Abstract
Non-native lake trout Salvelinus namaycush displaced native bull trout Salvelinus confluentus in Flathead Lake, Montana, USA, after 1984, when Mysis diluviana became abundant following its introduction in upstream lakes in 1968–1976. We developed a simulation model to determine the fishing mortality rate on lake trout that would enable bull trout recovery. Model simulations indicated that suppression of adult lake trout by 75% from current abundance would reduce predation on bull trout by 90%. Current removals of lake trout through incentivized fishing contests has not been sufficient to suppress lake trout abundance estimated by mark-recapture or indexed by stratified-random gill netting. In contrast, size structure, body condition, mortality, and maturity are changing consistent with a density-dependent reduction in lake trout abundance. Population modeling indicated total fishing effort would need to increase 3-fold to reduce adult lake trout population density by 75%. We conclude that increased fishing effort would suppress lake trout population density and predation on juvenile bull trout, and thereby enable higher abundance of adult bull trout in Flathead Lake and its tributaries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The lake trout Salvelinus namaycush is widely distributed across North America north of latitude 41° (Crossman, 1995), where the species co-dominates cold-water lakes with the lake whitefish Coregonus clupeaformis (Johnson, 1976). The lake trout is relatively slow growing (length = 33–83 cm at age 10) and late maturing (age at first maturity = 4–13 years), so is susceptible to over-fishing (Healey, 1978; Martin & Olver, 1980; Olver et al., 2004). Based on an extensive review of lake trout populations across the native range, Healey (1978) concluded that lake trout populations could not withstand total annual mortality higher than 50% or annual yield higher than 0.50 kg/ha. For example, the largest lake trout populations in the world were over-fished to extirpation in nearly all of the Laurentian Great Lakes (Hansen, 1999; Krueger & Ebener, 2004; Muir et al., 2012). Similarly, the lake trout population in the western arm of Great Slave Lake, Northwest Territories, Canada, failed after only 10 years of commercial fishing (Keleher, 1972; Healey, 1978). Exploitation remains the most critical stress affecting lake trout populations in Precambrian Shield lakes of eastern Canada and northeastern United States (Olver et al., 2004).
Because of its popularity in its native range, the lake trout was widely stocked across North America in the late 1800s and early 1900s (Crossman, 1995). In USA, west of the continental divide, the species sometimes increased greatly in abundance to suppress other species through predation or competition (Donald & Alger, 1993). Where introduced, the lake trout was often of minor importance until Mysis diluviana, an opportunistic planktivore, was widely introduced to enhance growth of kokanee Oncorhynchus nerka (Martin & Northcote, 1991). Lake trout recruitment was typically limited in lakes with a lack of native prey in deep waters where juvenile lake trout resided, so population density increased after Mysis were introduced (Stafford et al., 2002). For example, shortly after Mysis became established, lake trout increased in numbers and subsequently preyed heavily on kokanee and bull trout Salvelinus confluentus, thereby leading to a decline in these species in Flathead Lake, Montana (Stafford et al., 2002) and Priest Lake, Idaho (Bowles et al., 1991).
In Flathead Lake, lake trout predation, combined with reductions in plankton density caused by Mysis, drove the kokanee population into collapse (Ellis et al., 2011), thereby culminating in a system dominated by lake trout and lake whitefish (Table 1). Lake trout were thought to threaten the bull trout population through competition or predation (Donald & Alger, 1993; Fredenberg, 2002), although lake trout may exert some other control over the food web (Ellis et al., 2011). To benefit native fishes such as the bull trout and westslope cutthroat trout Oncorhynchus clarkii lewisi, the Confederated Salish and Kootenai Tribes (Tribes) are endeavoring to reduce lake trout abundance in Flathead Lake (CSKT, 2014). Specifically, the Tribes are employing incentivized angling and targeted netting to reduce lake trout abundance and predation on bull trout and cutthroat trout enough to enable both species to recover to sustainable and possibly fishable levels (CSKT, 2014).
Our objective was to quantify levels of lake trout mortality associated with levels of bull trout recovery in Flathead Lake. We developed a density-dependent stochastic simulation model for lake trout, combined with a bioenergetics model of lake trout consumption on bull trout to quantify the interaction between the two species. We used the model to simulate the level of fishing effort required to reduce future abundance of adult lake trout (age 8+) by prescribed levels (25, 50, and 75%), along with associated reductions in consumption of bull trout by lake trout. Mark-recapture surveys and stratified-random gill-netting were used to monitor status of the lake trout population and effectiveness of the lake trout population suppression program.
Study area
Flathead Lake (47°54′6″N, 114°6′15″W) is the largest natural freshwater lake west of the Mississippi River in the United States outside of Alaska (surface area = 496 km2; shoreline length = 301.9 km, volume = 23.2 km3; http://flbs.umt.edu/lake/flatheadlake.aspx). The lake is relatively deep (mean depth = 50 m; maximum depth = 113 m) and fed by the 18,290-km2 watershed of the Flathead River (mean flow = 286 m3/s; flushing time = 3.4 years) that drains west from the Continental Divide to the Columbia River. The lake is oligotrophic and relatively free of pollutants, with a small human population of 85,000 permanent residents in the lake basin. The upper 3 m of the lake is regulated by Kerr Dam, which was built in 1938.
Flathead Lake supports a recreational fishery based on introduced species. Native fishes include the bull trout, westslope cutthroat trout, northern pikeminnow Ptychocheilus oregonensis, two whitefish species Prosopium spp., two sucker species Catostomus spp., three minnow species (Cyprinidae), and slimy sculpin Cottus cognatus. At least 17 other species have been introduced since 1910. Cutthroat trout predominated in the recreational fishery before 1920, but were replaced by the kokanee from 1940 until 1985, the year before Mysis peaked in abundance (Ellis et al., 2011). The rapid collapse of the kokanee population was likely caused by a combination of predation by the rapidly growing lake trout population and a Mysis-induced decline in the plankton community (Spencer et al., 1991; Ellis et al., 2011). The lake trout is diversifying morphologically, through the appearance of a dwarf morph that exploits deep-water habitat (Stafford et al., 2014).
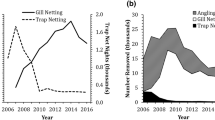
The lake trout population supports a recreational angling fishery, supplemented by subsidized fishing contests, called Spring and Fall Mack Days, to suppress lake trout numbers in Flathead Lake (CKST, 2014). Between 1994 and 2014, harvest of lake trout 762–914 mm was prohibited, to increase catch rates of large fish. The creel limit per angler was incrementally increased from 15 to 100 fish per day in 2004–2010. The number of rods allowed per angler was also increased from one to two in 2004. Lake trout harvest in contests grew from fewer than 1,000 fish in 2002 to nearly 53,000 fish in 2012, but stabilized at 47,000 fish per year in 2010–2014 (Fig. 1).
Methods
Response of the lake trout population to increased fishing mortality in 2010–2014 was evaluated using simulation modeling and fishery independent surveys. An age-structured density-dependent stochastic simulation model was based on biological attributes of the lake trout population in Flathead Lake. Model parameters were derived from data collected in annual mark-recapture and gill-net surveys, which were also used to monitor lake trout population response to increased fishing mortality implemented in 2010.
Abundance
Mark-recapture surveys were used to estimate lake trout abundance in Flathead Lake in 2008–2014. For each survey, angling and gillnetting were used to capture fish, which were marked and released for recapture in fishing contests. Fish were marked with 12.5-mm PIT tags inserted with plastic syringes into tissue at the top of the gill plate in a downward direction for at least 13 mm. Fish were also marked by removal of an adipose fin, to indicate tag presence in recapture sampling. Fish judged as likely to survive were tagged and released. Captured fish that were unable to swim downward after release were placed in a bottomless cage that was submerged to the recompression depth, where fish were allowed to swim away. Fish unable to swim or only able to swim at the surface were not tagged or released. During recapture sampling, all captured fish were checked for clipped adipose fins. To adjust for growth between marking and recapture, the relationship between length at marking and length at recapture of marked fish was used to estimate the length at marking of each unmarked fish that was examined for marks.
Recapture samples were obtained through fishing contests in which all recaptured lake trout were removed from the population, so sampling was without replacement, thereby making Chapman’s modification of the Petersen estimator applicable (Eq. 3.7 in Ricker, 1975). We computed 95% confidence limits for estimated abundance from exact binomial confidence limits for the fraction marked in recapture samples (Eqs. 24.28, 24.29 in Zar, 1999). Standard error (SE) and coefficient of variation (CV) were estimated from the variance of estimated abundance (Eq. 3.8 in Ricker, 1975). Estimates of abundance and associated statistics were generated for the pooled sample of all lake trout sampled, although lake trout longer than the minimum of the slot-length limit (762 mm) were not accepted in the contests, so estimated abundance did not include lake trout longer than 762 mm. The mean mark-recapture estimate of abundance in 2010–2012 was converted into number density (number/ha) for comparison to other lake trout populations in North America. Annual angler harvest in numbers from Mack Days contests and the regular angling season was converted into yield using the weight-length equation from the gill-net assessment fishery (see below) and expressed as yield density (kg/ha) for comparison to other lake trout populations in North America.
Relative abundance
A stratified-random gill-net survey was used to index lake trout population status in Flathead Lake in autumn, 1998–2014. Gill-net effort was distributed throughout the lake in proportion to the amount of surface area in five areas of the lake and up to four depth zones in each area (0–30.5, 30.5–61.0, 61.0–91.4, and >91.4 m). Each net was soaked overnight, with 48 sets/year in 1998–2007, 72 sets/year in 2008–2012 and 2014, and 94 sets in 2013. Gill nets were constructed of 12 mesh sizes to encompass the range of plausible lake trout lengths, with bar-measure panels of 9.7- and 12.7-mm to 76.2-mm in 6.4-mm increments. Based on the relationship between lake trout length and girth, lake trout ranging in total length from 130 mm to 1,086 mm were vulnerable to capture by wedging in the range of meshes used for sampling lake trout in Flathead Lake, although the linear progression of mesh sizes biases length and age frequencies higher than the true population length and age frequencies (Hansen et al., 1997b). Lake trout were measured in total length (mm) and weight (g). Sex (male or female) and maturity status (mature or immature) were determined by examining gonads.
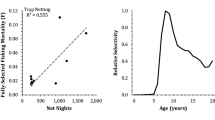
Relative abundance was indexed as geometric mean annual catch/net (±95% confidence limits) in Flathead Lake in 1998–2014. To estimate mean relative abundance for each year of the gill-net survey, we fit a general linear model with log e (catch + 1/net) as the dependent variable, and year, area, and depth stratum as independent variables. To account for 6% of nets that caught no lake trout, a constant of one fish was added to each net catch. To normalize residuals from the general linear model, (catch + 1)/net was transformed into natural logarithms. Least-squares means (±95% confidence limits) from the general linear model were then back-transformed as the exponent of log e (catch + 1/net), and the constant was subtracted, to express relative abundance as geometric-mean catch/net. Annual geometric-mean catch/net was estimated for all fish, number of adult fish (age-8-and-older), and numbers of fish shorter and longer than the lower limit of the protected slot-length limit (762 mm). Relative abundance could decrease as harvest increased, so we tested the significance of the correlation between catch/net and harvest in Mack Days contests (all fish, adult fish, fish < 762 mm, and fish ≥ 762 mm) in 1998–2014.
Biological attributes
Population size structure was indexed as proportional size distribution (PSD; Guy et al., 2007), the percentage of fish longer than 300 mm (stock length = minimum size vulnerable to capture) that are also longer than 500 mm (quality length = minimum size anglers like to catch; Hubert et al., 1994; Neumann et al., 2012), from gillnet samples each year in autumn 1998–2014. Gill-net size selectivity would bias low the sample size structure (Hansen et al., 1997b), but we assumed that the bias was consistent in all years surveyed. Population size structure could change if lake trout population density declined due to harvest, so we tested significance of the correlation between PSD and catch/net in 1998–2014.
Body condition was indexed as relative weight (W r; Neumann et al., 2012), the ratio of observed weight of individual fish (W) to a standard weight for the species (W s) from samples of weight and length data across the species’ range (W r = W/W s × 100; Piccolo et al., 1993), of lake trout caught in gill nets each year in 1998–2014. We first computed W r for each individual lake trout (≥280 mm TL) caught during standardized gillnet surveys, and for which length and weight were measured. We then computed mean W r (±95% confidence limits) for each year, as an index of body condition in 1998–2014. Body condition could change if lake trout population density declined due to harvest, so we tested significance of the correlation between relative weight and catch/net (total and ≥762 mm) in 1998–2014.
Annual mortality (A) was estimated from the descending limb of the age frequency for each year in 1998–2014 (catch-curve method of Ricker 1975). The age frequency of each annual sample was estimated from the length frequency of each annual sample using an age-length key derived from samples of 266 lake trout ages from 2005 and 2008 (Ricker, 1975). Estimated numbers at age (N t ) were converted into natural logarithms, and the slope (Z = instantaneous rate of total mortality ± 95% confidence limits) of the relationship between log e (N t) and age (t) was estimated using linear regression. Residuals were examined to ensure the model fit the data. The annual mortality rate (±95% confidence limits) was estimated as A = 1−e −Z (Ricker, 1975). Gill-net size selectivity would cause catch-curve mortality to be biased low (Hansen et al., 1997b), but we assumed that the bias was consistent in all years surveyed. An increase in fishing mortality caused by Mack Days contests could increase total mortality, so we tested significance of the correlation between annual mortality and harvest in Mack Days contests in 1998–2014. Further, mortality could change in response to increased harvest, so we tested significance of the correlation between mortality and catch/net (total and ≥762 mm) in 1998–2014.
Length at 50% maturity was estimated using logistic regression on the maturity status of individual fish sampled annually at each length or age (immature = 0; mature = 1) during gill-net surveys in 1998–2014. Males and females were combined for maturity analysis, because the simulation model operated on both sexes in the total population. Model intercept b 0 and slope b 1 and their standard errors were estimated for the logit transformation of the logistic equation (Hosmer & Lemeshow, 2000) and length at 50% maturity (L 50) was estimated as the absolute value of the ratio of the intercept to the slope |b 0/b 1|. Age at 50% maturity (A 50) was estimated from L 50 using the length-age model described above. Size at maturity could change if lake trout population density declined due to harvest, so we tested significance of the correlation between length at 50% maturity and catch/net (total and ≥ 762 mm) in 1998–2014.
Growth in length with age was modeled using a modified version of the Von Bertalanffy length-age model fit to length at age of capture of individual fish (Mooij et al., 1999):
The length-age model describes length L t (mm) at age t (years) as a function of length at age = 0 (L 0 = mm; length at emergence from the egg), early annual growth rate (ω = L ∞ × K = mm/year; Gallucci and Quinn 1979), average asymptotic length (L ∞ = mm), and multiplicative error (ε). We modeled lake trout growth from a sample of length at age of capture of lake trout captured in a period of low lake trout population density (n = 152, 3 December 1986–23 August 1995) and samples from 2 years in a period of higher population density (n = 153, 18 October 2005–9 November 2005; n = 114, 7 May 2008–27 June 2008 and 14 October 2008–2 December 2008). Model parameters and standard errors were estimated using nonlinear regression. To identify model parameters that accounted for year-specific differences in growth models, we compared L 0, ω, and L ∞, between each pair of periods using unpaired t-tests for unequal variances (Zar, 1999).
Simulation model
A stochastic age-structured population model was used to simulate effects of a range of fishing mortality on the lake trout population in Flathead Lake (Nieland et al., 2008; Schueller et al., 2008; Hansen et al., 2010). Age-specific starting abundance was derived from length-specific mark-recapture estimates in 2008–2012. Numbers present in each age class were subjected to fishing mortality that would induce 25, 50, and 75% reductions in abundance of adult (age-8-and-older) lake trout. Each age class was subjected to a rate of fishing mortality based on relative vulnerability to angling in Spring and Fall Mack Days contests. Natural mortality was set as a fixed rate that was estimated indirectly from growth parameters for lake trout in Flathead Lake. Recruitment of age-0 lake trout was simulated from a stock-recruit model for lake trout in western Lake Superior, scaled down to the smaller size of Flathead Lake. Stochastic variation in long-term simulations was driven by stock-recruit process error and parameter uncertainty. The model did not include assessment or implementation error as sources of model uncertainty.
Age-specific abundance was estimated from length-specific mark-recapture abundance estimates that were expanded to include lake trout not vulnerable to angling harvest because of the length limit. To account for large lake trout not vulnerable to angling during Mack Days contests (≥763 mm), we assumed the length frequency of lake trout caught in gill nets during 2008–2010 represented the population length frequency for lake trout ≥500 mm in length. For recapture samples from Mack Days contests in 2008–2010, we scaled each length frequency sample to the number of 500–549 mm lake trout caught by gillnetting each year, the smallest length class that was fully vulnerable to both angling and gill-netting. Each expanded sample length frequency was converted into an expanded sample age frequency using the age-length key described above. Age-specific starting abundance was then computed as average age-specific abundance in 2008–2010 (Supplementary Table 1).
From a starting abundance N ij at age j in year i = 2010, the number present N i+1, j+1 at the next age j + 1 in the next year i + 1 was modeled as a function of the total instantaneous mortality rate Z ij for each age class j in year i (Quinn & Deriso, 1999; Haddon, 2001):
The total instantaneous mortality rate Z ij for each age, j, and year, i, was the sum of instantaneous natural mortality (M = assumed constant across all ages and years) and instantaneous fishing mortality (F ij ) for each age, j, in year, i:
Instantaneous natural mortality (M = 0.1544) was estimated from Pauly’s equation (Pauly, 1980; Quinn & Deriso, 1999) using parameters of the Von Bertalanffy length-age model for pre-1996 samples (described above) and average monthly air temperature in Flathead Lake (T = 6.4°C). Total instantaneous fishing mortality F ij for each age j in year i was simulated from the relative selectivity S j of the gear for lake trout of age j and the fully selected fishing mortality rate F i that was specified as a model input for each year i:
Fully selected fishing mortality F i was specified as a model input for each simulation to cover a range of fishing mortality rates F i = 0.0–1.0. Relative selectivity, S j = s j /max(s j ), was estimated for Spring Mack Days and Fall Mack Days by dividing age-specific selectivity by the maximum age-specific selectivity, where age-specific selectivity, s j = C j /N j , was estimated for angling during Spring and Fall Mack Days from age-specific catches C j and abundances N j at age j in Flathead Lake (Supplementary Table 1). Length-frequencies of angling harvest during spring and fall Mack Days were converted to age frequencies using the age-length key described above.
Lake trout stock-recruitment is over-compensatory (Hansen et al., 1997a; Richards et al., 2004; Corradin et al., 2008), so the number of age-0 lake trout N i+1, j=0 that recruited to the population in each year i + 1 was predicted from the number of adult lake trout N i,j=8+ that spawned in the previous year i using a Ricker stock-recruitment model (Ricker, 1975):
In the stock-recruit model, α is the recruits per adult at low adult density, N i,j=8+ is the abundance of age-8-and-older adult lake trout in year i, β is the instantaneous decline in the recruitment rate as parental abundance increases, and ε is multiplicative recruitment error (Nieland et al., 2008; Hansen et al., 2010). To account for parameter uncertainty, a different set of model parameters (α, β, and ε) were selected for each year and simulation from a Markov Chain Monte Carlo (MCMC) sample of the joint posterior probability distribution of parameter estimates (Nieland et al., 2008). Age-8 and older lake trout were used to index spawning stock density, based on growth and maturity analyses described above for Flathead lake. To account for different carrying capacities of Flathead Lake, where the stock-recruit model was applied, and western Lake Superior, where the stock-recruit model was developed (as suggested by Myers, 2002), the estimated number of age-0 lake trout in the model was scaled downward using the ratio of estimated numbers of age-4-and-older lake trout in Flathead Lake (this study) and eastern Lake Superior (ratio = 0.357; Nieland et al., 2008).
Outputs of proposed lake trout population reduction scenarios included simulated abundance of age classes 1–30 of lake trout, the likelihood of population collapse, and the number of years to population collapse. Abundance was simulated 1,000 times for the average of years 51–200 to reflect the equilibrium effect of each fishing mortality rate. Suppression scenarios were developed by gradually adjusting fully-selected fishing mortality until age-8-and-older lake trout abundance was reduced 25, 50, and 75% (Suppression scenarios) from the baseline abundance associated with no change in fishing mortality (Status Quo scenario). Reduced fishing mortality was also simulated for elimination of all Mack Days contests (No Mack Days scenario) and for elimination of all fishing mortality (No Fishing scenario). Uncertainty of simulated abundance was quantified as the 2.5 and 97.5 percentiles of average abundance among 1,000 simulations. Population collapse was defined as a 90% decline in abundance of age-8-and-older lake trout. The likelihood of population collapse was quantified as the frequency of a 90% decline in abundance among 1,000 simulations. The number of years to collapse was quantified as the median number of years when abundance of age-8-and-older lake trout declined to ≤10% of present abundance, with 95% confidence intervals defined as for years to collapse.
Bull trout consumption
Lake trout feed predominantly on Mysis up to a length of ≥500 mm TL, but become increasingly piscivorous as they grow from 375 to 625 mm TL, after which fish prey are 70–100% of their diet by weight (Supplementary Tables 1, 2, 3; CSKT, 2014). Cannibalism was believed to self-regulate the lake trout population until the Mysis invasion (Ellis et al., 2011). Lake trout feed predominantly on juvenile bull trout that recruit to the lake at a mean weight of 90 g and a mean length of 229 mm TL (range 180–270 mm TL). The smallest sizes of bull trout caught by gill-netting and angling confirm the same size range. Three of four bull trout recovered from lake trout gut samples were 199–245 mm TL, whereas one was smaller (78 mm), which we assume was an uncommon precocious recruit. Lake trout are capable of eating fish prey up to 50% of their body length, although prey size is typically only 30% of their length (Beauchamp et al., 2007), which suggests a 600-mm TL lake trout would consume 180-mm TL bull trout, which is very close to the minimum size of the predominant size of bull trout recruits to the lake.
For each simulated level of lake trout abundance (±95% confidence limits), we estimated lake trout consumption of bull trout using a bioenergetics model of predator-prey dynamics in Flathead Lake (Supplementary Tables 1, 2, 3; CSKT, 2014). Inputs of bioenergetics modeling simulations for lake trout consumption included: (1) length-at-age from the length-age model described above; (2) weight-at-age estimated from length-at-age using a weight-length relationship from data sampled during autumn gill netting; (3) percentage of body mass lost during spawning; and (4) mass-dependent energy density estimated from weight-at-age and standard energy density for lake trout (Stewart et al., 1983). Size- and age-specific thermal experience and seasonal diet were from sampling in June 1998–August 2001 (CSKT, 2014). Consumption rates were fitted to annual body-mass increments for each age class of lake trout (ages 1–30) for 365 daily time steps from April 1 through the following March 31. From age-specific estimates of bull trout consumption by lake trout, we estimated the number and biomass of 90-g bull trout consumed by the simulated number of lake trout present for each management scenario described above. Lake trout > 625 mm TL were the smallest predators containing bull trout in their diet. We then computed the percentage change in bull trout biomass consumed in relation to consumption at current levels of lake trout harvest (status quo scenario).
Results
Abundance of lake trout estimated by mark-recapture averaged 773,000 fish (15.2 fish/ha) based on recaptures in Spring Mack Days contests and 482,000 fish (9.5 fish/ha) based on recaptures in Fall Mack Days contests in Flathead Lake during 2008–2014 (Table 2; Fig. 2). In mark-recapture surveys, numbers marked averaged 1,027, numbers examined for marks averaged 22,823, numbers recaptured averaged 38, and numbers estimated averaged 606,541. Estimated lake trout abundance was 21% to 55% higher when based on tag recaptures in Spring Mack Days than in Fall Mack Days. Estimated abundance based on recaptures in Spring Mack Days 2010 was 29–64% higher than any other abundance estimate, whereas other estimates varied only 10% among years based on recaptures during Spring Mack Days and 12% among years based on recaptures during Fall Mack Days.
Relative abundance of lake trout sampled in standardized autumn gillnetting varied independent of harvest in Mack Days contests in Flathead Lake in 1998–2014 (Fig. 3). For all lake trout captured during annual gillnet surveys, catch/net varied 2.6-fold among years, and was not related to harvest in Mack Days contests (r = 0.180; P = 0.489). For adult lake trout captured during annual gillnet surveys, catch/net varied 2.6-fold among years, and was not related to harvest in Mack Days contests (r = 0.086; P = 0.743). For lake trout shorter than the minimum of the slot-length limit (≥762 mm), catch/net varied 2.4-fold among years, and was not related to harvest in Mack Days contests (r = 0.343; P = 0.178). For lake trout longer than the minimum of the slot-length limit (≥762 mm), varied 6.1-fold among years, and was not related to harvest in Mack Days contests (r = 0.315; P = 0.219).
Geometric-mean catch/net (±95% confidence limits) of all lake trout (upper-left panel), adult (age-8+) lake trout (upper-right panel), lake trout shorter than a protected slot-length limit (lower-left panel), and longer than a protected slot-length limit (lower-right panel) caught in standardized gillnet surveys in Flathead Lake, Montana during 1998–2014
Size structure of the lake trout population was bimodal, with peaks at 500 mm and 850 mm, and varied independent of density in Flathead Lake in 1998–2014. The length frequency of lake trout vulnerable to capture in standardized gillnets was strongly peaked at 500 mm, with a secondary peak at 850 mm (Fig. 4). Proportional size distribution (PSD) varied 1.4-fold among years, generally increased from 1988 to 2009, and decreased from 2009 to 2014 (Fig. 5). Proportional size distribution (PSD) was not related to catch/net of all lake trout (r = 0.208; P = 0.423), but was directly related to catch/net of large lake trout ≥ 762 mm protected by the slot-length limit (r = 0.631; P = 0.0002).
Length frequency (upper left panel; n = 7,025), relative weight frequency (upper right panel; n = 5,065), age frequency with mortality curves (A = annual mortality rate; lower left panel; gillnets = solid curve and solid bars for ages 8–30; angling = dashed curve and open bars for ages 8–24), and proportion mature in relation to 25-mm length class (lower right panel; dashed line = logistic curve; dotted lines = length and associated age at 50% maturity) for lake trout caught in standardized-gillnet surveys during 1998–2014 and angling during 2008–2014 (open bars) in Flathead Lake, Montana
Percentage of fish ≥500 mm (upper-left panel), mean annual relative weight (upper-right panel), mean annual mortality rate (lower-left panel), and length at which 50% of fish were mature (lower-right panel) for lake trout caught in standardized-gillnet surveys in Flathead Lake, Montana during 1998–2014. Error bars depict 95% confidence limits
Relative weight (W r) of lake trout caught during standardized autumn gillnetting in Flathead Lake was low for the species and varied independent of density in 1998–2014. Relative weight (W r ) of lake trout averaged 85, with 50% of all lake trout ranging between 77 and 92, and 95% ranging between 65 and 108 (Fig. 4). Mean annual relative weight (W r ) varied 1.1-fold among years, generally decreased from 1988 to 2008, and increased from 2008 to 2014 (Fig. 5). Mean annual relative weight (W r) was inversely related to catch/net of all lake trout (r = − 0.612; P = 0.009) and to catch/net of large lake trout ≥762 mm protected by the slot-length limit (r = − 0.565; P = 0.018).
Annual mortality (A) of lake trout, indexed from catches during standardized autumn gillnetting, averaged 13.5%, and varied nonlinearly through time in Flathead Lake in 1998–2014. Average annual mortality (A) was much lower when estimated from the age frequency of gillnet samples (A = 14.7%; 95% CI = 12.6–16.8%) than when estimated from the age frequency of angling harvest (A = 26.1%; 95% CI = 18.1–33.3%; Fig. 4). The index of annual mortality (A) varied 1.4-fold among years, generally increased from 1998 to 2008, and generally decreased from 2008 to 2014 (Fig. 5). The annual mortality (A) index was not related to catch/net of all lake trout (r = 0.108; P = 0.680), but was inversely related to catch/net of lake trout ≥ 762 mm (r = − 0.614; P = 0.009).
Length at 50% maturity (L 50) of lake trout caught during standardized autumn gillnetting averaged 497 mm and varied nonlinearly through time in Flathead Lake in 1998–2014. Across all years, length at 50% maturity (L 50) averaged 497 mm (95% CI = 493–502 mm), which corresponded to an age of 8.8 years based on length-age data from 2008 (Fig. 4). Annual estimates of length at 50% maturity (L 50) varied 1.2-fold among years, generally increased from 1998 to 2006, and generally decreased from 2006 to 2014 (Fig. 5). Length at 50% maturity (L 50) was not related to catch/net of all lake trout (r = −0.305; P = 0.233) or to catch/net of lake trout longer than 762 mm (r = −0.011; P = 0.966).
Growth of lake trout differed among samples (1996, 2005, and 2008) in Flathead Lake, Montana (Fig. 6; F 6, 410 = 29.67; P < 0.001). Growth differed less between 2005 and 2008 (F 3, 261 = 2.54; P = 0.057) than between 1996 and 2008 (F 3, 260 = 31.80; P < 0.001) or between 1996 and 2005 (F 3, 299 = 55.80; P < 0.001). Asymptotic length (L ∞ = mm) was lower in 2005 than 1996 (t 216 = 9.21; P < 0.001) or 2008 (t 256 = 8.13; P < 0.001), but did not differ between 1996 and 2008 (t 159 = 1.37; P = 0.17; Table 3). The early growth rate (ω = mm/year) was lower in 2005 than 2008 (t 264 = 4.69; P < 0.001) or 1996 (t 254 = 27.7; P < 0.001), and was lower in 2008 than 1996 (t 209 = 23.83; P = < 0.001; Table 3). Length at age zero (L 0 = mm) was lower in 2008 than 2005 (t 262 = 8.93; P < 0.001) or 1996 (t 131 = 18.58; P < 0.001), and was lower in 2005 than 1996 (t 174 = 6.02; P = < 0.001; Table 3).
Length versus age (symbols) and Von Bertalanffy length-age models (curves) for lake trout sampled in Flathead Lake, Montana, during 3 December 1986–23 August 1995 (triangles and dotted line), 18 October 2005–9 November 2005 (circles and dashed line), and 7 May 2008–27 June 2008 and 14 October 2008–2 December 2008 (squares and solid line)
Simulated lake trout abundance changed less for young fish than old fish at all levels of fishing mortality, with simulated abundance of older fish increasing greatly at reduced fishing mortality rates and decreasing greatly at increased fishing mortality rates in Flathead Lake (Fig. 7). If fishing ceased, abundance would increase 6% for age-1+ lake trout, 22% for age-4+ lake trout, and 67% for age-8+ lake trout. If Mack Days ceased, abundance would increase 5% for age-1+ lake trout, 12% for age-4+ lake trout, and 29% for age-8+ lake trout. If current fishing mortality is sustained, abundance would increase 6% for age-1+ lake trout and 8% for age-4+ lake trout, but would decrease 9% for age-8+ lake trout. At a fishing mortality rate that would suppress age-8+ abundance 25%, abundance would decline 9% for age-1+ lake trout and 13% for age-4+ lake trout. At a fishing mortality rate that would suppress age-8+ abundance 50%, abundance would decline only 25% for age-1+ lake trout and 32% for age-4+ lake trout. At a fishing mortality rate that would suppress age-8 + abundance 75%, abundance would decline 50% for age-1+ lake trout and 57% for age-4+ lake trout. The time required to suppress abundance of age-8 + lake trout 90% decreased sharply from more than 200 years at A = 35% to 19 years (95% CI 11–29 years) at A = 45%. Coincidentally, likelihood of suppression increased sharply from 0% at A = 32 to 100% at A = 39%. Fishing effort would need to increase 2.1-times to cause A = 32%, 2.3-times to cause A = 35%, 2.8-times to cause A = 39%, and 3.7-times to cause A = 45%.
Long-term (years 51–200) simulated abundance of age-1+ , age-4+ , and age-8+ lake trout (upper panel, ± 95% confidence limits), years to collapse age-8 + lake trout (lower panel, dashed lines ± 95% confidence limits), and likelihood of collapse (lower panel, solid line) in relation to total annual mortality in Flathead Lake, Montana. In the upper panel, vertical lines connect points of abundance associated with 0, 25, 50, and 75% reductions in numbers of lake trout in each age range
Predation by lake trout on bull trout would likely increase substantially if fishing mortality is reduced by eliminating Mack Days contests, but would decrease substantially at higher levels of lake trout suppression in Flathead Lake (Table 4). Consumption of bull trout by lake trout would increase 60% if Mack Days contests are eliminated as a source of fishing mortality on suitably sized (age-14+; ≥626 mm) lake trout. In contrast, consumption of bull trout would decrease 90%, as numbers of suitably sized (age-14+; ≥626 mm) lake trout are suppressed from 67,348 fish at current fishing mortality rates (Status Quo) to 6,101 under a fishing mortality rate that would suppress age-8+ lake trout by 75%.
Discussion
We found through simulation modeling that suppression of adult lake trout by 75% below current abundance would reduce predation on bull trout by 90%. Evidently, current fishery removals of lake trout through incentivized fishing contests have not been sufficient to suppress lake trout abundance based on mark-recapture or stratified-random gill-netting surveys. In contrast, size structure, body condition, mortality, and maturity were consistent with a density-dependent reduction in lake trout abundance. Based on simulation modeling, total fishing effort would need to increase 3-fold to reduce adult lake trout population density by 75%. We therefore conclude that increased fishing effort could suppress lake trout population density, and thereby, reduce predation on juvenile bull trout to enable higher abundance of adult bull trout in the Flathead Lake basin.
Population density
The population density of lake trout in Flathead Lake was relatively high in relation to other native and introduced populations in North America. Compared to other introduced lake trout populations in North America, average spring population density of age-4-and-older lake trout in Flathead Lake in 2010–2014 (this study) was 16-times higher than in Lake Pend Oreille, Idaho in 2005 (Hansen et al., 2008), and nearly 3-times higher than in Yellowstone Lake, Wyoming in 2011 (Syslo, 2015). Compared to other native lake trout populations in North America, average spring lake trout population density in Flathead Lake was more than 2-times higher than a native restored population in western Lake Superior (Nieland et al., 2008) and 2.6-times higher than the average density of 37 native populations from across North America (Healey, 1978; Martin & Olver, 1980; Mills et al., 2002). Similarly, average yield density of lake trout from Flathead Lake in 2010–2012 was 2-times higher than the average yield density of 72 populations from across North America (Martin and Olver, 1980; Marshall, 1996).
Lake trout abundance varied independently from harvest of lake trout in Mack Days contests, whether indexed by mark-recapture estimates in 2008–2014 or gill-net catch/net in 1998–2014, so exploitation was likely at equilibrium with production of vulnerable-sized lake trout in Flathead Lake (Ricker, 1975; Hilborn & Walters, 1992; Quinn & Deriso, 1999; Allen & Hightower, 2010). If true, fishing mortality exerted in 2010–2014 was unlikely to reduce lake trout population density enough to cause persistent changes through time in abundance, size structure, body condition, total annual mortality, or size at maturity, other than those caused by annual variation in recruitment and survival (Ricker, 1975; Hilborn & Walters, 1992; Quinn & Deriso, 1999; Allen & Hightower, 2010). Increased fishing mortality exerted through Mack Days contests after 2010 was evidently insufficient to reduce lake trout population abundance, as suggested by our population modeling that indicated fishing effort would need to increase 2–4 times to induce a 50% to 75% decline in adult lake trout abundance. To achieve such increased fishing mortality, targeted gill netting began in 2014 to supplement spring and fall Mack Days contests to suppress adult lake trout abundance by 75% from the level in 2010 (CSKT, 2014).
Biological attributes
Size structure, body condition, annual mortality, and size at maturity of the lake trout population in Flathead Lake were all consistent with high density (Rose et al., 2001). First, size structure of the lake trout population in Flathead Lake was dominated by many more large fish than 13 other lake trout populations in North America (Martin & Olver, 1980). Next, body condition (relative weight) of the lake trout population in Flathead Lake was only in the 10th percentile of 58 other populations in North America (Hubert et al., 1994). Next, annual mortality of the lake trout population in Flathead Lake was lower than nearly all of 75 other populations in North America (Dubois & Lageaux, 1968; Healey, 1978; Martin & Olver, 1980; Trippel, 1993; Hansen et al., 2008, 2012; Syslo, 2010, 2015; Syslo et al., 2011), but similar to nine unexploited populations in Ontario (Mills et al., 2002). Last, length at 50% maturity of the lake trout population in Flathead Lake was higher but age at 50% maturity was similar to 134 other populations in North America (Dubois & Lageaux, 1968; Trippel, 1993; Hansen et al., 2008, 2012; McDermid et al., 2010).
Size structure of lake trout was directly related to catch/net of large lake trout, whereas relative weight, annual mortality, and length at 50% maturity were inversely related to catch/net in Flathead Lake during 1998–2014, which are collectively consistent with density-dependent responses to high lake trout population density (Rose et al., 2001). Population density should decline as indices of total annual mortality increase, and body condition of an average fish in a population should increase as population density declines (in the absence of increased prey availability) because prey resources available for each individual should be less at high density than at low density (Murphy et al., 1991; Pope & Kruse, 2007; Neumann et al., 2012). Increased body condition enables faster growth, shorter length at maturity, and greater fecundity (Murphy et al., 1991; Pope & Kruse, 2007; Neumann et al., 2012). When population density declines as a consequence of high fishing mortality, body condition foretells coincident changes in growth, length (age) at maturity, and fecundity that collectively serve as indices of exploitation stress (Spangler et al., 1977). These apparent density-dependent responses in size structure, body condition, mortality, and maturity are inconsistent with lack of detectable changes in either mark-recapture estimates of abundance or gill-net indices of relative abundance, which may reflect different time lags or power to detect changes in abundance and relative abundance, compared to biological indices related to size structure, body condition, mortality, and maturity.
Period-specific estimates of the early growth rate (ω) declined from pre-1996 to 2005 to 2008, consistent with a density-dependent decline in growth rate (Spangler et al., 1977; DeVries & Frie, 1996; Isely & Grabowski, 2007; Allen & Hightower, 2010). The decline in the growth rate may have caused a coincident large decrease in asymptotic length that was evident in the 2005 age sample, possibly because lake trout population density increased 14-fold after Mysis colonized Flathead Lake (Ellis et al., 2011). Alternatively, the similarity of asymptotic length (L ∞) between pre-1996 and 2008 samples suggests that this attribute of the lean lake trout morph has not changed since the increase in Mysis density enabled expansion of the lake trout population (assuming samples were representative of the lean morph in both periods; Stafford et al., 2014). Rather, shorter asymptotic length in 2005 than in either pre-1996 or 2008 may be an artifact of sampling that included more dwarf morphs in 2005 than in either pre-1996 or 2008, or conversely, more lean morphs in the 2008 sample than the 2005 sample (Stafford et al., 2014). The dwarf morph that was more evident in the 2005 sample may have emerged in response to high inshore population density of the lean morph (Stafford et al., 2014).
Population Suppression
We found that simulated adult lake trout abundance collapsed (declined 90%) when total annual mortality increased from 32 to 39% and time to collapse declined from 200 years to only 11 years as total annual mortality increased from 35 to 45%, like other studies of lake trout populations in North America (Nieland et al., 2008; Hansen et al., 2010). For example, angling and netting in Lake Pend Oreille, Idaho, exerted enough mortality (>50%) to collapse the simulated lake trout abundance while simultaneously reducing predation mortality enough to preserve the kokanee population (Hansen et al., 2010). Similarly, simulated lake trout abundance in Western Lake Superior suggested that the population could sustain a total annual mortality rate no higher than 40% (Nieland et al., 2008), which was lower than the maximum sustainable rate of 50% predicted by meta-analysis of native populations in North America (Healey, 1978) or the range of total annual mortality for a wide range of populations in North America (Martin & Olver, 1980). Collectively, these findings support a common view of the lake trout as a species that cannot withstand high rates of exploitation and is therefore susceptible to over-exploitation (Healey, 1978; Martin & Olver, 1980). Despite the vulnerability of lake trout to overharvest, current exploitation on the lake trout population in Flathead Lake was only enough to reduce adult lake trout abundance by 9%, so fishing effort would need to triple to cause a 75% reduction in lake trout abundance.
We found that any targeted level of suppression of the adult lake trout population would deliver a much higher level of reduction in predation losses of bull trout, because predation in Flathead Lake was limited to larger (≥626 mm), older (≥age 14) lake trout than the adults targeted for reduction (≥age 8; ≥500 mm). Lake trout predation on bull trout is constrained by the large size at which juvenile bull trout recruit to the lake from tributaries, and by the prey–predator size relationship for lake trout in Flathead and many other lakes (Beauchamp et al., 2007). Size at recruitment is a plastic trait in adfluvial bull trout, so predation by lake trout on bull trout will be sensitive to changes in age and size of bull trout recruiting to the lake as bull trout abundance increases in tributaries (Beauchamp & Van Tassell, 2001). In contrast, increased fishing would presumably cause a reduction in lake trout size that would lead to reduced lake trout predation on bull trout, the reverse of which occurred in Lake Chelan when cessation of lake trout stocking led to increased average size of lake trout and consequent predation on kokanee (Schoen et al., 2012). Countervailing responses to those predicted by modeling may result from (1) bycatch of native trout during suppression of lake trout, (2) difficulty in sustaining a perpetual suppression program, (3) inherent sensitivity of bull trout to a wide range of stressors, and (4) the potential for bull trout to respond to suppression differently than expected from bioenergetics simulation modeling, especially if competition between bull trout and lake trout is equally or more important than predation (Ferguson et al., 2012). Large experimental manipulations of lake trout population density would enable researchers to more fully evaluate the mechanisms between lake trout and bull trout. Tribal co-managers of the fishery in Flathead Lake relied on these modeling results when setting a course to suppress lake trout abundance by 75% from current levels because those results indicate that recovery of native bull trout and cutthroat trout can only occur after predation by lake trout has been reduced (CKST, 2014).
References
Allen, M. S. & J. E. Hightower, 2010. Fish population dynamics: mortality, growth, and recruitment. In Hubert, W. A. & M. C. Quist (eds.), Inland fisheries management in North America, 3rd ed. American Fisheries Society, Bethesda, Maryland: 43–79.
Beauchamp, D. A. & J. J. Van Tassell, 2001. Modeling trophic interactions of bull trout in Lake Billy Chinook, Oregon. Transactions of the American Fisheries Society 130: 204–216.
Beauchamp, D. A., D. Wahl & B. M. Johnson, 2007. Predator-prey interactions. In Guy, C. S. & M. L. Brown (eds.), Analysis and Interpretation of inland fisheries data. American Fisheries Society, Bethesda: 765–842.
Bowles, E. C., B. E. Rieman, G. R. Mauser, & D. H. Bennett, 1991. Effects of introductions of Mysis relicta on fisheries in northern Idaho. In Nesler, T. P., & E. P. Bergersen (eds), Mysids in fisheries: hard lessons from headlong introductions. American Fisheries Society Symposium 9, Bethesda: 65–74.
Corradin, L. M., M. J. Hansen, D. R. Schreiner & M. Seider, 2008. Recruitment dynamics of lake trout in western Lake Superior during 1980–1995. North American Journal of Fisheries Management 28: 663–677.
CSKT (Confederated Salish and Kootenai Tribes), 2014. Final environmental impact statement: proposed strategies to benefit native species by reducing the abundance of lake trout Flathead Lake, Montana. Federal Register, Volume 79, Number 35, Friday, February 21, 2014, Notices. http://www.mackdays.com.
Crossman, E. J., 1995. Introduction of the lake trout (Salvelinus namaycush) in areas outside its native distribution: a review. Journal of Great Lakes Research 21(Supplement 1): 17–29.
DeVries, D. R. & R. V. Frie, 1996. Determination of age and growth. In Murphy, B. R. & D. W. Willis (eds.), Fisheries techniques, 2nd ed. American Fisheries Society, Bethesda: 483–512.
Donald, D. B. & D. J. Alger, 1993. Geographic distribution, species displacement, and niche overlap for lake trout and bull trout in mountain lakes. Canadian Journal of Zoology 71: 238–247.
Dubois, A., & R. Lagueux, 1968. Etude comparée de l’âge scalaire et de l’âge otolithique de la touladi (Salvelinus namaycush), Lac Mistassini, Quebec. [Comparative study of the age as determined by scales and otoliths of the lake trout (Salvelinus namaycush) of Lake Mistassini, Quebec.] Naturaliste Canadien (Quebec) 95: 907–928.
Ellis, B. K., J. A. Stanford, D. Goodman, C. P. Stafford, D. L. Gustafson, D. A. Beauchamp, D. W. Chess, J. A. Craft, M. A. Deleray & B. S. Hansen, 2011. Long-term effects of a trophic cascade in a large lake ecosystem. Proceedings of the National Academy of Sciences of the United States of America 108: 1070–1075.
Ferguson, J. M., M. L. Taper, C. S. Guy & J. M. Syslo, 2012. Mechanisms of coexistence between native bull trout (Salvelinus confluentus) and non-native lake trout (Salvelinus namaycush): inferences from pattern-oriented modeling. Canadian Journal of Fisheries and Aquatic Sciences 69: 755–769.
Fredenberg, W., 2002. Further evidence that lake trout displace bull trout in mountain lakes. Intermountain Journal of Sciences 8: 143–152.
Guy, C. S., R. M. Neumann, D. W. Willis & R. O. Anderson, 2007. Proportional size distribution (PSD): a further refinement of population size structure index terminology. Fisheries 32: 348.
Haddon, M., 2001. Modelling and quantitative methods in fisheries. Chapman & Hall/CRC, Boca Raton.
Hansen, M. J., 1999. Lake trout in the Great Lakes: basin-wide stock collapse and binational restoration. In Taylor, W. W. & C. P. Ferreri (eds.), Great Lakes Fishery Policy and Management: a Binational Perspective. Michigan State University Press, East Lansing: 417–453.
Hansen, M. J., J. R. Bence, J. W. Peck & W. W. Taylor, 1997a. Evaluation of the relative importance of hatchery-reared and wild fish in the restoration of Lake Superior lake trout. In Hancock, D. A., D. C. Smith, A. Grant & J. P. Beumer (eds.), Developing and Sustaining Word Fisheries Resources: The State of Science and Management: 2nd World Fisheries Congress Proceedings. CSIRO Publishing, Collingwood: 492–497.
Hansen, M. J., C. P. Madenjian, T. E. Helser & J. H. Selgeby, 1997b. Gillnet selectivity of lake trout (Salvelinus namaycush) in Lake Superior. Canadian Journal of Fisheries and Aquatic Sciences 54: 2483–2490.
Hansen, M. J., N. J. Horner, M. Liter, M. P. Peterson & M. A. Maiolie, 2008. Dynamics of an increasing lake trout population in Lake Pend Oreille, Idaho, USA. North American Journal of Fisheries Management 28: 1160–1171.
Hansen, M. J., D. Schill, J. Fredericks & A. Dux, 2010. Salmonid predator–prey dynamics in Lake Pend Oreille, Idaho, USA. Hydrobiologia 650: 85–100.
Hansen, M. J., N. A. Nate, C. C. Krueger, M. S. Zimmerman, H. G. Kruckman & W. W. Taylor, 2012. Age, growth, survival, and maturity of lake trout morphotypes in Lake Mistassini, Quebec. Transactions of the American Fisheries Society 141: 1492–1503.
Healey, M. C., 1978. The dynamics of exploited lake trout populations and implications for management. Journal of Wildlife Management 42: 307–328.
Hilborn, R. & C. J. Walters, 1992. Quantitative Fisheries Stock Assessment: Choice, Dynamics and Uncertainty. Chapman and Hall, New York.
Hosmer, D. W. & S. Lemeshow, 2000. Applied Logistic Regression, 2nd ed. Wiley, New York.
Hubert, W. A., R. D. Gipson & R. A. Whaley, 1994. Interpreting relative weights of lake trout stocks. North American Journal of Fisheries Management 14: 212–215.
Isely, J. J. & T. B. Grabowski, 2007. Age and growth. In Guy, C. S. & M. L. Brown (eds.), Analysis and Interpretation of Freshwater Fisheries Data. American Fisheries Society, Bethesda: 187–228.
Johnson, L., 1976. Ecology of Arctic populations of lake trout, Salvelinus namaycush, lake whitefish, Coregonus clupeaformis, Arctic char, S. alpinus, and associated species in unexploited lakes of the Canadian Northwest Territories. Journal of the Fisheries Research Board of Canada 33: 2459–2488.
Keleher, J. J., 1972. Great Slave Lake: effects of exploitation on the salmonid community. Journal of the Fisheries Research Board of Canada 29: 741–753.
Krueger, C. C. & M. P. Ebener, 2004. Rehabilitation of lake trout in the Great Lakes: past lessons and future challenges. In Gunn, J. M., R. J. Steedman & R. A. Ryder (eds.), Boreal Shield Watersheds: Lake Trout Ecosystems in a Changing Environment. Lewis Publishers, Boca Raton: 37–56.
Marshall, T. R., 1996. A hierarchical approach to assessing habitat suitability and yield potential of lake trout. Canadian Journal of Fisheries and Aquatic Sciences 53(Supplement 1): 332–341.
Martin, N. V., & T. G. Northcote, 1991. Kootenay Lake: an inappropriate model for Mysis relicta introduction in north temperate lakes. In Nesler, T. P. & E. P. Bergersen (eds), Mysids in fisheries: hard lessons from headlong introductions. American Fisheries Society Symposium 9, Bethesda: 23–29.
Martin, N. V. & C. H. Olver, 1980. The lake charr, Salvelinus namaycush. In Balon, E. (ed.), Charrs: salmonid fishes of the genus Salvelinus. Dr W, Junk: 205–277.
McDermid, J. L., B. J. Shuter & N. P. Lester, 2010. Life history differences parallel environmental differences among North American lake trout (Salvelinus namaycush) populations. Canadian Journal of Fisheries and Aquatic Sciences 67: 314–325.
Mills, K. H., S. M. Chalanchuk & D. J. Allan, 2002. Abundance, annual survival, and recruitment of unexploited and exploited lake charr. Salvelinus namaycush, populations at the Experimental Lakes Area, northwestern Ontario Environmental Biology of Fishes 64: 281–292.
Mooij, W. M., J. M. Van Rooij & S. Wijnhoven, 1999. Analysis and comparison of fish growth from small samples of length-at-age data: detection of sexual dimorphism in Eurasian Perch as an example. Transactions of the American Fisheries Society 128: 483–490.
Muir, A. M., C. C. Krueger & M. J. Hansen, 2012. Re-establishing lake trout in the Laurentian Great Lakes: the past, present, and future. In Taylor, W. W., A. J. Lynch & N. J. Leonard (eds.), Great Lakes Fishery Policy and Management: A Binational Perspective, 2nd ed. Michigan State University Press, East Lansing: 533–588.
Murphy, B. R., D. W. Willis & T. A. Springer, 1991. The relative weight index in fisheries management: status and needs. Fisheries (Bethesda) 16: 30–38.
Myers, R. A., 2002. Recruitment: understanding density-dependence in fish populations. In Hart, P. J. B. & J. D. Reynolds (eds.), Handbook of Fish Biology and Fisheries, Vol. 1., Fish Biology Blackwell Publishing, Oxford: 123–148.
Neumann, R. M., C. S. Guy & D. W. Willis, 2012. Length, weight, and associated indices. In Zale, A. V., D. L. Parrish & T. M. Sutton (eds.), Fisheries Techniques, 3rd ed. American Fisheries Society, Bethesda: 637–676.
Nieland, J. L., M. J. Hansen, M. J. Seider & J. J. Deroba, 2008. Modeling the sustainability of lake trout fisheries in eastern Wisconsin waters of Lake Superior. Fisheries Research 94: 304–314.
Olver, C. H., D. Nadeau & H. Fournier, 2004. The control of harvest in lake trout fisheries on Precambrian Shield lakes. In Gunn, J. M., R. J. Steedman & R. A. Ryder (eds.), Boreal Shield Watersheds: Lake Trout Ecosystems in a Changing Environment. Lewis Publishers, Boca Raton: 193–218.
Pauly, D., 1980. On the interrelationships between natural mortality, growth parameters and mean environmental temperature in 175 fish stocks. Journal du Conseil International pour l’Exploration de la Mer 39: 175–192.
Piccolo, J. J., W. A. Hubert & R. A. Whaley, 1993. Standard weight equation for lake trout. North American Journal of Fisheries Management 13: 401–404.
Pope, K. L. & C. G. Kruse, 2007. Condition. In Guy, C. S. & M. L. Brown (eds.), Analysis and Interpretation of Freshwater Fisheries Data. American Fisheries Society, Bethesda: 423–471.
Quinn II, T. J. & R. B. Deriso, 1999. Quantitative Fish Dynamics. Oxford University Press, New York.
Richards, J. M., M. J. Hansen, C. R. Bronte & S. P. Sitar, 2004. Recruitment dynamics of the 1971–1991 year-classes of lake trout in Michigan waters of Lake Superior. North American Journal of Fisheries Management 24: 475–489.
Ricker, W. E., 1975. Computation and interpretation of biological statistics of fish populations. Bulletin 191 of the Fisheries Research of Canada, Ottawa, Ontario.
Rose, K. A., J. H. Cowan Jr, K. O. Winemiller, R. A. Myers & R. Hilborn, 2001. Compensatory density dependence in fish populations: importance, controversy, understanding and prognosis. Fish and Fisheries 2: 293–327.
Schoen, E. R., D. A. Beauchamp & N. C. Overman, 2012. Quantifying latent impacts of an introduced piscivore: pulsed predatory inertia of Lake Trout and decline of Kokanee. Transactions of the American Fisheries Society 141: 1191–1206.
Schueller, A. M., M. J. Hansen & S. P. Newman, 2008. Modeling the sustainability of walleye populations in northern Wisconsin lakes. North American Journal of Fisheries Management 28: 1916–1927.
Spangler, G. R., N. R. Payne, J. E. Thorpe, J. M. Byrne, H. A. Regier & W. J. Christie, 1977. Responses of percids to exploitation. Journal of the Fisheries Research Board of Canada 34: 1983–1988.
Spencer, C. N., B. R. McClelland & J. A. Stanford, 1991. Shrimp stocking, salmon collapse and eagle displacement: cascading interactions in the food web of a large aquatic ecosystem. Bioscience 41: 14–21.
Stafford, C. P., M. V. McPhee, L. A. Eby & F. W. Allendorf, 2014. Introduced lake trout exhibit life history and morphological divergence with depth. Canadian Journal of Fisheries and Aquatic Sciences 71: 10–20.
Stafford, C. P., J. A. Stanford, F. R. Hauer & E. B. Brothers, 2002. Changes in lake trout growth associated with Mysis relicta establishment: a retrospective analysis using otoliths. Transactions of the American Fisheries Society 131: 994–1003.
Stewart, D. J., D. Weininger, D. V. Rottiers & T. A. Edsall, 1983. An energetics model for lake trout, Salvelinus namaycush: application to the Lake Michigan population. Canadian Journal of Fisheries and Aquatic Sciences 40: 681–698.
Syslo, J. M., 2010. Demography of lake trout in relation to population suppression in Yellowstone Lake, Yellowstone National Park. Master’s thesis, Montana State University, Bozeman.
Syslo, J. M., 2015. Dynamics of Yellowstone cutthroat trout and lake trout in the Yellowstone Lake ecosystem: a case study for the ecology and management on non-native fishes. Doctoral dissertation, Montana State University, Bozeman.
Syslo, J. M., C. S. Guy, P. E. Bigelow, P. D. Doepke, B. D. Ertel & T. M. Koel, 2011. Response of non-native lake trout (Salvelinus namaycush) to 15 years of harvest in Yellowstone Lake, Yellowstone National Park. Canadian Journal of Fisheries and Aquatic Sciences 68: 2132–2145.
Trippel, E. A., 1993. Relations of fecundity, maturation, and body size of lake trout, and implications for management in northwestern Ontario lakes. North American Journal of Fisheries Management 13: 64–72.
Zar, J. H., 1999. Biostatistical Analysis, 4th ed. Prentice Hall, Upper Saddle River.
Acknowledgments
Edward B. Brothers provided age estimates for otolith samples collected in 1986–1995 and Craig P. Stafford provided age estimates for otolith samples collected in 2005 and 2008. Use of trade, product, or firm names is for descriptive purposes and does not imply endorsement by the U.S. Government. This article is Contribution 1990 of the U.S. Geological Survey, Great Lakes Science Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: M. Power, R. Knudsen, C. Adams, M. J. Hansen, J. B. Dempson, M. Jobling & M. Ferguson / Advances in Charr Ecology and Evolution
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hansen, M.J., Hansen, B.S. & Beauchamp, D.A. Lake trout (Salvelinus namaycush) suppression for bull trout (Salvelinus confluentus) recovery in Flathead Lake, Montana, North America. Hydrobiologia 783, 317–334 (2016). https://doi.org/10.1007/s10750-016-2703-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2703-0