Abstract
Invasive dreissenid mussels have altered plankton abundance and nutrient cycling in the Great Lakes. In this study, a 1-D hydrodynamic-biogeochemical coupled model is developed to investigate their effects at a mid-depth offshore site in Lake Michigan. Model simulation shows that water surface temperature and vertical thermal structure can be well reproduced. Driven by the simulated vertical mixing, the biological model solves the transport and transformation of nutrients, plankton and detritus in the water column. Mussel grazing and excretion are added at the bottom boundary. The biological model predicts a notable decline of phytoplankton biomass and considerable increase of dissolved phosphorus (DP) in the entire water column at the end of spring. However, the reduction of phytoplankton and the increase of DP are limited to the bottom 20 m in summer as a result of the strong stratification. Model results also show that mussels can maximize particle delivery to the benthos, as the modeled benthic diffusive flux of particulate phosphorus exceeds the passive settling rate by 4.2× on average. Model simulation over a 10-month period indicates that profundal mussels have the potential to significantly change the distribution of energy and nutrients in the water column, even in a deep and stratified environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past several decades, dreissenid mussels have successfully established dense population in the benthos of most of the Laurentian Great Lakes (Bunnell et al., 2009; Nalepa et al., 2010). Profound impacts on the ecosystem of Great Lakes have been documented, especially in shallow nearshore areas (Hecky et al., 2004). It is estimated that the spring chlorophyll has dropped by 50% and primary production has decreased by 70% since the mid-1990s in Lake Michigan (Fahnenstiel et al., 2010). Vanderploeg et al. (2010) indicated that the grazing effects of dreissenid mussels played an important role in the disappearance of the spring phytoplankton bloom in Lake Michigan. This decline of phytoplankton appears to have had negative consequences for higher trophic levels, including planktivorous fish (Vanderploeg et al., 2002; Strayer et al., 2004; Bunnell, et al., 2009; Nalepa et al., 2010). As consumers of a large fraction of plankton production, mussels appear to also have a large influence on the spatial and temporal dynamics of nutrients. In the particle-depleted boundary layer above mussel colonies in nearshore areas, high concentrations of dissolved nutrients have been observed (Dayton et al., 2014). In addition, particulate nutrients (primarily feces and pseudofeces) that are egested by mussels may be stored in the sediment, resulting in reallocation of nutrients from the water column to the profundal benthos and the nearshore zone (Hecky et al., 2004; Bootsma & Liao, 2013; Ozersky et al., 2015; Waples et al., 2016).
Studies of the role of dreissenid mussels in ecosystem dynamics of Great Lakes have focused primarily on shallow (< 10 m) systems (Ackerman et al., 2001; Hecky et al., 2004; Boegman et al., 2008). Conventional thought is that only in shallow systems are areal grazing rates comparable to areal phytoplankton production rates, because in these systems vertical mixing is sufficient to provide grazers access to the entire water column on relatively short time scales (Officer et al., 1982; Koseff et al., 1993; Boegman et al., 2008). However, there is evidence that quagga mussels, which cover a large portion of the bottom of Lake Michigan (Nalepa et al., 2014), may have fundamentally changed the dynamics of nutrients and phytoplankton, with effects being propagated through the entire food web (Fahnenstiel et al., 2010; Mida et al., 2010; Vanderploeg et al., 2010; Turschak et al., 2014). One of the apparent effects of these benthic filter feeders is an increase in the effective settling velocity of phosphorus (Dolan & Chapra, 2012). This is supported by recent field measurements in Lake Michigan which indicate that profundal quagga mussel phosphorus grazing rates may be several times greater than passive particulate phosphorus sinking rates (Mosley & Bootsma, 2015). This is feasible if vertical mixing delivers particles to the benthic boundary layer at a rate faster than the passive settling rate. Under these conditions, in the absence of mussels a small fraction of particles delivered to the benthic boundary layer settle to the lake bottom, with a large fraction remaining in suspension and mixing back up into the water column. However, when mussels are present in sufficient densities, filter feeding enhances the retention of particles on the lake bottom and reduces the return flux from the benthic boundary layer to the overlying water, thereby increasing the effective settling velocity.
Observations in shallow systems, and the observations described above for Lake Michigan, highlight the importance of understanding hydrodynamic processes when evaluating the effects of dreissenid mussels on energy flow and nutrient dynamics in lakes. Bottom Chl-a depletion was noted at sites with moderate or high mussel biomass and sufficient thermal stratification to impede vertical mixing in Lake Simcoe (Schwalb et al., 2013). Several recent studies have applied 3-D physical and biogeochemical models to explore the interactions between dreissenids and phytoplankton in the Great Lakes (Leon et al., 2011; Bocaniov et al., 2014; Schwalb et al., 2015). Compared to general 3-D hydrodynamic circulation models, a 1-D mixing model is more computationally efficient and easy to calibrate. Although the effects of bathymetry, horizontal gradients, and horizontal advection cannot be resolved by a 1-D model, it is potentially useful to study the mixing and mass transport in a deep water environment, where flow is predominantly along isobaths and cross-isobath exchanges are limited to episodic events of upwelling/downwelling events or internal waves (Troy et al., 2012). Moreover, in most popular 3-D circulation models, vertical mixing processes are usually decoupled from horizontal advection and dispersion by solving 1-D transport equations for mass, momentum and energy implicitly along the water column (i.e., the “internal mode”). It is also of value to develop 1-D vertical mixing models for large lakes to determine whether the parameterizations that are commonly used for ocean models are applicable to these systems. Ivey & Patterson (1984) proposed a simple 1-D two-layer model to simulate the hydrodynamics in the central basin of Lake Erie in summer and model results showed good agreement with observed measurements. Likewise, Rucinski et al. (2010) successfully applied a linked 1-D thermal-dissolved oxygen model based on the Mellor–Yamada 2.5 level scheme for the central basin of Lake Erie.
In this study, a 1-D turbulence model coupled with biogeochemical simulations has been developed to investigate the effects of profundal quagga mussel grazing on nutrient and phytoplankton dynamics in the offshore waters of Lake Michigan. The biogeochemical component of the model is developed based on a conventional Nutrient–Phytoplankton–Zooplankton–Detritus (NPZD) approach, with mussels added as source and sink terms on the bottom boundary. The primary objective is to explore the parameterization of a general 1-D mixing model specifically for the hydrodynamic environment of Lake Michigan. The model is calibrated with field measurements of lake currents, turbulence, thermal structure, and phosphorus distribution, as well as field and lab measurements of quagga mussel metabolism. A specific objective of this modeling exercise is to examine how vertical mixing and filter feeding by profundal quagga mussels interact to regulate the transport of particles from the water column to the lake bottom, and the fate of nutrients recycled by mussels.
Methods
Model description
The coupled 1-D hydrodynamic-biogeochemical model is developed in Matlab and adopts a staggered grid arrangement in the vertical direction (z), similar to that of most popular ocean circulation models. The hydrodynamic component of the model solves the mean (non-turbulent) easterly and northerly velocity components (U and V, respectively), and the temperature T distribution over the water column as they vary in time (t). The turbulent kinetic energy (TKE) and mixing length scale, q2 and l respectively, are solved based on the Mellor–Yamada 2.5 closure scheme (Mellor & Yamada, 1982). Considering the idealized condition of horizontal homogeneity, the following one-dimensional transport equations can be applied:
where KV is the eddy viscosity; DV is the eddy diffusivity; K q is the mixing coefficient for TKE; f is the Coriolis factor; c is a sink term to balance the artificial increase of kinetic energy due to the lack of lateral transport (Pollard & Millard, 1970); the ρ is the water density; CP is heat capacity; I is shortwave radiation, and E1, B1 are the empirical coefficients which are set as 1.8 and 16.6, respectively (Mellor & Yamada, 1982).
Surface waves can be an important source of turbulence production in oceans and large lakes, and recent studies suggest that including wave effects into ocean/lake mixing models can improve model performance (Mellor & Blumberg, 2004; Huang & Qiao, 2010; Babanin & Onorato, 2012; Bai et al., 2013). As described by Mellor & Blumberg (2004), a modified surface boundary condition for Eqs. (4) and (5) was applied to represent surface wave breaking:
where u* is the water side surface friction velocity, and αCB is an empirical constant which is set to be 100 (Mellor & Blumberg, 2004).
To model the effect of wave-current interaction, the turbulent kinetic energy dissipation rate (εw) and a vertical mixing coefficient (Bv), both of which are functions of surface wave parameters and water depth, were introduced in the mixing model following Huang & Qiao (2010). Hence the modified eddy diffusivity \((D_{\rm v}^{\prime})\) and viscosity \((K_{\rm v}^{\prime})\) are applied for transport equations:
where
in which, δ is the wave steepness; us0 is the Stokes drift at water surface; z is the water depth.
The biological component of the model solves the transport and transformation of nutrients (N), phytoplankton (P), zooplankton (Z), and detritus (D) and generally follows the lower trophic level food web model described by Chen et al. (2002) and Luo & Wang (2012). Because Lake Michigan phytoplankton are strongly phosphorus limited (Bootsma et al., 2012), only phosphorus was considered in the nutrient pool. Effects of quagga mussels’ grazing and excretion are incorporated as the bottom source and sink terms. The governing equations are given as
The dynamics and fluxes of the four components and mussels are illustrated in Fig. S1. Growth rate of phytoplankton depends on nutrient limitation, light intensity as well as water temperature and is calculated by Eq. (15):
where μmax is the maximum phytoplankton growth rate; N is the nutrient concentration; Ks is the half saturation constant; I is available photosynthetically active radiation (PAR); KI is the half saturation constant for PAR; αT is the temperature dependence coefficient, and Topt is the optimal water temperature. An average of 0.43 for ratio of surface PAR and shortwave radiation is applied (Olofsson et al., 2007) and subsurface PAR is calculated with an attenuation coefficient (Kd),
where aw, ap, and ad are empirical attenuation coefficients for water, phytoplankton, and detritus, respectively. Model parameters applied in this study are listed in Table 1. The initial condition of N, P, Z, D was set as 0.12 mmol P m−3, 0.5 mmol C m−3, 0.5 mmol C m−3, and 6.0 mmol C m−3 following Luo & Wang (2012).
Direct mussel effects are limited to the bottom layer of the computation cells in the model and their grazing on phytoplankton and detritus is modeled as the product of their individual clearance rate, the population density, and the local concentration of phytoplankton and detritus. Mussel clearance rate (MP) is generally modeled as a function of temperature and mussel body length. In this study, since temperature in the hypolimnion is nearly constant, MP is determined as a constant of 0.28 L mg DW−1 day−1 based on the measurement results reported by Tyner et al. (2015); to derive clearance rate, O2 consumption was converted to organic C grazing rate, which was then divided by the ambient concentration of suspended organic C. The egestion and excretion effects of mussels were calculated assuming a recycling efficiency of 80% (Berg et al., 1996). Excretion is accounted as an input of dissolved P to the bottom computational cell. Egested feces are taken as additional input for bottom detritus pool following Rowe et al. (2017)’s approach.
1-D model site
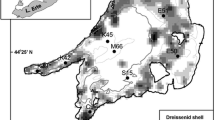
The model test site is located in a mid-depth region (55 m) of Lake Michigan (42.9797N, 87.6658W), southeast of the city of Milwaukee, WI (Fig. 1). Since 2012 we have conducted a number of field experiments at this site, including the deployment of a thermistor chain in 2012 and 2013 (4-m vertical intervals recorded every 5 min) and a bottom-mounted Acoustic Doppler Current Profiler (ADCP, 250 kHz) that measured current velocities at 2-m intervals from near-surface to near-bottom (Troy et al., 2016). To validate surface temperature simulations, the lake surface temperature at this site was obtained as satellite-based measurements from NOAA’s Coast Watch Program (http://coastwatch.glerl.noaa.gov/ftp/glsea/) on a daily basis. In 2013, mean mussel biomass density at this site was 34.95 ± 17.20 g m−2 (std. dev.) (Mosley & Bootsma, 2015). During 2013, vertical profile water samples were collected monthly between April and October and analyzed for soluble reactive phosphorus (SRP), total dissolved P (TDP), particulate P (PP), and particulate C (PC). Phosphorus concentrations were measured using the molybdate–ascorbic acid method, preceded by digestion for TDP and PP samples, while particulate C concentrations were measured on a continuous flow isotope ratio mass spectrometer interfaced with an elemental analyzer (see Mosley & Bootsma, 2015 for complete description of analytical methods).
Model drivers
A bulk aerodynamic formulation was applied to estimate the heat and momentum fluxes over the water surface as primary driving forces for the 1-D model. The calculation of fluxes is based on meteorological data. However, no direct meteorological measurements are available for the model site. Overland meteorological data available through NOAA’s stations, including wind, air temperature, cloud cover, and dew point, were interpolated to the study site following the method described by Beletsky & Schwab (2001). The locations of the overland stations used for the interpolation are shown in Fig. 1. Different to the other three heat flux terms (longwave, sensible, and latent heat), the shortwave radiation is the major source of heat which can penetrate into the water body and is treated as an internal source term in the temperature transport Eq. (3). The shortwave solar radiation reaching the water surface is generally influenced by the geographic location, time of the year, and cloud cover. To account for the variation in attenuation among wavelengths, 55% of the shortwave radiation was assigned an extinction coefficient of 2.85 m−1 (representing radiation in the yellow to infrared region) and 45% was assigned an extinction coefficient of 0.28 m−1 (representing the green to ultraviolet region) (Rucinski et al., 2010).
Transport equations for both physical and biological variables are solved with a Crank–Nicolson-based implicit finite-difference method, using a tridiagonal matrix algorithm to update the variables at each model time step, which was set at 30 s. The water column was discretized into 55 vertical uniform cells with 1-m resolution. The model was initialized with a uniform temperature distribution on March 1st when no ice cover was observed for Lake Michigan. Initial current velocity was set to zero as a cold start. Simulation was then conducted for 10 months until December 31 (year 2009–2013).
Results
Water temperature
Water surface temperatures simulated over a five-year period (2009–2013) were compared with that obtained from GLSEA’s satellite images (Fig. S2). As demonstrated in the figure, there is a good agreement between simulation results and satellite observations. The difference represented as the Root Mean Square error (RMSE) is generally small, with the maximum RMSE value of 1.39°C in year 2013 and the minimum of 0.89°C in year 2010. These differences may be due to the fact that our 1-D model does not account for horizontal transport, which can result in surface temperature variability that is not directly associated with lake-atmosphere heat flux.
Simulated vertical distribution of water temperature appears to capture the seasonal variation of the internal thermal structure of Lake Michigan, clearly showing the development of stable stratification from spring to summer, mixed layer deepening in the fall and complete mixing from winter through spring (Fig. S3). The comparison of the simulated vertical thermal structure and the individual measurements by the thermistor string in 2012, from 21 June to 18 September, is also illustrated in Fig. S3. As can be expected, isothermal lines predicted by the model lack fluctuations that are shown by the measurements, likely due to basin-scale internal waves which cannot be resolved by a 1-D model. Measured temperature profiles also indicated a sudden deepening of the thermocline around 17 August, which was due to a strong west basin downwelling event during that time period (with a corresponding upwelling on the east side) (Troy et al., 2016), as revealed by satellite images. Such a western basin downwelling is unusual since the western coast of Lake Michigan is more predisposed to upwelling due to the prevailing summer wind conditions and rotation effects of the Earth (Troy et al., 2012). Episodic events like this cannot be reproduced by the simple 1-D model. In addition to these limitations, simulation results of the 1-D model displayed a rather diffusive thermocline, a problem that has also been observed in 3-D hydrodynamic models for the Great Lakes (Beletsky et al., 2006). A possible explanation is that some vertical mixing mechanisms, such as Langmuir circulation, are not accurately simulated in general 3-D hydrodynamic circulation models (Beletsky et al., 2006). Time series of modeled and measured temperature at different depths are shown in Fig. S4. Good agreements are found in the surface mixed layer and near the lake bottom, with RMSE values of 1.63 and 0.46°C, respectively. Differences between modeled and simulated temperatures are higher near the thermocline, due to the diffusive nature of the model simulation. These differences were most pronounced at a depth of ~ 20 m, where the model overestimated the temperature by nearly 5°C for much of the simulation period.
Lake current and turbulent mixing
In the 1-D model presented here, a sink term is introduced in the momentum transport equations to suppress the kinetic energy build up in the water column. This approach was proposed by Pollard & Millard (1970) in ocean models to account for the missing horizontal transport terms in a 1-D model. In this study, the sink term is set to 1/3 (day−1) for the surface mixed layer, which has been shown to produce lake current speeds comparable to observed ones.
The modeled current velocity results at 6 m and 40 m depth were compared with ADCP measurements at the same site from the end of July to the beginning of August (Fig. S5). Simulated lake currents generally match well with observed results in magnitude, with some shifting of phase. The difference between the simulation and observations may partially be explained by lateral transport processes that cannot be accounted for by a 1-D model. The model site is located at the mid-depth region between the nearshore and offshore regions, where coastal processes may have some significant impacts as well. The time variability of the velocity was also examined by calculating the velocity spectra of measured and modeled result at 10-m and 40-m depths (Fig. S6), representing the dynamics in the surface mixed layer and the hypolimnion, respectively. Results at other depths show very similar spectral features. Both simulated and observed spectra have energy peaks concentrated in a time band between 17 and 18 h. In the high frequency range (< 10 h), observed results contain higher energy than the simulated, and the difference is more significant in the hypolimnion. A closer inspection of the spectra for the observed data indicates that energy peaks at 17.5 h, which can be attributed to the near-inertial Poincaré wave in the southern basin of Lake Michigan during the strongly stratified period. Similar to the simple slab model (Choi et al., 2012), the present 1-D model results featured a peak at the inertial period of 17.8 h which can be attributed to the fundamental inertial oscillation response of governing equations. The difference of the peak period explains the velocity phase shift in the model simulation compared to the observations shown in Fig. S5.
Since the focus of this research is to investigate the importance of hydrodynamic mixing on the exchange of biological and chemical constituents in the water column, it is critical to determine if the turbulent diffusion can be modeled correctly. While the “eddy” diffusion coefficient cannot be directly measured, other related parameters of turbulence can be used to evaluate the model performance. The production of turbulent kinetic energy (TKE) due to shearing lake currents can be estimated from both modeled and measured results as
where modeled “eddy” viscosity Kv is used along with velocity gradients to estimate both the modeled and measured turbulence production. Vertical profiles of daily-averaged turbulence production on the 20th day of June, July, August, and September are shown in Fig. S7. Modeled turbulence production matches well with that estimated from measurements in the surface mixed layer, suggesting that velocity gradients are simulated well in that layer. However, the model generally underestimates turbulence production in the deeper hypolimnion, particularly during the period of strong stratification, i.e., in July and August. The measured velocity spectrum (Fig. S6) also shows higher energy content in the deep water (i.e., at 40 m depth) compared with model results. The energetic hydrodynamics in the hypolimnion are likely due to boundary layer currents induced by basin-scale internal waves, which cannot be reproduced by the 1-D model, and so the model may produce an underestimate of deep water turbulence mixing during the stratification season.
The dissipation rate of TKE can also be used as an indication of turbulent mixing. Very few data are available for Great Lakes to evaluate TKE dissipation. For this study, the vertical distribution of dissipation was measured on June 20th, 2012 with an Underwater Particle Image Velocimetry (PIV) system (Liao et al., 2009). The PIV system was deployed from a research vessel to profile the 2D velocity field at different depths along the entire water column near the modeling site of this study. Dissipation rates are calculated from the measured 2D flow field following a “direct” method (Liao et al., 2009; Wang et al., 2013). At the same time, a Self-Contained Autonomous Micro Profiler (SCAMP) was deployed to measure the dissipation rate at a nearby location. The SCAMP can estimate dissipation based on the spectrum of the measured temperature microstructure. Modeled dissipation rate is calculated from turbulence transport terms in the model (Mellor & Yamada, 1982) as
Effects of surface waves through both wave breaking and non-breaking wave-current interaction were turned on and off in this study to examine their impact on turbulent mixing.
Profiles of modeled and measured dissipation are shown in Fig. S8. As demonstrated in the figure, model results agree well with the measurement in the surface mixed layer. The maximum dissipation rate occurs on the surface, and dissipation rate generally decreases with depth in the mixed layer, reaching a minimum value near the bottom of epilimnion (about 17-m below water surface). An increase of dissipation around the depth of the thermocline (between 15-m and 20-m depth) is found in both model results and field observations. Modeled dissipation in the hypolimnion is uniform as it is limited by the defined minimum value for q2 in the model. SCAMP measurements are noisy in the hypolimnion since the temperature there is highly uniform with very weak fluctuations. Dissipation rate measured by PIV shows a gradual increase towards the lake bottom in the hypolimnion. The difference between model and PIV measurements in the hypolimnion provides further evidence that the 1-D model may not be able to accurately reproduce the mixing process in the deep benthic boundary layer, which again may be related to the inability to simulate currents and shear there.
While model results with wave effects seem to increase dissipation near the surface and slightly deepen the mixed layer, the differences between modeled results with and without waves are minor. Similarly, adding wave components in the model does not significantly change simulation results in current speed, temperature structure, and concentrations of biological variables. Surface waves in the Great Lakes are generally shorter in length and lower in amplitude than ocean waves, and hence they may not have major impacts on lake mixing, except very close to the water surface.
Biological results
To assess mussel grazing effects on phytoplankton and nutrient cycles, the NPZD model was implemented with and without mussel filtration and excretion. Time series of the vertical distribution of phytoplankton, dissolved P (DP) and zooplankton in year 2013 are shown in Fig. 2. Biological simulation results at the 55-m site, with and without mussels, clearly demonstrate the general seasonal variability. During the spring, phytoplankton concentration gradually increases with very little vertical gradient over the entire water column. With the increased light intensity, water temperature, and sufficient nutrient supply at the end of spring and early summer, an abrupt plankton bloom can be observed, and the timing of the variation is consistent with previous field observations (Fahnenstiel et al., 2010; Rowe et al., 2015). In the summer when thermal stratification suppresses vertical mixing, phytoplankton in the hypolimnion is kept at a low concentration due to grazing by zooplankton and mussels, as well as self-mortality and sinking. Phytoplankton concentration in the surface mixed layer remains high until mid-June. Similarly, both DP and zooplankton are well distributed through the water column in the early spring. The spatio-temporal variation of zooplankton closely tracks that of phytoplankton. A dramatic decrease of DP is observed in late spring and early summer due to the phytoplankton bloom. Then it continues to decrease in the summer to a very low concentration in the surface mixed layer due to continuous uptake by phytoplankton. A relatively high DP concentration is found in the hypolimnion near the bottom, which can be attributed to microbial remineralization of detritus and mussel excretion.
The impact of mussel grazing on phytoplankton is most significant during the spring/early summer season, as the strong turbulent mixing provides bottom mussels sufficient access to phytoplankton throughout the water column. At the end of April, the average reduction of phytoplankton due to mussel grazing for the top and bottom 5 m was 24% and 28%, respectively, compared to the scenario without mussels. The effect of mussel grazing is strongest during the bloom period in early May, when the reduction in phytoplankton biomass reaches 42% and 47% in the top and bottom 5 m, respectively. By contrast, mussel filtration effects are limited during the stratified period, when there is little difference of phytoplankton biomass in the surface mixed layer under the two scenarios. However, in the hypolimnion, a layer of significant phytoplankton reduction (28%) is observed in the presence of mussels, primarily in the bottom 10 m of the water.
Model results highlight the importance of mussel excretion as a benthic nutrient source term. Compared to the case without mussels in the simulation, DP in the well-mixed water column increases by 5% during the spring period, increasing to 20% at the end of spring. During the stratified period, DP in the surface mixed layer is low due to uptake by phytoplankton, either with or without mussels. However, the near bottom (5 m) concentration increases by 62% on average due to mussel excretion.
Model dissolved P results are compared with field measurements that were made on 29 April, 19 June, 16 July, and 21 August, 2013. All biological components are modeled in terms of carbon mass concentration and they are converted to phosphorus concentration with a constant C:P ratio of 150:1. Measured and modeled profiles are shown in Figs. 3 and 4. Modeled PP profiles with mussel grazing effect match the measured ones very well, particularly for the well-mixed condition in the spring. During the stratified summer season, modeled PP concentration near the lake bottom (35–55 m depth) also agree with measurements when mussel grazing is included. In both spring and summer, PP concentrations are significantly lower than model results without mussels (Fig. 3). Moreover, the model appears to be able to simulate the observed peak PP concentration near the thermocline. While the concentration level of the peak agrees well with measurements, the exact depth of the peak differs from the observed. As the measured SRP showed an elevated concentration in the hypolimnion in the summer, the vertical variability seems to be greater than that simulated by the model. The simulated near-bottom DP concentration with mussels present agrees better with observations than the simulation without mussels. It should also be noted that the model over predicts the DP concentrations throughout the water column in late spring (Fig. 4a) which may be because the model does not account for the adsorption and desorption of DP by bottom sediments (Brooks & Edgington, 1994).
To examine the impact of mussel grazing and turbulent mixing on the effective settling rate of phosphorus, vertical fluxes of PP were calculated from the model results. The total vertical fluxes of PP can be considered as the sum of passive settling FS and the turbulent diffusive flux FD. They can be calculated as
where wS is the passive settling speed of PP (0.6 m day−1); \(\bar{P}\) is the temporally averaged PP concentration (averaged over 24 h in this study), and Dv is the “eddy” diffusivity. Field samples collected at the 55 m modeling site suggest that the mussel grazing rate can be 4–11 times higher than the passive settling rate as measured by sediment traps (Mosley & Bootsma, 2015; Tyner et al., 2015). We used our model to calculate the daily FD to FS ratio at 53 m for cases with and without mussel grazing (Fig. 5). While diffusion-to-settling ratios show strong daily variation during the well-mixed spring season for both cases, they become fairly constant when the lake stratifies. According to model results, the averaged ratio (from March to August) is about 4.2, which means the overall particle delivery rate is 5.2 times greater than the passive settling rate. However, under the same vertical mixing condition in the absence of mussels, the average flux due to turbulent diffusion is reduced by about 50%. Hence, the model analysis supports the conclusion that mussels accelerate the rate of particle transfer from the water column to the lake bottom, as inferred by the field observations of Mosley & Bootsma (2015) and Tyner et al. (2015).
Figure 6 shows the vertical profiles of monthly averaged settling and diffusive fluxes of PP in April, May, June, and July of 2013. Profiles of fluxes also suggest that in the stratified hypolimnion, mussel filtration combined with vertical mixing can significantly increase diffusive flux, which exceeds the sedimentation rate within a layer of about 20 m above the mussel colony (35–55 m depth). Diffusion effects diminish quickly above 35 m, where settling is the dominant process for vertical PP transport. In addition, the enhanced diffusive transport in the benthic boundary layer approximately equals the passive settling near the thermocline (20–30 m depth). This observation suggests that food delivery to benthic mussels is ultimately limited by settling from the epilimnion into the hypolimnion. Figure 6 also shows that without mussels’ grazing effects, transport due to turbulent diffusion is consistently lower than the settling flux in the stratified benthic water.
Analysis of modeling results indicates that dreissenid mussels in the mid-depth offshore water of Lake Michigan can maximize the food capture rate by creating a strong vertical gradient of phytoplankton concentration within the hypolimnion. Although vertical mixing in the hypolimnion is suppressed by stratification, it is adequate to promote a food delivery rate to profundal mussels that is faster than that due to passive sinking.
Time series of depth-averaged concentrations of phytoplankton, DP, and zooplankton are shown in Fig. S9 to demonstrate the annual cycling of energy and nutrients with and without mussels. The 1-D model is currently configured to run starting on 1 March and ending on 31 December of a given year. The first two months of the year are not included, as the simulation of biogeochemical processes under ice-covered conditions has yet to be developed. Simulated depth-averaged phytoplankton and zooplankton biomass variation was similar to the model results of Pilcher et al. (2015). Without model simulation results for the full winter period (due to ice cover), it is not clear if an equilibrium state can be reached after a full annual simulation cycle. While concentrations of phytoplankton and DP at the end of the simulation period differ only slightly from their corresponding initial values, the zooplankton population seems to drop by about 50% after 10 months of simulation. However, the range of zooplankton biomass produced by the model is similar to the range of observed values following the establishment of quagga mussels in Lake Michigan (Vanderploeg et al., 2010; Pothoven & Fahnenstiel, 2013; Driscoll & Boostma, 2015). Taking the average over the entire simulation period as an approximation of the annual average value, the model results suggest that dreissenid mussels are able to (1) decrease the average concentration of phytoplankton by 18%; (2) increase the average DP concentration by 20%; and (3) decrease zooplankton biomass by 11.5%.
Discussion
Many advanced, fully 3D biophysical models have recently been developed to study the impact of mussels on the cycling of nutrient and phytoplankton in Great Lakes. The much simpler 1-D model described here presents a convenient alternative tool that can be applied to the offshore water of Great Lakes, where lateral gradients and transport are relative weak compared with those in nearshore areas. Comparison of turbulence production and dissipation between model results and field observations, which is rarely done even for more advanced 3D models, suggested that a well-constructed 1-D model can provide realistic mixing parameters for the subsequent particle and nutrient transport modeling. However, the weakness is also clear that it underestimates the benthic boundary layer mixing due to basin-scale internal waves during summer stratification. As a result, the model may underestimate cycling rates of phytoplankton mass and dissolved nutrient under stratification. In our ongoing research, a 3-D coupled physical-biological model does predict a higher “eddy” viscosity and diffusivity in the benthic boundary layer which was about 10 meters thick above the lake bed (manuscript in preparation). The 1-D model reported a 5.2 times increase of particle delivery rate on average due to mussels’ filter feeding, while the 3D model suggested an 8-fold increase, which agrees better with the field observation (Mosley & Bootsma, 2015).
Field data acquired in southeastern Lake Michigan indicate that the summer mean chlorophyll concentration in the surface mixed layer did not differ between the pre- and post-mussel period (Pothoven & Fahnenstiel, 2013). Model simulations presented here are largely consistent with the field observations as the phytoplankton concentration in the epilimnion does not seem to be affected by mussel grazing in later summer. It is interesting to note that the model with mussels actually predicted a slight increase (5%) of phytoplankton in the mixed layer in early summer compared to the case without mussels. Pilcher et al. (2017) also found comparable phytoplankton increase (0–20%) with mussel filtration included in their biophysical model for Lake Michigan. A closer inspection indicates that modeled zooplankton biomass is reduced by about 25% during the spring bloom period, likely due to competition for food with dreissenid mussels. Meanwhile dissolved P is higher before the onset of stratification, likely due to mussels’ P recycling (see Fig. 2). The combination of lower zooplankton grazing pressure and higher availability of dissolved P in the early summer, along with limited mussel grazing due to stratification may explain the phytoplankton increase in the early summer. The 1-D model simulation also shows that mussels graze nearly twice as much as zooplankton do in spring when mussels can access the entire water column. By contrast, zooplankton grazing exceeds mussel grazing by nearly 5 times in the stratified summer period. Previous studies have highlighted the importance of zooplankton grazing as a sink for phytoplankton. Scavia & Fahnenstiel (1987) determined that summer zooplankton grazing rates in Lake Michigan were comparable to phytoplankton growth rates prior to the presence of dreissenids in the lake. Zhang et al. (2011) concluded that phytoplankton loss to zooplankton grazing in the central basin of Lake Erie remains much greater than loss to dreissenid grazing, while the two loss rates are similar in the shallower western basin. Model results presented here suggest that the relative importance of zooplankton and dreissenids as phytoplankton grazers depends on the degree of water column stratification. The results also suggest that feedback mechanisms may lead to complex interactions among mussels, zooplankton, and phytoplankton. If mussel grazing in the spring isothermal period does indeed result in lower zooplankton biomass in the early stratified period, then phytoplankton production during that time may be less efficiently transferred to higher trophic levels. Further modeling efforts will need to consider how diel migration of zooplankton, which is not accounted for in this study, may affect zooplankton grazing rates and the vertical distribution of phytoplankton and dissolved nutrients.
Fahnenstiel et al. (2010) analyzed field data from two offshore stations in southeastern Lake Michigan and found an 87% reduction of phytoplankton biomass in the surface mixed layer during the spring isothermal period following the establishment of quagga mussels. Further observations at 4 additional offshore stations indicated that while chlorophyll concentration in the surface mixed layer had not changed in summer after mussels became established, the bottom chlorophyll concentration declined by nearly 60% (Pothoven & Fahnenstiel, 2013). The 1-D model presented here generally agrees with these field observation, although the simulated reductions in phytoplankton biomass in the spring (about 45% reduction) and near the lake bottom in the summer (28% reduction) are not as extreme as those previously reported. This is reasonable as the model compares the change of biomass only in a one-year time scale for the scenarios with and without mussels at bottom. Spring total phosphorus concentration has declined during the time period of the quagga mussel invasion, while the initial condition for total phosphorus in our model is likely lower than that before mussel invasion. Rowe et al. (2017) indicated that net primary productivity in Lake Michigan is much more sensitive to the initial nutrient concentration compared with filtration of mussel, especially during summer time. Also, our recent measurements of particulate organic carbon concentrations ranged from 4 to 6 mmol C m−3 in the spring (April) of 2013, and 14–15.4 mmol C m−3 in the surface mixed layer in the stratified period of 2013. By contrast, Fahnenstiel et al. (2010) estimated a phytoplankton carbon concentration of ~ 0.6 mmol C m−3 in the spring and a surface mixed layer concentration of 2.5–3.2 mmol C m−3 during the stratified period. The higher values in 2013 are likely due in part to the fact that our method (filtration on GF/F filter followed by direct measurements of carbon mass) includes non-phytoplankton carbon. However, in a large, oligotrophic lake like Lake Michigan, living phytoplankton likely make up 30% to 50% of total POC (Hessen et al., 2003), and so the 5-fold difference between our measured POC concentrations and the concentrations derived from cell counts by Fahnenstiel et al. (2010) cannot be due entirely to detrital PC. While Fahnenstiel et al. (2010) reported an 8-fold difference between spring PC concentrations in 1995–1998 versus 2007–2008, the difference in chlorophyll a concentrations was 3-fold, which is closer to the with- vs. without-mussel difference in phytoplankton C produced by the model, i.e., about a 2-fold difference.
To evaluate the impact of mussels’ filtration, uncertainty analysis is conducted by varying mussel filtration rate and/or biomass density. Our field sampling at the 55-m station indicated noticeable mussel biomass density variation, i.e., 34.95 ± 17.20 g m−2 (std. dev.). Mussel filtration rate can also vary significantly as it depends on several factors, including mussel size, water temperature, species, food source (Vanderploeg et al., 2010). Under scenarios of 2-fold/4-fold increase of mussel biomass, which is equivalent to 2-fold/4-fold increase of filtration rate, an additional 18%/25% phytoplankton reduction is found in spring compared to the base scenario. However, the increased mussel biomass or filtration rate has little influence (within 5%) on summer surface phytoplankton, which again illustrates the importance of physical mixing which can regulate mussels’ grazing efficiency.
Based on our model results and empirical observations, it appears that the net effect of quagga mussels in Lake Michigan is to increase the rate of transfer of particles from the water column to the lake bottom, thereby reducing the water column particle residence time. Grazing and processing of these particles by profundal mussels also accelerate the recycling of particulate P to dissolved P. Prior to the arrival of quagga mussels in Lake Michigan, recycling of P from the sediment to the water column was relatively slow, and may have been dictated by the kinetics of apatite dissolution (Brooks & Edgington, 1994). Quagga mussels have short circuited this process (Mosley & Bootsma, 2015). However, during the stratified period, much of the dissolved P released by mussels appears to remain sequestered in the hypolimnion, and while phytoplankton may have access to some of this recycled P, on a seasonal time scale phytoplankton are regulated more by mussel grazing than by mussel nutrient recycling. During the spring isothermal period, the average irradiance that phytoplankton are exposed to is relatively low, due to moderate levels of surface radiation and mixing throughout the entire water column, and so the ability of phytoplankton to take advantage of the P recycled by mussels is limited. This is supported by the observation by Fahnenstiel et al. (2010) that the phytoplankton C:total P ratio is relatively low in the spring isothermal period. By contrast, during the stratified period phytoplankton in the surface mixed layer experience higher light levels, but limited mixing between this layer and the hypolimnion restricts access to P recycled by profundal mussels, and as a result phytoplankton C: total P ratios increase (Fahnenstiel et al., 2010). If the date of onset of stratification in Lake Michigan becomes earlier as the lake continues to warm (Austin & Colman, 2007; Dobiesz & Lester, 2009), average water column irradiance prior to stratification will become even less, which may further limit the ability of phytoplankton to utilize P recycled by mussels in the spring.
The apparent importance of profundal mussels as both grazers and nutrient recyclers highlights the need to quantify vertical mixing rates, as these will influence both the delivery of particles to the mussel bed and the distribution of dissolved P excreted by mussels. If the hypolimnion is reasonably well mixed, the interaction between profundal mussels and phytoplankton will be strengthened; grazing rates will be greater, but phytoplankton production rates in the upper layers of the hypolimnion will also be greater due to an optimal combination of light from above and mussel-recycled P from below. However, if the hypolimnion is stagnant then the interaction between zooplankton and phytoplankton will be relatively more important. This tradeoff between mussels and zooplankton is most obvious when comparing model results between the isothermal and stratified periods. But profundal mussel grazing and nutrient recycling rates during the stratified period (Mosley & Bootsma, 2015; Tyner et al., 2015) suggest that they remain as an important phytoplankton sink, and the magnitude of this sink may vary according to short-term (Troy et al. 2016) and long-term variation in the strength of vertical stratification.
In addition to improved measurement and modeling of vertical mixing, other measurements needed to refine the model include the role of dissolved organic phosphorus, which can make up a significant proportion of the P recycled by quagga mussels, and the fate of quagga mussel biodeposits, which represent ~ 50% of the food ingested (Mosley & Bootsma, 2015).
Despite the limitations, the simplicity of the 1-D model makes it a convenient tool to investigate pelagic physical and biogeochemical processes in large lakes. With the expansion of dreissenid mussels from the nearshore to offshore areas, the 1-D model can be an attractive alternative to study the long-term impacts of profundal mussels at the whole lake scale. The analysis presented here suggests that dreissenid mussels have the potential to significantly redistribute organic carbon and nutrients in the water column, even in a deep and stratified environment. Mussel populations in the Great Lakes appear to still be in a state of transition. The 1-D model may help to predict the long-term response of the lakes to these benthic filter feeders as their abundance and distribution continue to change.
References
Ackerman, J. D., M. R. Loewen & P. F. Hamblin, 2001. Benthic–Pelagic coupling over a zebra mussel reef in western Lake Erie. Limnology and Oceanography 46: 892–904.
Austin, J. A. & S. M. Colman, 2007. Lake Superior summer water temperatures are increasing more rapidly than regional air temperatures: a positive ice-albedo feedback. Geophysical Research Letters 34: L06604.
Babanin, A., M. Onorato & F. Qiao, 2012. Surface waves and wave-coupled effects in lower atmosphere and upper ocean. Journal of Geophysical Research: Ocean 117: C00J01.
Bai, X., J. Wang, D. Schwab & Y. Yang, 2013. Modelling 1993-2008 climatology of seasonal general circulation and thermal structure in the Great Lakes using FVCOM. Ocean Modelling 65: 40–63.
Beletsky, D. & D. Schwab, 2001. Modeling circulation and thermal structure in Lake Michigan: annual cycle and interannual variability. Journal of Geophysical Research: Ocean 106(C9): 19745–19771.
Beletsky, D., D. J. Schwab & M. McCormick, 2006. Modeling the 1998–2003 summer circulation and thermal structure in Lake Michigan. Journal of Geophysical Research: Ocean 111: C10010.
Bennington, V., G. A. McKinley, N. Urban & C. McDonald, 2012. Can spatial heterogeneity explain the perceived imbalance in Lake Superior’s carbon budget? A model study. Journal of Geophysical Research: Biogeosciences 117: G03020.
Berg, D. J., S. W. Fisher & P. F. Landrum, 1996. Clearance and processing of algal particles by zebra mussels (Dreissena polymorpha). Journal of Great Lakes Research 22: 779–788.
Bocaniov, S. A., R. E. H. Smith, C. M. Spillman, M. R. Hipsey & L. Leon, 2014. The nearshore shunt and the decline of the phytoplankton spring bloom in the Laurentian Great Lakes: insights from a three-dimensional lake model. Hydrobiologia 731: 151–172.
Boegman, L., M. R. Loewen, D. A. Culver, P. F. Hamblin & M. N. Charlton, 2008. Spatial-dynamic modelling of algal biomass in Lake Erie: relative impacts of dreissenid mussels and nutrient loads. Journal of Environmental Engineering 134(6): 456–468.
Bootsma, H. A. & Q. Liao, 2013. Nutrient cycling by dreissenid mussels. In: Quagga and Zebra Mussels. CRC Press, Boca Raton: 555–574.
Bootsma, H. A., J. T. Waples & Q. Liao, 2012. Identifying Major Phosphorus Pathways in the Lake Michigan Nearshore Zone. MMSD Contract.
Brooks, A. S. & D. N. Edgington, 1994. Biogoechemical control of phosphorus cycling and primary production in Lake Michigan. Limnology and Oceanography 39: 961–968.
Bunnell, D. B., C. P. Madenjian, J. D. Holuszko, J. V. Adams & J. R. P. French, 2009. Expansion of Dreissena into offshore waters of Lake Michigan and potential impact on fish populations. Journal of Great Lakes Research 35: 74–80.
Chen, C., R. Ji, D. J. Schwab, D. Beletsky, G. L. Fahnenstiel, M. Jiang, T. H. Johengen, H. A. Vanderploeg, B. Eadie, J. W. Budd, M. H. Bundy, W. Gardner, J. Cotner & P. J. Lavrentyev, 2002. A model study of the coupled biological and physical dynamics in Lake Michigan. Ecological Modelling 152: 145–168.
Choi, J., C. D. Troy, T. Hsieh, N. Hawley & M. J. McCormick, 2012. A year of internal Poincaré waves in southern Lake Michigan. Journal of Geophysical Research: Ocean 117(C7): 16.
Dayton, A. I., M. T. Auer & J. F. Atkinson, 2014. Cladophora, mass transport, and the nearshore phosphorus shunt. Journal of Great Lakes Research 40: 790–799.
Dobiesz, N. E. & N. P. Lester, 2009. Changes in mid-summer water temperature and clarity across the Great Lakes between 1968 and 2002. Journal of Great Lakes Research 35: 371–384.
Dolan, D. M. & S. C. Chapra, 2012. Great Lakes total phosphorus revisited: 1. Loading analysis and update (1994–2008). Journal of Great Lakes Research S3: 104–114.
Driscoll, Z. & H. A. Boostma, 2015. Zooplankton trophic structure in Lake Michigan as revealed by stable carbon and nitrogen isotopes. Journal of Great Lakes Research 36: 20–29.
Fahnenstiel, G., S. Pothoven, H. Vanderploeg, D. Klarer, T. Nalepa & D. Scavia, 2010. Recent changes in primary production and phytoplankton in the offshore region of southeastern Lake Michigan. Journal of Great Lakes Research 36: 20–29.
Hecky, R. E., R. E. H. Smith, D. R. Barton, S. J. Guildford, W. D. Taylor, M. N. Charlton & T. Howell, 2004. The nearshore phosphorus shunt: a consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Canadian Journal of Fisheries and Aquatic Science 61: 1285–1293.
Hessen, D. O., T. Andersen, P. Brettum & B. A. Faafeng, 2003. Phytoplankton contribution to sestonic mass and elemental ratios in lakes: implications for zooplankton nutrition. Limnology and Oceanography 48: 1289–1296.
Huang, C. J. & F. L. Qiao, 2010. Wave-turbulence interaction and its induced mixing in the upper ocean. Journal of Geophysical Research: Ocean 115: C04026.
Ivey, G. N. & J. C. Patterson, 1984. A model of vertical mixing in Lake Erie in summer. Limnology and Oceanography 29: 553–563.
Koseff, J. R., J. K. Holen, S. G. Monismith & J. E. Cloern, 1993. Coupled effects of vertical mixing and benthic grazing on phytoplankton populations in shallow, turbid estuaries. Journal of Marine Research 51: 843–868.
Leon, L. F., R. E. H. Smith, M. R. Hipsy, S. A. Bocaniov, S. N. Higgins, R. E. Hecky, J. P. Antenucci, J. A. Inberger & S. J. Guildford, 2011. Application of a 3D hydrodynamic-biological model for seasonal and spatial dynamics of water quality and phytoplankton in Lake Erie. Journal of Great Lakes Research 37: 41–53.
Liao, Q., H. A. Boostma & J. E. Xiao, 2009. Development of an in situ underwater particle image velocimetry (UWMPIV) system. Limnology and Oceanography: Methods 7: 169–184.
Luo, L. & J. Wang, 2012. Simulating the 1998 spring bloom in Lake Michigan using a coupled physical-biological model. Journal of Geophysical Research: Ocean 117: C10011.
Mellor, G. & A. Blumberg, 2004. Wave breaking and ocean surface layer thermal response. Journal of Physical Oceanography 34: 693–698.
Mellor, G. & T. Yamada, 1982. Development of a turbulent closure model for geophysical fluid problems. Reviews of Geophysics and Space Physics 20: 851–875.
Mida, J. L., D. Scavia, G. L. Fahnenstiel, S. A. Pothoven, H. A. Vanderploeg & D. M. Dolan, 2010. Long-term and recent changes in southern Lake Michigan water quality with implications for present trophic status. Journal of Great Lakes Research 36: 42–49.
Mosley, C. & H. A. Bootsma, 2015. Phosphorus recycling by profunda quagga mussels (Dreissena rostriformis bugensis) in Lake Michigan. Journal of Great Lakes Research S3: 38–48.
Nalepa, T. F., D. L. Fanslow & S. A. Pothoven, 2010. Recent changes in density, biomass, recruitment, size structure, and nutritional state of Dreissena populations in southern Lake Michigan. Journal of Great Lakes Research 36: 5–19.
Nalepa, T. F., D. L. Fanslow, G. A. Land, K. Mabrey & M. Rowe, 2014. Lake-wide benthic surveys in Lake Michigan in 1995-95, 2000, 2005, and 2010: Abundances of the amnphipod Diporeia spp. and abundances and biomass of the mussels Dreissena polymorpha and Dreissena rostriformis bugensis. NOAA Technical Memorandum GLERL-164.
Officer, C. B., T. J. Smayda & R. Mann, 1982. Benthic filter feeding: a natural eutrophication control. Marine Ecological Progress Series 9: 203–210.
Olofsson, P., E. V. Laake & E. Lars, 2007. Estimation of absorbed PAR across Scandinavia from satellite measurements: Part I: Incident PAR. Remote Sensing of Environment. 110: 252–261.
Ozersky, T., D. O. Evans & B. K. Ginn, 2015. Invasive mussels modify the cycling, storage and distribution of nutrients and carbon in a large lake. Freshwater Biology 60: 827–843.
Parsons, T. R., M. Takahashi & B. Hargrave, 1984. Biological Oceanographic Process, 3rd ed. Pergamon Press, New York.
Pilcher, D. J., G. A. McKinley, H. A. Bootsma & V. Bennington, 2015. Physical and biogeochemical mechanisms of internal carbon cycling in Lake Michigan. Journal of Geophysical Research: Oceans 120: 2112–2128.
Pilcher, D. J., G. A. McKinley, J. Kralj, H. A. Bootsma & E. D. Reavie, 2017. Modeled sensitivity of Lake Michigan productivity and zooplankton to changing nutrient concentrations and quagga mussels. Journal of Geophysical Research: Oceans. Physical and biogeochemical mechanisms of internal carbon cycling in Lake Michigan. Journal of Geophysical Research: Biogeosciences 122: 2032–2107.
Pollard, R. T. & R. C. Millard, 1970. Comparison between observed and simulated wind-generated inertial oscillations. Deep-Sea Research 17: 813–821.
Pothoven, S. A. & G. L. Fahnenstiel, 2013. Recent change in summer chlorophyll a dynamics of southeastern Lake Michigan. Journal of Great Lakes Research 39: 287–294.
Rowe, M. D., E. J. Anderson, J. Wang & H. A. Vanderploeg, 2015. Modelling the effect of invasive quagga mussels on the spring phytoplankton bloom in Lake Michigan. Journal of Great Lakes Research 41: 49–65.
Rowe, M. D., E. J. Anderson, H. A. Vanderploeg, S. A. Pothoven, A. K. Elgin & J. Wang, 2017. Influence of invasive quagga mussels, phosphorus loads, and climate on spatial and temporal patterns of productivity in Lake Michigan: a biophysical modelling study. Limnol. Oceanogr. 62: 2629–2649.
Rucinski, D. K., D. Beletsky, J. V. DePinto, D. J. Schwab & D. Scaiva, 2010. A simple 1-dimensional, climate based dissolved oxygen model for the central basin of Lake Erie. Journal of Great Lakes Research 36: 465–476.
Scavia, D. & G. Fahnenstiel, 1987. Dynamics of Lake Michigan phytoplankton: mechanisms controlling epilimnetic communities. Journal of Great Lakes Research 13: 103–120.
Schwalb, A. N., D. Bouffard, T. Ozersky, L. Boegman & R. H. Smith, 2013. Impacts of hydrodynamics and benthic communities on phytoplankton distributions in a large, dreissenid-colonized lake (Lake Simcoe, Ontario, Canada). Inland Waters 3: 269–284.
Schwalb, A. N., D. Bouffard, L. Boegman, L. Leon, J. Winter, L. Molot & R. H. Smith, 2015. 3D modelling of dreissenid mussel impacts on phytoplankton in a large lake supports the nearshore shunt hypothesis and the importance of wind-driven hydrodynamics. Aquatic Science 77: 95–114.
Strayer, D. L. & K. A. Hattala, 2004. Effects of an invasive bivalve (Dreissena polymorpha) on fish in the Hudson River estuary. Canadian Journal of Fisheries and Aquatic Science 61: 924–941.
Troy, C. D., S. Ahmed, N. Hawley & A. Goodwell, 2012. Cross-shelf thermal variability in southern Lake Michigan during the stratified periods. Journal of Geophysical Research: Ocean 117(C2): 27.
Troy, C., D. Cannon, Q. Liao & H. A. Bootsma, 2016. Logarithmic velocity structure in the deep hypolimnetic waters of Lake Michigan. Journal of Geophysical Research: Oceans 121: 949–965.
Turschak, B. A., D. Bunnell, S. Czesny, T. O. Höök, J. Janssen, D. Warner & H. A. Bootsma, 2014. Nearshore energy subsidies support Lake Michigan fishes and invertebrates following major changes in food web structure. Ecology 95: 1243–1252.
Tyner, E. H., H. A. Bootsma & B. M. Lafrancois, 2015. Dreissenid metabolism and ecosystem-scale effects as revealed by oxygen consumption. Journal of Great Lakes Research 41: 27–37.
Vanderploeg, H. A., T. F. Nalepa, D. J. Jude, E. L. Mills, K. T. Holeck, J. R. Liebig, I. A. Grigorovich & H. Ojaveer, 2002. Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Canadian Journal of Fisheries and Aquatic Science 59: 1209–1228.
Vanderploeg, H. A., J. R. Liebig, T. F. Nalepa, G. L. Fahnenstiel & S. A. Pothoven, 2010. Dreissena and the disappearance of the spring phytoplankton bloom in Lake Michigan. Journal of Great Lakes Research 36: 50–59.
Wang, B., Q. Liao, J. Xiao & H. A. Bootsma, 2013. A free-floating PIV system: measurements of small scale turbulence under the wind wave surface. Journal of Atmospheric and Oceanic Technology 30: 1494–1509.
Waples, J. T., H. A. Bootsma & J. V. Klump, 2016. How are coastal benthos fed? Limnology and Oceanography: Letters 2(1): 18–28.
Zhang, H. Y., D. A. Culver & L. Boegman, 2011. Dreissenids in Lake Erie: an algal filter or a fertilizer? Aquatic Invasions. 6: 175–194.
Acknowledgements
This study was funded by the Wisconsin SEA Grant under project number R/HCE-02-10, and by the National Science Foundation under project number NSF-OCE 1658390.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Alex Elliott
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shen, C., Liao, Q., Bootsma, H.A. et al. Regulation of plankton and nutrient dynamics by profundal quagga mussels in Lake Michigan: a one-dimensional model. Hydrobiologia 815, 47–63 (2018). https://doi.org/10.1007/s10750-018-3547-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-018-3547-6