [juvenile, 25 mm standard length (SL)]. Neuromast number and distribution is the same in Tramitichromis, b µCT reconstruction of A. stuartgranti indicating the location of the supraorbital (SO), IO, MD, and PO canals in dermatocranial bones (adult, 78 mm SL). c Ventral view of the head of A. stuartgranti, revealing canal neuromasts in the MD and PO canals (juvenile, 28 mm SL). d A. stuartgranti (adult, 78 mm SL) and e Tramitichromis (adult, 79 mm SL) in ventral view. Asterisks denote the locations of the MD and PO canal neuromasts, as visualized in c; canal neuromasts are found in floor of the canal, between canal pore positions in the canal roof. Note the much larger pores in A. stuartgranti (d) than in Tramitichromis (e). f MD canal neuromast in a juvenile A. stuartgranti. Sensory hair cells are evident in an elongate sensory strip in the middle of the diamond-shaped neuromast. Double-headed arrow indicates the axis of physiological sensitivity of the hair cells, as well as the long axis of the canal in which the neuromast is found. Scale bar 10 µm. g A. stuartgranti and h Tramitichromis in frontal-ventral view with the pores of the SO, IO, MD, and PO canals that are directed ventrally, toward the source of stimuli generated by benthic prey. The pores on the right side of each fish in g and h have been enhanced to increase their visibility
Aulonocara and Tramitichromis species appear to share a food resource (benthic invertebrates in sandy substrates), but they occupy different depth ranges (Aulonocara species at depths of 5–120 m and Tramitichromis species at depths of <15 m; Fryer and Iles 1972; Konings 1990, 2007) and use different strategies to detect and capture benthic invertebrate prey in the field. Species of Aulonocara swim just above the substrate to sense hydrodynamic flows generated by benthic invertebrates in or on the substrate (Konings 2007). In contrast, species of Tramitichromis typically capture invertebrate prey by plunging into the substrate, filling their mouth with sand, and sifting out prey with their gill rakers in the field (=“sand sifting,” Fryer 1959). This sand sifting behavior appears to be synonymous with the “winnowing” behaviors observed in some surfperches (Laur and Ebeling 1983) and vision likely contributes to the ability to locate patches of high-quality food resources (Holbrook and Schmitt 1984; Schmitt and Holbrook 1984).
This study uses the same methods used in prior studies (Schwalbe et al. 2012; Schwalbe and Webb 2014) to test the hypothesis that variation in light intensity (0–800 lx) will have different effects on the detection of live (mobile) and dead (immobile) benthic invertebrate prey by A. stuartgranti and Tramitichromis sp.
Materials and methods
Study species
Adult Aulonocara stuartgranti and Tramitichromis sp. (unidentifiable to species level, J. Stauffer, pers. commun.; referred to as Tramitichromis throughout) were reared in the laboratory from breeding stock originally acquired from commercial suppliers (A. stuartgranti: Bluegrass Aquatics, Louisville, KY, USA; Tramitichromis: Old World Exotic Fish, Inc., Homestead, FL, USA and Life Fish Direct, Draper, UT, USA). They were housed in small groups by species in 190 L aquaria at 26 ± 1 °C and 1.0 ± 0.2 p.p.t. salinity (using Cichlid Lake Salt, Seachem Laboratories, Inc., Madison, GA, USA) with standard white fluorescent light on a 12 h:12 h diurnal cycle and equipped with appropriate mechanical and biological filtration. Fish were fed daily with cichlid pellets (New Life Spectrum Cichlid Formula; New Life International, Inc., Homestead, FL) and supplemented with live adult brine shrimp. Individual fish were not used in feeding experiments if breeding behavior was observed. Animal care and all experimental procedures followed an approved IACUC protocol.
Light environment in the experimental tank
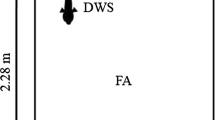
Light in the experimental tank was provided by two fluorescent light fixtures (Lithonia Lighting, Model GRW 2 14 CSW CO M4, Conyers, GA, USA) fitted with full spectrum bulbs (BlueMax lamps, Full Spectrum Solutions, Jackson, MI, USA) positioned above the tank and within a four-sided opaque curtain enclosure. The curtain (black canvas) was suspended from a rectangular plywood frame placed 2 m above the top of the tank to exclude ambient light from entering the set-up during all behavioral trials (Fig. 2a). Light intensity was varied by changing the height of the lights above the water surface and by using combinations of different neutral density filters covering the lights (Lee Filters, Burbank, CA, USA). Light intensity [in lux (lx), lumen/m2, and photosynthetically active radiation (PAR), µmol photons/m2/s] and color spectrum were measured using a spectrometer (range 340–1,028 nm, Jaz spectrometer, Ocean Optics, Dunedin, FL, USA) connected to a 2-m optical fiber (QP400-2-UV/VIS, Ocean Optics) fitted with a cosine corrector (CC-3, Ocean Optics). Water temperature was monitored during experiments and the fluorescent bulbs did not raise the temperature of the experimental tank.
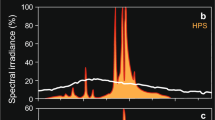
Experimental setup and light conditions used to record feeding behavior of A. stuartgranti and Tramitichromis. a Diagram of experimental tank with front panel of light curtain removed. Light is provided by two fluorescent light fixtures (full spectrum light) and two infrared (IR) lights. b Sample light spectra generated by fluorescent lights in behavioral trials. The y-axis is compressed to illustrate peaks in the visual spectrum (400–700 nm) in 1–800 lx trials. These peaks were consistent when light intensity was decreased with the addition of neutral density filters among trials (see Table 1). The peak at 840 nm is from two IR lights used in 0 and 1 lx trials only. c Mean (±s.e.m.) light intensities measured before and after trials did not differ at any of the light intensities used (Students t-test, P > 0.05)
Light intensities used in this study were based on the following data and calculations: first, light levels present during sunrise/sunset to darkness are known for other freshwater habitats (Harden Jones 1956; Ali 1959) and can range from 1,000 lx (early sunset/late sunrise) to 0 lx (new moon, Table 1). Second, few direct measurements of light intensities at different depths in Lake Malawi are available, so the light intensity at specific depths was estimated using the following equation:
where I s and I t are the light intensities at the surface (S) and at depth (T); and ε is the light extinction coefficient. The average light intensity at the surface of Lake Malawi at midday on a clear sunny day is approximately 2,000 µmol photons/m2/s (~108,000 lx). This photon flux was derived from cloudless surface irradiance for Lake Malawi (Guildford et al. 2000). Using light extinction coefficient of either 0.10 m−1 (Patterson et al. 2000), 0.13 m−1 (Guildford et al. 2007), or 0.43 m−1 (Guildford et al. 2007) depending on location and season, the light intensity at many depths can be estimated under these conditions (Table 1).
Full spectrum bulbs were used because they provide the range of wavelengths that correspond to the range of known absorption peaks of retinal photopigments in species of Aulonocara and Tramitichromis. For instance, absorption peaks for A. hueseri are at 415 nm (violet), 484 nm (blue–green), and 526 nm (green; Jordan et al. 2006) and absorption peaks for T. intermedius are at 455 nm (blue), 532 nm (green), and 569 nm (red; Parry et al. 2005). In the experimental tank, full spectrum bulbs generated major and minor peaks at 404, 435, 487, 545, 587, and 611 nm, and the addition of neutral density filters used to change light intensity did not appreciably change the light spectrum in the experimental tank (Fig. 2b).
Experiments
Behavioral trials and video analysis of six well-defined behavioral parameters [number of prey strikes, detection distance, detection angle, detection-to-strike velocity, swimming phase (glide, pause) at detection and, prey type preference (order of prey strikes)] were carried out as in Schwalbe et al. (2012) and Schwalbe and Webb (2014) with slight modifications. A total of 60 trials were conducted using A. stuartgranti [30 trials, n = 6 fish, 75–85 mm total length (TL), 4 females, 2 males] and Tramitichromis (30 trials, n = 6 fish, 75–98 mm TL, 1 female, 5 males) to quantify variation in behavioral responses to live (mobile) and dead (immobile) prey (=tethered adult brine shrimp) at five light intensities between 0 and 800 lx.
Trials were conducted in an experimental tank (120 × 75 × 60 cm; 560 L) with 5 cm of sand covering the bottom of the tank. Light intensity and spectral measurements (with ±0.01 accuracy, measured in lx and PAR) were taken directly above the center of each mesh platform (to which live and dead prey were tethered, see below) before and after each trial, and light intensity and spectrum were found to be consistent at all six platforms and trials (Fig. 2b, c). Each fish was acclimated to a particular light intensity in the experimental tank for at least 30 min prior to a trial. The transition between photopic (cone-mediated) and scotopic (rod-mediated) vision occurs at approximately 1 lx, and light-adapted fish may take 30 min (and up to 3 h) to become dark-adapted (Ali 1959). Thus, the 30+ min light adaptation period was judged to be sufficient to allow the fish’s visual system to adjust to the light level for a given trial.
Before each trial, 12 adult brine shrimp (Artemia sp.) were tethered in pairs (1 live and 1 dead, freshly frozen) on each of six mesh platforms (10 × 10 cm), which were positioned in a 2 × 3 matrix so that the top of each platform flush with the sand surface. The water filtration system for the experimental tank was then turned off to eliminate acoustic and hydrodynamic noise. At the start of a trial, a fish was released into the experimental arena from behind an opaque divider and feeding behavior was recorded for 30 min using an HD digital video camera (Sony © HDR-CX550V, 30 frames per second) mounted directly above the tank, which provided a dorsal view of the experimental arena. Trials were carried out with standard fluorescent room lights on for all but the lowest light levels (1–12 lx). Dark trials (0 lx) and trials at 1 lx were conducted with room lights off, but with infrared illumination (peak = 840 nm, range 800–880 nm; SpecoProvideo, IR-200/24, Amityville, NY) to allow video recording of behavior.
Each fish was run through five trials, one trial per day each at a single light intensity, progressing from highest to lowest intensity on subsequent days (e.g., 800, 112, 12, 1, and then 0 lx). Trials were carried out in this order to increase the likelihood that a fish would respond to prey at lower light intensities (especially in the dark, 0 lx), as was suggested by preliminary results. Trials were conducted over 4 months and the mean time between the first (800 lx) and last (0 lx) trial for a given fish was 11 days (range 6–19 days).
“Light” trials (1–800 lx) started midday to late afternoon (11:00–17:00) and “dark” trials (0 lx) took place shortly after sunset (19:00–21:00; soon after room lights had automatically shut off; as in Schwalbe et al. 2012; Schwalbe and Webb 2014). Dark trials (0 lx) were not carried out during the day (during the light phase of the lab’s light:dark cycle) to avoid the introduction of extraneous light. In addition, it was known that placing fish in low light or darkness during normal daylight hours would disrupt feeding behavior (M.A.B. Schwalbe and A. Mensinger, pers. obs.) and that species that normally feed both in full light during the day and at night (e.g., during the dark phase of a lab’s light:dark cycle) were unresponsive in dark (0 lx) trials carried out during the day.
To assess the number of prey detections that lead to prey strikes, unconsumed prey were counted at the end of each 30-min trial and strike success was also confirmed in video recordings. Video sequences leading to each prey strike were exported to Premier Pro (Adobe, CS5) for further analysis. Analysis of sequential video frames was used to identify the phase of swimming behavior (thrust, glide, or pause) during which prey detections occurred. Detection distance and detection angle were measured in these images using ImageJ (NIH, v. 1.410). Detection distance was defined as the distance from the tip of a fish’s mouth to the prey, measured in the frame immediately before the fish oriented towards it (e.g. before a turn defining detection). For each prey strike, detection-to-strike velocity was calculated by dividing detection distance by the time interval between detection and initiation of a strike. Detection angle was measured in the same video frame in which detection distance was measured and was defined as the angle between a line extending anteriorly along midline of the fish (body axis) and a line drawn from the prey to the tip of the fish’s mouth.
Statistical analysis
Four of the six behavioral parameters were analyzed using generalized linear mixed models (GLMMs; number of prey strikes, detection distance, detection-to-strike velocity, phase of search behavior during which detections occurred). In addition, a ranking method (Taplin 2007) was used to analyze prey preferences (live versus dead prey) and circular statistics were used to analyze detection angles. All continuous data (e.g. detection distance and detection-to-strike velocity) were tested for normality (Kolmogorov–Smirnov test) and were log10 transformed to achieve normality (detection distance and detection-to-strike velocity). All statistical tests were considered significant at P < 0.05 and values are given as mean ± s.e.m.
Start time (=time of day, 0–24 h) for trials conducted at the five different light intensities was analyzed with nonparametric tests (e.g. Kruskal–Wallis test and Mann–Whitney U test) to determine whether time of day affected feeding behavior. This analysis showed that the times at which light trials (1–800 lx) and dark trials (0 lx) started did not differ between species (Mann–Whitney U test, P > 0.05), but trial start time varied among light intensities in each species (Kruskal–Wallis test, A. stuartgranti: K = 22.804, P < 0.001; Tramitichromis: K = 20.141, P < 0.001). Thus, time of day (=trial start time) was included in all GLMM analyses.
Four-way GLMM analyses (SPSS, IBM, v. 22) were used to test whether species (A. stuartgranti, Tramitichromis), light intensity (0–800 lx), prey type (live, dead), and/or trial start time (0–24 h) predict differences in each of four behavioral parameters (number of prey strikes, detection distance, detection-to-strike velocity, and phase of search behavior during which detections occurred). Three-way GLMM analyses were used to further examine whether light intensity, prey type, and/or trial start time predict differences in the four behavioral parameters in each species separately. The selection of random (individual) and fixed effects (species, light intensity, prey type, and trial start time), including repeated measures for the same individual, was addressed in all analyses. Different types of GLMMs were used to account for the different types of data collected in this study (summarized in Table 2) and the most parsimonious model was selected for each behavioral parameter based on the corrected Akaike information criterion (AICC).
The order in which live (mobile) and dead (immobile) prey were struck was analyzed in each species following Taplin (2007). This method assumes that when presented with equal numbers of two or more types of prey, the order in which prey are consumed provides information about prey preference—that prey consumed first are more highly preferred than prey consumed second, third, etc. and the last prey consumed is the least preferred. While differences in handling time, encounter rates, and relative mobility of prey can potentially complicate the results of this sort of analysis (Durham et al. 2012; McWilliam et al. 2013), such variation was minimized in the current study by offering equal numbers of live and dead brine shrimp tethered to platforms in the same 2 × 3 matrix in all trials. The null hypothesis for this analysis was that live and dead prey would be consumed randomly during a trial. Videos were analyzed so that each prey consumed was assigned a rank number (first prey consumed = 1, second prey consumed = 2, etc.), and any remaining prey were assigned an average of the remaining preference scores, and considered “tied for last.” A pair of preference scores for live and dead prey at each light intensity was calculated for each fish. The pairs of scores from all of the fish were considered independent samples and thus grouped by light intensity and species for analysis. A score of 6.5 (based on presentation of six live and six dead prey, 12 total prey in a trial) indicated no preference, a score of <6.5 revealed a preference for that prey type, and a score of >6.5 indicated no preference or that prey type was ignored or avoided. Scores for live and dead prey at each light intensity and for each species were compared separately using paired t tests (SPSS, IBM, v. 22).
Detection angles were analyzed with circular statistics (Oriana v. 3, Kovach Computing Services, Anglesey, UK). Rayleigh tests were performed for each species to test whether detections of live and dead prey at each of the five light intensities (0–800 lx) occurred at uniformly or non-uniformly distributed positions around the fishes’ body relative to the body axis (e.g. to define the receptive field). Watson’s U 2 tests were used to determine if detection angles differed with prey type and with light intensity within and between species.
Results
Aulonocara stuartgranti and Tramitichromis sp. actively swam around the experimental tank during trials at all light intensities, including darkness (0 lx), and used a saltatory search strategy (a cyclic sequence of a caudal fin thrust, glide, and pause) while exploring the tank. Of the 360 total prey presented to fish during all 60 trials, A. stuartgranti struck at 299 prey (=83 %) and Tramitichromis struck at 231 prey (=64 %; see Figs. 3, 4). Prey were detected by both species during a glide or a pause, but never during a thrust (see Fig. 5).
Total number of prey detections by prey type (white bars live tethered brine shrimp, gray bars dead tethered brine shrimp) for a A. stuartgranti (n = 6 fish) and b Tramitichromis (n = 6 fish) at five different light intensities. Maximum number of possible prey detections = 72 for each light intensity
Three behavioral parameters defining prey detection in A. stuartgranti (n = 6 fish) and Tramitichromis (n = 6 fish) at five different light intensities. a, c, e Mean (±s.e.m.) number of prey detections (maximum 6 live, 6 dead tethered brine shrimp), detection distance, and detection-to-strike velocity, for A. stuartgranti feeding on live ( ) and dead (
) and dead ( ) prey, and (b, d, f) Tramitichromis feeding on live (
) prey, and (b, d, f) Tramitichromis feeding on live ( ) and dead (
) and dead ( ) prey. See text for statistics
) prey. See text for statistics
Four-way GLMM analyses (Table 3) indicated that species alone did not predict differences in any of the four behavioral parameters (number of prey detections, detection distance, detection-to-strike velocity, or swimming phase at prey detection). However, the interaction of species, light intensity, and prey type had a significant effect on number of prey detections and the interaction of light intensity and time of day predicted differences in all four behaviors. Light intensity alone predicted differences in all four behaviors, and time of day predicted differences in three behavioral parameters (number of prey detections, detection distance, detection-to-strike velocity), but not in swimming phase at prey detection. Separate three-way GLMM analyses for each species (Table 4, see below) revealed interesting trends that are indicative of species differences in prey detection behavior. Analyses of prey preference (live vs. dead prey) and prey detection angle, carried out using other statistical methods, also indicated differences in behavior between species, but did not consider time of day.
Feeding behavior of Aulonocara stuartgranti
Generalized linear mixed model (GLMM) analyses (Table 4) showed that light intensity did not significantly predict any of the four behavioral parameters in A. stuartgranti (number of prey detections, detection distance, detection-to-strike velocity, or swimming phase at prey detection; GLMMs, P > 0.05; Table 4). However, time of day predicted the number of prey detections, and the interaction of time of day and prey type predicted both detection distance and detection-to-strike velocity (GLMMs, P < 0.05; Table 4). Neither light intensity, time of day, prey type, nor their interactions predicted swimming phase at prey detection.
An examination of data for each of the behavioral parameters revealed informative trends. A. stuartgranti struck at high numbers of both live (mobile) and dead (immobile) prey at light intensities of 1–800 lx (Figs. 3a, 4a). They detected prey during a pause about half of the time, but detected 61 % of prey in a pause at the highest light intensity (800 lx; Fig. 5a). Detection distance and detection-to-strike velocity appeared to not vary among light intensities of 12–800 lx, but fish tended to detect live prey from greater distances (mean = 8.7–9.6 cm) than dead prey (6.0–6.9 cm) and to detect live prey at higher detection-to-strike velocities (9.7–10.4 cm/s) than dead prey (6.9–7.4 cm/s; Fig. 4c, e) at these light intensities. At 1 lx, fish tended to detect live and dead prey from similar distances (mean = 6.5 and 6.3 cm, respectively; Fig. 4c) and at similar detection-to-strike velocities (6.8 and 7.8 cm/s, respectively; Fig. 4e). Live and dead prey (combined) were detected at non-uniformly distributed positions around the fishes’ bodies at light intensities ≥1 lx (Rayleigh test, P < 0.001; ±90° from body axis) with no differences in the distribution of angles among pairs of light intensities with the exception of the two highest light intensities (112 lx vs. 800 lx; Watson’s U 2 test, U 2 = 0.19, P < 0.05; Fig. 6a). Finally, fish tended to prefer live prey at all light intensities, but only demonstrated a statistically significant preference for live prey at 112 lx (and not at 800 lx; Table 5; Fig. 7a), which is not easily explained.
Detection angle for live and dead prey combined (=12 total prey/trial) at five different light intensities for a A. stuartgranti (n = 6 fish) and b Tramitichromis (n = 6 fish). Black lines represent the proportion of prey detections grouped into 20 intervals. Fish snout is at the center and fish is facing 0° (indicated by the gray arrow in the top plot in a). The thin line represents the mean angle for all trials. Results for Tramitichromis at 0 lx were not included here due to the small number of strikes (n = 3)
Mean (±s.e.m.) prey preference scores (following Taplin 2007) for a A. stuartgranti (n = 6 fish) and b Tramitichromis (n = 6 fish) feeding on six live (white bars) and six dead (gray bars) tethered adult brine shrimp in trials at five different light intensities. Preferences scores were calculated by taking the mean of the rank order in which prey were captured. The dotted line (=6.5) indicates the mean preference score with no preference for either prey type. Scores <6.5 (below dotted line) indicate a preference. Significantly different preference scores between live and dead prey indicated by an asterisk (*, paired t test, P < 0.05, Table 5)
In the dark (0 lx), prey detection behavior of A. stuartgranti was different than at light intensities ≥1 lx. Fish struck at only 22 prey in the dark (=30.6 % of total prey presented; Fig. 3a), and tended to detect prey at even shorter distances (Fig. 4c) and at slower detection-to-strike velocities (Fig. 4e) than when at least some light was present. In the dark, fish tended to detect more live prey than dead prey (mean of 2.7 and 1.0, respectively; Figs. 3a, 4a), showed a statistically significant preference for live prey (Table 5; Fig. 7a), and detected live prey from more than twice the distance than dead prey (3.2 and 1.4 cm, respectively; Fig. 4c). In addition, detection-to-strike velocity at 0 lx was about one-half of that at higher light intensities (~3.5–5 cm/sat 0 lx vs. ~7–10 cm/s at ≥1 lx), but fish tended to detect live prey at somewhat higher detection-to-strike velocities than dead prey (Fig. 4e). In the dark, 95 % of prey were detected during a glide and only a few prey (5 %) were detected during a pause (Fig. 5a). Prey (live and dead combined) were detected at positions uniformly distributed around fishes’ bodies (Rayleigh test, P > 0.05) at a wide range of angles (±180° from body axis, Fig. 6a), but the distribution of detection angles did not differ for live versus dead prey (Watson’s U 2 test, P > 0.05).
Feeding behavior of Tramitichromis
GLMM analyses (Table 4) showed that, in contrast to A. stuartgranti, the interaction of light intensity and time of day predicted three of four behavioral parameters (number of prey detections, detection distance, and detection-to-strike velocity). As in A. stuartgranti, neither light intensity, time of day, nor prey type, or their interactions, predicted swimming phase at prey detection. Prey type did not predict any of the four behavioral parameters in Tramitichromis, and the interaction of light intensity and prey type predicted only detection distance (Table 4).
An examination of trends for each of the behavioral parameters revealed that Tramitichromis tended to strike at high numbers of prey (Figs. 3b, 4b), and >60 % of prey (live and dead, combined) were detected during a pause at light intensities of 1–800 lx (Fig. 5b). At light intensities of 12–800 lx, fish struck at live and dead prey from similar, long detection distances (means = 9.8–10.1 and 8.5–10.0 cm, respectively) and at high detection-to-strike velocities (9.6–10.5 and 8.7–9.3 cm/s, respectively). In contrast, at 1 lx, fish tended to strike at both live and dead prey at similar, but shorter detection distances (6.9 and 6.3, respectively) and lower detection-to-strike velocities (7.0 and 6.1, respectively; Fig. 4d, f) than at higher light intensities. Both live and dead prey were detected at non-uniform positions around the body (Rayleigh test, P < 0.001), which defined a very narrow range of detection angles from the body axis (±40°); distributions were the same for live prey and dead prey at light intensities of 1–800 lx (Watson’s U 2, P > 0.05; Fig. 6b). Fish tended to prefer live prey at the different light intensities, but only showed a statistically significant preference for live prey at the highest light intensity (800 lx; Table 5; Fig. 7b).
Despite being active in the dark (0 lx), Tramitichromis only struck at 3 prey (=4.2 % of the 72 prey presented in all trials). These strikes are likely to have been the result of random encounters with prey as opposed to being the result of active search and directed strikes.
Discussion
The multiple statistical analyses presented here and the detailed examination of trends in the detection of live and dead prey at different light intensities in each species indicate that light intensity affects prey detection behavior in different ways in Aulonocara stuartgranti and Tramitichromis.
Feeding behavior of Aulonocara stuartgranti and Tramitichromis
Prey type and/or time of day, but not light intensity, were predictors of A. stuartgranti behavior with respect to three of the four behavioral parameters (number of prey detections, detection distance, and detection-to-strike velocity) analyzed using GLMMs. The lack of significance for light intensity (0–800 lx) is consistent with the use of lateral line cues (see also Schwalbe et al. 2012), but also suggests that A. stuartgranti may use a light-independent circadian rhythm to interpret time of day. Aulonocara species occur at depths up to 120 m in Lake Malawi where light is limited or absent (Konings 1990, 2007) and in caves where spawning has been reported (Grant et al. 1987), and thus where normal diurnal variation in light intensity may not be a consistent or reliable cue for the regulation of behavior. The significance of prey type as a predictor of detection distance and detection-to-strike velocity is illustrated by their tendency to detect different numbers of live and dead prey at the same light intensities (Fig. 4; see also Schwalbe et al. 2012; Schwalbe and Webb 2014), the tendency to prefer live prey at all light intensities, and the statistically significant preference for live prey in the dark.
In contrast, in Tramitichromis, it is the interaction of light intensity and time of day that predicts these same three behavioral parameters. The importance of light intensity is not surprising because Tramitichromis uses visual, but not lateral line cues, for prey detection and because it does not feed in the dark (Schwalbe and Webb 2014). Furthermore, light intensity and time of day are correlated both in the lab where the fish were reared (on a 12:12 h light/dark cycle) as well as in the relatively shallow waters in their natural habitat in Lake Malawi (just 9°–17° south of the equator, with 11–13 h of daylight per day on an annual basis; http://astro.unl.edu). Thus, these fish have evolved and have been reared in environments where light intensity and time of day are tightly correlated. The independent roles of these two factors in predicting behavior would need to be addressed in additional experiments, which were out of the scope of this study.
Swimming phase (glide, pause) during which prey were detected was predicted neither by light intensity nor by time of day in either species. The ability to detect prey during a glide or pause will affect both the stabilization of the visual field (for vision-mediated detection) and/or the magnitude of environmental and self-generated hydrodynamic noise (for lateral line-mediated detection). A. stuartgranti and Tramitichromis both detected between 40 and 70 % of prey during a pause at light intensities of ≥1 lx, suggesting the importance of stabilizing the visual field for prey detection at these light intensities. Prey type (which defines the presence or absence of an additional visual motion stimulus) did not predict swimming phase at detection for Tramitichromis (P < 0.053), but a larger sample size may have yielded a different statistical outcome. Prey type also did not predict swimming phase at prey detection for A. stuartgranti, but the shift to 95 % of prey detections during a glide in the dark (where stabilization of visual field is irrelevant), and their preference for live prey (that generate hydrodynamic flows detected in the dark; Schwalbe and Webb 2014), are important indicators of the overall importance of prey type. A. stuartgranti detected live prey at distances of less than half of a body length and at lower detection-to-strike velocities at a low light intensity (1 lx) and in the dark (0 lx). Lower detection-to-strike velocities would also reduce self-generated hydrodynamic noise (Montgomery et al. 2009), enhancing lateral line-mediated prey detection, which suggests that fish should tend to detect prey during a pause. However, the high proportion of detections (95 %) at relatively low detection-to-strike velocities during a glide, while not eliminating self-generated noise, would bring a fish into the vicinity of potential prey that are generating detectable hydrodynamic flows (Schwalbe et al. 2012).
Roles of vision and critical light intensity
The importance of vision in A. stuartgranti and Tramitichromis is further supported by a consideration of critical light intensities and the potential differences in the use of binocular vision. Prey detection at relatively long distances is consistent with vision-mediated prey detection in fishes (Vinyard and O’Brien 1976; Confer et al. 1978; Henderson and Northcote 1985; Mazur and Beauchamp 2003), and at higher light intensities detection of free swimming prey generally occurs at longer distances (Vinyard and O’Brien 1976; Richmond et al. 2004; Bergstrom and Mensinger 2009). In this study, both A. stuartgranti and Tramitichromis tended to demonstrate the longest detection distances at the highest light intensities, which is thus consistent with vision-mediated prey detection. Detection distances may not increase as light intensity increases further in a given species (Schmidt and O’Brien 1982), but may decrease sharply below a “critical light intensity” (Confer et al. 1978). Trends in behavioral parameters in the current study reveal that the critical light intensity for both A. stuartgranti and Tramitichromis feeding on tethered adult brine shrimp is between 12 and 1 lx. This is comparable to the critical light intensities for other freshwater teleosts in studies feeding on free-swimming Daphnia (11–50 lx, in bluegill, Vinyard and O’Brien 1976; in lake trout, brook trout, and bluegill, Confer et al. 1978), amphipods (5–25 lx, in round goby, logperch, slimy sculpin, and spoonhead sculpin, Bergstrom and Mensinger 2009), or on small fish (~6–18 lx, in largemouth bass, Howick and O’Brien 1983; lake trout, Vogel and Beauchamp 1999). Below the critical intensity (e.g., at 1 lx), the ability of A. stuartgranti to detect more prey than Tramitichromis, but at comparable distances, suggests that A. stuartgranti may have superior visual abilities for prey capture at these lower light intensities. This is consistent with their distribution over a wider depth range than Tramitichromis and the observation of their reproductive behaviors in caves (Grant et al. 1987), but whether Aulonocara species possess adaptations for increased visual sensitivity and/or acuity as found in known crepuscular or nocturnal teleosts (reviewed in Warrant 2004; Schmitz and Wainwright 2011) requires further study.
The potential for binocular vision can be revealed by looking at behavioral evidence for differences in the size of visual fields under different light conditions and between species. While visual predators may respond differently to stimuli in different portions of their visual fields (Collin 1989; McComb and Kajiura 2008; Miyazaki et al. 2011), it is detection angle that reflects the overall size of the visual field, which is defined by the size, shape, and position of the eyes (Collin and Shand 2003). A. stuartgranti demonstrates a wide range of detection angles at light intensities ≥1 lx (±90° from body axis) and an even wider range of angles in darkness (0 lx, ±180° from body axis). This shift is correlated with differences in behavioral parameters at 1 versus 0 lx, which are interpreted as a shift between primarily vision-mediated prey detection to lateral line-mediated prey detection. Lateral line-mediated detection of prey around the body is enabled by the more sensitive widened cranial lateral line canals that characterize Aulonocara species, and by the broad distribution of canal and superficial neuromasts on the skin of the head, trunk and tail, which is typical of cichlids and of most teleosts (reviewed in Webb 2014). In contrast, Tramitichromis detected prey at a range of angles (±40° from body axis) that was less than half of that for A. stuartgranti (±90° from body axis) at light intensities of 1–800 lx, with one exception (Watson’s U 2 test, P < 0.05). This suggests that Tramitichromis, but likely not A. stuartgranti, uses binocular vision and depth perception to detect prey at a distance (as demonstrated in other teleosts, Sivak 1978; Blanco-Vives et al. 2011; Miyazaki et al. 2011). Furthermore, Tramitichromis tends to swim higher above the substrate than A. stuartgranti when searching for prey in the laboratory (Schwalbe and Webb 2014). Coupled with the use of binocular vision, this search strategy could explain the tendency for Tramitichromis to detect benthic prey at somewhat longer distances than A. stuartgranti (Fig. 4c, d).
The movements of the appendages of the live prey used in this study presumably generate a visual motion stimulus, and an enhanced dispersal of an odor plume (not evaluated here), in addition to a hydrodynamic stimulus, which addresses the importance of multimodal integration in the formulation of prey detection behavior. However, prey type predicted detection distance and detection-to-strike velocity only in A. stuartgranti, which tended to strike at live prey at longer detection distances and at higher velocities than for dead prey at the same light intensities (12–800 lx). At 1 lx, detection distances were about one body length or less, which is within the effective range of the lateral line system (Coombs 1999). Behavior is consistent with the use of the lateral line system in addition to vision for detection of live prey by A. stuartgranti in full light.
In contrast, in Tramitichromis, prey type did not predict any of the four behavioral parameters analyzed using GLMMs, although the interaction of prey type and light intensity did predict detection distance. However, the examination of data trends showed that Tramitichromis demonstrates comparable values and trends for live and dead prey with reference to number of prey detections, detection distance (despite the significance of its interaction with prey type), and detection-to-strike velocity at light intensities of 1–800 lx. These results also substantiate results of a prior laboratory study (Schwalbe and Webb 2014) that showed that Tramitichromis is a visual predator, which is not dependent on the detection of hydrodynamic stimuli generated by live prey. However, the lack of significance of prey type indicates that Tramitichromis does not respond to a visual motion stimulus that is likely to have been generated by live (but not dead) prey. This is surprising given the feeding strategies that these fish employ in nature. In the relatively shallow, well-lit waters of Lake Malawi, Tramitichromis species typically capture prey by plunging into the substrate, filling their mouth with sand, and sifting out prey with their gill rakers (=sand sifting, Fryer 1959). The sensory basis for the initiation of the plunge and sift feeding behavior needs to be determined experimentally, but the results of this study suggest that it is a visual stimulus and not necessarily an associated motion stimulus generated by live prey that influences where Tramitichromis initiates feeding behavior in the field.
The connection between experimental light conditions and light levels in Lake Malawi
As in other lakes, the photic conditions in Lake Malawi are dynamic and many factors influence the light environment, including habitat type, water depth, and proximity to the lake bottom (Sabbah et al. 2011), as well as meteorological events, eutrophication, turbidity, and both diurnal and seasonal changes in light quality and quantity. In shallow water, full spectrum light is typically present and middle wavelengths transmit best, but shorter and longer wavelengths attenuate rapidly (Dalton et al. 2010). Further, the irradiance spectrum differs between waters overlying sandy and rocky substrates, where light transmission in water above sand is shifted to longer wavelengths compared to that above rocky habitats (Sabbah et al. 2011).
The extent to which species of Aulonocara and Tramitichromis forage using vision at different depths can be approximated by comparing behavioral data from the current study to estimates of depths at which particular light intensities are predicted to occur in Lake Malawi. The light extinction coefficients in Table 1 are representative of pelagic (ε = 0.10 m−1, Patterson et al. 2000; ε = 0.13 m−1, Guildford et al. 2007) and nearshore (ε = 0.43 m−1, Guildford et al. 2007) habitats in Lake Malawi, but disparities in water clarity between these areas are likely influenced by nutrient loading and sedimentation from deforestation, intense agricultural practices, and erosion in nearshore areas (Bootsma and Jorgensen 2004). Estimations based on low light extinction coefficients (e.g. ε = 0.10 or 0.13 m−1) suggest that Aulonocara species could visually detect prey at 71–92 m (≥12 lx) and with some visual limitations at ~89–115 m where light levels are at ~1 lx. Some Aulonocara species are found to depths of 120 m (Konings 2007), so they may be able to visually detect prey in these depths at midday when light intensities are highest. Alternatively, when light extinction coefficients are used (ε = 0.43 m−1), the maximum depths at which Aulonocara species could reliably detect prey are greatly reduced (to 21 and 27 m, respectively). In the lab, Tramitichromis was able detect prey at a light intensity of 1 lx, which translates to depths of 89–115 m if the light extinction coefficient is low. However, these fish are typically found in shallower waters (<15 m, Konings 1990, 2007), so the ability of Tramitichromis to find prey at 1 lx is more relevant for the potential for feeding early or late during the day. Given its dependence on vision for prey detection (Schwalbe and Webb 2014), Tramitichromis species may be limited to shallow habitats so that the visual detection of prey is not compromised. In contrast, Aulonocara species can feed at low light intensities and in the dark, which can explain the wider range of depths at which they occur in Lake Malawi. They may also be crepuscular or nocturnal in habit, which may also facilitate other behaviors (e.g., social interactions) at low light intensities.
Conclusions
A. stuartgranti fed on prey at a range of ecologically relevant light intensities, including darkness, and Tramitichromis was also able to feed at low light intensities, but not in darkness. In A. stuartgranti, the influence of time of day on several aspects of its behavior suggests that it may use circadian rhythms to regulate behavior in nature where diurnal light cues may not be available (e.g. at greater depth, in caves). The integration of visual and non-visual (e.g., lateral line) sensory modalities can explain the statistically non-significant trends in behavior. Similarly, the dramatic change in behavior from 1 to 0 lx is consistent with a transition from primarily vision-mediated to exclusively lateral line-mediated prey detection behavior. In contrast to A. stuartgranti, Tramitichromis depends on vision-mediated prey detection (Schwalbe and Webb 2014); in this study its behavior was significantly affected by the interaction of light intensity with time of day, but these two factors could not be teased apart. Finally, in an ecological context, the tendency of Tramitichromis species to live in shallower, well-lit habitats, in contrast to Aulonocara species, which live at a wide range of depths and light environments, suggests that sensory capabilities may allow Aulonocara species to escape competition with Tramitichromis species for prey resources, thus facilitating niche differentiation between these taxa. Field observations in Lake Malawi are needed to test this hypothesis, which would provide an important link between the morphology, feeding behavior, and ecology of cichlid fishes.
Abbreviations
- AICC:
-
Akaike information criterion
- GLMM:
-
Generalized linear mixed model
- Lx:
-
Lux
- PAR:
-
Photosynthetically active radiation
- SL:
-
Standard length
- TL:
-
Total length
References
Ali MA (1959) The ocular structure, retino motor and photo behavioral responses of juvenile pacific salmon. Can J Zool 37:965–996
Atema J (1971) Structures and functions of the sense of taste in the catfish (Ictalurus natalis). Brain Behav Evol 4:273–294
Batty RS, Blaxter JHS, Libby DA (1986) Herring (Clupea harengus) filter-feeding in the dark. Mar Biol 91:371–375
Bergstrom MA, Mensinger AF (2009) Interspecific resource competition between the invasive round goby and three native species: logperch, slimy sculpin, and spoonhead sculpin. Trans Am Fish Soc 138:1009–1017
Blanco-Vives B, Aliago-Guerrero M, Cañavate JP, García-Mateos G, Martín-Robles AJ, Herrera-Pérez P, Muñoz-Cueto JA, Sánches-Vázquez FJ (2011) Metamorphosis induces a light-dependent switch in Senegalese sole (Solea senegalensis) from diurnal to nocturnal behavior. J Biol Rhythms 27:135–144
Bootsma H, Jorgensen SE (2004) Lake Malawi/Nyasa: experience and lessons learned brief. In: companion CD-ROM for: ILEC (2005). Managing lakes and their basins for sustainable use: a report for lake basin managers and stakeholders. International Lake Environment Committee Foundation, Kusatsu, Japan, pp 259–276
Collin SP (1989) Topographic organization of the ganglion cell layer and intraocular vascularization in the retinae of two reef teleosts. Vision Res 29:765–775
Collin SP, Shand J (2003) Retinal sampling and the visual field in fishes. In: Collin SP, Marshall NJ (eds) Sensory processing in aquatic environments. Springer, New York, pp 139–169
Confer JL, Howick GL, Corzette MH, Kramer SL, Fitzgibbon S, Landesberg R (1978) Visual predation by planktivores. Oikos 31:27–37
Coombs S (1999) Signal detection theory, lateral-line excitation patterns and prey capture behaviour of mottled sculpin. Anim Behav 58:421–430
Dalton BE, Cronin TW, Marshall NJ, Carleton KL (2010) The fish eye view: are cichlids conspicuous? J Exp Biol 213:2243–2255
Denton EJ, Gray JAB (1988) Mechanical factors in the excitation of the lateral lines of fish. In: Atema J, Fay RR, Popper AN, Tavolga WN (eds) Sensory biology of aquatic animals. Springer, New York, pp 595–617
Denton EJ, Gray JAB (1989) Some observations on the forces acting on neuromasts in fish lateral line canals. In: Coombs S, Gorner P, Münz H (eds) The mechanosensory lateral line: neurobiology and evolution. Springer, New York, pp 229–246
Diehl S (1988) Foraging efficiency of three freshwater fishes: effects of structural complexity and light. Oikos 53:207–214
Dunbrack RL, Dill LM (1984) Three-dimensional prey reaction field of the juvenile coho salmon (Oncorhynchus kisutch). Can J Fish Aquat Sci 41:1176–1182
Durham SR, Dietl GP, Visaggi CC (2012) The mismeasure of behavior: a natural history revision of prey preference in the banded tulip snail. J Shellfish Res 31:101–109
Fryer G (1959) The trophic interrelationships and ecology of some littoral communities of Lake Nyasa with especial reference to the fishes, and a discussion of the evolution of a group of rock-frequenting Cichlidae. Proc Zool Soc Lond 132:153–281
Fryer G, Iles TD (1972) The cichlid fishes of the great lakes of Africa: their biology and evolution. Oliver and Boyd, Edinburgh
Gardiner JM, Motta PJ (2012) Largemouth bass (Micropterus salmoides) switch feeding modalities in response to sensory deprivation. Zoology 115:78–83
Grant SM, Dieckhoff HW, Mayland HJ, Meyer MK (1987) Ecology of Aulonocara REGAN, 1922 in Lake Malawi. Cour Forsch Inst Senckenberg 94:131–139
Guildford SJ, Bootsma HA, Fee EJ, Hecky RE, Patterson G (2000) Phytoplankton nutrient status and mean water column light intensity in Lakes Malawi and superior. Aquat Ecosyst Health 3:35–45
Guildford SJ, Bootsma HA, Taylor WD, Hecky RE (2007) High variability of phytoplankton photosynthesis in response to environmental forcing in oligotrophic Lake Malawi/Nyasa. J Great Lakes Res 33:170–185
Harden Jones FR (1956) The behaviour of minnows in relation to light intensity. J Exp Biol 33:271–281
Henderson MA, Northcote TG (1985) Visual prey detection and foraging in sympatric cutthroat trout (Salmo clarki clarki) and dolly varden (Salvelinus malma). Can J Fish Aquat Sci 42:785–790
Hofmann CM, O’Quin KE, Marhsall NJ, Cronin TW, Seehausen O, Carleton KL (2009) The eyes have it: regulatory and structural changes both underlie cichlid visual pigment diversity. PLoS Biol 7(12):e1000266
Holbrook SJ, Schmitt RJ (1984) Experimental analyses of patch selection by foraging black surfperch (Embiotoca jacksoni Aggasiz). J Exp Mar Biol Ecol 79:39–64
Howick GL, O’Brien WJ (1983) Piscivorous feeding behavior of largemouth bass: experimental analysis. Can J Fish Aquat Sci 42:785–790
Janssen J (1997) Comparison of response distance to prey via the lateral line in the ruffe and yellow perch. J Fish Biol 51:921–930
Jordan R, Kellogg K, Howe D, Juanes F, Stauffer J, Loew E (2006) Photopigment spectral absorbance of Lake Malawi cichlids. J Fish Biol 68:1291–1299
Kirk JTO (2011) Light and photosynthesis in aquatic ecosystems, 3rd edn. Cambridge University Press, New York
Konings A (1990) Koningʼs book of cichlids and other fishes of Lake Malawi. TFH Publications Inc., Neptune City
Konings A (2007) Malawi cichlids in their natural habitat, 4th edn. Cichlid Press, El Paso
Laur DR, Ebeling AW (1983) Predator-prey relationships in surfperches. Env Biol Fish 8:217–229
Lythgoe JN (1979) The ecology of vision. Clarendon Press, Oxford
Mazur MM, Beauchamp DA (2003) A comparison of visual prey detection among species of piscivorous salmonids: effects of light and low turbidities. Environ Biol Fish 67:397–405
McComb DM, Kajiura SM (2008) Visual fields of four batoid fishes: a comparative study. J Exp Biol 211:482–490
McWilliam RA, Minchinton TE, Ayre DJ (2013) Despite prolonged association in closed populations, an intertidal predator does not prefer abundant local prey to novel prey. Biol J Linn Soc 108:812–820
Miyazaki T, Iwami T, Meyer-Rochow VB (2011) The position of the retinal area centralis changes with age in Champsocephalus gunnari (Channichthyidae), a predatory fish from coastal Antarctic waters. Polar Biol 34:1117–1123
Moller P (2002) Multimodal sensory integration in weakly electric fish: a behavioral account. J Physiol Paris 96:547–556
Montgomery JC, Coombs S (1992) Physiological characterization of lateral line function in the Antarctic fish Trematomus bernacchii. Brain Behav Evol 40:209–216
Montgomery JC, Diebel C, Halstead MBD, Downer J (1999) Olfactory search tracks in the Antarctic fish Trematomus bernacchii. Polar Bio 21:151–154
Montgomery JC, McDonald F, Baker CF, Carton AG, Ling N (2003) Sensory integration in the hydrodynamic world of rainbow trout. Roy Soc Lond B Bio 270(2):S195–S197
Montgomery JC, Windsor S, Bassett D (2009) Behavior and physiology of mechanoreception: separating signal and noise. Integr Zool 4:3–12
Montgomery JC, Bleckmann H, Coombs S (2014) Sensory ecology and neuroethology of the lateral line. In: Coombs S, Bleckmann H, Fay RR, Popper AN (eds) The lateral line system. Springer, New York, pp 121–150
Parry JWL, Carleton KL, Spady T, Carboo A, Hunt DM, Bowmaker JK (2005) Mix and match color vision: tuning spectral sensitivity by differential opsin gene expression in Lake Malawi cichlids. Curr Biol 15:1734–1739
Partridge BL, Pitcher TJ (1980) The sensory basis of fish schools: relative roles of lateral line and vision. J Comp Physiol 135:315–325
Parzefall J (1993) Behavioural ecology of cave-dwelling fishes. In: Pitcher T (ed) Behaviour of teleost fishes, 2nd edn. Chapman & Hall, London, pp 573–608
Patterson G, Hecky RE, Fee EJ (2000) Effect of hydrological cycles on planktonic primary productivity in Lake Malawi/Niassa. Adv Ecol Res 31:421–430
Richmond HE, Hrabik TR, Mensinger AF (2004) Light intensity, prey detection and foraging mechanisms of age 0 year yellow perch. J Fish Biol 65:195–205
Rickel A, Genin A (2005) Twilight transitions in coral reef fish: the input of light-induced changes in foraging behaviour. Anim Behav 70:133–144
Ryer CH, Olla BL (1999) Light-induced changes in the prey consumption and behavior of two juvenile planktivorous fish. Mar Ecol Prog Ser 181:41–51
Sabbah S, Gray SM, Boss ES, Fraser JM, Zatha R, Hawryshyn CW (2011) The underwater photic environment of Cape Maclear, Lake Malawi: comparison between rock- and sand-bottom habitats and implications for cichlid fish vision. J Exp Biol 214:487–500
Schmidt D, O’Brien WJ (1982) Planktivorous feeding ecology of arctic grayling (Thymallus arcticus). Can J Fish Aquat Sci 39:475–482
Schmitt R, Holbrook SJ (1984) Ontogeny of prey selection by black surfperch Embiotoca jacksoni (Pisces: Embiotocidae): the roles of fish morphology, foraging behavior, and patch selection. Mar Ecol Prog Ser 63:6–12
Schmitz L, Wainwright PC (2011) Nocturnality constrains morphological and functional diversity in the eyes of reef fishes. BMC Evol Biol 11:338
Schwalbe MAB, Webb JF (2014) Sensory basis for detection of benthic prey in two Lake Malawi cichlids. Zoology 117:112–121
Schwalbe MAB, Bassett DK, Webb JF (2012) Feeding in the dark: lateral-line-mediated prey detection in the peacock cichlid Aulonocara stuartgranti. J Exp Biol 215:2060–2071
Sivak JG (1978) The functional significance of the aphakic space of the fish eye. Can J Zool 56:513–516
Taplin RH (2007) Experimental design and analysis to investigate predator preferences for prey. J Exper Mar Biol Ecol 344:116–122
Townsend CR, Risebrow AJ (1982) The influence of light level on the functional response of a zoo planktonivorous fish. Oecologia 53:293–295
Vinyard GL, O’Brien WJ (1976) Effects of light and turbidity on reaction distance of bluegill (Lepomis macrochirus). J Fish Res Board Can 33:2845–2849
Vogel JL, Beauchamp DA (1999) Effects of light, prey size, and turbidity on reaction distances of lake trout (Salvelinus namaycush) to salmonid prey. Can J Fish Aquat Sci 56:1293–1297
Warrant EJ (2004) Vision in the dimmest habitats on Earth. J Comp Physiol A 190:765–789
Webb JF (2014) Morphological diversity, development, and evolution of the mechanosensory lateral line system. In: Coombs S, Bleckmann H, Fay RR, Popper AN (eds) The lateral line system. Springer, New York, pp 17–72
Webb JF, Montgomery JC, Mogdans J (2008) Bioacoustics and the lateral line system of fishes. In: Webb JF, Fay RR, Popper AN (eds) Fish bioacoustics. Springer, New York, pp 145–182
Acknowledgments
We thank Drs. Karen Carleton (University of Maryland) for her expertise in cichlid vision, Stephanie Guildford (University of Minnesota Duluth) for her expertise on light levels in Lake Malawi, and Graham Forrester (University of Rhode Island) and Rebeca Rosengaus (Northeastern University) for statistical expertise. We also thank Edward Baker (Facilities Manager, RI NSF EPSCoR Marine Life Science Facility), Emily Becker, Brandon Fuller, Christopher Holland, Callie Veelenturf, Rebecca Scott, and Benjamin Sevey for assistance with fish husbandry, Dr. Christopher Kenaley (Harvard University) for doing the µCT scans, and Benjamin Sevey for generating the 3D images in Fig. 1. This work was carried out under an approved University of Rhode Island IACUC protocol (#AN08-11-005). This research was assisted by funds provided by NSF EPSCoR Cooperative Agreement EPS-0554548 and EPS-100405, the College of the Environment and Life Sciences (University of Rhode Island) and was supported by NSF grant IOS 0843307 to JFW.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwalbe, M.A.B., Webb, J.F. The effect of light intensity on prey detection behavior in two Lake Malawi cichlids, Aulonocara stuartgranti and Tramitichromis sp.. J Comp Physiol A 201, 341–356 (2015). https://doi.org/10.1007/s00359-015-0982-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-015-0982-y