Abstract
The nonnative lake trout (Salvelinus namaycush Walbaum, 1792) population in Lake Pend Oreille, Idaho increased exponentially during 1999–2006. This led to an unsustainable level of predation mortality on kokanee (Oncorhynchus nerka Walbaum, 1792), increased the conservation threat to native bull trout (Salvelinus confluentus Suckley, 1859), and jeopardized the popular recreational fishery for kokanee and rainbow trout (Oncorhynchus mykiss Walbaum, 1792). In response, lake trout were suppressed since 2006 using incentivized angling, gill netting, and trap netting. From 2006 through 2016, 193,982 lake trout were removed (50% by gill netting; 44% by angling; 6% by trap netting). During this period, age-8 + (adult) lake trout abundance declined by 64%, age-3 (recruit) abundance declined by 56%, and mean total annual mortality (A) was 31.1%. Lake trout did not show evidence of a density-dependent response. Kokanee did not collapse and rebounded to abundances not observed since before lake trout expansion. Bull trout abundance declined during suppression, but the population was sustained. Lake trout suppression allowed a harvest fishery for kokanee and trophy fishery for rainbow trout to be restored. We conclude that suppression can be an effective management action for mitigating effects of nonnative lake trout in a large, deep lake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-native fish introductions have occurred worldwide for centuries (Li & Moyle, 1999), often with the intent of enhancing recreational fisheries (Rahel, 2000; Eby et al., 2006). Negative outcomes from these introductions have been common, including declines in many native freshwater fish species (Miller et al., 1989; Li & Moyle, 1999; Jelks et al., 2008). Introductions of upper trophic level predators have been particularly prevalent because of their recreational value and ecological outcomes include increased species richness of predators, replacement of native predators, and altered food web and ecosystem processes (Eby et al., 2006). Control programs for introduced predators, such as lake trout Salvelinus namaycush in the western United States, are increasingly being implemented to conserve native fish populations and sustain socially valued recreational fisheries (Martinez et al., 2009; Syslo et al., 2011).
The lake trout evolved in cold, oligotrophic lakes within a native range spanning much of northern North America (Crossman, 1995; Behnke, 2002) and is typically the apex predator in these waters (Scott & Crossman, 1973; Shuter et al., 1998). Widespread introductions of lake trout in the late 1800s and early 1900s (Crossman, 1995), eventually led to the establishment of naturalized populations in over 200 lentic waters in the western United States (Martinez et al., 2009). In their native range, lake trout support socially and economically valuable recreational fisheries (Healy, 1978; Sellers et al., 1998; Hansen et al., 1999), and similar fisheries now exist in the western United States (Martinez et al., 2009; Pate et al., 2014). However, native and other valued sport fishes are often jeopardized by predation from or competition with nonnative lake trout (Donald & Alger, 1993; Fredenberg, 2002; Ruzycki et al., 2003; Martinez et al., 2009). For example, native bull trout populations generally cannot be sustained following lake trout introductions (Donald & Alger, 1993; Fredenberg, 2002). More broadly, nonnative lake trout can alter food webs (Ruzycki et al., 2001; Vander Zanden et al., 2003; Ellis et al., 2011), such as the trophic cascade that was caused by lake trout predation effects in Yellowstone Lake, Wyoming (Tronstad et al., 2010).
In many western United States waters, lake trout did not become predominant until Mysis diluviana (Audzijonyte & Väinölä, 2005; hereafter, Mysis) were widely stocked to enhance kokanee Oncorhynchus nerka growth (Martin & Northcote, 1991). Mysis provided an important food source in deep waters occupied by juvenile lake trout, which apparently removed a recruitment bottleneck and allowed populations to increase (Stafford et al., 2002; Ellis et al., 2011). Wherever introduced lake trout co-existed with Mysis their negative effects on other fishes were exacerbated, particularly for kokanee and bull trout Salvelinus confluentus (e.g., Flathead Lake, Montana: Stafford et al., 2002; Priest Lake, Idaho: Bowles et al., 1991; Glacier National Park lakes, Montana: Fredenberg, 2002). The effects of introduced lake trout in the presence of Mysis have been repeated in enough waters to confirm negative ecological and fishery outcomes (Martinez et al., 2009).
Ecological problems caused by lake trout populations in the western United States have increasingly prompted fishery managers to implement strategies to control or reduce their abundance, including mechanical removal (Martinez et al., 2009; Pate et al., 2014; Hansen et al., 2016; Fredenberg et al., 2017). The lake trout is recognized as vulnerable to over-fishing in its native range because of a life history characterized by relatively slow growth and late maturity (Healey, 1978; Martin & Olver, 1980; Shuter et al., 1998; Olver et al., 2004). An extensive review by Healey (1978) concluded that lake trout populations in their native range could not be sustained above thresholds of 50% total annual mortality or 0.5 kg/ha annual yield. Overharvest is typically the limiting factor for lake trout populations in Precambrian Shield lakes of eastern Canada and the northeastern United States (Olver et al., 2004). Moreover, over-fishing was the primary factor leading to collapse of some of the world’s largest lake trout populations in nearly all of the Laurentian Great Lakes (Hansen et al., 1999; Krueger & Ebener, 2004; Muir et al., 2012).
In Lake Pend Oreille, Idaho, the exponential population growth of lake trout during 1999–2005 (Hansen et al., 2008) threatened to quickly collapse the kokanee population, cause the decline or eventual collapse of the native bull trout population, and severely alter a highly valued recreational fishery targeting primarily kokanee and rainbow trout. Lake trout were introduced in 1925 but existed at low density and exerted little predation mortality on kokanee (2% of production in 1999; Vidergar, 2000) prior to 1999. The introduction of Mysis during 1966–1969 allowed for lake trout population expansion, including an exponential increase in abundance after 1995 (Corsi et al., 2019). Rainbow trout Oncorhynchus mykiss and bull trout, both prominent kokanee predators, were already abundant at that time (Vidergar, 2000) and the added predation from a rapidly expanding lake trout population combined to produce an unsustainable predation demand on kokanee (Maiolie et al., 2006; Hansen, 2007). In response, the kokanee fishery was closed to harvest in 2000, and harvest limits were removed from lake trout and rainbow trout in 2000 and 2006, respectively. Additionally, research began in 2003 to evaluate lake trout population dynamics (Hansen et al., 2008, 2010), while simultaneously engaging with outside experts, stakeholders, and funding entities to assess suppression feasibility.
After three years of evaluation and public outreach, a lake trout suppression program was implemented on Lake Pend Oreille. From 2006 to 2016, lake trout were removed using gill nets, trap nets, and incentivized angler harvest to allow for kokanee recovery and sustain bull trout, contributing to both recreational fishery management and native species conservation goals. Simultaneous population monitoring was conducted to allow effectiveness of the suppression program to be evaluated. Thus, the objectives of this study were to determine if (1) suppression resulted in a 67% reduction in lake trout abundance by 2015, as predicted by Hansen et al. (2010); (2) attributes of the lake trout population changed in a manner consistent with 11 years of suppression (e.g., reduced size and age structure, increased growth and mortality); (3) kokanee avoided collapse and increased in abundance; and (4) bull trout abundance was sustained.
Methods
Study area
Lake Pend Oreille is located in northern Idaho within the Upper Columbia River Basin at an elevation of 625 m. It is the largest lake in Idaho (38,300 ha surface area) and the fifth deepest lake in the United States (357 m maximum depth). Much of the lake is deep (164 m mean depth) and is dominated by steep, rocky shorelines with limited littoral habitat. The lake has two major basins; the northern basin represents about 25% of the lake surface area and is shallower (primarily < 100 m) than the comparatively larger and deeper southern basin (primarily > 200 m). Although it is a natural lake, the upper 3.5 m of Lake Pend Oreille is regulated by Albeni Falls Dam. Inflow to the lake is primarily from the Clark Fork River and the only outflow is the Pend Oreille River. The lake is temperate, oligotrophic, and thermal stratification typically occurs from June to October (Steed, 2011).
The contemporary Lake Pend Oreille fish assemblage includes ten native species and at least 13 nonnative species that have resulted from intentional and illegal introductions (Hoelscher, 1992). Management objectives focus on native species conservation, particularly for bull trout and westslope cutthroat trout Oncorhynchus clarkii lewisi (Richardson, 1836), and providing recreational fisheries for valued introduced species that are compatible with native fishes. Kokanee have been highly influential in Lake Pend Oreille since becoming established in the 1930s. Shortly after their introduction, a high-yield kokanee fishery developed and both native (e.g., bull trout) and introduced (e.g., Gerrard-strain rainbow trout) pelagic predators benefitted from the abundant food source kokanee provided. From the 1940s to the early 1970s, Lake Pend Oreille was the most popular fishery in Idaho (Bowles et al., 1987), in part fueled by the international notoriety of the trophy fishery after the lake produced world records for bull trout (14.5 kg) and rainbow trout (16.8 kg). Most angling effort continues to be directed at kokanee (41%) and rainbow trout (38%; Bouwens & Jakubowski, 2016), even with numerous other recreational species now established.
Lake trout response
Lake Trout were suppressed in Lake Pend Oreille during 2006–2016 using gill netting, trap netting, and incentivized angling at a combined annual cost of US$307,000 to $796,000. Netting conducted over the 11-year period fit into one of three strategies: large-mesh gill netting (102–140 mm stretch measure) to target adult lake trout primarily during spawning, small-mesh gill netting (32–89 mm stretch measure) to target immature lake trout, and trap netting to target adult lake trout. A description of suppression methods is provided in Online Resource 1.
All lake trout captured were enumerated, processed to collect biological data, and then most fish were distributed to local food banks. Total length (TL; mm) was measured for all lake trout captured by trap netting and gill netting during 2008–2016. Head length (HL) was recorded for angler-caught lake trout and converted into TL based on a HL–TL regression model (y = 4.3268x + 62.419; r2 = 0.92) for 147 lake trout (range 283–880 mm TL) sampled in 2009 from Lake Pend Oreille. Head length was measured from the tip of the snout to the posterior edge of the operculum. Few HL measurements were taken during 2006–2008, but HL was measured for > 98% of angler-caught lake trout from 2009 through 2016. Otoliths were collected for age estimation from subsamples of fish in 2006, 2008–2012, and 2015 (≤ 10 fish were randomly subsampled from each 50-mm length class). Age was estimated from otolith thin sections using methods previously described by Hansen et al. (2010).
Annual age-specific abundance Nj was estimated by cohort analysis from the number of lake trout caught from each age class by gill netting, trap netting, and angling during 2006–2016 (Pope, 1972; Quinn & Deriso, 1999). Cohort analysis was used because a census of lake trout harvest was available. First, length frequencies of annual harvest by gill netting, trap netting, and angling were converted into age frequencies of annual harvest for each fishery using age-length keys (Ricker, 1975) and summed across fisheries within age classes and years. Age-length keys were created for each age subsample (2006, 2008–2012, and 2015) by tabulating age (columns) against 50-mm length classes (rows) for subsampled ages, and then calculating age frequencies (proportions of the total number sampled in each length class) within each length class. For years without age subsamples, age-length keys were created by combining adjoining years. The age-length key was then used to convert the length frequency into an age frequency of all fish sampled (gill nets, trap nets, and angling) for each year sampled. Next, terminal abundance in 2016 was estimated by dividing age-specific total harvest in 2016 by the estimated exploitation rate in 2016 (u = 0.179; 95% CI 0.128–0.227; where u = F × A/Z, F = Z − M, Z = catch-curve estimate (Ricker, 1975) of instantaneous total mortality Z from the age frequency of the harvest in all gill-net meshes fished in 2016, M = instantaneous natural mortality rate M described below, and A = 1 − e−Z). Last, abundance at age in 2006–2015 Ni,j was estimated recursively using Pope’s method (Pope, 1972; Quinn & Deriso, 1999) from age- and year-specific catch Ci,j and the instantaneous natural mortality rate M (described below): Ni,j = Ni+1,j+1eM + Ci,jeM/2.
Cohort abundance estimates were used to index adult lake trout abundance and recruitment. Age-8 + lake trout were used to index adult abundance (stock) based on the average age at 50% maturity of lake trout in Lake Pend Oreille (Hansen et al., 2010). The recruitment response to changes in adult abundance was evaluated using estimates of age-3 (recruit) abundance. Age-3 lake trout were used as an index to recruitment because this was the youngest age class fully vulnerable to gill nets.
Growth in length with age was modeled using the Von Bertalanffy length-age model fit to length at age of capture of individual fish subsampled for age in 2004, 2006, 2008–2012, and 2015 (Mooij et al., 1999):
In the length-age model, L∞ = asymptotic length (mm) for an average fish in the population, L0 = length at age = 0 (mm; length at emergence from the egg), ω = early annual growth rate (L∞ × K = mm/year; Gallucci & Quinn, 1979), and εj = multiplicative error (Ricker, 1975). Parameters and standard errors for the length-age model were estimated using nonlinear least-squares for each annual subsample of ages.
Annual mortality rates were estimated from harvest and cohort estimates of abundance. Exploitation rates for each fishery were computed by dividing total catch for each fishery by age-3 + abundance estimated by cohort analysis (u for each fishery). Total instantaneous natural mortality (M) was estimated from Pauly’s equation (Pauly, 1980) from annual asymptotic length L∞ and instantaneous growth rate K of Von Bertalanffy length-age models during 2004–2015, and the average annual air temperature T at Sandpoint, Idaho during 2006–2016 (Hansen et al., 2019). Instantaneous natural mortality M was converted into an annual expectation of natural death (v = 1 − e−M; at these rates, this approximation is very close to unbiased). Annual mortality rates are additive when expressed in these units (A = uA + uG + uT + v; where uA = angling exploitation, uG = gill net exploitation, and uT = trap net exploitation).
Population size structure was indexed as proportional size distribution (PSD; Guy et al., 2007), the percentage of fish longer than 300 mm (stock length) that are also longer than 500 mm (quality length). To overcome size selectivity of capture methods, PSD was estimated from cohort estimates of age-specific abundance and reverse age-length keys. First, annual subsamples of age estimates described above for use in age-length keys were converted into reverse age-length keys by calculating length frequencies (proportions of the total number sampled in each age class) within each age class. Next, the reverse age-length key was used to convert annual age-specific abundance into annual length-specific abundance within 50-mm length bins. Proportional size distribution was then estimated as the fraction of fish ≥ 300 mm TL that were also ≥ 500 mm TL. Confidence limits were computed as exact binomial confidence limits (Zar, 1999).
Population age structure was estimated as the mean age of annual cohort estimates of abundance. First, annual mean age of vulnerable age groups (age-3 +) was computed by dividing the sum product of annual age-specific abundance and age (+ 0.5 years) by the sum of all ages. Next, variance of annual mean age was computed using the method for frequency data (Zar, 1999). Last, 95% confidence intervals around mean age were computed as 1.96 × SE, where SE = (variance/n)0.5.
Kokanee response
Kokanee abundance in Lake Pend Oreille was estimated from annual hydroacoustic surveys conducted at night during August or September. The lake was stratified into three sections (northern, central, and southern basins) and six to eight transects were surveyed in each lake section using a Simrad EK60 echosounder. Kokanee abundance was estimated with echo integration techniques using Echoview software version 6.1.60.27483 (Echoview Software Pty Ltd, Hobart, Tasmania). Age-0 kokanee (recruitment index) was separated from age-1 + kokanee (abundance index) using hydroacoustic target strength, thus allowing mean age-0 and age-1 + kokanee density to be estimated (fish/hectare) separately for each lake section (Wahl et al., 2010). For each of these estimates, mean density in each lake section was then multiplied by area to estimate abundance by lake section. Finally, abundance in each of the three sections was summed and divided by total lake area to estimate mean lakewide density. Geometric-mean confidence intervals (90%) were calculated for kokanee density estimates using a stratified-random sampling estimator (Cochran, 1977).
Bull trout response
Bull trout captured incidentally during gill netting and trap netting were enumerated, measured for TL, and released if alive. Direct mortalities were recorded, and bull trout mistakenly turned in by anglers as lake trout for an incentive payment were enumerated.
Bull trout redds were counted to index trends in abundance. Surveys were conducted annually in 18 tributaries to Lake Pend Oreille and the Clark Fork River below Cabinet Gorge Dam. Redds were counted by trained personnel. Surveys were conducted within standardized tributary reaches in October. Bull trout population abundance trends were evaluated for 12 of 18 tributaries with continuous counts starting in 1992, although two tributaries were not surveyed in 2016 due to high flows. Redd counts were summed across tributaries to index aggregate trends in adult bull trout abundance. Trends were stratified by time, with 1992–2005 representing a pre-suppression period and 2006–2016 representing a suppression period. The estimated population growth rate µ was estimated for each time period by the slope of a regression with loge(Ni+1/Ni) as the response variable, where N is the annual aggregate redd abundance in 12 index tributaries, against the time interval (1 year). The intercept was set to zero so there can be no population change if there is no time change (Morris & Doak, 2002). Positive values of µ indicated population growth, while negative values indicated population decline.
Results
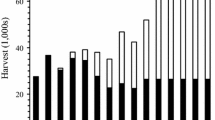
In total, 5.7 million meters of gill net were fished, and trap nets were fished for 6,628 nights. The amount of gill net fished was not recorded in 2006, but occurred during 17 weeks, while 22–30 weeks of netting took place in other years. Gill net effort increased annually to a peak of 831,000 m in 2014 (Fig. 1a). Trap net effort was highest during 2006–2009 (Fig. 1a) and occurred during 21–25 weeks annually. From 2010 to 2016, trap nets were fished during 7–11 weeks annually, and effort was low relative to prior years (Fig. 1a).
A total of 193,982 lake trout was harvested by gill netting, trap netting, and angling from 2006 to 2016 (Fig. 1b). Gill netting accounted for 50% (97,124 fish) of the harvest and was closely followed by angling at 44% (85,097), with trap netting contributing only 6% (11,761 fish) of the total harvest. From 2006 to 2008, angling was responsible for most lake trout harvest (71%), and trap netting made a larger relative contribution (15%) to harvest than in subsequent years (Fig. 1b). After 2008, most lake trout were harvested by gill netting (66%; Fig. 1b).
The combined harvest from gill netting, trap netting, and angling was well distributed across all sizes (> 150 mm) of lake trout in the population. Small lake trout (< 350 mm) made up 40% of the harvest, while intermediate (350–500 mm) and large (> 500 mm) lake trout were each 30% of the harvest (Fig. 2). Gill netting was most effective for removing small lake trout (Fig. 2), which resulted from targeting concentrations of these fish with small meshes. Angling removed the most intermediate size lake trout (Fig. 2). Large lake trout were best targeted with large-mesh gill netting at spawning areas, although trap netting also was productive (Fig. 2).
Exponential lake trout population growth ended soon after suppression began, and abundance declined during the suppression period at a rate similar to the 67% decline predicted by Hansen et al. (2010). Total lake trout abundance (age-3 +) was 136,000 fish (95% CI 119,000–158,000) in 2006 when suppression began. Adult abundance (age-8 +) declined by 64% during the suppression period, varying from a high of 25,300 fish (95% CI 23,200–28,100 fish) in 2007 to a low of 9,200 fish (95% CI 7,800–10,300 fish) in 2015 (Fig. 3b). Lake trout recruitment, as indexed by age-3 abundance, declined by 56% during the suppression period, with peak abundance occurring in 2009 (36,900 fish; 95% CI 31,800–44,400 fish) and the lowest abundance (10,500 fish; 95% CI 9,200–12,500 fish) being reached in 2014 (Fig. 3a).
Lake trout demographic responses were largely inconsistent with expectations for a population subjected to increased fishing mortality. Lake trout growth rate did not increase in response to declining abundance during the suppression period. Instead, asymptotic length and early growth rate varied independent of abundance (Fig. 4a, b). Similarly, population size structure and age structure did not decline, both varying independent of abundance during suppression (Fig. 5a,b ). However, annual mortality (A) of lake trout increased, as expected, in response to increased fishing effort during suppression (Fig. 6). Mean annual mortality (A) was 31.1% during 2006–2016 (range 23.0–35.9%). Angling was the largest source of fishing mortality from 2006 to 2008, and gill netting was the highest mortality source in all subsequent years (Fig. 6). Trap netting had the greatest influence on fishing mortality during 2006–2008, but was a minor mortality source for all years combined relative to gill netting and angling (Fig. 6).
The kokanee population did not collapse, and instead, rapidly increased during the period of lake trout suppression. Age-1 + kokanee density steadily declined prior to lake trout suppression (1995–2005), reached a low of 101 fish/ha (90% CI 88–114 fish/ha) in 2008, and then increased to a high of 377 fish/ha (90% CI 320–434 fish/ha) by 2016 (Fig. 7). Age-0 kokanee density was variable before suppression began (1995–2005), but reached one of its lowest levels (219 fish/ha; 90% CI 190–248) in 2008. After that time, age-0 density increased to a high of 741 fish/ha (90% CI 699–783) in 2016 (Fig. 7).
Incidental by-catch of bull trout was common when targeting lake trout. In total, 15,209 bull trout were captured in gill nets and trap nets during 2006–2016 and had a mean TL of 464 mm (SE = 1.0; range 144–939 mm TL). More bull trout were captured in gill nets (14,165; 93%) than in trap nets (1,044; 7%), and the direct mortality rate of 26.2% (4,020 mortalities) resulted in most bull trout being released. Bull trout had a higher direct mortality rate when caught in gill nets (27.4%) than in trap nets (13.6%). A total of 226 bull trout were mistakenly turned in by anglers for the lake trout harvest incentive.
Bull trout abundance increased (µ = 0.07, 95% CI − 0.12–0.25) prior to lake trout suppression and decreased (µ = − 0.14, 95% CI − 0.35–0.08) during suppression. Aggregate redd counts for 12 index tributaries averaged 644 redds (range 288–1,144; Fig. 8). For the entire period (1992–2016), counts exceeded 500 redds in 21 of 25 years (Fig. 8) and µ = − 0.02 (95% CI − 0.15–0.12).
Discussion
The multiple lake trout suppression techniques used were complementary and caused higher fishing mortality than would have been possible using a single technique, thereby halting lake trout population growth more quickly and causing greater reductions in abundance. For instance, incentivized angling was the most effective early when netting techniques were being developed, but became a lesser source of mortality later. While valuable, angling alone would have been insufficient to reach suppression targets because of reduced effectiveness as lake trout abundance and angling success declines. Using angling as a supplemental suppression method has been recommended (Tiberti et al., 2017), and a similar strategy that combines angling and gill netting is being used to suppress lake trout in Flathead Lake, Montana (Hansen et al., 2016). Similarly, trap netting is most effective when used in conjunction with other suppression techniques given its low relative contribution to fishing mortality. The utility of trap nets is greatest when large lake trout are abundant and nets can be set in high-use habitats. Gill netting was the most effective and versatile technique used because mesh size selection allowed lake trout of any size to be targeted and fishing could occur throughout the lake at many depths. This versatility was particularly valuable when lake trout density was low because gill netting, more than other techniques, could be adapted to target remaining fish. Not surprisingly, other lake trout suppression programs rely heavily on gill netting (Syslo et al., 2011; Syslo et al., 2013; Hansen et al., 2016; Fredenberg et al., 2017).
The suppression strategy for Lake Pend Oreille was designed to rapidly and adaptively respond to a growing lake trout population, while maximizing the amount and efficiency of fishing effort to increase the likelihood of recruitment overfishing. Population suppression programs are often more effective when implemented in the early phases of population growth (Simberloff, 2003), but only if more individuals are removed than are produced (Bomford & O’Brien, 1995). Suppression was initiated soon after lake trout were detected in Yellowstone Lake, Wyoming, but limited resources and population-specific knowledge to guide suppression failed to slow lake trout population growth (Syslo et al., 2011). Similarly, suppression began shortly after lake trout introduction in Swan Lake, Montana, but has been insufficient to reduce abundance (Syslo et al., 2013). Evaluations to understand lake trout distribution patterns and population characteristics have been recommended to improve the likelihood that suppression programs will succeed (Dux et al., 2011). In Lake Pend Oreille, population dynamics and distribution studies conducted before and during the suppression period (Hansen et al., 2008; Schoby et al., 2009; Hansen et al., 2010; Wahl & Dux, 2010; Wahl et al. 2011; Rust et al., 2018) increased program efficiency and success. In particular, identifying spawning aggregations and areas where immature lake trout were concentrated allowed small and large fish to be targeted with gill nets and increased suppression efficiency (Hansen et al., 2019).
The amount and type of fishing efforts directed at lake trout in Lake Pend Oreille were evidently sufficient to quickly halt exponential population growth by causing recruitment overfishing. When suppression began, lake trout density was still low relative to many native populations (Healey, 1978; Martin & Olver, 1980; Mills et al., 2002; Neiland et al., 2008). Relative to introduced populations in lakes of similar size, lake trout density in Lake Pend Oreille during 2006 was nearly four times lower than that in Flathead Lake, Montana during 2008–2014 (Hansen et al., 2016) and five times lower than that in Yellowstone Lake, Wyoming in 2013 (Syslo, 2015). Historical overharvest of large native lake trout populations suggests that suppression is still achievable once higher density is reached (Keleher, 1972; Hansen et al., 1999; Krueger & Ebener, 2004). However, suppression of nonnative lake trout populations has been more difficult at higher densities (Syslo et al., 2011; Hansen et al., 2016) and suggests that starting suppression in Lake Pend Oreille when lake trout density was still low likely aided the rate and magnitude of decline.
A simulation model was useful for evaluating the initial rate of lake trout population growth in Lake Pend Oreille (Hansen et al., 2008), and similar approaches have since been used elsewhere to predict lake trout responses to suppression (Syslo et al., 2011; Cox et al., 2013; Syslo et al., 2013; Hansen et al., 2016; Ng et al., 2016; Fredenberg et al., 2017). A subsequent simulation model for Lake Pend Oreille predicted a 67% decline in lake trout abundance by 2015 (Hansen et al., 2010), only slightly higher than observed for age-8 + (adult; 64%) and age-3 (recruit; 56%) fish in this study. Thus, our results validate the Hansen et al. (2010) model for Lake Pend Oreille and demonstrate that simulation modeling can be valuable for predicting effectiveness of lake trout suppression.
In Lake Pend Oreille, increased fishing effort produced higher fishing mortality, but size and age structure and growth did not respond to suppression as hypothesized. Annual mortality (A) averaged 31.1% and caused lake trout population decline despite being considerably lower than the commonly accepted threshold (A = 50%) for overharvest of lake trout populations (Healey, 1978). However, simulations for lake trout populations have predicted population declines below A = 50% for native (Nieland et al., 2008) and nonnative (Hansen et al., 2010; Syslo et al., 2011, 2013; Hansen et al., 2016; Ng et al., 2016) populations. Empirical data from Lake Pend Oreille demonstrate that lake trout declines can occur below A = 50% and further highlights their vulnerability to overharvest (Healey, 1978; Martin & Olver, 1980). Size and age structure often become truncated following size-selective harvest (Hutchings, 2000; Hutchings & Reynolds, 2001). Lake trout size and age structure declined in response to suppression targeting juveniles and adults in Quartz Lake, Montana (Fredenberg et al., 2017). In contrast, size and age structure was variable in Lake Pend Oreille and difficult to explain, but may be consistent with low population density. Fish populations commonly exhibit compensatory density-dependent responses to increased fishing mortality (Lorenzen & Enberg, 2002; Rose et al., 2001), including increased body condition and faster growth (Ferreri & Taylor, 1996; Hansen et al., 2016). In Lake Pend Oreille, lake trout density was low when suppression began, and observed growth was among the fastest for western United States populations (Martinez et al., 2009). Further, western United States populations are typified by fast growth rates for the species because of relatively high productivity and extended growing seasons (Johnson & Martinez, 2000). Thus, an observation of further increased growth was unlikely for lake trout in Lake Pend Oreille, despite the increased fishing mortality.
Kokanee did not collapse in Lake Pend Oreille, which was an unlikely outcome if lake trout suppression had not occurred. Kokanee faced a 65% likelihood of collapse by 2016 because of excessive predation mortality (Hansen et al., 2010), and kokanee populations collapsed over similar time periods elsewhere in response to increasing lake trout abundance (Bowles et al., 1991; Stafford et al., 2002; Martinez et al., 2009). Because kokanee were sustained in Lake Pend Oreille, suppression fishing likely reduced lake trout abundance to such an extent that predation mortality no longer limited kokanee population growth.
Even with sustained high fishing mortality on lake trout, Hansen et al. (2010) predicted that a combination of low predation and high kokanee production would be needed for kokanee to avoid collapse in Lake Pend Oreille. As predicted, lake trout suppression alone did not explain the rapid increase in kokanee abundance. Interestingly, as predation by lake trout was declining during suppression, a simultaneous increase in kokanee production occurred starting in 2011 and coincided with the sudden and inexplicable collapse of Mysis (Corsi et al., 2019). Low density of Mysis, a kokanee competitor (Rieman & Falter, 1981; Martin & Northcote, 1991; Ashley et al., 1997), likely played a large role in the rate at which kokanee rebounded in Lake Pend Oreille (Corsi et al., 2019). However, low Mysis density alone would not likely have prevented the collapse of kokanee in the absence of reduced predation mortality. Despite competition from Mysis, kokanee have been sustained in Lake Pend Oreille for decades, albeit at lower density, following Mysis introduction (Corsi et al., 2019).
The conservation benefit to bull trout from lake trout suppression was difficult to assess because of potential effects from by-catch mortality, an inability to quantitate the indirect benefits to bull trout from decreased lake trout abundance and increased kokanee abundance, and the confounding role of large-scale bioclimatic conditions that may influence bull trout populations (Copeland & Meyer, 2011). Because bull trout declined during the suppression period, it seems reasonable to question whether by-catch mortality was the primary driver. While it may have contributed, it seems unlikely that by-catch mortality played a major role since direct mortality of bull trout from by-catch in 2008 was only 1.6% of estimated abundance during that year (12,513 fish; 95% CI 7,456–22,521; McCubbins et al., 2016). Even the highest annual direct by-catch mortality (539 in 2013) would have resulted in low exploitation of bull trout based on the observed population trend. Similar gill netting to suppress lake trout in Quartz Lake, Montana did not appear to negatively affect bull trout (Fredenberg et al., 2017).
While limitations exist to determining the bull trout conservation benefit during the study period, lake trout suppression has likely contributed to the long-term conservation of bull trout in Lake Pend Oreille. Based on the numerous documented collapses or severe declines of bull trout following lake trout introductions elsewhere (Bowles et al., 1991; Donald & Alger, 1993; Fredenberg, 2002; Stafford et al., 2002), bull trout abundance in Lake Pend Oreille would likely be much lower if suppression had not occurred. Despite their decline during lake trout suppression, bull trout remain abundant in Lake Pend Oreille relative to other regional populations (Kovach et al., 2016). This is encouraging given that nearly 20 years have passed since exponential growth of lake trout began. In contrast, it took only 10–15 years for bull trout to collapse in Priest Lake, Idaho (Bowles et al., 1991) and suffer a major decline in Flathead Lake, Montana (Ellis et al., 2011) in response to increasing lake trout abundance and declining kokanee abundance. A recent regional analysis concluded that bull trout using three lakes where lake trout suppression occurred (including Lake Pend Oreille) were more abundant and had higher population growth rates than bull trout using lakes and reservoirs where competitors and predators were not suppressed (Kovach et al., 2016). This lends support to our contention that lake trout suppression has provided a conservation benefit for bull trout in Lake Pend Oreille.
Conclusions
We conclude that suppression can be an effective management action for mitigating effects of nonnative lake trout in large, deep lakes. It is likely that reduced predation mortality from lake trout prevented kokanee collapse, contributed to the conservation of native bull trout, and allowed a popular recreational fishery to be restored, including re-opening of a kokanee harvest fishery in 2013. To date, Lake Pend Oreille represents the largest effective suppression of a nonnative lake trout population. The only other example of suppression resulting in nonnative lake trout population decline is Quartz Lake, Montana, a small (352 ha) mountain lake (Fredenberg et al., 2017). Despite the drastically different scales of implementation, these two programs provide valuable insight into critical factors influencing suppression effectiveness. In both cases, suppression was initiated early after population growth began, small and large lake trout were targeted with gill nets, spawning aggregations were identified and targeted, and routine monitoring data were collected that allowed progress to be evaluated (Fredenberg et al., 2017). In Lake Pend Oreille, the long-term management target of a 90% reduction from peak abundance has yet to be achieved. Simulation modeling will help to understand how long it will take to reach that target, how to optimally allocate netting effort, and what it will take to maintain target abundance in the future (Hansen et al., 2019). Maximizing suppression efficiency will increase the likelihood of reaching the management target, thus allowing eventual reductions in fishing effort and costs. Attempting to transition into maintenance suppression to sustain low lake trout density will continue to make Lake Pend Oreille an informative model for long-term management of nonnative lake trout populations elsewhere.
On a broader-scale, our efforts to suppress lake trout highlight the tremendous management complexity and costs that can be required to mitigate the effects of introduced fishes. While it may be possible to eventually reduce suppression costs substantially, an adequate initial investment of resources and a long-term commitment are necessary since eradication of introduced species is rarely possible. In our case, sufficient long-term funding was available, and the cost of suppression was deemed a worthwhile investment given the high social and economic values of the recreational fishery and the native fish conservation value. The costs and benefits, available resources, and reality of making a long-term management commitment should be considered before initiating a suppression program.
References
Ashley, K., L. C. Thompson, D. C. Lasenby, L. McEachern, K. E. Smokorowski & D. Sebastian, 1997. Restoration of an interior lake ecosystem: the Kootenay Lake fertilization experiment. Water Quality Research Journal of Canada 32: 295–323.
Behnke, R. J., 2002. Trout and salmon of North America. The Free Press, New York.
Bomford, M. & P. O’Brien, 1995. Eradication or control for vertebrate pests. Wildlife Society Bulletin 23: 249–255.
Bouwens, K. A. & R. Jakubowski, 2016. 2014 Lake Pend Oreille creel survey. Idaho Department of Fish and Game, Boise.
Bowles, E. C., V. L. Ellis, D. Hatch & D. Irving, 1987. Kokanee stock status and contribution of Cabinet Gorge Hatchery, Lake Pend Oreille, Idaho, Annual Progress Report FY 1986. Idaho Department of Fish and Game, Agreement No. DE-AI79-85BP22493, Project 85-339, Boise, Idaho.
Bowles, E. C., B. E. Rieman, G. R. Mauser & D. H. Bennett, 1991. Effects of introductions of Mysis relicta on fisheries in northern Idaho. In T. P. Nesler & E. P. Bergersen (eds), Mysids in fisheries: hard lessons from headlong introductions. American Fisheries Society Symposium 9, Bethesda, Maryland: 65–74.
Cochran, W. G., 1977. Sampling techniques, 3rd ed. John Wiley & Sons, New York.
Copeland, T. & K. A. Meyer, 2011. Interspecies synchrony in salmonid densities associated with large-scale bioclimatic conditions in central Idaho. Transactions of the American Fisheries Society 140: 928–942.
Crossman, E. J., 1995. Introduction of the lake trout (Salvelinus namaycush) in areas outside its native distribution: a review. Journal of Great Lakes Research 21(Supplement 1): 17–29.
Corsi, M. P., M. J. Hansen, M. C. Quist, D. J. Schill, & A. M. Dux, 2019. Influences of lake trout (Salvelinus namaycush) and Mysis diluviana on Kokanee (Oncorhynchus nerka) in Lake Pend Oreille, Idaho. Hydrobiologia, this issue. https://doi.org/10.1007/s10750-019-3889-8.
Cox, B. S., C. S. Guy, W. A. Fredenberg & L. R. Rosenthal, 2013. Baseline demographics of a non-native lake trout population and inferences for suppression from sensitivity-elasticity analyses. Fisheries Management and Ecology 20: 390–400.
Donald, D. B. & D. J. Alger, 1993. Geographic distribution, species displacement, and niche overlap for lake trout and bull trout in mountain lakes. Canadian Journal of Zoology 71: 238–247.
Dux, A. M., C. S. Guy & W. A. Fredenberg, 2011. Spatiotemporal distribution and population characteristics of a nonnative lake trout population, with implications for suppression. North American Journal of Fisheries Management 31: 187–196.
Eby, L. A., W. J. Roach, L. B. Crowder & J. A. Standford, 2006. Effects of stocking-up freshwater food webs. Trends in Ecology and Evolution 21: 576–584.
Ellis, B. K., J. A. Stanford, D. Goodman, C. P. Stafford, D. L. Gustafson, D. A. Beauchamp, D. W. Chess, J. A. Craft, M. A. Deleray & B. S. Hansen, 2011. Long-term effects of a trophic cascade in a large lake ecosystem. Proceedings of the National Academy of Sciences of the United States of America 108: 1070–1075.
Ferreri, C. P. & W. W. Taylor, 1996. Compensation in individual growth rates and its influence on lake trout population dynamics in the Michigan waters of Lake Superior. Journal of Fish Biology 49: 763–777.
Fredenberg, W., 2002. Further evidence that lake trout displace bull trout in mountain lakes. Intermountain Journal of Sciences 8: 143–152.
Fredenberg, C. R., C. C. Muhlfeld, C. S. Guy, V. S. D’Angelo, C. C. Downs & J. M. Syslo, 2017. Suppression of invasive Lake Trout in an isolated backcountry lake in Glacier National Park. Fisheries Management and Ecology 24: 33–48.
Gallucci, V. F. & T. J. Quinn, 1979. Reparameterizing, fitting, and testing a simple growth model. Transactions of the American Fisheries Society 108: 14–25.
Guy, C. S., R. M. Neumann, D. W. Willis & R. O. Anderson, 2007. Proportional size distribution (PSD): a further refinement of population size structure index terminology. Fisheries 32: 348.
Hansen, M. J., 1999. Lake trout in the Great Lakes: basin wide stock collapse and binational restoration. In Taylor, W. W. & C. P. Ferreri (eds), Great Lakes Fishery Policy and Management: A Binational Perspective. Michigan State University Press, East Lansing, Michigan: 417–453.
Hansen, M. J., 2007. Predator-prey dynamics in Lake Pend Oreille. Idaho Department of Fish and Game, Fishery Research Report 07-53, Boise, Idaho.
Hansen, M. J., N. J. Horner, M. Liter, M. P. Peterson & M. A. Maiolie, 2008. Dynamics of an increasing lake trout population in Lake Pend Oreille, Idaho, USA. North American Journal of Fisheries Management 28: 1160–1171.
Hansen, M. J., D. Schill, J. Fredericks & A. Dux, 2010. Salmonid predator-prey dynamics in Lake Pend Oreille, Idaho, USA. Hydrobiologia 650: 85–100.
Hansen, M. J., B. H. Hansen & D. A. Beauchamp, 2016. Lake trout (Salvelinus namaycush) suppression for bull trout (Salvelinus confluentus) recovery in Flathead Lake, Montana, North America. Hydrobiologia 783: 317–334.
Hansen, M. J., M. P. Corsi & A. M. Dux, 2019. Long-term suppression of the lake trout (Salvelinus namaycush) population in Lake Pend Oreille, Idaho. Hydrobiologia, this issue. https://doi.org/10.1007/s10750-019-3890-2.
Healey, M. C., 1978. The dynamics of exploited lake trout populations and implications for management. Journal of Wildlife Management 42: 307–328.
Hoelscher, B., 1992. Pend Oreille Lake fishery assessment 1951 to 1989. Idaho Department of Health and Welfare, Division of Environmental Quality Community Programs, Boise, Idaho.
Hutchings, J. A., 2000. Collapse and recovery of marine fishes. Nature 46: 882–885.
Hutchings, J. A. & J. D. Reynolds, 2001. Marine fish population collapses: consequences for recovery and extinction risk. BioScience 54: 297–309.
Jelks, H. L., S. J. Walsh, N. M. Burkhead, S. Contreras-Balderas, E. Diaz-Pardo, D. A. Hendrickson, J. Lyons, N. E. Mandrak, F. McCormick, J. S. Nelson, S. P. Platania, B. A. Porter, C. B. Renaud, J. J. Schmitter-Soto, E. B. Taylor & M. L. Warren Jr., 2008. Conservation status of imperiled North American freshwater and diadromous fishes. Fisheries 33: 372–407.
Johnson, B. M. & P. J. Martinez, 2000. Trophic economic of lake trout management in reservoirs of differing productivity. North American Journal of Fisheries Management 20: 127–143.
Keleher, J. J., 1972. Great Slave Lake: effects of exploitation on the salmonid community. Journal of the Fisheries Research Board of Canada 29: 741–753.
Kovach, R. P., R. Al-Chokhachy, D. C. Whited, D. A. Schmetterling, A. M. Dux & C. C. Muhlfeld, 2016. Climate, invasive species and land use drive population dynamics of a cold-water specialist. Journal of Applied Ecology. https://doi.org/10.1111/1365-2664.12766.
Krueger, C. C. & M. P. Ebener, 2004. Rehabilitation of lake trout in the Great Lakes: past lessons and future challenges. In Gunn, J. M., R. J. Steedman & R. A. Ryder (eds), Boreal shield watersheds: Lake trout ecosystems in a changing environment. Lewis Publishers, Boca Raton: 37–56.
Li, H. W. & P. B. Moyle, 1999. Management of introduced fishes. In Kohler, C. C. & W. A. Hubert (eds), Inland Fisheries Management in North America, 2nd ed. American Fisheries Society, Bethesda, Maryland: 345–374.
Lorenzen, K. & K. Enberg, 2002. Density-dependent growth as a key mechanism in the regulation of fish populations: evidence from among-population comparisons. Proceedings of the Royal Society of London B: Biological Sciences 269: 49–54.
Maiolie, M. A., M. P. Peterson, W. J. Ament & W. Harryman, 2006. Kokanee Response to Higher Winter Lake Levels in Lake Pend Oreille During 2005. Idaho Department of Fish and Game, Fishery Research Report 08-06, Boise, Idaho.
Martin, N. V., & T. G. Northcote, 1991. Kootenay Lake: an inappropriate model for Mysis relicta introduction in north temperate lakes. In T. P. Nesler & E. P. Bergersen (eds), Mysids in fisheries: hard lessons from headlong introductions. American Fisheries Society Symposium 9, Bethesda, Maryland: 23–29.
Martin, N. V. & C. H. Olver, 1980. The lake charr, Salvelinus namaycush. In Balon, E. (ed.), Charrs: salmonid fishes of the genus Salvelinus. Junk Publishers, The Hague, The Netherlands: 205–277.
Martinez, P. J., P. E. Bigelow, M. A. Deleray, W. A. Fredenberg, B. S. Hansen, N. J. Horner, S. K. Lehr, R. W. Schneidervin, S. A. Tolentino & A. E. Viola, 2009. Western lake trout woes. Fisheries 34: 424–442.
McCubbins, J. L., M. J. Hansen, J. M. DosSantos & A. M. Dux, 2016. Demographic characteristics of an adfluvial bull trout population in Lake Pend Oreille, Idaho. North American Journal of Fisheries Management 36: 1269–1277.
Miller, R. R., J. D. Williams & J. E. Williams, 1989. Extinctions of North American fishes during the past century. Fisheries 14: 22–38.
Mills, K. H., S. M. Chalanchuk & D. J. Allan, 2002. Abundance, annual survival, and recruitment of unexploited and exploited lake charr, Salvelinus namaycush, populations at the Experimental Lakes area, northwestern Ontario. Environmental Biology of Fishes 64: 281–292.
Mooij, W. M., J. M. Van Rooij & S. Wijnhoven, 1999. Analysis and comparison of fish growth from small samples of length-at-age data: detection of sexual dimorphism in Eurasian Perch as an example. Transactions of the American Fisheries Society 128: 483–490.
Morris, W. F. & D. F. Doak, 2002. Quantitative conservation biology: Theory and practice of population viability analysis. Sinauer Associates Inc, Sunderland.
Muir, A. M., C. C. Krueger & M. J. Hansen, 2012. Re-establishing lake trout in the Laurentian Great Lakes: the past, present, and future. In Taylor, W. W., A. J. Lynch & N. J. Leonard (eds), Great Lakes Fishery Policy and Management: A Binational Perspective, 2nd ed. Michigan State University Press, East Lansing: 533–588.
Nieland, J. L., M. J. Hansen, M. J. Seider & J. J. Deroba, 2008. Modeling the sustainability of lake trout fisheries in eastern Wisconsin waters of Lake Superior. Fisheries Research 94: 304–314.
Ng, E. L., J. P. Fredericks & M. C. Quist, 2016. Population dynamics and evaluation of alternative management strategies for nonnative lake trout in Priest Lake, Idaho. North American Journal of Fisheries Management 36: 40–54.
Olver, C. H., D. Nadeau & H. Fournier, 2004. The control of harvest in lake trout fisheries on Precambrian Shield lakes. In Gunn, J. M., R. J. Steedman & R. A. Ryder (eds), Boreal shield watersheds: lake trout ecosystems in a changing environment. Lewis Publishers, Boca Raton, Florida: 193–218.
Pate, W. M., B. M. Johnson, J. M. Lepak & D. Brauch, 2014. Managing for coexistence of kokanee and trophy lake trout in a montane reservoir. North American Journal of Fisheries Management 34: 908–922.
Pauly, D., 1980. On the interrelationships between natural mortality, growth parameters and mean environmental temperature in 175 fish stocks. Journal du Conseil International pour l’Exploration de la Mer 39: 175–192.
Pope, J. G., 1972. An investigation of the accuracy of virtual population analysis using cohort analysis. In International Commission for the Northwest Atlantic Fisheries, Research Bulletin Number 9: 65–74.
Quinn II, T. J. & R. B. Deriso, 1999. Quantitative Fish Dynamics. Oxford University Press, New York.
Rahel, F. J., 2000. Homogenization of fish faunas across the United States. Science 288: 854–856.
Ricker, W. E., 1975. Computation and interpretation of biological statistics of fish populations. Bulletin 191 of the Fisheries Research Board of Canada, Ottawa, Canada.
Rieman, B. E. & C. M. Falter, 1981. Effects of the establishment of Mysis relicta on the macrozooplankton of a large lake. Transactions of the American Fisheries Society 110: 613–620.
Rose, K. A., J. H. Cowan Jr., K. O. Winemiller, R. A. Myers & R. Hilborn, 2001. Compensatory density dependence in fish populations: importance, controversy, understanding and prognosis. Fish and Fisheries 2: 293–327.
Rust, P., N. C. Wahl, M. P. Corsi, W. J. Ament & W. H. Harryman, 2018. Lake Pend Oreille Reserach, 2015. Idaho Department of Fish and Game, Fishery Research Report 18–01, Boise, Idaho.
Ruzycki, J. R., W. A. Wurtsbaugh & C. L. Luecke, 2001. Salmonine consumption and competition for endemic prey fishes in Bear Lake, Utah-Idaho. Transactions of the American Fisheries Society 130: 1175–1189.
Ruzycki, J. R., D. A. Beauchamp & D. L. Yule, 2003. Effects of introduced lake trout on native cutthroat trout in Yellowstone Lake. Ecological Applications 13: 23–37.
Schoby, G. P., N. C. Wahl & A. M. Dux, 2009. Lake trout spawning locations in Lake Pend Oreille, 2007. Idaho Department of Fish and Game, Fishery Research Report 09-13, Boise, Idaho.
Scott, W. B. & E. J. Crossman, 1973. Freshwater fishes of Canada. Bulletin of the Fisheries Research Board of Canada 184: 1–996.
Sellers, T. J., B. R. Parker, D. W. Schindler & W. M. Tonn, 1998. Pelagic distribution of lake trout (Salvelinus namaycush) in small Canadian Shield lakes with respect to temperature, dissolved oxygen, and light. Canadian Journal of Fisheries and Aquatic Sciences 55: 2161–2177.
Shuter, B. J., M. L. Jones, R. M. Korver & N. P. Lester, 1998. A general, life history based model for regional management of fish stocks: the inland lake trout (Salvelinus namaycush) fisheries of Ontario. Canadian Journal of Fisheries and Aquatic Sciences 55: 2161–2177.
Simberloff, D., 2003. How much population biology is needed to manage introduced species? Conservation Biology 17: 83–92.
Stafford, C. P., J. A. Stanford, F. R. Hauer & E. B. Brothers, 2002. Changes in lake trout growth associated with Mysis relicta establishment: a retrospective analysis using otoliths. Transactions of the American Fisheries Society 131: 994–1003.
Steed, R., 2011. Pend Oreille Lake monitoring results, 2006–2009. Idaho Department of Environmental Quality, Water Quality Report, Coeur d’Alene, Idaho.
Syslo, J. M., 2015. Dynamics of Yellowstone cutthroat trout and lake trout in the Yellowstone Lake ecosystem: a case study for the ecology and management on non-native fishes. Doctoral dissertation, Montana State University, Bozeman.
Syslo, J. M., C. S. Guy, P. E. Bigelow, P. D. Doepke, B. D. Ertel & T. M. Koel, 2011. Response of non-native lake trout (Salvelinus namaycush) to 15 years of harvest in Yellowstone Lake, Yellowstone National Park. Canadian Journal of Fisheries and Aquatic Sciences 68: 2132–2145.
Syslo, J. M., C. S. Guy & B. S. Cox, 2013. Comparison of harvest scenarios for the cost-ffective suppression of Lake Trout in Swan Lake, Montana. North American Journal of Fisheries Management 33: 1079–1090.
Tiberti, R., M. Ottino, S. Brighenti, R. Iacobuzio, M. Rolla, A. Von Hardenberg & B. Bassano, 2017. Involvement of recreational anglers in the eradication of alien brook trout from high-altitude lakes. Journal of Mountain Ecology 10: 13–26.
Tronstad, L., R. O. Hall Jr., T. M. Koel & K. G. Gerow, 2010. Introduced lake trout produced a four-level trophic cascade in Yellowstone Lake. Transactions of the American Fisheries Society 139: 1536–1550.
Vander Zanden, M. J., S. Chandra, B. C. Allen, J. E. Reuter & C. R. Goldman, 2003. Historical food web structure and restoration of native aquatic communities in the Lake Tahoe basin. Ecosystems 6: 274–288.
Vidergar, D. T., 2000. Population estimates, food habits and estimates of consumption of selected predatory fishes in Lake Pend Oreille, Idaho. Master’s thesis. University of Idaho, Moscow, Idaho.
Wahl, N. C. & A. M. Dux, 2010. Evaluation of lake trout spawning locations in Lake Pend Oreille, 2008. Idaho Department of Fish and Game, Fishery Research Report 10-03, Boise, Idaho.
Wahl, N. C., A. M. Dux, W. J. Ament & W. Harryman, 2010. Kokanee and rainbow trout research, Lake Pend Oreille, 2008. Idaho Department of Fish and Game, Fishery Research Report 10-02, Boise, Idaho.
Wahl, N. C., A. M. Dux, W. J. Ament & W. Harryman, 2011. Lake Pend Oreille Research, 2010. Idaho Department of Fish and Game 11–22, Boise, Idaho.
Zar, J. H., 1999. Biostatistical analysis, 4th ed. Prentice-Hall, Upper Saddle River, New Jersey.
Acknowledgements
Funding for lake trout suppression was provided by the Avista Corporation and the U.S. Department of Energy, Bonneville Power Administration. We thank the many Idaho Department of Fish and Game staff, volunteers, and other collaborators who assisted with this project. We also thank Hickey Brothers Research, LLC personnel who conducted all netting operations. Use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: C. E. Adams, C. R. Bronte, M. J. Hansen, R. Knudsen & M. Power / Charr Biology, Ecology and Management
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dux, A.M., Hansen, M.J., Corsi, M.P. et al. Effectiveness of lake trout (Salvelinus namaycush) suppression in Lake Pend Oreille, Idaho: 2006–2016. Hydrobiologia 840, 319–333 (2019). https://doi.org/10.1007/s10750-019-3913-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-3913-z