Abstract
Water level fluctuation by artificial drawdown is one management activity that has the potential to control macrophyte growth, but there is little knowledge of how this operational procedure affects other biotic components of the ecosystem. This study investigated zooplankton dynamics in response to artificial drawdown over a short timeframe (13 days) in a Brazilian reservoir, by examining the impact on zooplankton communities in two shallow lakes (Lake Pedra Branca-LPB, and Guaritá-LG) connected to a reservoir (Salto Grande) that undergo sudden and remarkable fluctuations in their water levels. Zooplankton communities were sampled in both lakes before (pre-drawdown), during (low water), and after (post-drawdown) the artificial drawdown procedure. In LPB, drawdown resulted in an increase in zooplankton density, and temporarily changed the community in association with an increase in water conductivity and presence of non-planktonic organisms during the low water phase. In LG, drawdown had no significant effect on zooplankton community between the phases before and during the drawdown event. The results from this study suggest that artificial drawdown over a short timeframe in reservoir systems do not negatively affect the overall density, richness, and diversity of zooplankton communities in marginal shallow lakes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The excessive growth of aquatic macrophytes poses a major threat to the management of many river systems around the world (Hershner & Havens, 2008; Monterroso et al., 2011). Aquatic plants can become a nuisance in highly regulated river systems where reservoirs have been constructed and the hydrology has been dramatically altered (Boschilia et al., 2012). The massive occurrence of macrophytes can result in a number of ecological, social, and/or economic impacts on river systems (Thomaz, 2002). For example, two Hydrocharitaceae species native to South America, Egeria densa Planch. and Egeria najas Planch. (Cook & Urmi-Köning, 1984), have been implicated in the clogging of hydropower intake turbines, and disrupting navigation and recreational activities in tropical reservoirs (Bini & Thomaz, 2005; Boschilia et al., 2012).
The difficulties associated with the management of aquatic macrophytes in reservoir systems have increased the need for the development and implementation of novel and specific solutions to control their growth (Thomaz, 2002). Herbicides and mechanical methods of harvesting and cutting have both been used to control macrophytes in reservoir systems (Velini et al., 2005; Richardson, 2008), but such methods often adversely affect water quality, and subsequently have negative impacts on the aquatic biota (Pieterse & Murphy, 1990; Monahan & Caffrey, 1996; Richardson, 2008). Another potential method of dealing with these challenges is through artificial water level drawdown (Thomaz, 2002; Thomaz et al., 2006; Cook et al., 2012). This involves the management of water level regimes in reservoirs during a winter season, in order to expose shore macrophytes to dehydration with the aim of decreasing their biomass (Pieterse & Murphy, 1990; Rørslett & Johansen, 1996). The dead plants are partially removed before restoration of the normal water level (Pieterse & Murphy, 1990; Rørslett & Johansen, 1996), thereby maintaining ecological values with minimal impact on the aquatic biodiversity (Thomaz, 2002; Cook et al., 2012).
The management of water level regimes by artificial drawdown often occurs much more rapidly than a natural drying event in tropical regions, and may directly affect physical processes (e.g., geomorphologic processes of erosion and sedimentation) and water quality, and in turn, negatively impact on the aquatic biota (Leira & Cantonati, 2008; Wantzen et al., 2008). The impact of water level drawdown events on aquatic macrophytes has been a topic of considerable research (Rørslett and Johansen, 1996; Thomaz et al., 2006; Boschilia et al., 2012; Ning et al., 2012); however, the effects on many other biotic components are less known. Previous studies have shown that lake level changes may affect littoral biota, such as periphyton (Hawes & Smith, 1993) and benthic macroinvertebrates (Baumgärtner et al., 2008). Unpredictable and extreme changes to water levels over a short time period can affect phytoplankton biomass and species composition by influencing both light availability (Valeriano-Riveiros et al., 2014; Da Costa et al., 2016) and nutrient dynamics (Kimmel et al., 1990). Such water level changes may also affect zooplankton by influencing their water quality conditions (Duggan et al., 2002; Watkins et al., 2013; Perbiche-Neves et al., 2013a), competition for resources, and vulnerability to predation by fish and invertebrates during the low water phase (Havens et al., 2007). Indeed, these processes can interactively affect the taxonomic structure of zooplankton communities, with subsequent changes to species dominance and community composition (Danielsdottir et al., 2007; Deboer et al., 2016).
Artificial drawdowns are now commonly used for the management of aquatic macrophytes in many tropical reservoirs (Thomaz, 2002; Thomaz et al., 2006), and studies evaluating the success of applying these practices have warranted greater attention in recent years (Havens et al., 2007; Deboer et al., 2016). This study assessed the broader ecological implications of using an artificial drawdown over a short timeframe (13 days) to reduce the abundance and distribution of Egeria in a reservoir. Specifically, it examined the impact of artificial drawdown on the zooplankton communities in two shallow lakes connected to the reservoir, which undergo fluctuations in their water levels mainly caused by the operation of the dam. It focused on the response of zooplankton, since firstly they are usually the most important food source for invertebrates and juvenile fish; and secondly they respond rapidly to short-term changes in hydrology, and thus are useful indicators of the trophic status and water quality of aquatic systems (Fernando, 1994; Perbiche-Neves et al., 2013a).
The specific objective of this study was to examine temporal changes in the density, taxon richness, diversity, and structure of the zooplankton communities in the two marginal shallow lakes, by assessing changes in these attributes before drawdown (i.e., the pre-drawdown phase), during drawdown (i.e., the low water phase), and after re-filling (i.e., the post-drawdown phase).physical and chemical characteristics of water were also assessed to examine the environmental changes in both lakes that may have been affecting the zooplankton communities during the drawdown. We predicted that the artificial drawdown over a short timeframe would cause a decrease in the density, richness, and diversity of zooplankton, and alter the community structure by causing losses as a result of advection and water quality alterations.
Materials and methods
Study sites
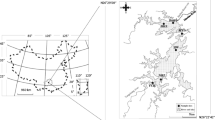
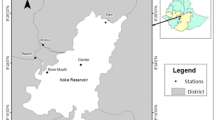
The study was conducted in Salto Grande Reservoir watershed (Fig. 1) (22°57′S49°57′W). The Salto Grande Reservoir is located on the Paranapanema River (southeast Brazil), and has an area of 12 km2, a volume of 44.2 hm3, a mean water retention time of 1.5 days, and a mean water discharge of 395 m3 s−1 (Nogueira et al., 2012). The land in the Salto Grande Reservoir watershed is intensively used for agricultural activities (corn (Zea mays), sugarcane (Saccharum spp.), soy beans (Glycine max)), and livestock grazing. As a consequence, the tributaries of this reservoir are highly turbid and transport considerable nutrient loads (a detailed limnological description can be found in Nogueira et al., 2012).
Two shallow lakes, connected to Salto Grande Reservoir, were selected for sampling to evaluate the effects of artificial drawdown on the zooplankton community (Fig. 1). There are only two shallow lakes connected to this reservoir, and they are strategically important for the maintenance of the regional vertebrate and invertebrate diversity in this river basin (Ferrareze & Nogueira, 2011). The first, Lake Pedra Branca (LPB), has a surface area of 0.44 km2 and a mean depth of 2 m, which varies in response to the water levels in the reservoir. The second, Lake Guaritá (LG), has a surface area of 0.23 km2 and a mean depth of 2.5 m, which also varies in response to the water level in the reservoir. There are dense stands of emergent aquatic macrophytes in both lakes, but they are particularly dense in LPB.
The management of water levels by artificial drawdown in the Salto Grande Reservoir is currently being undertaken as part of a long-term management program by the hydropower energy company to mitigate the detrimental effects of the Egeria stands. This procedure often causes declines in the water level of the reservoir and associated declines in the water levels of the two lakes. Indeed, although the two lakes undergo climatically-driven annual fluctuations in their water levels, their water levels are still predominantly influenced by the operation of the Salto Grande Reservoir (Fig. 2a–c).
The Salto Grande Reservoir was drawn down by ~2 m over a period of 13 days, between August 21, 2011 and September 2, 2011 (Fig. 2b). Water levels in both LG and LPB changed rapidly during the drawdown (Fig. 2c), but LG still remained connected to the main channel river over the course of the drawdown event, whereas LPB became disconnected following three days of drawdown.
Zooplankton sampling and identification
Samples were collected from each lake on sampling days undertaken during the pre-drawdown phase (August 19, 2011), low water phase (23 and 30 August), and post-drawdown phase (September 2 and 19, 2011, and October 22, 2011). Zooplankton samples and environmental variables were collected from three sites at different locations in the limnetic region of each lake (Fig. 1). These sites were selected to represent different ecosystem conditions of the lakes. For both lakes, site 1 was located in an open area, which had a relatively narrow permanent connection to the river. Site 2 was located in the middle of each lake, which was densely colonized by submerged aquatic macrophytes, especially E. densa and E. naja (though LPB had a higher density of these plants than LG). Site 3 was more isolated from each lake’s connection to the river, represented preferential fish habitat, and was characterized by the presence of different physiognomic macrophytes groups in the littoral region (e.g., floating, submerged), mainly in LPB. The three sites in each lake were distributed approximately 0.40 km from one another.
Zooplankton samples were collected at each site with a 20-L bucket, with five samplings undertaken on each sampling occasion to form a 100 L composite sample. The samples were filtered through a zooplankton net (55 µm) and preserved in 4% formalin. All zooplankton were identified using specialized identification guides (e.g., Koste, 1978; Reid, 1985; Elmoor-Loureiro, 1997). A minimum of 200 individuals were quantified per replicate, and the final density was expressed as individuals per cubic meter. Samples with low zooplankton densities were counted in full and not subsampled.
Environmental variables
The environmental conditions within each lake were also assessed at each site and on each sampling day. The depth of each lake was measured using a Speedtech sonar (depthmate Portable Sounder), and water transparency was determined using a Secchi disk. Water temperature, pH, conductivity, and dissolved oxygen were measured using a calibrated Multiprobe Horiba (Model U22- Horiba Ltd. Japan) water analyzer. Water samples were obtained from the surface (Van Dorn bottle) for assessing the concentrations of pelagic phytoplankton—chlorophyll-a (Talling & Driver, 1963), total nitrogen (Mackereth et al., 1978), total phosphorus (Strickland & Parsons, 1960), and suspended matter (mineral and organic) (Cole, 1979).
Data analysis
All analyses were undertaken separately for each lake because of their distinct zooplankton communities, and differences in their morphology and degree of physical connection to the main channel (Debastiani-Júnior & Nogueira, 2015).
Since measurements taken in each lake over time violated the ANOVA assumption of independence (see online resource 1—Table S1), we performed a repeated measures ANOVA (RM-ANOVA) to examine the effects of drawdown on the zooplankton community and environment variables. Repeated measures designs assume that observations on the same unit are correlated, with measures taken close together in time being more highly correlated than measures taken further apart in time (Field, 2013).
Prior to performing all analyses, all environmental variables were log10 (x + 1) transformed data (except pH). With the exception of the taxon richness data, which were already normally distributed, all density and diversity data were square-root transformed to reduce the influence of outliers and to fulfill the requirements of parametric ANOVA. The normality of the data was assessed using the Shapiro–Wilks Test, and homogeneity of variances was assessed using Levene’s Test. Secchi transparency was found to be 100% of total depth during all sampling phases, and consequently, this variable was excluded from the statistical analyses.
Difference between means of the density, taxon richness, and Shannon–Wiener diversity index of the total zooplankton, rotifer, and microcrustacean communities for each lake were tested using one-way RM-ANOVA, with the sampling day (19 August, 23 and 30 August, 02 and 19 September, and 22 October) as the repeated factor. Environmental variables were analyzed using the same RM-ANOVA model. Data were also evaluated by the Mauchley sphericity test to validate RM-ANOVAs (Field, 2013). If there was a significant violation of sphericity, an adjustment (Greenhouse–Geisser) univariate procedure for repeated measures was used (Field, 2013). Where significant factors were identified, post hoc tests were carried out using Bonferroni pairwise comparisons. Statistical analyses were undertaken using SPSS version 20.0 (SPSS Inc., Chicago, IL, U.S.A.).
Variation in zooplankton community structure among the pre-drawdown (August 19, 2011), low water (23 and 30 August), and post-drawdown (September 2 and 19, 2011, and October 22, 2011) phases was assessed using Principal Coordinates Analyses (PCoA) [PERMANOVA + for PRIMER (Anderson et al., 2008)] on the basis of Bray-Curtis similarities derived from square-root transformed density data (Anderson et al., 2008). PERMANOVA was then used to determine whether the variation in zooplankton community structure was significant. The Distance-based Linear Model routine [DistLM in PERMANOVA + for PRIMER (Anderson et al., 2008)] was performed (Clarke & Warwick, 2001; Anderson et al., 2008) to analyze and model the relationship between zooplankton community structure and the environmental variables. The DistLM model was constructed using the stepwise selection procedure and the adjusted R 2 as a selection criterion, to enable the fitting of the best explanatory variables to the model (Anderson et al., 2008). Prior to undertaking DistLM, the full set of nine available environmental variables was tested for collinearity using Draftsman plots and Spearman correlation matrices, and redundant variables with correlations (r 2) > 0.8 were omitted from the analysis. For LPB, the environmental variables selected included depth, water temperature, water transparency, suspended matter, conductivity, chlorophyll-a, total nitrogen, and total phosphorus, while for LG, all nine environmental variables were selected. Finally, the similarity percentage procedure (SIMPER in PRIMER v6.0 (Clarke & Warwick, 2001) was used to identify those taxa contributing most to similarities within each phase of the drawdown. All analyses were undertaken on site averages from each lake within each sampling day. Significant differences were inferred at an α-level of 0.05.
Results
Environmental variables
In LPB, pH and dissolved oxygen (30 August) increased significantly during the low water phase before declining during the post phase (Tables 1 and 2; see Bonferroni post hoc analysis P < 0.05 in Online resource 2—Table S2). Also, conductivity was significantly greater during the low water phase and soon after artificial drawdown (2 September) than pre-drawdown event (Tables 1 and 2; Table S2).
In LG, water temperature increased during the low water phase (30 August) before declining during the post-drawdown phase (Tables 1 and 2; Table S2). Dissolved oxygen increased during the low water phase (23 August) before declining during the post-drawdown phase and remained at concentrations similar to those prior to artificial drawdown, while chlorophyll-a concentration increased during the post-drawdown phase (22 October), eight weeks after the artificial drawdown was applied (Table 1 and 2; Table S2).
Zooplankton community in the Lake Pedra Branca
RM-ANOVA indicated that there were significant differences in the density of total zooplankton, microcrustaceans, and rotifers among sampling days in LPB (Table 2). Total zooplankton and microcrustacean densities increased significantly during the low water and post-drawdown phase (Fig. 3a; see Bonferroni post hoc analysis P < 0.05 in Online resource 2—Table S3). Rotifer densities were uniform during the pre-drawdown and low water phases, but they were significantly greater in post-drawdown (19 September) than pre-drawdown events (Fig. 3a; Table S3).
Mean (SD) density, taxon richness, and diversity of total zooplankton (filled circle), microcrustaceans (filled triangle), and rotifers (filled square) during artificial drawdown in (a) LPB (shaded symbols) and (b) LG (unshaded symbols). Note, the dotted line indicates the timing of drawdown for the low water phase (23 August and 30 August)
Total zooplankton and rotifer taxon richness differed significantly among sampling days (Table 2). Total zooplankton richness was similar among sampling days during the pre-drawdown and low water phases, but was greater soon after artificial drawdown (2 September) than pre-drawdown event (Fig. 3a; Table S3). Rotifer richness increased significantly during the low water (23 August) and post-drawdown (2 and 19 September) phases than pre-drawdown event (Fig. 3a; Table S3). Microcrustacean richness did not vary significantly over time (Table 2; Fig. 3a).
There were significant differences in the diversity of total zooplankton, microcrustaceans, and rotifers among sampling days in LPB (Table 2). Total zooplankton diversity did not differ significantly among sampling days during the pre-drawdown and low water phases; however, it increased significantly soon after artificial drawdown on 2 September, and was greater on that day than on 19 September or on 22 October (Fig. 3a; Table S3). Rotifer diversity was significantly greater during the low water phase (23 August) and after re-filling of the lake on 2 September than pre-drawdown phase (Fig. 3a; Table S3). Although microcrustacean diversity varied significantly over time, the variation could not be detected using Bonferroni post hoc analysis (Fig. 3a; Table S3).
Results from the PCoA plot corroborated the differences among phases in LPB (Fig. 4a). The two first axes explained 49.6% of the variation in zooplankton community structure. Plots of the first two axes showed separation of treatments before drawdown (pre-drawdown = 19 August) to the treatments after the drawdown event (low water = 23 August and 30 August; post-drawdown phase = 2 September, 19 September and 22 October). PERMANOVA indicated that zooplankton community structure during the pre-drawdown phase differed significantly from that during the low water and post-drawdown phases in LPB (Table 3). Marginal tests from the DistLM analysis revealed that depth and conductivity were both significant in explaining the variation in community structure among drawdown phases (Table 4). The sequential (i.e., cumulative) test from the DistLM analysis showed that depth and water temperatures were responsible for explaining 27% of the variation in zooplankton community structure (Table 4). Three taxa (bolded in Table 5) accounted for up to 36.53% of the community similarity during the pre-drawdown phase; namely Polyarthra vulgaris Carlin, 1943, Collotheca sp. and Euchlanis sp. In comparison, Bosmina freyi De Melo & Hebert, 1994, Thermocyclops decipiens Kiefer, 1929, and Ceriodaphnia silvestrii Daday, 1902 were dominant during the low water phase, and the former two species remained dominant during the post-drawdown phase (along with Polyarthra vulgaris) (Table 5).
PCoA plots showing ordinated sampling phases and sampling days based on community structure and composition data in (a) LPB (Lake Pedra Branca) and (b) LG (Lake Guaritá). Vectors visualize the fitted environmental variables as suggested by the DistLM model. Symbols represent average PCoA scores for replicates of each site by sampling day
Zooplankton community in the Lake Guaritá
Total zooplankton and microcrustacean densities did not vary significantly during the artificial drawdown in LG (Table 2; Fig. 3b). Rotifer densities varied significantly over time and were uniform during the pre-drawdown and low water phases; however, they were greater during post-drawdown phase (2 and 19 September) than low water phase (Table 2; Fig. 3b; see Bonferroni post hoc analysis P < 0.05 in Online resource 2—Table S3).
RM-ANOVA indicated that there were significant differences in the taxon richness of total zooplankton and microcrustaceans among sampling days in LG (Table 2). Total zooplankton richness was uniform during the pre-drawdown and low water phases; however, it was significantly greater during post-drawdown (19 September) than pre-drawdown phase, whereas microcrustacean richness increased during low water (23 August) than post-drawdown phase (2 and 19 September) (Fig. 3b; Table S3). Rotifer richness did not vary significantly over time (Table 2; Fig. 3b).
There were significant differences in the diversity of total zooplankton, microcrustaceans, and rotifers among sampling days in LG (Table 2; Fig. 3b). Overall total zooplankton, microcrustacean, and rotifer diversities were uniform during pre-drawdown and low water phases (Fig. 3b). Nevertheless, total zooplankton and rotifer diversity were significantly greater on 19 September than on 19 August, 23 August, and 22 October (Fig. 3b; Table S3), whereas microcrustacean diversity was greater during low water phase than soon after artificial drawdown on 2 September (Fig. 3b; Table S3).
The first two axes of the PCoA plot explained 45.3% of the total variation, and showed how zooplankton community structure and composition varied during the drawdown event in LG (Fig. 4b). PERMANOVA analysis indicated that zooplankton community structure during the post-drawdown phase differed significantly from that during the pre-drawdown and low water phases (Table 3). The DistLM analyses revealed that total nitrogen, dissolved oxygen, and chlorophyll-a concentrations were all significant in explaining the variation in community structure among drawdown phases (Table 4, marginal tests). The influence of total nitrogen concentration remained significant in the sequential DistLM tests, along with conductivity (Table 4). The pre-drawdown phase zooplankton community was mainly characterized by Chydorus pubescens Sars, 1901, Euchlanis sp. and Polyarthra vulgaris; whereas the low water phase community was mainly characterized by Cephalodella sp., Chydorus pubescens and Lepadella sp. By contrast, the post-drawdown phase community was mainly characterized by the rotifers, Bdelloidea, Trichotria tetractis (Ehrenberg, 1830), and Lecane bula (Gosse, 1851) (Table 5).
Discussion
In disagreement with hypothesis, artificial drawdown over a short timeframe (13 days) significantly increased the density, taxon richness, and diversity of total zooplankton, microcrustaceans, and rotifers in LPB, and the composition of the zooplankton during the pre-drawdown phase differed significantly from that during the low water and post-drawdown phases. In comparison, no significant drawdown alterations to total zooplankton, microcrustacean, and rotifer density, taxon richness, and diversity were detected in LG; however, zooplankton community composition differed during the post-drawdown phase from that during the pre- drawdown and low water phases, which partially supports the hypothesis for this lake. Nevertheless, the overall collection of taxa present in both lakes during the pre-drawdown phase was not significantly affected in subsequent phases of the drawdown event (as shown in Table 5). The results from this study suggest that artificial drawdown could potentially be used to control the excessive growth of E. densa and E. najas in reservoir systems without negatively affecting the overall density, richness, and diversity of zooplankton communities in marginal shallow lakes.
Although both lakes were exposed to identical drawdown regimes, their zooplankton communities differed in their response patterns. The most likely explanation for this variation relates to the morphology of these marginal lakes and their associated differences in water quality (Debastiani-Júnior & Nogueira, 2015). LPB becomes completely disconnected from the main channel of the Paranapanema River during a drawdown event, whereas LG still maintains water exchange with the main channel of the river. Hydrological connectivity and disturbance caused by water level fluctuations are important drivers of zooplankton population dynamics via their influence on abiotic and biotic factors, such as water quality, competition, and predation (Baranyi et al., 2002; Paggi & Paggi, 2008).
Changing water levels typically influence complex processes observed in shallow lakes, especially those involving nutrients and biological productivity (Leira & Cantonati, 2008; Wantzen et al., 2008; Lake, 2011). Artificial drawdown had a significant effect on the water quality in LPB. During the period of declining water levels, there was an increase in conductivity, combined with a decline in dissolved oxygen and a deviation from non-neutral pH (from 8.37 to 6.41) toward the post-drawdown phase. These results may be explained by the biological processes associated with submerged macrophytes that were not removed during periods of low water levels, such as their intense respiration and the decomposition of their labile organic matter (Carvalho et al., 2005). After lakes refill, the dead biomass from macrophytes becomes rehydrated, releasing organic matter and nutrients from the dead portion of the plants back into the system (Carvalho et al., 2005). All these water quality changes are therefore potential factors relating to the environmental trophic status (Pinto-Coelho et al., 2005) and food resource availability, which influence the zooplankton species composition in LPB.
The water quality changes occurred in such a way that contributed to peaks in the densities of rotifer populations and the populations of some microcrustacean taxa in LPB. The zooplankton community was numerically dominated by small zooplankton organisms, including Bosmina freyi and Thermocyclops decipiens, Polyarthra vulgaris, Keratella cochlearis, and species of the Lecanide family. Based on records and comparisons of lakes of differing trophic status, several workers suggested that more eutrophic conditions favor the dominance of these taxa (Gannon & Stemberger, 1978; Duggan et al., 2002; Perbiche-Neves et al., 2013a), owing to their ability to effectively avoid typically abundant cyanobacteria and feed on smaller algal particles (Danielsdottir et al., 2007). DeBoer et al. (2016) similarly observed a rapid increase in total zooplankton density (especially Bosmina spp.) and changes in zooplankton community structure following a water level drawdown at Red Willow Reservoir, and they attributed this response to a decline in the dissolved oxygen concentration and an increase in algal biomass (as estimated by chlorophyll-a concentration). In this study, the high variability of abiotic conditions similarly led to changes in species dominance and community composition and contributed to the high variability of zooplankton communities, which continued until soon after the re-filling of LPB Lake on 2 September. It is possible that the artificial drawdown over a short timeframe produced a temporary disturbance effect, which the water quality changes enhanced the availability of resources, increasing community variability between the phases before and during the drawdown event (Connell, 1978; Dodson et al., 2000; Angeler & Moreno, 2007). However, zooplankton community structure appeared altered again after drawdown was applied (22 October), which could be associated with seasonal variation by changes in precipitation (Nogueira et al., 2006).
Moreover, analyses of fish assemblages in tropical reservoirs of Brazil (Pelicice et al., 2005; Pelicice & Agostinho, 2006) have shown that Egeria spp. support a particular fish fauna composed of small-sized individuals (<5 cm), many of which feed almost exclusively medium and large-sized zooplankton (Iglesias et al., 2011). Even though there is no fish data in this study, this may also explain the development, maintenance and high abundance of small zooplankton species, such as Bosmina freyi, in LPB during the low water and post-drawdown phases.
Most taxa recorded during the low water phase in LPB are commonly linked to aquatic macrophytes in tropical lakes (Maia-Barbosa & Guimarães, 2008; Lansac-Tôha et al., 2009; Nadai & Henry, 2009). The increase in zooplankton taxon richness and consequent change in composition could have also been at least partly due to the dispersal of non-planktonic species during the low water phase. This was supported by the presence of non-planktonic species, such as Alona sp., Macrothrix sp, Lecane cf. leontina, Lecane arcula Harring, 1914, Lecane decipiens (Murray, 1913), Lecane cf. elsa, Macrochaetus sp. and Trichotria tetractis (Ehrenberg, 1830) in LPB during the low water phase (Table 5). These taxa together contributed to >25% to the total similarity of community composition (58%) during the low water phase. In shallower water, light conditions at the lake bottom are better; some rooted emergent macrophytes (e.g., Egeria sp.) can more easily grow to the surface layer of the water, thus reducing the separation between macrophyte beds and open water (pelagic) within the lake (as shown in the pictures of lakes in the Online resource 3). Many epiphytic and benthic zooplankton organisms often exhibit vertical movements in subtropical vegetated shallow lakes to avoid unsuitable physical and chemical features, such as low dissolved oxygen within macrophytes stands and potentially also to avoid predation (Meyers, 1980; Miranda & Hodges, 2000; Tavşanoğlu et al., 2012). In addition, non-planktonic rotifers would be able to vertically migrate to upper layers, where phytoplankton is abundant and available to feed on (José de Paggi, 1995). Thus, we cannot discard the possibility that the reduced separation between macrophyte beds and open water probably led to a more active replacement of species and allowed for a great number of non-planktonic species in the water column throughout our sampling period. A similar situation has been observed in other shallow subtropical lakes on the Paraná River floodplain (José de Paggi, 1993; José de Paggi et al., 2012), where Panicum elephantipes, Cyperus alternifolius, Thypa sp., and Paspalum repens were the dominant macrophytes and they supported the diversity of non-planktonic organisms in the open water regions of these lakes.
In LG, the density and diversity of microcrustacean and rotifer communities remained similar during the pre-drawdown and low water phases, and were largely influenced by the resident populations of the main channel of the Paranapanema River, inhabiting in the Salto Grande Reservoir (Nogueira et al., 2008; Perbiche-Neves & Nogueira, 2010; Perbiche-Neves & Nogueira, 2013b). In agreement with the ecological attribute results, overall community structure did not vary significantly between the pre-drawdown and low water phases. Watkins et al. (2013) also found no effect of water level drawdown on zooplankton communities. Their results indicated that a management regime involving a partial drawdown did not compromise zooplankton diversity in experimental wetlands located on the floodplain of a temperate Australian river (Watkins et al., 2013). In this study, changes in the post-drawdown phase zooplankton community varied with nutrient (total nitrogen), dissolved oxygen, and chlorophyll-a concentrations, in addition to conductivity. The water quality and associated zooplankton community changes occurred approximately eight weeks after the artificial drawdown was applied (Fig. 4b), coinciding with the increasing trophic status of Paranapanema Reservoir at the beginning of the rainy season (Fig. 2a) which typically occurs in early October (Nogueira et al., 2006; Minuzzi et al., 2007). Thus, the temporary changes to zooplankton community structure observed may have been solely due to direct and indirect effects of seasonal variation on the limnological conditions rather than artificial drawdown.
Conclusion
Our results suggest that artificial drawdown over a short timeframe may have enhanced zooplankton richness and diversity in one shallow, less-connected lake, but had no significant impact on zooplankton community structure in the other also shallow, but more-connected lake. However, other effects might be considered when the community interactions are measured over a long term (Lake, 2011). Havens et al. (2007) observed significantly changes in zooplankton community composition in Lake Okeechobee between the pre- to the post-drawdown periods, which persisted for five years after the drawdown event. They argued that the water level drawdown had positive effects on plant growth of the macrophyte, Chara, in Lake Okeechobee, which became more favorable for the survival of small planktivorous fish and contributed to their predation on zooplankton among the plants. Environmental consequences of an artificial drawdown can appear in very different timescales, ranging from days to seasons or even years (Hellsten et al., 1996; Leira & Cantonati, 2008). All these findings support the need for more research on the effects of drawdown to macrophyte growth/communities into the response of zooplankton and other communities to drawdown events—particularly in relation to their extent, frequency, and duration.
References
Anderson, M. J., R. N. Gorley & K. R. Clarke, 2008. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods. National Environment Research Council, Plymouth.
Angeler, D. G. & J. M. Moreno, 2007. Zooplankton community resilience after press-type anthropogenic stress in temporary ponds. Ecological Application 17: 1105–1115.
Baranyi, C., T. Hein, C. Holarek, S. Keckeis & F. Schiemer, 2002. Zooplankton biomass and community structure in a Danube River floodplain system: effects of hydrology. Freshwater Biology 47: 473–482.
Baumgärtner, D., M. Mörtl & K. Rothhaupt, 2008. Effects of water-depth and water-level fluctuations on the macroinvertebrates community structure in the littoral zone of Lake Constance. Hydrobiologia 613: 97–107.
Bini, L. M. & S. M. Thomaz, 2005. Prediction of Egeria najas and Egeria densa occurrence in a large subtropical reservoir (Itaipu Reservoir, Brazil-Paraguay). Aquatic Botanic 83: 227–238.
Boschilia, S. M., E. F. De Oliveira & A. Schwarzbold, 2012. The immediate and long-term effects of water drawdown on macrophytes assemblages in a large subtropical reservoir. Freshwater biology 57: 2461–2651.
Carvalho, P., S. M. Thomaz & L. M. Bini, 2005. Effects of temperature on decomposition of potential nuisance species: the submerged aquatic macrophyte Egeria najas Planchon (Hydrocharitaceae). Brazilian Journal of Biology 65: 51–60.
Clarke, K. R. & R. M. Warwick, 2001. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 2nd ed. National Environment Research Council, Plymouth.
Cole, G. A., 1979. Textbook of Limnology, 2nd ed. The C.V. Mosby Company, Saint Louis.
Connell, J. H., 1978. Diversity in tropical rain forests and coral reefs. Science 199: 1302–1310.
Cook, C. D. K. & K. Urmi-König, 1984. A revision of the genus Egeria (Hydrocharitaceae). Aquatic Botanic 19: 73–96.
Cook, N. C., R. W. Carter, R. A. Fuller & M. Hockings, 2012. Managers consider multiple lines of evidence important for biodiversity and management decisions. Journal of Environmental Management 113: 341–346.
Da Costa, M. R. A., J. L. Attayde & V. Becker, 2016. Effects of water level reduction on the dynamics of phytoplankton functional groups in tropical semi-arid shallow lakes. Hydrobiologia 778: 75–89.
Danielsdottir, M. G., M. T. Brett & G. B. Arhonditsis, 2007. Phytoplankton food quality control of planktonic food web processes. Hydrobiologia 589: 29–41.
Debastiani-Junior, J. R. & M. G. Nogueira, 2015. How water level management affetcs cladoceran assemblages in lakes lateral to a reservoir. Marine and Freshwater Research 67: 1853–1861.
DeBoer, J. A., C. M. Webber, T. A. Dixon & K. L. Pope, 2016. The influence of a severe reservoir drawdown on springtime zooplankton and larval fish assemblages in Red Willow Reservoir, Nebraska. Journal of Freshwater Ecology 31: 131–146.
Dodson, S. I., S. E. Arnott & K. L. Cottingham, 2000. The relationship in lake communities between primary productivity and species richness. Ecology 81: 2662–2679.
Duggan, I. C., J. D. Green & R. J. Shiel, 2002. Distribution of rotifers in North Island, New Zealand lakes: relationships to environmental and historical factors. Freshwater Biology 47: 195–206.
Elmoor-Loureiro, L. M. A., 1997. Manual de Identificação de Cladóceros Límnicos do Brasil. Universa, Taguatinga.
Fernando, C. H., 1994. Zooplankton, fish and fisheries in tropical freshwaters. Hydrobiologia 272: 105–123.
Ferrareze, M. & M. G. Nogueira, 2011. Importance of lateral lagoons for the zooplankton assemblages (Cladocera and Copepoda) in a large tropical reservoir. Oecologia Australis 15: 673–687.
Field, A. P., 2013. Discovering statistics using IBM SPSS Statistics: and sex and drugs and rock ‘n’ roll. Sage publications, London.
Gannon, J. E. & R. S. Stemberger, 1978. Zooplankton (especially crustaceans and rotifers) as indicators of water quality. Transactions of the American Microscopical Society 97: 16–35.
Havens, K. E., T. L. East & J. R. Beaver, 2007. Zooplankton response to extreme drought in a large subtropical lake. Hydrobiologia 589: 187–198.
Hawes, L. & R. Smith, 1993. The effect of localized nutrient enrichment on the shallow, epilithic periphyton of oligotrophic lake Taupo, New Zealand. New Zealand Journal of Marine and Freshwater Research 27: 365–372.
Hellsten, S., M. Marttunen, R. Palomaeki, J. Riihimaeki & E. Alasaarela, 1996. Towards an ecologically based regulation practice in Finnish hydroelectric lakes. Regulated Rivers: Research and Management 12: 535–545.
Hershner, C. & K. J. Havens, 2008. Managing invasive aquatic plants in a changing system: strategic consideration of ecosystem services. Conservations Biology 22: 544–550.
Iglesias, C., N. Mazzeo, M. Meerhoff, G. Lacerot, J. M. Clemente, F. Scasso, C. Kruk, G. Goyenola, J. García-Alonso, S. L. Amsinck, J. C. Paggi, S. José de Paggi & E. Jeppesen, 2011. High predation is of key importance for dominance of small-bodied zooplankton in warm shallow lakes: evidence from lakes, fish exclosures and surface sediments. Hydrobiologia 667: 133–147.
José de Paggi, S., 1993. Composition and seasonality of planktonic rotifers in limnetic and littoral regions of a floodplain lake (Paraná river system). Revue d’Hydrobiologie Tropicale 26: 53–63.
José de Paggi, S., 1995. Vertical distribution and diel migration of rotifers in a Parana River floodplain lake. Hydrobiologia 301: 87–94.
José de Paggi, S., S. Muñoz, D. Frau, J. C. Paggi, P. Scarabotti, M. Devercelli & M. Meerhoff, 2012. Horizontal distribution of rotifers in a subtropical shallow lake (Paraná floodplain, Argentina). Fundamental and Applied Limnology 180: 321–333.
Kimmel, B. L., O. T. Lind & L. J. Paulson, 1990. Reservoir primary production. In Thornton, K. W., B. L. Kimmel & F. E. Payne (eds), Reservoir Limnology: Ecological Perspectives. Wiley, New York: 109–131.
Koste, W., 1978. Rotatoria die Rädertiere Mitteleuropas begründet von Max Voight. Monogononta, Gebrüder Borntraeger.
Lake, P. S., 2011. Drought and Aquatic Ecosystems: effects and responses. Wiley-Blackwell, Oxford.
Lansac-Tôha, F. A., C. C. Bonecker, L. F. M. Velho, N. R. Simões, J. D. Dias, G. M. Alves & E. M. Takahashi, 2009. Biodiversity of zooplankton communities in the Upper Paraná River floodplain: interannual variation from long-term studies. Brazilian Journal of Biology 69: 539–549.
Leira, M. & M. Cantonati, 2008. Effects of water-level fluctuations on lakes: an annotated bibliography. Hydrobiologia 613: 171–184.
Mackereth, F. J. H., J. Heron & F. J. Talling, 1978. Water Analysis: Some Revised Methods for Limnologists. Titus Wilson and Sons Ltd., Kendall.
Maia-Barbosa, P. M. & A. S. Guimarães, 2008. Zooplankton in littoral waters of a tropical lake: a revisited biodiversity. Brazilian Journal of Biology 68: 1069–1078.
Meyers, D. G., 1980. Diurnal vertical migration in aquatic microcrustacea: light and oxygen responses of littoral Zooplankton. In Kerfoot, W. C. (ed.), Evolution and Ecology of Zooplankton Communities. University Press of New England, England: 80–90.
Minuzzi, R. B., G. C. Sediyama, E. M. Barbosa & J. C. F. De Melo Júnior, 2007. Climatologia do comportamento do período chuvoso da região sudeste do Brasil. Revista Brasileira de Meteorologia 22: 338–344.
Miranda, L. E. & K. B. Hodges, 2000. Role of aquatic vegetation on hypoxia and sunfish abundance in bays of a eutrophic reservoir. Hydrobiologia 427: 51–57.
Monahan, C. & J. M. Caffrey, 1996. The effect of weed control practices on macroinvertebrate communities in Irish canals. Hydrobiologia 340: 205–211.
Monterroso, I., R. Binimelis & B. Rodríguez-Labajos, 2011. New methods for the analysis of invasion processes: multi-criteria evaluation of the invasion of Hydrilla verticillata in Guatemala. Journal of Environmental Management 92: 494–507.
Nadai, R. & R. Henry, 2009. Temporary fragmentation of a marginal lake and its effects on zooplankton community structure and organization. Brazilian Journal of Biology 69: 819–835.
Ning, N. S. P., S. C. Watkins, B. Gawne & D. L. Nielsen, 2012. Assessing the potential for using wetlands as intermediary storages to conjunctively maintain ecological values and support agricultural demands. Journal of Environmental Management 107: 19–27.
Nogueira, M. G., A. Jorcin, N. C. Vianna & Y. C. T. Britto, 2006. Reservatórios em cascata e os efeitos na limnologia e organização das comunidades bióticas (fitoplâncton, zooplâncton e zoobentos) – um estudo de caso no rio Paranapanema. In Nogueira, M. G., R. Henry & A. Jorcin (eds), Ecologia de reservatórios: impactos potenciais, ações de manejo e sistema em cascata. Rima, São Carlos: 83–125.
Nogueira, M. G., P. C. R. Oliveira & Y. T. De Britto, 2008. Zooplankton assemblages (Copepoda and Cladocera) in a cascade of reservoirs of a large tropical river (SE Brazil). Limnetica 27: 151–170.
Nogueira, M. G., G. Perbiche-Neves & D. A. O. Naliato, 2012. Limnology of two contrasting hydroelectric reservoirs (storage and run-of-river) in southeast Brazil. In Samadi-Boroujeni, H. (ed.), Hydropower, practice and application. Rijeka, Croatia: 167–184.
Paggi, S. B. J. & J. C. Paggi, 2008. Hydrological connectivity as a shaping force in the zooplankton community of two lakes in the Paraná River Floodplain. International Review of Hydrobiology 93: 659–678.
Pelicice, F. & A. Agostinho, 2006. Feeding ecology of fish associated with Egeria spp. Patches in a tropical reservoir. Brazil. Ecology Freshwater Fish 15: 10–19.
Pelicice, F., A. Agostinho & S. Thomaz, 2005. Fish assemblages associated with Egeria in a tropical reservoir: investigating the effect of plant biomass and diel period. Acta Oecologica 27: 9–16.
Perbiche-Neves, G. & M. G. Nogueira, 2010. Multi-dimesional effects on Cladoceran (Crustacea, Anomopoda) assemblages in two cascade reservoirs in Southeast Brazil. Lakes & Reservoirs: Research & Management 15: 139–152.
Perbiche-Neves, G. & M. G. Nogueira, 2013. Reservoir design and operation: effects on aquatic biota – a case study of planktonic copepods. Hydrobiologia 707: 187–198.
Perbiche-Neves, G., C. Fileto, J. L. Portinho, A. Troguer & M. Serafim-Júnior, 2013. Relations among planktonic rotifers, cyclopoid copepods, and water quality in two brazilian reservoirs. Latin American Journal of Aquatic Research 41: 138–149.
Pieterse, A. H. & K. J. Murphy, 1990. Aquatic Weeds. The ecology and management of Nuisance Aquatic Vegetation. Oxford University Press, Oxford.
Pinto-Coelho, R. M., J. F. Bezerra-Neto & C. A. Morais Jr., 2005. Effects of eutrophication on size and biomass of crustacean zooplankton in a tropical reservoir. Brazilian Journal of Biology 65: 325–338.
Reid, J. W., 1985. Key of identification for free-living continental species of South America of order Cyclopoida (Crustacea, Copepoda) (in Portuguese). Boletim de Zoologia, Universidade de São Paulo 9: 17–143.
Richardson, R. J., 2008. Aquatic plant management and the impact of emerging herbicide resistance issues. Weed Techology 22: 8–15.
Rørslett, B. & S. W. Johansen, 1996. Remedial measures connected with aquatic macrophytes in Norwegian regulated rivers and reservoirs. River Research and Application 12: 509–522.
Strickland, J. D. & T. R. Parsons, 1960. A manual of sea water analysis. Bulletin of Fishery Research Board of Canada 125: 1–185.
Talling, J. F. & D. Driver, 1963. Some problems in the estimation of chlorophyll a in phytoplankton. In Proceedings, Conference of primary productivity measurements in marine and freshwater. USAEE, Hawaii: 142–146.
Tavşanoğlu, Ü. N., A. I. Cakiroğlu, S. Erdogan, M. Meerhoff, E. Jeppesen & M. Beklioğlu, 2012. Sediments, no plants, offer the preferred refuge for Daphnia against fish predation in Mediterranean shallow lakes: an experimental demonstration. Freshwater Biology 57: 765–802.
Thomaz, S. M., 2002. Ecological factors associated to aquatic macrophytes colonization and growth and management challenges. Planta Daninha 20: 21–33.
Thomaz, S. M., T. A. Pagioro, L. M. Bini & K. J. Murphy, 2006. Effects of reservoir drawdown on biomass of three species of aquatic macrophytes in a large sub-tropical reservoir (Itaipu, Brazil). Hydrobiologia 570: 53–59.
Valeriano-Riveros, M. E., G. Vilaclara, F. S. Castillo-Sandoval & M. Merino-Ibarra, 2014. Phytoplankton composition changes during water levels fluctuations in high-altitude, tropical reservoir. Inland Waters 4: 337–348.
Velini, E. D., M. R. Corrêa, R. H. Tanaka, L. F. Bravin, U. R. Antuniassi, F. T. Carvalho & M. L. B. T. Galo, 2005. Operational evaluation of mechanical control of aquatic macrophytes submerged in the Jupia Reservoir. Planta Daninha 23: 277–285.
Wantzen, K. M., K. Rothhaupt, M. Mörtl, M. Cantonati, L. G. Tóth & P. Fischer, 2008. Ecological effects of water-level fluctuations in lakes: an urgent issue. Hydrobiologia 613: 1–4.
Watkins, S. C., N. S. P. Ning, B. Gawne & D. L. Nielsen, 2013. Managing wetlands as off-river storages: impacts on zooplankton communities. Hydrobiologia 701: 51–63.
Acknowledgements
We are grateful to CNPq (Brazilian National Counsel of Technological and Scientific Development) for the scholarship to JLP (CNPq process 152109/2012-9) and Duke Energy for financial support. We also thank Virgínia Sanches Uieda, Raoul Henry, Gilmar Perbiche-Neves, Daryl Nielsen, and Nathan Ning for constructive comments on earlier versions of the manuscript, as well as Danilo A.O. Naliato and Diogo F. Souza for help with the fieldwork. Dr. Douglas D. Kane improved the grammar of the final manuscript. We thank the anonymous reviewers for their careful reading of our manuscript and their many insightful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Mariana Meerhoff
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Portinho, J.L., Nogueira, M.G. Does artificial drawdown affect zooplankton structure in shallow lakes? A short-term study in a tropical reservoir. Hydrobiologia 797, 303–318 (2017). https://doi.org/10.1007/s10750-017-3193-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3193-4