Abstract
Dreissenid mussels have been hypothesized to cause selective decreases of phytoplankton in nearshore areas (nearshore shunt hypothesis) as well as the near-complete loss of the offshore phytoplankton spring bloom in some Laurentian Great Lakes. To evaluate whether mussels can reasonably be expected to mediate such changes, we extended the three-dimensional hydrodynamic-ecological model (ELCOM-CAEDYM) to include mussels as a state variable and applied it to Lake Erie (USA-Canada). Mussel-mediated decreases in mean phytoplankton biomass were highly sensitive to the assigned mussel population size in each basin. In the relatively deep east basin, mussels were predicted to decrease phytoplankton in both nearshore and offshore zones, even during periods of thermal stratification but especially during the spring phytoplankton maximum. Spatially, impacts were associated with mussel distributions but could be strong even in areas without high mussel biomass, consistent with advection from areas of higher mussel biomass. The results supported the nearshore shunt hypothesis that mussel impacts on phytoplankton should be greater in nearshore than offshore waters and also supported suggestions about the emerging importance of deep water offshore mussels. The results of this study provide an important insight into ecological role of mussels in lowering plankton productivity in some world’s largest lakes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dreissenid mussels are invaders that can have large ecological impacts in lakes (Karatayev et al., 1997; Higgins & Vander Zanden, 2010). In the very large Laurentian Great Lakes, advent of dreissenid mussels was associated with decreased phytoplankton abundance in shallower and/or nearshore areas such as inner Saginaw Bay (Fahnenstiel et al., 1995), nearshore Lake Erie and Ontario (Nicholls et al., 1999) and west basin Lake Erie (Charlton et al., 1999; Makarewicz et al., 1999). Changes associated with colonization in the deeper waters of outer Saginaw Bay (Fahnenstiel et al., 1995) and the offshore waters of central and eastern basins of Lake Erie (Makarewicz et al., 1999; Charlton et al., 1999) were smaller or non-existent. The summer Chl–TP relationship, which changed significantly with dreissenid arrival in many shallower locations (e.g. Nicholls et al., 1999) had not changed as of 1997 in offshore waters of Lake Erie (Smith et al., 2005) even though quagga mussels (Dreissena bugensis) had already colonized the profundal zone of the east basin by 1992–1993. As the mussel population continued to expand in deep waters (Haltuch et al., 2000), evidence of decreased spring phytoplankton biomass in offshore east basin appeared (Barbiero et al., 2006). More recently, the expansion of quagga mussels to deeper waters of Lakes Huron and Michigan has been associated with large decreases of phytoplankton biomass and production, particularly during the spring diatom maximum period (Fahnenstiel et al., 2010; Vanderploeg et al., 2010; Evans et al., 2011). Dreissenid mussels can be important in nutrient budgets in smaller lakes (Goedkoop et al., 2011) and may be important even in very large lakes (Evans et al., 2011). A meta-analysis suggests that dreissenids can strongly affect phytoplankton even where deep and relatively cold waters might be expected to limit the direct grazing impact (Higgins et al., 2011). Given the evidence for ecosystem-wide impacts of dreissenid mussels, even in very large and deep lakes, there is need for an improved capacity for predictive modelling of their role in lake ecology.
The characterization of mussel effects on phytoplankton can be greatly complicated by factors such as coincident changes in external nutrient loads and variations in the choice of metric (especially Chl a vs phytoplankton biovolume) for phytoplankton (Conroy et al., 2005a), posing serious problems for predictive modelling and management. Hydrodynamic processes also pose a number of challenges. In large lakes, shallower nearshore areas are generally more productive with a higher potential for new organic matter export (production > respiration; Bocaniov & Smith, 2009) and may afford mussels favourable substrate and access to plankton (Hecky et al., 2004) but the often poorly characterized exchange between such areas and deeper offshore areas (Rao & Schwab, 2007) may alter the resulting spatial pattern of phytoplankton in surface waters, as exemplified for cases of water transport across mussel-colonized reefs (MacIsaac et al., 1999; Ackerman et al., 2001). Even in shallower waters that do not normally form strong thermal stratification, mixing strength may frequently be insufficient to deliver plankton at a rate that satisfies the filtering demand of the mussels (Ackerman et al., 2001; Boegman et al., 2008a). Effective grazing rates may be substantially less than expected from approaches that assume a well-mixed system and laboratory-derived values for mussel filtering performance (Yu & Culver, 1999; Boegman et al., 2008a). Nutrient regeneration by mussels can represent a very substantial internal load (Arnott & Vanni, 1996; Conroy et al., 2005b) but the availability of the nutrients to phytoplankton (or other potential users such as benthic algae) will depend on patterns of thermal stratification, vertical mixing and horizontal advection (Zhang et al., 2008). Spatially resolved models that include essential features of the system hydrodynamics, as well as important processes such as external and internal nutrient loading, are necessary tools for developing a predictive understanding of dreissenid mussel effects in large lakes.
Considerable progress has been made in spatially resolved modelling of physical and ecological variables in lakes, including Lake Erie. Boegman et al. (2008b) presented the first spatially resolved and hydrodynamically linked model to include a depiction of dreissenid mussels. They extended a two-dimensional reservoir model for hydrodynamics and water quality variables (CE-QUAL-W2) to include dreissenid mussels and zooplankton. The model was used to analyse the implications of variable P loading and mussel presence for the vertical and longitudinal variations of nutrients and phytoplankton in Lake Erie, with an emphasis on west basin. Zhang et al. (2008) used the same two-dimensional modelling framework but increased the detail of the lower food web model. These modelling efforts have confirmed conclusions from earlier modelling studies (e.g. Bierman et al., 2005) that mussels can have major impacts on phytoplankton but have also revealed some of the complexities that arise from variations in depth, stratification and mixing strength. Indirect effects of mussel-mediated nutrient cycling are predicted to be important (Zhang et al., 2008) and are also affected by hydrodynamic conditions.
Mussel distributions are notoriously heterogeneous (Patterson et al., 2005; Naddafi et al., 2011), and the often large populations residing in the nearshore will experience different conditions from populations residing in deeper waters (Vanderploeg et al., 2010). To capture these and other pertinent realities requires a three-dimensional (3D) approach, which we present here. It is the first lake ecosystem model we know of to include the 3D hydrodynamics and a biological model that treats mussels as a state variable, with relatively realistic descriptions of mussel energetics and feeding. Here we use simulations and observations of Lake Erie in 2002 to assess model performance and evaluate hypotheses about spatio-temporal variations of mussel effects on phytoplankton, including sensitivity of effects to variations of mussel biomass. Questions of particular interest were whether mussels could be expected to have a major impact on the spring bloom in deep, cold waters (Fahnenstiel et al., 2010; Vanderploeg et al., 2010; Evans et al., 2011) and whether impacts in shallower waters are likely to be greater than in deeper offshore waters (Hecky et al., 2004).
Materials and methods
Model structure and description
The model used in this study is a coupled hydrodynamic and water quality model (ELCOM-CAEDYM, or ELCD) that consists of two interdependent models. One is the 3D Estuary and Lake Computer Model (ELCOM) that is used as the hydrodynamic driver and simulates the Coriolis forcing, advection, mixing, turbulence and the effects of inflows/outflows, in addition to simulating the water body temperature (including surface thermodynamics), salinity, tracers and velocity dynamics (Hodges et al., 2000; Laval et al., 2003). The model has been described and validated in detail in many studies (e.g. Hodges et al., 2000) including Lake Erie (Leon et al., 2005). The second model is the Computational Aquatic Ecosystem Dynamics Model (CAEDYM) that is able to simulate the carbon (C), nitrogen (N), phosphorus (P), dissolved oxygen (DO) and silica (Si) cycles, along with inorganic suspended solids and phytoplankton. In the current study, phytoplankton are represented as five groups to represent some of the functional diversity. A detailed description of the CAEDYM parameterization is given in Leon et al. (2011), where evidence is presented that the model was able to reproduce major features of phytoplankton and nutrient dynamics in Lake Erie.
Mussel sub-model description
The equations for mussel energetics are of the same form as for the clam Tapes philippinarum growing in a temperate estuarine lagoon (Spillman et al., 2008) and are listed in Appendix A—Supplementary Material. The interactions of mussels with other model state variables follow the schematic in Fig. 1. Parameter values were changed to reflect knowledge of Dreissena polymorpha and D. bugensis; where available, parameters suitable to D. bugensis were used (Appendix B in Supplementary Material) as it was expected to be the dominant species in most parts of Lake Erie in our year of simulation, 2002 (Patterson et al., 2005). There is no harvest or stocking of dreissenid mussels so that aspect of the T. philippinarum model was not used. As in Spillman et al. (2008) the model recognizes three size classes (Fig. 1) but differs in treating the classes as distinct cohorts rather than simulating the transfer of biomass among classes by growth. This allows for greater computational efficiency while still permitting use of size-dependent parameters for energetics.
Bivalve biomass (g tissue C) and numbers (density) are simulated concurrently. Dynamics of bivalve biomass (B C j for size class j) reflect the difference between ingestion of phytoplankton and detrital particles (G A + G POC) and losses to respiration (R DIC), excretion (E DOC), and a combined egestion and mortality term (E POC). Bivalve nitrogen and phosphorus concentrations are also explicitly modelled in a similar way (though without a respiration term), by assuming a fixed C:N:P ratio for mussel tissue (Appendix B in Supplementary Material). As a result, simulated excretion of N and P is sensitive to the balance between mussel C, N and P demand and the relative supply of those elements in their food, in contrast to previous approaches that used fixed biomass-specific nutrient excretion rates. Bivalve numbers (B Num j ) within each size class j are adjusted for size-dependent mortality. Reproduction (i.e. gamete production) and larval dynamics are not simulated.
Grazing rate (G) is a function of filtration rate, which is influenced by temperature, bivalve size and seston concentration. Grazing rates in the model are reduced under high food concentrations due to bivalve ingestion limitations and satiety thresholds or in the presence of detrimentally high inorganic loads. Assimilation ratios are used to derive the fraction of filtered food which is egested or excreted (E). A user-defined fraction of ingested food is egested as particulate organic matter in the model, with excretion then calculated dynamically to ensure nutrient homeostasis within the bivalve. Respiration (R) is a function of bivalve size and temperature, with oxygen consumed via respiration calculated by applying a stoichiometric factor associated with respired carbon (Spillman et al., 2008, 2009). Mortality increases with both temperature extremes and low DO.
In CAEDYM, mussels are assumed to have direct interactions (e.g. filtration of particles) only with the bottom vertical grid cell. The particle and nutrient fluxes through grazing, defecation, excretion and mortality interact with the larger elemental balances within CAEDYM (Fig. 1) through exchange with overlying cells. Dissolved and particulate organic matter contributions to the water column by mussels (E POC(B j ), E PON(B j ), E POP(B j ), E DOC(B j ), E DON(B j ) and E DOP(B j ) are summed over the mussel size classes and added to the detrital pools represented by state variables POC, PON, POP, DOC, DON and DOP (Spillman et al., 2008). Similarly, the algal and POM pools have corresponding terms to account for grazing losses. Mussels graze on both algae and detritus. The implications of selective feeding by mussels are a topic of interest to lake ecologists but, for this stage of the modelling, we felt the literature was inadequate to support robust specifications of preference among algal groups so no selective preferences were designated. The implications of this decision will be considered in the discussion section.
Model setup
The coupled ELCD model was run for Lake Erie for 2002, primarily for two main reasons: (i) since the most comprehensive validation data as well data on distribution of biomass and densities of mussels set exists for this period; and, (ii) ELCD results for 2002 have been recently validated against the field observations (Leon et al., 2011).
A 2-km horizontal grid and 40 vertical layers of variable thickness were used to represent the Lake Erie bathymetry as in Leon et al. (2005, 2011). To account for the spatial variability of meteorological conditions across the entire lake it was divided into three sections (west, central and east basins, Fig. 2) and different measured meteorological data from the three stations located in each basin were applied to a given section. The meteorological data for 2002 were retrieved from two sources of archived data, the National Water Research Institute (NWRI) and the NOAA National Data Buoy Center. The lake was simulated for 190 days (April 10 to October 17) with a time step of 5 min.
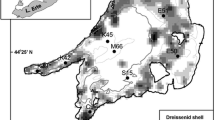
Initial conditions for Lake Erie in respect to physical (e.g. water temperatures), biological (phytoplankton concentrations, major groups of phytoplankton) and chemical parameters (e.g. nutrients, suspended solids, DO, etc.) were the same as in Leon et al. (2011) and derived from observations at some selected stations (Fig. 2; west basin: 965, 966, 968, 969, 970, 971, 973 and 974; central basin: 945; 946, 949, 952, 953, 954, 961 and 962; east basin: 879, 886, 934 and 940). The initial spatial distribution of mussel biomass was estimated using the lake substrate distribution (Haltuch & Berkman, 1998) and a regression model, based on Patterson et al. (2005), that predicts biomass for each defined depth from depth range based on substrate and basin. Three mussel size classes of 0.000874, 0.0131 and 0.046 g shell-free dry weight per individual (equivalent to shell lengths of 5, 15 and 25 mm) were defined and assigned relative abundance of 55, 30 and 15% of total numbers based on information in Patterson et al. (2005). The resulting carbon biomass of the individual size classes was mapped onto the bathymetry of Lake Erie (Fig. 3) using GIS mapping solutions (ArcView 8) and assuming carbon is 45% of shell free dry weight (Walz, 1978). Table 1 summarizes the assigned mussel population levels for the three main basins of the lake.
Model parameters
The configuration and parameters for each of five simulated phytoplankton groups were taken as in Leon et al. (2011). All other biological and chemical parameters were also adopted from Leon et al. (2011). Sediment oxygen demand and nutrient flux parameters were also set based on historical data available that was specific to Lake Erie. Parameters for quagga mussels were taken from literature and are presented in Appendix B—Supplementary Material. Note that once the parameter values were estimated from literature values, or assumed in the case of unknown values, then no further parameter adjustments were made.
Model validation
Data on water temperature and phytoplankton Chl a concentrations in Lake Erie in 2002 for model validation came from several different sources, such as an Environment Canada-University of Waterloo study in the east basin (Depew et al., 2006; North et al., 2012), “Star Database” housed by the National Water Research Institute (NWRI) of Environment Canada, and U.S. E.P.A. survey cruises on Lake Erie. Stations used in the model validation are shown in Fig. 2. To evaluate the ability of our model to predict the observed data we used a combination of graphical techniques and quantitative statistics that included tests for statistical significance (pared t test and linear regression analysis) and calculation of statistical measures of fit. Because statistical measures of fit capture different aspects of model performance, we used simultaneously several commonly used measures with the published ranges of performance ratings for a better and more comprehensive assessment of model performance and comparison between two different models to indicate whether there is a statistically measured improvement.
The selected measures included six different indices: relative error (RE), root mean squared error (RMSE), Nash–Sutcliffe efficiency (NSE), RMSE-observation standard deviation ratio (RSR), percent bias (PBIAS) and a cost function (CF) (Table 2). In the literature, RE values are usually in the range between 0.3 and 0.6 with a median value of 0.4 in the phytoplankton studies using simple biochemical models but RE tends to increase with the model complexity (Arhonditsis & Brett, 2004). RMSE is another frequently used error index in model evaluations. The smaller values of RMSE indicate smaller errors and better model performance with a value of 0 indicating a perfect fit. The NSE is a dimensionless coefficient indicating how well the plot of simulated versus observed data fits the 1:1 line (Moriasi et al., 2007). It ranges between −∞ and 1. Values between 0 and 1 are generally considered as acceptable levels of model performance (Moriasi et al., 2007) but the closer the NSE is to 1, the more accurate the model is. RSR combines an error index and a scaling factor so that it can be applied for comparison of different constituents. The optimal value is 0 that indicates a perfect fit but all values that are ≤0.7 can be considered as acceptable level of model performance (Moriasi et al., 2007). PBIAS measures the average tendency of the model predictions to be smaller or larger than the observations (Gupta et al., 1999). Positive and negative values of PBIAS indicate model underestimation or overestimation bias, respectively. PBIAS has an optimal value of 0 with the low magnitude values indicating accurate predictions. The range of acceptable values for PBIAS depends on the constituent being evaluated. It was recommended that values within ±70% to be considered satisfactory for nutrient simulation studies (nitrogen and phosphorus) (Moriasi et al., 2007). CF is a measure of goodness of fit between model and observations with the following reported performance rating: |CF| < 1 (very good), 1 < |CF| < 2 (good), 2 < |CF| < 3 (reasonable) (Radach & Moll, 2006).
The statistical significance tests included the paired t tests that were conducted using non-transformed data and the linear regression analysis that was performed using the log10-transformed data to comply with the assumption of homoscedasticity. The ordinary least squares (OLS) regressions are good for predictive purposes but they are known to underestimate the slope between two variables. Therefore, the ranged major axis (RMA) regression was also used to uncover the true slope between two variables.
Results
The patterns in predicted mussel dynamics will be the subject of detailed examination in a following paper. In brief, the model predicted that mussel biomass could increase or decrease over the simulation period, depending on the size class of mussel and the lake basin concerned (Table 1). The lake-wide mussel biomass over all size classes decreased by about 30% during the simulation period. While mussel biomass decreased in east basin, it increased in central and west basins (Table 1). The biomass of class 1 increased in all three basins, while biomass for class 2 also increased in west and central basins but decreased in east basin. The biomass of class 3 decreased in west and east basins but was stable in central basin. Lake-wide, the biomass of class 1 increased while two other classes decreased in biomass (Table 1).
The ability of ELCD model to capture the major spatial and seasonal dynamics of temperature and Chl a distribution in Lake Erie has been demonstrated previously (Leon et al., 2011). Predicted and observed temperatures were similar and the seasonal thermocline that forms in offshore locations in central and east basins was evident in the simulated temperatures (e.g. Fig. 4a). Shallower stations that are not normally observed to display pronounced thermal stratification were also predicted to have little vertical temperature structure (e.g. Fig. 5). Lake-wide comparison of the predicted surface and near-bottom temperatures over time and space showed a good agreement with observations (Fig. 6a, b). The statistical measures of fit (Table 3) were all in the acceptable range (Arhonditsis & Brett, 2004) and together with the regression analysis (Table 3), indicated a good ability of the model to reproduce surface and near-bottom temperatures. The model had an average tendency to predict surface temperatures that were 4% cooler than the observations (Table 3; PBIAS = 4.04) and near-bottom temperatures that were 1.2% warmer than the observations (Table 3; PBIAS = −1.19).
Scatter plot of the observed values versus predicted values for lake-wide comparison of surface temperatures (a; depth from 0 to 6 m, N = 324), near-bottom temperatures (b; 0 to 1 m above bottom, N = 324) and Chl a concentrations in the surface mixed layer (depth 0 to 6 m, N = 400; c, d) with c and d showing non-transformed and log10-transformed data, respectively. N is the number of the compared pairs
The model with the added code to simulate mussels continued to provide satisfactory simulations and showed statistically measurable improvements in model performance (Tables 4, 5). For example, smaller SE, RMSE, RSR, CF, larger NSE, as well as PBIAS that was closer to 0 (Table 4). The tests for statistical significance and linear regression analysis also indicated the improvements in new model performance (Tables 4, 5). In the relatively deep and transparent east basin, a deep Chl a maximum is often observed and such features were also evident in simulated Chl a (Fig. 4). The deep Chl a maximum predicted by the model is a result of differential growth, loss and settling, as variations of phytoplankton Chl:C and phytoplankton vertical migration were not simulated. At shallower and better mixed locations, a distinct deep maximum is not usually observed but observed profiles are often non-uniform, with an increasing trend towards the lower depths. The model predicted that there would be vertical variations, although the location and magnitude of Chl a peaks did not always coincide with available observations (Fig. 5). With closer (1–3 m) approach to bottom, the model predicted a pronounced decrease of Chl a at stations with appreciable mussel biomass and relatively well-mixed conditions (Fig. 5). Observed profiles usually did not approach bottom closely enough to clearly resolve such features but, where they did, a near-bottom decrease of Chl a was commonly apparent. As evident from Figs. 4 and 5, snapshot comparisons for individual sites and times indicated variable agreement between simulations and observations with some deviations being observed between model and data. For example, the model underestimated the depth of the deep Chl a maximum by more than 5 m for station 23 (Fig. 4a; June 12) and over-predicted Chl a by almost 100% for station 442 (Fig. 5b; June 10). However, overall predicted Chl a concentrations were in reasonable agreement with the observed values (e.g. lake-wide results; Fig. 6c, d; Tables 4, 5).
For the east basin, observations in 2002 suggested a bimodal seasonal pattern of Chl a, with a spring or early summer maximum, a summer minimum, and intermediate values in late summer and early autumn (Fig. 7a, b). The seasonal pattern of predicted Chl a had a similar bimodal pattern. The pattern was apparent whether mussels were simulated or not, but there was a systematic shift to lower values when mussels were simulated. The predicted spring-early summer maximum was diminished and delayed when mussels were simulated, both for nearshore and offshore zones. For the offshore zone (Fig. 7b), predicted Chl a captured the major seasonal dynamics very well, particularly the early maximum and summer minimum, and agreement was improved when mussels were simulated and predicted values were lower. Agreement between predicted and observed was not as close in the nearshore zone (Fig. 7a), as the model underestimated the early increase (early May) of observed Chl a, and predicted even less Chl a when mussels were simulated, and slightly overestimated Chl a in the summer. Predicted nearshore values for summer through fall were nonetheless closer to observations when mussels were simulated than when they were not.
Time series output of the predicted basin average nearshore (a) and offshore (b) Chl a concentrations with the observed values in east basin, showing model results with mussels OFF and ON. Difference in Chl a between mussels ON and OFF for nearshore and offshore zones in east basin (c). Negative values in c denote lower Chl a for mussels ON results
The most dramatic predicted effect on Chl a in east basin was during the early spring maximum (Fig. 7c), which was diminished by about 2 mg m−3 in both nearshore and offshore zones compared to maximum values of 4–5 mg m−3 in simulations without mussels (Fig. 7a, b). During the summer minimum, values from simulations with mussels were similar to, or even slightly larger than, those from simulations without mussels, but in late summer and fall predicted values with mussels were mostly 0.5–1 mg m−3 lower than without mussels. Despite persistent thermal stratification, the offshore zone was predicted to display lower Chl a in the presence of mussels throughout the late summer and early fall (Fig. 7c).
Lakewide, the predicted Chl a in the surface layer in simulations with mussels showed strong seasonal and spatial dynamics, which are illustrated here with emphasis on the period preceding and during the early season maximum (Fig. 8a, c, e). In early May, predicted Chl a was highest in western parts of the lake when mussels were not simulated. By June 1, highest Chl a was predicted to occur in central basin, especially along the north shore, with high values in the northern nearshore of east basin as well. By July 1, highest values were predicted to be within boundary zone between west and central basins (e.g. Sandusky Bay). Simulations that did not include mussels (results are not shown here but see Fig. 8a–f) produced a generally similar picture of the major spatial and temporal variability of Chl a but some of local maxima predicted with mussels were elevated or even appearing as new (e.g. east basin on June 1) compared to the scenario when mussels were not simulated. These localized areas of elevated phytoplankton Chl a and new maxima were short lived and appeared at places where nutrients were limited (e.g. east basin) meaning that mussel grazing, and therefore, mussel-driven nutrient recycling, can be responsible for that.
Model predictions of the spatial distribution of Chl a with mussels ON in the surface layer at 2 m depth of Lake Erie for May 1 (a), June 1 (c) and July 1 (e). Model predictions of the difference in Chl a (note the scale) in the surface layer at 2 m depth between simulations with mussels ON and mussels OFF for May 1 (b), June 1 (d) and July 1 (f)
The difference in predicted Chl a for the surface layer when mussels were included (Fig. 8b; note the scale) shows that on May 1 the largest decreases were in the east basin, especially in the northern nearshore. Decreases approached 1.0 mg m−3 in parts of east basin, and 0.5 mg m−3 in localized parts of west basin. Central basin showed very little difference and even some patches of increased Chl a. On June 1, very large decreases (up to 4 mg m−3 or more) of Chl a were predicted in the northern nearshore of east basin (Fig. 8d; note the scale). Decreases of 0.5–1 mg m−3 were predicted for much of west basin and parts of central basin. On July 1 (Fig. 8f; note the scale) the largest predicted decreases (1 mg m−3 or more) were in the boundary area between west and central basins but a sizable areas of strongly diminished Chl a occurred along the southern margin of the central basin and in its northwestern corner, where the assigned local mussel biomass was minimal. The east basin continued to show appreciable (>0.5 mg m−3) predicted decreases of Chl a in easterly areas but lesser decreases, and even localized increases, in western parts of the basin.
Basin-average simulated Chl a as well as differences between mussels ON and OFF scenarios provided a picture of the predicted sensitivity to presence or absence of mussels, and to hypothetical variations in the initial mussel biomass (Figs. 9, 10). Compared to east basin, west basin was predicted to experience a smaller decrease of Chl a with mussels included, and the central basin to experience very little decrease. Similar to east basin, predicted decreases in west basin were largest during the early maximum (May and June), negligible throughout much of the summer, and larger again in autumn. The summer period of little or no mussel effect appeared longer in west basin than east. On a basin-average basis, inclusion of mussels in the simulations did not result in any substantial Chl a increases in any basin, although, there were some episodes of Chl a increases in east basin (0.5 mg m−3; mid to late June, Fig. 10a) and smaller increases in central basin (0.3 mg m−3; early June, Fig. 10c). The increases were as much as 20% (east) and 5% (central) of values with mussels off (Fig. 10b, d) but were brief. Unlike east and central basins, west basin did not show any increases in Chl a when mussels were turned on (Fig. 10e). In all basins (Fig. 10b, d, f), there were time periods when the effects of including mussels were nearly proportional to the hypothetical ±50% range of mussel biomass (e.g. east basin: early July to early August; central basin: mid June to mid July; west basin: April to late July). There were also episodes of non-proportional effects, more pronounced in east basin than in any other basin (e.g. east basin: spring to early summer, late summer to fall; central basin: late summer to fall; west basin: late August to mid September). At times, there was little or no predicted mussel impact regardless of the assigned biomass values (e.g. east basin: early August; central basin: end of July; west basin: early to late August), implying that factors other than mussel biomass were limiting the effects of the mussels on the phytoplankton. These episodes coincided with predicted water temperatures exceeding the upper limit for optimal feeding (T max, 18°C) and, in west basin, approached the upper limit for feeding activity (T max, 25°C, Appendix B in Supplementary Material). However, on average, in west and central basins the effects of including mussels were nearly proportional to the hypothetical variations, ±50% range, in mussel biomass (Table 6) while the effect was non-proportional in the east basin.
Time series output of the predicted basin average Chl a concentrations in east (a), central (b) and west (c) basins showing a sensitivity of phytoplankton to initial mussel biomass and densities: comparison of 4 scenarios (mussels OFF, and mussels ON with 100, 150 and 50% of best estimate for initial biomass)
Magnitude of the predictive change in Chl a for different mussel biomass scenarios (100, 50, and 150% of best estimate for initial biomass) relative to the base scenario with mussels OFF, expressed in absolute (mg m−3) and relative (%) values for each basin: east basin (a, b), central basin (c, d) and west basin (e, f)
Simulations with and without mussels indicated that early diatoms, flagellates, and the Others group were the major phytoplankton groups while late diatoms were of appreciable but lesser importance (Fig. 11). Cyanobacteria reached their greatest abundance in west basin but even there were <0.5 mg m−3 Chl a equivalent. The dominant patterns of seasonal succession were similar with or without mussels but early diatoms, which dominated the spring bloom, were particularly diminished when mussels were included, especially in east basin. In west basin, flagellates and the Others group accounted for much of the predicted decrease of total Chl a when mussels were turned on. Both early and late diatoms were predicted to have brief periods of enhanced biomass when mussels were turned on while the Others group at the selected west basin station showed short period oscillations between increased and decreased biomass for much of the summer. Although not shown in Fig. 11, cyanobacteria in west basin during late summer were predicted to be more abundant when mussels were turned on, but the effect was small (0.5 mg m−3 Chl a equivalent or less).
Model predictions of algal group dynamics at 2 m depth with mussels ON in east basin, station 23 (a); west basin, station er91 (c) and lake-wide average (e). Corresponding differences in algal groups between mussels ON and OFF, as percent of total Chl a in simulations with mussels OFF, are shown in b, d and f. Negative percentages indicate decreased abundances when mussels are ON. Cyanobacteria were predicted to remain <0.5 mgChl a m−3 and are not plotted
Discussion
While sufficiently complex to require simulation run times of a week or so, ELCD as used here is still a highly simplified and incomplete representation of Lake Erie but it has been shown to capture some of the major features of Chl a, N and P dynamics (Leon et al., 2011). In the present study, we extend the previous Lake Erie ELCD model (Leon et al., 2011) to include mussels as a state variable. All previously published studies either simulated mussels with a constant biomass or only accounted for their grazing effect. To the best of our knowledge, the present study presents the first model that includes mussels as a state variable with their own dynamics (Table 1). An analysis of model predictions concerning mussel energetics and implications for nutrient cycling and algal productivity in the lake is in preparation and is the subject of a following paper. Here we suggest that the predicted mussel dynamics were in a reasonable range, forecasting neither a collapse nor an explosion of the population. The mussel biomass increased in west and central basins (Table 1) but decreased in east basin meaning that the mussel growth was negative there. A recent study on mussels in Lake Ontario (Malkin et al., 2012) shows that mussel growth may indeed be limited over much of the period we simulate, at least in a part of Lake Ontario that is comparable to east basin of Lake Erie, and can be negative. Lacking any published measurements of mussel growth on the bottom in Lake Erie, particularly for 2002, the model results are within a reasonable range for seasonal gain and loss of biomass.
In this study, we focus on patterns in phytoplankton biomass and note that, with addition of mussels, the predictions of Chl a spatial dynamics showed an improved agreement with observations (Tables 4, 5). It can be that the model with mussels ON shows better statistics than the model with mussels OFF mainly because phytoplankton abundance without mussels is overestimated in the summer. However, any factor increasing mortality of phytoplankton that is similar to mussel grazing, e.g. zooplankton grazing, would result in a similar improvement. Our model with both mussels OFF and ON did include a mortality term for the effect of zooplankton grazing but it will not properly account for the seasonal changes in zooplankton abundance. Nonetheless, the improvement in the statistics supports the idea that representation of the mussels may be required for accurate simulation of phytoplankton abundance. In general, model predictions were much closer to observations when mussels were turned on, the main exception was the spring Chl a increase in the east basin nearshore. Observations suggested the spring increase occurred earlier than the model predicted, and the disparity worsened when mussels were turned on. It is likely that spring warming and Chl a increases at the beginning of our simulation period had advanced further in nearshore than offshore waters (Holland & Kay, 2003) but we had only offshore data for initialization. The earliest samples did not suggest a strong bias but the near-exponential Chl a dynamics in spring (Fig. 7) would lend great weight to even moderate initialization errors.
The reproduction of vertical Chl a concentrations was also imperfect. It might be that the model is still unable to capture the full dynamics of such a large and dynamic lake as Lake Erie where conditions at any fixed station are at constant change because of the water movement, strong currents, upwelling and downwelling events. For example, Fig. 5a shows that two profiles taken at the same station within 1 h showed a remarkable difference of almost 50% in Chl a. Comparisons with observations are also complicated by the fact that Chl a:C ratios are fixed for each algal group in the model, so that model variations of Chl a mainly reflect biomass changes. By comparison, field observations of Chl a are affected by photoacclimation and other physiological processes that can have a strong influence on the spatial dynamics including vertical distributions (White & Matsumoto, 2012). There is scope for including photoacclimation in ELCD (Hipsey & Hamilton, 2008) so future model applications could be more refined. Other desirable refinements could include explicit modelling of zooplankton (e.g. Zhang et al., 2008, Boegman et al., 2008b), although the model as used here did include temperature- and oxygen-dependent phytoplankton mortality terms, and associated dissolved and particulate nutrient cycling terms, that can capture some of the expected zooplankton impacts.
The desirability as mussel (or zooplankton) food of algal groups can also be varied, and in our application of ELCD the group-specific mortality terms did vary to reflect presumed grazer preferences. For example, the cyanobacteria group, configured to represent the larger colonial forms, was assigned a lower mortality constant to reflect lower edibility, whereas flagellates were assigned a higher mortality (Leon et al., 2011). Some cyanobacteria, notably Microcystis, are often rejected as food by dreissenid mussels, particularly when they occur in colonial form, although selectivity can be variable (Vanderploeg et al., 2001, 2009; Fishman et al., 2009). Ecosystem models have supported a role for mussel grazing in promoting increased abundance of inedible groups, particularly Microcystis (Noonburg et al., 2003; Bierman et al., 2005; Zhang et al., 2008; Fishman et al., 2009). In the current application we did not feel we had sufficient information to properly assign explicit feeding preferences for mussels, although information to support such decisions is growing (e.g. Naddafi et al., 2007) and could allow future model refinement. In our simulations, cyanobacteria remained low in biomass (<0.5 mgChl a m−3) even with mussels turned on, but they did increase by nearly 50% in summer at stations in west basin near higher mussel biomass areas (e.g. er91, Fig. 2). The low sinking rates assigned to cyanobacteria (Leon et al., 2011) may have lessened their vulnerability to mussel grazing (Zhang et al., 2008) while nutrient competition from other groups was alleviated. The west basin of Lake Erie has been famous for its late summer cyanobacterial blooms in recent years (Millie et al., 2009) but in our simulation year (2002) cyanobacteria did not appear to form a bloom that was extensive enough to elevate average summer cyanobacteria above 0.5 mgChl a m−3 (Ghadouani & Smith, 2005).
Comparing model predictions of mussel impacts with evidence from field studies is greatly complicated by uncertainties in the observational estimates but it is of interest to know whether model predictions are at all reasonable. Although we simulated 2002, the sudden appearance of mussels in the model (with no attempt to account for any of the coincident and longer-term benthic community changes that have been observed in invaded lakes, e.g. Karatayev et al., 1997; Patterson et al., 2005; Higgins & Vander Zanden, 2010; Gergs et al., 2011), should be most comparable to the initial effects of the rapid colonization of the lake in 1989–1993. In east basin, our assigned initial mussel biomass of 11.6 gC m−2 was somewhat higher than the reported average of 7.7 for the immediate post-colonization years of 1992–1993 (Jarvis et al., 2000; with unit conversions according to Patterson et al. 2005, and assuming C is 45% of dry weight). The model predicted a decrease of spring-summer average Chl a of 22%, within the range of 15–25% inferred by field studies (Charlton et al., 1999; Makarewicz et al., 1999; Barbiero & Tuchman, 2004; results from latter two sources for summer only as their spring data were for dates earlier than simulated here).
By contrast, the model predicted only a 6.9% decrease of Chl a in west basin, whereas the field studies suggested decreases of 10–46% upon mussel colonization. Our model assumed a low average mussel biomass in west basin (0.32 gC m−2) compared to a survey estimate of 4.8 gC m−2 for 1992–1993 (converted from Jarvis et al., 2000). Our biomass estimates for west basin and central basin (where simulated impacts were again lower than suggested by field studies) were likely too low, particularly in west basin (see Patterson et al., 2005), but it is hard to know what a truly accurate value would be. For example, the average mussel biomass in west basin was reported as 0.034 gC m−2 in 1992, 9.6 in 1993, and 0.085 in 1998 (Jarvis et al., 2000). Surveys reveal strong variability of mussel biomass even within basins and substrate categories (e.g. Patterson et al., 2005) and both physical and biotic processes can have quick and dramatic effects on mussel population sizes (e.g. Werner et al., 2005). With a nearly direct proportionality between assigned mussel biomass and predicted degree of impact in both west and central basins (Table 6), predicted impacts could probably be reconciled with those inferred from field studies by adjusting the assigned mussel biomass within the observed range. To explore the model ability to reasonably predict the phytoplankton biomass in central and west basins with much higher initial biomass observed in the 1992–1993 survey of Jarvis et al. (2000), we performed an extra sensitivity test. The initial values in west and central basins were assigned to be the average biomass values observed in 1992 and 1993 (Jarvis et al., 2000; west basin: 4.8 gC m−2; central basin: 2.9 gC m−2), 15 times larger those used in the present study. The new results indicated that the model was still able to reproduce reasonable Chl a dynamics in both basins. Compared to west basin, central basin experienced smaller decreases in Chl a. The model predicted average decreases in Chl a (expressed as a % relative to the base scenario with mussels OFF) of 19% in central basin and 43% in west basin. Such changes are more consistent with the decreases inferred from studies of post-colonization years (e.g. Makarewicz et al., 1999). There is uncertainty in the model parameters for mussel energetics that could also affect predictions, but the effect of uncertainty in mussel biomass is obviously great. Strong tests of models and predictions of possible future conditions demand better knowledge of mussel biomass distributions and their controlling factors (cf Zhang et al., 2008). Progress in identifying physico-chemical (Naddafi et al., 2011) as well as biotic (Patterson et al., 2005; Werner et al., 2005; Naddafi et al., 2010) factors associated with dreissenid mussel population dynamics is very important to success of ecosystem modelling efforts.
The model also predicts, as might be expected but has not to date been shown with 2D horizontal resolution, that the impact of mussels on surface layer phytoplankton is highly variable in space and time (Fig. 8b, d, f). Impacts are related to the assigned mussel biomass distribution (Fig. 3) but only loosely, because of the strong effects of water mass advection. The strong spatio-temporal variability poses challenges to observational programs that have to characterize basin characteristics with limited numbers of stations and observation dates. It is easy to understand from Fig. 8 how two studies, using somewhat different sampling dates and locations, could reach different conclusions (e.g. 9.8 vs 46% decrease of west basin summer Chl a upon mussel colonization; Makarewicz et al., 1999; Barbiero & Tuchman, 2004). However, a model such as the current one could be used to better choose stations and/or interpret results from them. For example, simulations suggest that stations along the south-central coast of the central basin, where assigned mussel biomass was low, may frequently display depressions of Chl a resulting from effects of “upstream” mussels in the southwest central, and even west, basins.
The most sophisticated previous modelling study of mussel effects (the 2D Lake Erie model of Zhang et al., 2008) concluded that, while mussels in the years 1997–1999 consumed an appreciable fraction of the phytoplankton, the biomass of non-diatom edible algae biomass nonetheless increased as mussel biomass assigned in the model increased. Biomass of diatoms (also edible) either increased (west basin) or showed decreases far less than proportional to the mussel biomass increases (central and east basins). It was suggested that concentration boundary layers [which the hydrodynamic models of Zhang et al. (2008) and Boegman et al. (2008b) were able to capture] intensified as mussel biomass increased, thus constraining mussel grazing rates, while excreted nutrients were still able to diffuse upward and support faster phytoplankton growth. This mechanism was proposed to explain why simulated impacts on phytoplankton biomass were so much less, or even opposite to, those suggested by simpler previous modelling studies (MacIsaac et al., 1999; Noonburg et al., 2003; Bierman et al., 2005).
Nutrient excretion by dreissenid mussels is an important process (Conroy et al., 2005b; Naddafi et al., 2007, 2009) and can reasonably be expected to stimulate phytoplankton growth. The current model, as in Zhang et al. (2008) and Boegman et al. (2008b), did predict higher growth rates in the presence of mussels. However, we found no evidence that increased mussel biomass would promote a sustained increase of edible algae groups or of total biomass. Increased mussel biomass gave decreased average biomass of phytoplankton in each basin (Table 6), including non-diatom edible algae (our “flagellate” and “others” groups). Enhancements of early and late diatoms with inclusion of mussels (Fig. 11) were ephemeral and small compared to the decreases of phytoplankton biomass. It may be important that feeding and nutrient excretion rates are coupled in our mussel model (Spillman et al., 2008). Excretion of a nutrient will decrease as the intake of the nutrient through grazing decreases, which seems consistent with observations that mussels grazing on P-limited phytoplankton excrete less P and can actually raise C:P ratios of seston (Naddafi et al., 2008). By comparison, mussel excretion rates were assumed to continue unabated when phenomena such as concentration boundary layers or poor food stoichiometry limited nutrient intake in the model of Zhang et al. (2008). Further sophistication of ecosystem models for mussels, for example to better allow for the flexibility of mussel tissue nutrient stoichiometry (Naddafi et al., 2009) and better characterize biodeposition processes (Gergs et al., 2009) is desirable, but the relatively realistic mussel model used here suggested that sustained elevation of edible phytoplankton biomass through mussel activity is unlikely in Lake Erie.
The nearshore shunt (Hecky et al., 2004) predicts a greater effect of mussels on plankton in nearshore than offshore zones, partly on the assumption that mussel biomass will often be greater in the more hospitable (for mussels) nearshore habitat. The spread of quagga mussels into the profundal zone of the Laurentian Great Lakes (e.g. Nalepa et al., 2010), including Lake Erie (Patterson et al., 2005), has greatly affected this assumption. However, the shallower waters, absence of strong and persistent thermal stratification and generally favourable temperature and oxygen conditions should still permit nearshore mussels to exert relatively more impact on the plankton in systems with high nearshore mussel biomass such as east basin Erie. The model did predict a greater impact of mussels on Chl a in nearshore than offshore east basin. Average surface layer Chl a was 2.4 mg m−3 in both zones with mussels off, compared to 1.7 nearshore and 2.0 offshore with mussels on. Observational studies in east basin have documented lower Chl a and primary production in nearshore than offshore east basin, including in the year (2002) simulated here (Depew et al., 2006; North et al., 2012). Phytoplankton nutrient physiology can also differ in a pattern consistent with greater effects of mussel-mediated nutrient cycling in the nearshore (North et al., 2012). While model and observations both lend support to nearshore shunt effects on phytoplankton in east basin, however, the magnitude of nearshore-offshore differences was not large compared to seasonal and inter-basin differences. The spatial dynamics exemplified by the model snapshots given here (Fig. 8) show how easily incipient differences in surface layer characteristics due to local mussel effects can be altered by the dynamic circulation patterns, lessening differences between zones and complicating interpretation of field surveys.
Perhaps the more striking outcome of the nearshore-offshore comparisons was the prediction of strong mussel impacts on the offshore spring bloom (Fig. 7). In unstratified conditions mussels will in theory have good access to phytoplankton but the offshore water column is deep and, in spring, temperatures are low and may be strongly limiting. Temperature relationships of mussels, like most animals, are complex (McMahon, 1996) and mussel characteristics can vary among habitats within a lake (Vanderploeg et al., 2010). We had to adopt a single set of physiological parameters, which we based on what is known, or guessed, about quagga mussels. Temperature preferences were shifted lower than for zebra mussels but feeding and growth rates would still be temperature-limited below 8°C (Appendix B in Supplementary Material). This might be expected to limit impacts on the spring phytoplankton yet the model predicted large decreases when mussels were turned on (Figs. 7, 10a, b). Profundal quagga mussels may be capable of maintaining higher rates of activity at very low temperatures than we assumed in the model (Vanderploeg et al., 2010) so we may have underestimated the potential impact on the early stage spring bloom. Field studies have indeed suggested that mussels may have diminished the spring diatoms in east basin Lake Erie even more than our model would suggest (Barbiero et al., 2006).
The current model nonetheless supports the inference (Vanderploeg et al., 2010) that offshore mussels can have major impacts on spring phytoplankton despite the low temperatures, deep water column, and typically modest current speeds near bottom in deep water habitats. In Lake Michigan, negative effects of offshore mussels on Chl a and primary production in the surface layer appear to be minimal during summer stratification, consistent with the expected isolation of mussels from the surface layer (Fahnenstiel et al., 2010). However, our simulations predicted that mussels cause surface layer Chl a in offshore east basin during summer stratification to decrease, though less so than during spring mixing. Two factors likely contribute to the ability of mussels to influence surface layer Chl a in deep offshore waters of Lake Erie. Unlike many areas of Lake Michigan (Fahnenstiel et al., 2010; Nalepa et al., 2010), east basin of Lake Erie has extensive shallow areas with favourable substrates for mussels and a high biomass, while the small (for a Great Lake) size of the basin allows advection between nearshore and offshore to play a large role. As a result, nearshore mussels can diminish Chl a in the offshore much as mussels in the “mid-depth sink” of Lake Michigan (Vanderploeg et al., 2010) are proposed to affect conditions further offshore. Unlike the mussels of the mid-depth sink, however, the nearshore mussels of east basin are mostly in good contact with the surface layer throughout the summer. To quantify the relative importance of two effects, the advected nearshore water from the areas of nearshore mussels and deep-water mussels, on the observed reductions in the surface layer Chl a in the east basin offshore zone, we performed an additional simulation with the nearshore mussels (depth <20 m) switched off. The results revealed that for the reduction in the surface Chl a in deep offshore waters (depth ≥20 m) both effects were important. During the spring time and early summer (April 10 to June 15 inclusive), the advected water from the areas of neashore mussels was responsible for 60% of the observed reduction in the offshore Chl a, while the contribution of the deep-water mussels to the overall effect was 40%. This means that both nearshore and offshore mussels have sizable impacts on the surface layer Chl a in the offshore zone of Lake Erie.
Work in progress is examining model predictions of mussel energetics and phytoplankton processes to obtain more mechanistic insight into the effects on phytoplankton and nutrient cycling. The predicted effects on phytoplankton biomass were not always proportional to the assigned mussel biomass. Re-filtration in crowded natural populations of mussels (Yu & Culver, 1999) and formation of concentration boundary layers (e.g. Ackerman et al., 2001) may limit the ability of additional mussel biomass to consume phytoplankton, as suggested in previous modelling studies (Boegman et al., 2008b; Zhang et al., 2008). There may be additional physiological processes of importance. For example, predicted mussel impacts in west basin were very small and insensitive to mussel biomass in summer, a time when water temperatures may be supra-optimal for quagga and even zebra mussels (Appendix B in Supplementary Material; McMahon, 1996). The nutrient stoichiometry of seston in Lake Erie varies (e.g. North et al., 2012) and may be expected to influence mussel growth and excretion rates (Naddafi et al., 2008). Phytoplankton growth and biomass should be altered in turn (Zhang et al., 2008), depending partly on the flexibility of mussel tissue stoichiometry (Naddafi et al., 2009). A better understanding of such processes could help inform our understanding of the role of mussels in issues of oligotrophication (Evans et al., 2011; Barbiero et al., 2012) and harmful algal blooms (Vanderploeg et al., 2001; Millie et al., 2009), particularly in the complex biological and hydrodynamic setting of large lakes.
References
Ackerman, J. D., M. R. Loewen & P. F. Hamblin, 2001. Benthic pelagic coupling over a zebra mussel reef in western Lake Erie. Limnology and Oceanography 46: 892–904.
Arhonditsis, G. B. & M. T. Brett, 2004. Evaluation of the current state of mechanistic aquatic biogeochemical modeling. Marine Ecology Progress Series 271: 13–26.
Arnott, D. L. & M. J. Vanni, 1996. Nitrogen and phosphorus recycling by the zebra mussel (Dreissena polymorpha) in the western basin of Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 53: 646–659.
Barbiero, R. P. & M. L. Tuchman, 2004. Long-term dreissenid impacts in water clarity in Lake Erie. Journal of Great Lakes Research 30: 557–565.
Barbiero, R. P., D. C. Rockwell, G. J. Warren & M. L. Tuchman, 2006. Changes in spring phytoplankton communities and nutrient dynamics in the eastern basin of Lake Erie since the invasion of Dreissena spp. Canadian Journal of Fisheries and Aquatic Sciences 63: 1549–1563.
Barbiero, R. P., B. M. Lesht & G. J. Warren, 2012. Convergence of trophic state and the lower food web in Lakes Huron, Michigan and Superior. Journal of Great Lakes Research 38: 368–380.
Bierman, V. J. Jr., J. Kaur, J. V. DePinto, T. J. Feist & D. W. Dilks, 2005. Modeling the role of zebra mussels in the proliferation of blue-green algae in Saginaw Bay, Lake Huron. Journal of Great Lakes Research 31: 32–55.
Bocaniov, S. A. & R. E. H. Smith, 2009. Plankton metabolic balance at the margins of very large lakes: temporal variability and evidence for dominance of autochthonous processes. Freshwater Biology 54: 345–362.
Boegman, L., M. R. Loewen, P. F. Hamblin & D. A. Culver, 2008a. Vertical mixing and weak stratification over zebra mussel colonies in western Lake Erie. Limnology and Oceanography 53: 1093–1110.
Boegman, L., M. R. Loewen, D. A. Culver, P. F. Hamblin & M. M. Charlton, 2008b. Spatial–dynamic modeling of algal biomass in Lake Erie: relative impacts of dreissenid mussels and nutrient loads. Journal of Environmental Engineering 134: 456–468.
Charlton, M. N., M. N. LeSage & J. E. Milne, 1999. Lake Erie in transition: the 1990’s. In Munawar, M., T. Edsall & I. F. Munawar (eds), State of Lake Erie: Past. Present and Future. Ecovision World Monograph series. Backhuys Publishers, Leiden: 97–123.
Conroy, J. D., D. D. Kane, D. M. Dolan, W. J. Edward, M. N. Charlton & D. A. Culver, 2005a. Temporal trends in Lake Erie plankton biomass: roles of external phosphorus loading and dreissenid mussels. Journal of Great Lakes Research 31(Suppl. 2): 89–110.
Conroy, J. D., W. J. Edwards, R. A. Pontius, D. D. Kane, H. Y. Zhang, J. F. Shea, J. N. Richey & D. A. Culver, 2005b. Soluble nitrogen and phosphorus excretion of exotic freshwater mussels (Dreissena sp.): potential impacts for nutrient remineralisation in western Lake Erie. Freshwater Biology 50: 1146–1162.
Depew, D. C., S. J. Guildford & R. E. H. Smith, 2006. Nearshore-offshore comparison of chlorophyll-a and phytoplankton production in the dreissenid-colonized eastern basin of Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 63: 1115–1129.
Evans, M. A., G. A. Fahnenstiel & D. Scavia, 2011. Incidental oligotrophication of North American Great Lakes. Environmental Science and Technology 45: 3297–3303.
Fahnenstiel, G. L., T. B. Bridgeman, G. A. Lang, M. J. McCormick & T. F. Nalepa, 1995. Phytoplankton productivity in Saginaw Bay, Lake Huron: effects of zebra mussel (Dreissena polymorpha) colonization. Journal of Great Lakes Research 21: 465–475.
Fahnenstiel, G. L., S. Pothoven, H. Vanderploeg, D. Klarer, T. Nalepa & D. Scavia, 2010. Recent changes in primary production and phytoplankton in the offshore region of southeastern Lake Michigan. Journal of Great Lakes Research 36(Suppl. 3): 20–29.
Fishman, D. B., S. A. Adlerstein, H. A. Vanderploeg, G. L. Fahnenstiel & D. Scavia, 2009. Causes of phytoplankton changes in Saginaw Bay, Lake Huron, during the zebra mussel invasion. Journal of Great Lakes Research 35: 482–495.
Gergs, R., K. Rinke & K.-O. Rothhaupt, 2009. Zebra mussels mediate benthic–pelagic coupling by biodeposition and changing detrital stoichiometry. Freshwater Biology 54: 1379–1391.
Gergs, R., J. Grey & K.-O. Rothhaupt, 2011. Temporal variations in zebra mussel (Dreissena polymorpha) density structure the benthic food web and community composition. Biological Invasions 13: 2727–2738.
Ghadouani, A. & R. E. H. Smith, 2005. Phytoplankton distribution in Lake Erie as assessed by a new in situ spectrofluorometric technique. Journal of Great Lakes Research 31(Suppl. 2): 154–167.
Goedkoop, W., R. Naddafi & U. Grandin, 2011. Retention of N and P by zebra mussels (Dreissena polymorpha) and its quantitative role in the nutrient budget of eutrophic Lake Ekoln, Sweden. Biological Invasions 13: 1077–1086.
Gupta, H. V., S. Sorooshian & P. O. Yapo, 1999. Status of automatic calibration for hydrologic models: comparison with multilevel expert calibration. Journal of Hydrologic Engineering 4(2): 135–143.
Haltuch, M. E. & Berkman, P. A. 1998. The Lake Erie Geographic Information System: Bathymetry, Substrates and Mussels. Ohio State University, Ohio SeaGrant College Program, Publication number OHSU-GS-20.
Haltuch, M. A., P. A. Berkman & D. W. Garton, 2000. Geographic information system (GIS) analysis of an ecosystem invasion: exotic mussels in Lake Erie. Limnology and Oceanography 45: 1778–1787.
Hecky, R. E., R. E. H. Smith, D. R. Barton, S. J. Guildford, W. D. Taylor, M. N. Charlton & T. Howell, 2004. The nearshore phosphorus shunt: a consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 61: 1285–1293.
Higgins, S. N. & M. J. Vander Zanden, 2010. What a difference a species makes: a meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecological Monographs 80: 179–196.
Higgins, S. M., M. J. VanderZanden, L. N. Joppa & Y. Vadeboncoeur, 2011. The effects of dreissenid invasions on chlorophyll and the chlorophyll:total phosphorus ratio in north temperate lakes. Canadian Journal of Fisheries and Aquatic Sciences 68: 319–329.
Hipsey, M. R. & D. P. Hamilton, 2008. Computational aquatic ecosystems dynamics model: CAEDYM v3 Science Manual. Centre for Water Research Report, University of Western Australia.
Hodges, B. R., J. Imberger, A. Saggio & K. Winters, 2000. Modeling basin scale waves in a stratified lake. Limnology and Oceanography 45: 1603–1620.
Holland, P. R. & A. Kay, 2003. A review of the physics and ecological implications of the thermal bar circulation. Limnologica 33: 153–162.
Jarvis, P., J. Dow, R. Dermott & R. Bonnell, 2000. Zebra (Dreissena polymorpha) and quagga mussel (Dreissena bugensis) distribution and density in Lake Erie, 1992–1998. Canadian Technical Report of Fisheries and Aquatic Sciences No. 2304, Burlington, Ontario, Canada: 46 pp.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 1997. The effects of Dreissena polymorpha (Pallas) invasion on aquatic communities in Eastern Europe. Journal of Shellfish Research 16: 187–203.
Laval, B., J. Imberger, B. R. Hodges & R. Stocker, 2003. Modeling circulation in lakes: spatial and temporal variations. Limnology and Oceanography 48: 983–994.
Leon, L. F., J. Imberger, R. E. H. Smith, D. Lam & W. Schertzer, 2005. Modeling as a tool for nutrient management in Lake Erie. Journal of Great Lakes Research 31: 309–318.
Leon, L. F., R. E. H. Smith, M. R. Hipsey, S. A. Bocaniov, S. N. Higgins, R. E. Hecky, J. P. Antenucci, J. A. Imberger & S. J. Guildford, 2011. Application of a 3D hydrodynamic-biological model for seasonal and spatial dynamics of water quality and phytoplankton in Lake Erie. Journal of Great Lakes Research 37: 41–53.
MacIsaac, H. J., O. E. Johansson, J. Ye, W. G. Sprules, J. H. Leach, J. A. McCorquodale & I. A. Grigorovich, 1999. Filtering impacts of an introduced bivalve (Dreissena polymorpha) in a shallow lake: application of a hydrodynamic model. Ecosystems 2: 338–350.
Makarewicz, J. C., T. W. Lewis & P. Bertram, 1999. Phytoplankton composition and biomass in the offshore waters of Lake Erie: pre- and post-Dreissena introduction (1983–1993). Journal of Great Lakes Research 25: 135–148.
Malkin, S. Y., G. M. Silsbe, R. E. H. Smith & E. T. Howell, 2012. A deep chlorophyll maximum nourishes benthic filter feeders in the coastal zone of a large clear lake. Limnology and Oceanography 57: 735–748.
McMahon, R. F., 1996. The physiological ecology of the zebra mussel, Dreissena polymorpha, in North America and Europe. American Zoologist 36: 339–363.
Millie, D. F., G. L. Fahnenstiel, J. D. Bressie, R. J. Pigg, R. R. Rediske, D. M. Klarer, P. A. Tester & R. W. Litaker, 2009. Late summer phytoplankton in western Lake Erie (Laurentian Great Lakes): bloom distributions, toxicity and environmental influences. Aquatic Ecology 43: 915–934.
Moriasi, D. N., J. G. Arnold, M. W. Van Liew, R. L. Bingner, R. D. Harmel & T. L. Veith, 2007. Model evaluation guidelines for systematic quantification of accuracy in watershed simulations. Transactions of the ASABE 50: 885–900.
Naddafi, R., K. Pettersson & P. Eklöv, 2007. The effect of seasonal variation in selective feeding by zebra mussels (Dreissenia polymorpha) on phytoplankton community composition. Freshwater Biology 52: 823–842.
Naddafi, R., K. Pettersson & P. Eklöv, 2008. Effects of the zebra mussel, an exotic freshwater species, on seston stoichiometry. Limnology and Oceanography 53: 1973–1987.
Naddafi, R., P. Eklöv & K. Pettersson, 2009. Stoichiometric constraints do not limit successful invaders: zebra mussels in Swedish lakes. PLoS ONE 4(4): e5345.
Naddafi, R., K. Pettersson & P. Eklöv, 2010. Predation and physical environment structure the density and population size structure of zebra mussels. Journal of the North American Benthological Society 29: 444–453.
Naddafi, R., T. Blenckner, P. Eklöv & K. Pettersson, 2011. Physical and chemical properties determine zebra mussel invasion success in lakes. Hydrobiologia 669: 227–236.
Nalepa, T. F., D. L. Fanslow & S. A. Pothoven, 2010. Recent changes in density, biomass, recruitment, size structure and nutritional state of Dreissena populations in southern Lake Michigan. Journal of Great Lakes Research 36(Suppl. 3): 5–19.
Nicholls, K. H., G. J. Hopkins & S. J. Standke, 1999. Reduced chlorophyll to phosphorus ratios in nearshore Great Lakes waters coincide with the establishment of dreissenid mussels. Canadian Journal of Fisheries and Aquatic Sciences 56: 153–161.
Noonburg, E. G., B. J. Shuter & P. A. Abrams, 2003. Indirect effects of zebra mussels (Dreissena polymorpha) on the planktonic food web. Canadian Journal of Fisheries and Aquatic Sciences 60: 1353–1368.
North, R. L., R. E. H. Smith, R. E. Hecky, D. C. Depew, L. F. Leon, M. N. Charlton & S. J. Guildford, 2012. Distribution of seston and nutrient concentrations in the eastern basin of Lake Erie pre- and post-dreissenid mussel invasion. Journal of Great Lakes Research 38: 463–476.
Patterson, M. W. R., J. H. Ciborowski & D. R. Barton, 2005. The distribution and abundance of Dreissena species (Dreissenidae) in Lake Erie, 2002. Journal of Great Lakes Research 31(Suppl. 2): 223–237.
Radach, G. & A. Moll, 2006. Review of three-dimensional ecological modelling related to the North Sea shelf system. Part II: Model validation and data needs. Oceanography and Marine Biology – An Annual Review 44: 1–60.
Rao, Y. R. & D. J. Schwab, 2007. Transport and mixing between the coastal and offshore waters in the Great Lakes: a review. Journal of Great Lakes Research 33: 202–218.
Smith, R. E. H., V. P. Hiriart-Baer, S. N. Higgins, S. J. Guildford & M. N. Charlton, 2005. Planktonic primary production in the offshore waters of dreissenid-infested Lake Erie in 1996. Journal of Great Lakes Research 31: 50–62.
Spillman, C. M., D. P. Hamilton, M. R. Hipsey & J. Imberger, 2008. A spatially resolved model of seasonal variations in phytoplankton and clam (Tapes philippinarum) biomass in Barbamarco Lagoon, Italy. Estuarine, Coastal and Shelf Science 79: 187–203.
Spillman, C. M., D. P. Hamilton & J. Imberger, 2009. Management strategies to optimise sustainable clam (Tapes philippinarum) harvest in Barbamarco Lagoon, Italy. Estuarine Coastal and Shelf Science 81: 267–278.
Vanderploeg, H. A., J. R. Liebig, W. W. Carmichael, M. A. Agy, T. H. Johengen, G. L. Fahnenstiel & T. F. Nalepa, 2001. Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 58: 1208–1221.
Vanderploeg, H. A., T. H. Johengen & J. R. Leibig, 2009. Feedback between zebra mussel selective feeding and algal composition affects mussel condition: did the regime changer pay a price for its success? Freshwater Biology 54: 47–63.
Vanderploeg, H. A., J. R. Liebig, T. F. Nalepa, G. L. Fahnenstiel & S. A. Pothoven, 2010. Dreissena and the disappearance of the spring phytoplankton bloom in Lake Michigan. Journal of Great Lakes Research 36(Suppl. 3): 50–59.
Walz, N., 1978. The energy balance of the freshwater mussel Dreissena polymorpha Pallas in laboratory experiments and in Lake Constance. I. Pattern of activity, feeding and assimilation efficiency. Archiv für Hydrobiologie, Supplement 55: 83–105.
Werner, S., M. Mörtl, H.-G. Bauer & K.-O. Rothhaupt, 2005. Strong impact of wintering waterbirds on zebra mussel (Dreissena polymorpha) populations at Lake Constance, Germany. Freshwater Biology 50: 1412–1426.
White, B. & K. Matsumoto, 2012. Causal mechanisms of the deep chlorophyll maximum in Lake Superior: a numerical modeling investigation. Journal of Great Lakes Research 38: 504–513.
Yu, N. & D. A. Culver, 1999. Estimating the effective clearance rate and refiltration by zebra mussels, Dreissena polymorpha, in a stratified reservoir. Freshwater Biology 41: 481–492.
Zhang, H., D. A. Culver & L. Boegman, 2008. A two-dimensional ecological model of Lake Erie: application to estimate dreissenid impacts on large lake plankton populations. Ecological Modelling 214: 219–241.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: D. Straile, D. Gerdeaux, D. M. Livingstone, P. Nõges, F. Peeters & K.-O. Rothhaupt / European Large Lakes III. Large lakes under changing environmental conditions
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bocaniov, S.A., Smith, R.E.H., Spillman, C.M. et al. The nearshore shunt and the decline of the phytoplankton spring bloom in the Laurentian Great Lakes: insights from a three-dimensional lake model. Hydrobiologia 731, 151–172 (2014). https://doi.org/10.1007/s10750-013-1642-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1642-2