Abstract
This study aims to test whether environmental conditions including the trophic habitat and diet impact the biochemical composition of storage organs and affect the nutritional quality of eggs of Octopus vulgaris. Trophic habitat and gonad quality of neighbouring populations off the Portuguese coast, subject to different oceanographic regimes, were compared using the digestive gland and beaks as recorders of trophic and habitat preferences, and gonads as indicators of egg quality. Cholesterol, phospholipids and triacylglycerol content, essential fatty acid (EFA) profile of the digestive gland and stable isotopes, δ15N and δ13C, in the buccal mass flesh and beaks were indicators of the differences in the trophic habitat between populations. For gonad quality, the same bio-indicators were used to identify differences with maturation. The study shows that, although diet influences the EFA profile of the gonads to a certain degree, the main lipid content, phospholipids and cholesterol content in the gonads are not influenced by habitat conditions. This, therefore, suggests that O. vulgaris is able to influence the quality of egg content independent of diet. The species is believed to be an income breeder which attains maturity upon reaching a sufficient condition level, then channelling energy directly from food to gonad development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The common octopus, Octopus vulgaris Cuvier (1797), is an important fisheries resource and a key component of coastal food webs as a prey and generalist predator (Smith, 2003; Katsanevakis & Verripoulos, 2004). The species is believed to channel energy to growth and reproduction directly from food (Quetglas et al., 2011; Smith et al., 2011). It is a species with high feeding and turnover rates (Semmens et al., 2004), short life cycle and high plasticity to environmental conditions. Studies on the molecular and nutritional composition of reserve and reproductive organs reflect the benthic characteristics of the species. The habitat biodiversity and energy flow to which the populations are exposed are mirrored in the molecular and energetic composition of the tissues (Rosa et al., 2004a; Bandarra et al., 2006; Cherel et al., 2009; García-Garrido et al., 2010).
The diet of O. vulgaris depends on the life-cycle stage, size, depth of occurrence, habitat and seasonal availability of their prey (Nixon, 1985; Smith, 2003). The cephalopod hepatopancreas or digestive gland has different functions in the physiology of Octopus spp., including the synthesis and secretion of digestive enzymes, the reabsorption and metabolism of nutrients; synthesis and storage of lipids like cholesterol, lipoproteins, glycogen, pigments, vitamins and protein-bound Fe, Cu, Ca and non-physiological heavy metals; and excretion and rejection of waste products of the digestion and cell metabolism (Blanchier & Boucaud-Camou, 1984; Budelmann et al., 1997; Moltschaniwskyj & Johnston, 2006). Its function as a storage organ indicates its utility as the ideal source of dietary tracers such as essential fatty acids (EFA) (Phillips et al., 2001) and dietary lipids (e.g., triacylglycerol and cholesterol) that are deposited in this organ with little or no modification of the lipid content (Boucaud-Camou & Boucher-Rodoni, 1983; Phillips et al., 2003).
Lipids are important dietary constituents, providing energy, vitamins and EFA. Cholesterol is the predominant sterol in the cephalopod’s lipid reserves (Sieiro et al., 2006) and proxy of the production of hormones in marine invertebrates (Kanazawa, 2002). The endogenous synthesis of cholesterol seems to be absent in cephalopods, suggesting that it is an essential dietary nutrient (Villanueva & Norman, 2008). Triacylglycerol is a neutral lipid involved in fatty acid (FA) storage and metabolism (Lee et al., 2006). The FAs incorporated within the phospholipids act as building blocks for the membrane lipid bilayer (Dalsgaard et al., 2003; Bergé & Barnathan, 2005; Athenstaedt & Daum, 2006). The seasonal variability of triacylglycerols and phospholipids is related with cellular mechanisms under different environmental conditions and because of that, they are good indicators of the condition (Shulman & Love, 2006). The most important EFA are arachidonic acid C20:4n6 (ARA), eicosapentaenoic acid C20:5n3 (EPA) and docosahexaenoic acid C22:6n3 (DHA). The requirements on ARA, EPA and DHA and the balance between them are important for growth (Navarro & Villanueva, 2000; Shulman & Love, 2006). These EFA are related with the energy channelling, the cellular membrane structure and function, and are integral elements of phospholipids as components of lipid bilayers (Tocher, 2010).
Reproduction timing in O. vulgaris depends on the local oceanographic regime: a long season in productive systems, frequently with two reproductive peaks; and a shorter season (1–2 months) in oligotrophic systems (Lourenço et al., 2012). The formation of the yolk is extremely important during maturation and egg development, because the newly hatched paralarvae are not truly lecithotrophic depending to some extent on these reserves to survive (Boletzky, 1975; Villanueva & Norman, 2008). The nutritional content of the yolk is mainly protein, but lipids are also important for membrane formation and energetic supply (11–14% of dry weight) particularly that of polyunsaturated fatty acids (PUFA), phospholipids and cholesterol (Navarro & Villanueva, 2000).
The δ13C and δ15N signatures in different tissues of a predator like O. vulgaris reflect its habitat and trophic position, respectively (Cherel & Hobson, 2005). Consumers or predators are enriched in 15N relative to their food and consequently the δ15N measurement is an indicator of the consumer trophic position (Vander Zanden & Rasmussen, 2001; Hobson & Cherel, 2006). With little variation along the food chain, the δ13C is used to determine primary sources in the trophic web indicating the habitat of the organism, and the inshore versus offshore, or pelagic versus benthic contribution of food intake (Cherel & Hobson, 2007; Jackson et al., 2007). The determination of stable isotope δ13C and δ15N signatures both in muscle and in cephalopod beaks are complementary approaches to stomach content and fatty acid analysis in studies of trophic dynamics and feeding ecology, allowing the identification of ontogenic migration and feeding shift events (Stowasser, 2004; Jackson et al., 2007). In species like O. vulgaris, diet studies are difficult to carry out due to the diversity of preys (Smith, 2003); the fast digestion rate (Boucaud-Camou & Boucher-Rodoni, 1983); and the large number of empty stomachs. For these cases, an analytic approach combining fatty acids and stable isotopes analyses provides overall information on the average diet regarding both trophic level and habitat of a particular population.
Here we hypothesise that environmental conditions, including the trophic habitat, influence the biochemical composition of storage organs and the nutritional quality of the eggs (indirectly potentially affecting the next generation). The Portuguese coast presents an advantageous geographical setting where it is possible to follow O. vulgaris populations which are subjected to distinct environmental conditions: in the northwest coast, one of the populations is in a productive system integrated in the Western Iberia Upwelling System (Relvas et al., 2007); in the south, the other is integrated in the Gulf of Cadiz System influenced by the oligotrophic and warmer waters of the Huelva front where downwelling and upwelling events are weaker and not seasonal (García-Lafuente et al., 2006). To test our hypothesis, we followed the EFA profile and the content of total lipids, cholesterol, triacylglycerol and phospholipids as bio-indicators of variations in the digestive gland and in immature and fully developed gonads of females of both populations. We also followed the stable isotope δ13C and δ15N signatures in the upper and lower beaks and buccal mass tissue of immature and mature individuals to identify the possible differences in the overall diet between population and maturity stages.
Materials and methods
Sampling
All tissue samples were obtained from females captured in the small-scale O. vulgaris fisheries from March to April 2011 within the two study areas represented by landings in ports of Peniche (northwest coast area; lat: 39°21.40 N, long: 9°20.38 W) and Olhão (south coast area; lat: 36°59.34 N, long: 7°50.44 W). Within each area, five immature females and five mature females were selected and digestive gland tissue and gonads were collected, freeze-dried and stored at −20°C.
Cleaned upper and lower beaks and buccal mass muscular tissue (referred as flesh hereafter) samples were collected from frozen animals and kept in 70% ethanol for isotopic analysis. Sampling was conducted considering the same explanatory factors, area and maturity in an unbalanced sampling design, collecting 27 flesh samples (4 mature and 6 immature samples in the northwest coast and 5 mature and 12 immature samples in the south coast) and 33 beak samples (8 mature and 9 immature in the northwest coast and 5 mature and 12 immature in the south coast). The dorsal mantle length and individual weight were measured to the nearest 5 mm and 0.1 g, respectively. Immature and maturing females were classified as immature whereas mature and spawning females were classified as mature (according to Guerra, 1975).
Stable isotope analyses
Beaks and flesh samples were freeze-dried and homogenized prior to analysis. To avoid the depletion of δ13C values due to the presence of lipids, flesh samples were rinsed successively in a 2:1 chloroform–methanol solution (Cherel et al., 2005). Nitrogen and carbon isotope ratios were determined via Finningan conflo II interface to a Thermo Delta V S mass spectrometer coupled to a Flash EA1112 Series elemental analyser. Approximately 0.3 mg of each sample was combusted in a tin cup for the simultaneous determination of nitrogen and carbon isotope ratios. Isotope ratios are presented in the usual δ notation relative to the PeeDee Belemnite (PDB) for carbon and atmospheric N2 (AIR) for nitrogen, and expressed as ‰. Replicate measurements of internal laboratory standards (acetanilide) indicate a precision of <0.2‰ both for δ13C and δ15N. The C/N mass ratio was used to check the effectiveness of the lipid extraction in the flesh and in the beaks (Post et al., 2007; Cherel et al., 2009).
Prior to the statistical analysis, the assumptions of normality and sample variance homogeneity were assessed by Shapiro–Wilk’s test and Bartlett’s test, respectively. A paired t test was performed to assess possible differences in stable isotope signatures between upper and lower beaks. A two-way analysis of variance (ANOVA) was performed to assess the effect of area, maturity and the interaction between the two factors in the ratios δ13C and δ15N in the upper and lower beaks and flesh. The hypothesis for the two-way ANOVA was formulated under the assumption of: H0A: there is no main effect of the factor area on the stable isotope (δ13C or δ15N) mean value in the beaks upper and lower beaks and flesh; H0B: there is no main effect of the factor maturity on the stable isotope mean value in the upper and lower beaks and flesh; H0C: there is no additive effect of the interaction between area and maturity on the stable isotope mean value in the upper and lower beaks and flesh. If H0C was rejected, a post-hoc Tukey test for multiple comparisons was performed to determine which combination of factors were significantly different (P value < 0.05).
Lipid class analyses
The total lipid (TL) fraction was extracted by the Bligh & Dyer (1959) method. Samples of ≈1 and ≈2 g of dry tissue of digestive glands and gonads were used, respectively. The results were expressed as g lipid/100 g dry weight. Lipid classes were determined by different spectrophotometric methods. The phospholipids fraction was purified from the total lipid extract according to Auborg et al. (1996). Total phospholipids were quantified by measuring the organic phosphorus in total lipid extracts according to the Raheja et al. (1973) method based on a complex formation with ammonium molybdate. Results are expressed as g PL/100 g dry weight. Total cholesterol was determined in the total lipids extracts by the method of Huang et al. (1961) based on the Liebermann–Buchardt reaction. Results are expressed in g cholesterol/100 g dry weight. FA composition of lipids present in the Bligh & Dyer extract was determined by converting total lipids into fatty acid methyl esters (FAME), according to the method described by Lepage & Roy (1984). FAME were analysed by gas chromatography. Peaks corresponding to FAs were identified by comparison of their retention times with standard mixtures. Peak areas were automatically integrated with C19:0 being used as an internal standard for quantitative analysis. The concentration of each fatty acid or fatty acid group was expressed as g/100 g total FAME.
Prior to the statistical analysis, the assumptions of normality and sample variance homogeneity were assessed with Shapiro–Wilk’s and Bartlett’s tests, respectively. Whenever these assumptions failed the data were log-transformed to guarantee normality and variance homogeneity. Two-way ANOVA was performed to assess the effect of area, maturity and the interaction between the two factors in the lipid classes in the digestive gland and in the gonad. The hypothesis for the two-way ANOVA was formulated under the assumption of: H0A: there is no main effect of the factor area on the lipid class content in the tissue; H0B: there is no main effect of the factor maturity on the lipid class content in the tissue; H0C: there is no additive effect of the interaction between area and maturity on the mean lipid class content in the tissue. If H0C was rejected, a post hoc Tukey test for multiple comparisons was performed to determine which factor combinations were significantly different.
Major FAs were defined as those presenting concentration by area and by maturity stage higher than 1 g/100 g of FAME. For each FA, the assumption of sample normality and homogeneity was tested by the Shapiro–Wilk’s and Bartlett’s, respectively, by tissue and factor (area and maturity), when these assumptions failed, the variable was log-transformed. Differences in mean concentration of each FA between area and maturity stage were tested with t test (Zar, 1999). The major fatty acids profile was compared by explanatory factor, area and maturity and the interaction between area and maturity by means of Multivariate ANOVA (MANOVA), followed by Discriminant Function Analysis (DFA). The MANOVA and DFA are complementary approaches based in the separation of observation groups represented by their centroids obtained under the effect of two or more factors levels (Quinn & Keough, 2002). DFA is particularly useful to detect the variables that better discriminates between different observation groups (Zuur et al., 2007).
Results
Diet and habitat
According to the mean C:N mass ratio, lipids were effectively removed from flesh (C:N = 3.13 ± 0.06, mean ± standard deviation, SD) and from beaks (upper beak: 3.38 ± 0.07; lower beak: 3.33 ± 0.08). The flesh of O. vulgaris females presents a mean δ15N of 11.86 ± 0.66 (SD) ‰, and the mean δ13C value of −16.77 ± 0.74 (SD) ‰ (Fig. 1) independently of area, maturity stage or the interaction of both factors (Table 1). Upper and lower beaks present different δ15N and δ13C signatures between them (for δ15N: paired t = −2.12; df = 32, P value < 0.01; for δ13C: paired t = −4.14, df = 32, P value < 0.01). The factorial ANOVA shows that area has a significant effect in δ15N signature (Table 1), with both beaks presenting higher values in south coast (Fig. 1). The δ13C signature is not affected by area in both beaks (Table 1).
Octopus vulgaris flesh and upper and lower beaks stable isotope δ15N and δ13C signatures by explanatory factors area and maturity. The upper right and left graphics represent the stable isotope signatures for the northwest and south coast, and the lower left and right graphics represent the stable isotope signatures for immature and mature individuals. The dot indicates the mean value for the δ15N and δ13C for each tissue studied; the error bars indicate the magnitude of standard deviation on either axis
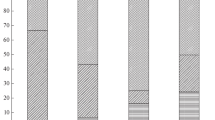
The mean individual weight of the females collected was 2340.30 ± 979.45 (SD) g in the northwest coast and 2637.40 ± 479.00 (SD) g in the south coast. Digestive gland and gonad presented different mean contents in total lipids and lipid classes, with the digestive gland presenting higher mean content of total lipids, cholesterol and triacylglycerol (Fig. 2). Despite that, the gonads were richer in phospholipids than the digestive gland (Fig. 2).
Mean lipid classes’ content in % dw (±SD) aggregated by tissue (digestive gland; gonad), by area (nw—Northwest coast; south—South coast) and by maturity stage (maturity; i—immature, m—mature). Different superscript letters indicate significant statistical differences (P < 0.05). The acronym cho stands for cholesterol, tag for triacylglycerol and phosp for phospholipids
In the digestive gland the mean total lipid content and mean cholesterol content depended on area. The digestive glands collected in the northwest coast presented a significantly lower content of total lipids (t = −2.42, df = 18, P value < 0.01), and higher mean content in cholesterol (t = 7.91, df = 18, P value < 0.01; see Fig. 2) when compared with south coast. The two-way ANOVA for the interaction between the factors area and maturity showed significant differences in the mean content of cholesterol and triacylglycerol due to the additive effect of the two factors (Table 1).
In the digestive gland, the fatty acids (FA) represented 31 and 46 μg/mg of the total lipid content in the northwest coast and the south coast, respectively. The most abundant FA were the saturated fatty acids (SFA), followed by the polyunsaturated fatty acids (PUFA) and the monounsaturated fatty acids (MUFA) (Table 2). The mean content of myristic acid, C14:0; palmitoleic acid, C16:1n7; gondoic acid, C20:1n9; ARA, C20:4n6 and DHA were significantly different between areas, as well as the ratio DHA/EPA mainly due to the increase in DHA content in digestive glands of the south coast (Table 2). Results of the MANOVA analysis showed that content levels depend both on area and maturity, with no effect on the interaction between both factors (Table 3). In the DFA, the first discriminant function maximizes the differences between groups. The DFA for region groups show that the first function explained 84% of the variability, with a good separation between the northwest coast group (class score = 0.92) and the south coast group (class score = −0.92). The FA that contributed the most to the group separation was the DHA which was positively correlated with the south coast and negatively correlated with northwest coast. The DFA for maturity groups showed a good separation between immature (class score = −0.85) and mature digestive glands (class score = 1.02) with 86% of explained variation. The FAs that most contributed to the group separation were EPA and DHA and C18:0. The DFA for area x maturity groups (Fig. 3, digestive gland) showed a poor separation between groups with an explained variance of 51% in accordance with MANOVA results. Nevertheless, the FAs that contributed the most to the group separation were: DHA, positively related with immature females of the south coast area and negatively related with the immature and mature females from northwest coast: EPA, positively related with the immature females, and negatively related with the northwest coast mature females.
Discriminant function analysis biplots of the fatty acid profiles of the digestive gland (on the left) and gonad (on the right). The acronym nw_i stands for the group immature females of northwest coast, nw_m stands for the group mature females of northwest coast, sth_i stands for the immature individuals of the south coast and sth_m stands for the mature individuals of south coast
Maturity
Stable isotope signatures showed that the δ13C signature is statistically significant different with the maturity stage (Table 1), with the beaks of immature females presenting significant higher δ13C signature (Fig. 1).
The effect of different nutritional levels on maturity was studied by assessing differences in the digestive gland and gonads with maturity. For the digestive gland, there was no effect of this factor in the lipid classes content studied (Table 1). Total lipid, cholesterol and triacylglycerol contents tended to increase from immature to mature females although this increase is not statistically significant. In relation to the phospholipid content, the digestive glands of mature females of south coast were significantly poor in phospholipids in relation to the immature ones (t = 3.50, df = 18, P value < 0.01) (Fig. 2). Nevertheless, when comparing the digestive glands lipid content between mature females of northern coast with mature females of south coast, some differences arose. Particularly, the total lipid content (t = −2.82, df = 18, P value = 0.01), the cholesterol content (t = 10.52, df = 18, P value < 0.01) and the phospholipids content (t = 6.04, df = 18, P value < 0.01) were significantly higher in the mature digestive glands of south coast (Fig. 2).
Mature gonads of the northwest coast present higher contents in triacylglycerol, cholesterol and phospholipids, although no significant differences were found for the mean content of those bio-indicators between immature and mature individuals (Fig. 2; Table 1). In the south coast, the gonads of mature individuals are significantly higher than immature individuals in triacylglycerol (t = −2.86, df = 18, P value < 0.01) and phospholipids (t = 3.50, df = 18, P value < 0.01).
The FA profile in the gonads was affected by area, maturity, and by the interaction of both factors (Table 3). Mature gonads were rich in SFA, showing the capacity to produce saturated fatty acids namely palmitic acid C16:0, increasing significantly from immature to mature females in the northwest coast. In opposition, the stearic acid C18:0 in the northwest coast decreased from immature to mature gonads (Table 2). MUFA were the second most abundant fatty acids in the gonads, with the presence of vaccenic acid C18:1n7 as a minor FA, presenting significant differences between the northwest coast and the south coast namely in the FA C17:1 and C20:1n9. The FA C16:1n7 was significantly more abundant in immature than in mature gonads from the northwest coast. The PUFA represented between 9 and 13% of the FAME present in the total lipid content of the gonads, with the content in ARA, DHA and EPA decreasing significantly from immature to mature gonads in both study areas. In particular, the significant decrease in DHA in the northwest coast led to significant differences between the DHA/EPA ratio of immature and mature females in that area (Table 1).
The DFA on the FA profile of the females’ gonads between areas showed that the first function explained 79% of the variability, with a good separation between the northwest coast group (class score = 0.82) and the south coast group (class score = −0.97). The FA that contributed the most to the group separation is the C18:0 which is positively correlated with the northwest coast. The DFA for maturity groups showed a good separation between immature (class score = −0.94) and mature individuals (class score = 0.79) with 74% of the variability explained. The FA that contributed the most to the group separation was the C18:0, positively correlated to the immature individuals. The DFA for area x maturity groups (Fig. 3, Gonad) showed a good separation between groups with an explained variability of 95% in concordance with MANOVA results. The FAs that contributed the most to the group separation were C16:0, 18:0 and ARA that were positively correlated with immature gonads from both areas.
Discussion
The δ15N and δ13C signatures in the flesh seem to be adequate short-term indicators of diet and habitat. Cherel & Hobson (2005), working on Psychroteuthis glacialis, suggested that the difference between the δ15N of the flesh relative to the beaks relates to the chitin synthesis and consequent N accretion in the beaks. In the more recently formed regions of the beaks, such as the wing, the δ13C signature of the predator closely matches that of the prey (Hobson & Cherel, 2006), also reflecting to an extent the recent dietary composition. The δ15N and δ13C of beaks are, therefore, used as indicators of habitat and diet preference (Post, 2002; Jackson et al., 2007). In our southern population of O. vulgaris, beak δ13C signature is different between maturity stages, probably a reflection of the recent trophic history of the different maturation stages. The higher δ15N ratio in the beaks from the south coast in relation to the northwest coast is probably related to the higher frequency of occurrence of crustacean prey (e.g., decapod crabs) found in the diet of O. vulgaris sampled (Lourenço, unpublished data). Rosa et al. (2004b) show that the decapod crabs are important prey of the O. vulgaris populations along the Portuguese coast and the presence of crustacean prey in the diet of Loligo forbesi is known to increase the δ15N content of the beaks and flesh of that species (Stowasser, 2004). Darnaude et al. (2004) relate increased δ15N to a significant input from river plumes, through the added input of δ15N-rich particulate organic matter. This, however, does not appear to be the case of our study, since river input is more important on the west than on the south coast.
Considering that the digestive gland is mainly a lipid reservoir and, therefore, has a high lipid content, it is the ideal organ to trace fatty acids as diet bio-indicators (Phillips et al., 2001; Moltschaniwskyj & Johnston, 2006; García-Garrido et al., 2010). In our study the total lipid content ranged between 14.60% in northwest coast immature females, in line with values obtained by Rosa et al. (2004a), and 24.42% in south coast mature females. Contrary to what might be expected for a nutrient-rich area, the digestive glands of the northwest coast population present relatively low lipid content in comparison to the south coast population, in particular to the storage lipid triacylglycerol. This is probably related to nutrient seasonality. In March and April the pre-upwelling conditions in the northeast coast determine a low-fat content in sardine Sardina pilchardus (Bandarra et al., 2006) and horse-mackerel Trachurus trachurus (Bandarra et al., 2001), while on the other hand phytoplankton peaks in the south coast are common in early spring associated with the warmer and nitrogen-rich river plumes. Conditions in the south are then relatively favourable to the development of earlier phytoplankton blooms dominated by dinoflagellates (Navarro & Ruiz, 2006; Crespo et al., 2012), which is corroborated by the higher relative content in ARA, EPA and DHA produced by the community of primary producers (Dalsgaard et al., 2003).
The high digestive gland content of C18:1n9 (≈11% in both study areas), which is indicative of deep waters prey species, and C16:0 (≈20% in both study areas) which is indicative of the presence of herbivorous preys, actually suggest a diverse diet (Piché et al., 2010). Comparing both areas, the DHA/EPA ratio is exceptionally low in the north-western population. According to Dalsgaard et al. (2003), the DHA content should be higher relative to the EPA for a carnivorous species. In their study Rosa et al. (2004b) observed the possibility of a carnivorous preys on the northwest coast, while this study highlights a dominance of a carnivorous preys towards the south coast. A higher triacylglycerol content in the South can in addition be related with the dominance of crustacean preys (Phillips et al., 2003) in the diet of the population of this area, which is also suggested by the δ15N and δ13C of the beaks.
It is, therefore, likely that during the period in analysis the diet of the south coast population had a relatively higher dominance of crustacean prey, those in turn recently fed on lipid-rich plankton.
As in the squids Sepioteuthis lessoniana and Photololigo sp. (Semmens, 1998), or the cuttlefish Sepia officinalis (Blanchier & Boucaud-Camou, 1984), the lipid class contents in the digestive gland do not correlate directly with the lipid contents of the oocytes in the gonad, especially for total lipid content, triacylglycerol and phospholipids (see Fig. 2). The lipid class contents of the digestive gland of mature females show significant differences between regions but this regional difference is not found in the gonads of the same females, which seems to indicate that the factors triggering oocytes maturity are independent of the nutritional value of reserves, even though females generally reach maturity beyond the optimum body mass and independently of gonad size (Lourenço et al., 2012). As income breeders that channel the energy directly from food to the gonad during maturation (Houston et al., 2006; Quetglas et al., 2011), the different levels in triacylglycerol and cholesterol noted in the O. vulgaris digestive glands between geographical areas should be expected to show in the gonads as a reflection of diet. Despite that, only the gonad fatty acid profile presents significant differences related with both area and maturity stage evidencing not only that the composition changes as maturation progresses but also that there are differences between areas at every stage of the process. The gonads of the south coast are richer in PUFA and in MUFA and the gonads of the northwest coast are richer in SFA especially C16:0 and C18:0. Nevertheless, the differences found in the ratio DHA/EPA between areas are marginally insignificant (t = −1.86, df = 18, P value = 0.08), indicating a low impact of trophic habitat in the quality of oocytes. These results partially aggree with the results obtained by Farías et al. (2011). In that study, the authors found a direct effect of diet in the DHA/EPA ratio for Patagonian red octopus Enteroctopus megalocyathus; here, although FA profile in the gonads revealed different between areas, the contents in DHA, EPA or DHA/EPA ratio are identical between the studied areas.
In conclusion, this study shows that, despite the variability observed in the stable isotopic signatures studied, δ15N signature in beaks is a valuable bio-indicator to follow the diet history of different O. vulgaris populations, and the δ13C signature has potential to assess feeding behaviour changes related with maturity in O. vulgaris females. Furthermore, specific lipid classes as the triacylglycerol and the cholesterol and its content in the digestive gland can be used as indicators of their nutritional availability in the local habitat. The fatty acid profile is a good indicator of both nutritional availability in the environment and also of gonads quality as shown by the main EFA content and the EPA/DHA ratio. The environmental availability of these EFA depends on seasonal cycles. Upwelling/downwelling events for example determine changes in the lipidic contents of the plankton and in turn of the prey of octopus, as evidenced by the higher total lipids and cholesterol content in the digestive glands of the animals from the south coast. In spite of their characteristics as income breeders, it is difficult to observe a direct relationship between the lipid class contents in the digestive gland and in the gonad, suggesting that a measure of regulation is exerted between food intake and oocyte formation.
References
Athenstaedt, K. & G. Daum, 2006. The life cycle of neutral lipids: synthesis, storage and degradation. Cellular and Molecular Life Sciences 63: 1355–1369.
Auborg, S., I. Medina & R. Pérez-Martin, 1996. Polyunsaturated fatty acids in tuna phospholipids: distribution in Sn – 2 location and changes during cooking. Journal of Agricultural and Food Chemistry 44: 585–589.
Bandarra, N., I. Batista, M. Nunes & J. Empis, 2001. Seasonal variation in the chemical composition of horse-mackerel (Trachurus trachurus). European Food Research and Technology 212: 535–539.
Bandarra, N. M., I. Batista, M. L. Nunes, J. M. Empis & W. W. Christie, 2006. Seasonal changes in lipid composition of sardine (Sardina pilchardus). Journal of Food Science 62: 40–42.
Bergé, J.-P. & G. Barnathan, 2005. Fatty acids from lipids of marine organisms: molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. In Ulber, R. & Y. Gal (eds), Marine Biotechnology I. Springer-Verlag, Heidelberg: 49–125.
Blanchier, B. & E. Boucaud-Camou, 1984. Lipids in the digestive gland and the gonad of immature and mature Sepia officinalis (Mollusca: Cephalopoda). Marine Biology 80: 39–43.
Bligh, E. & W. Dyer, 1959. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 37: 911–917
Boletzky, S. V., 1975. A contribution to the study of yolk absorption in the Cephalopoda. Zeitschrift für Morphologie der Tiere 80: 229–246.
Boucaud-Camou, E. & R. Boucher-Rodoni, 1983. Feeding and digestion in cephalopods. In Wilburn, K. M. (ed.), The Mollusca. Academic Press, New York: 149–187.
Budelmann, B. U., R. Schipp, & S. V. Boletzky, 1997. Cephalopoda. In Harrison, F. W. (ed.), Microscopic Anatomy of Invertebrates. Wiley-Liss, New York: 119–414.
Cherel, Y. & K. A. Hobson, 2005. Stable isotopes, beaks and predators: a new tool to study the trophic ecology of cephalopods, including giant and colossal squids. Proceedings of the Royal Society B: Biological Sciences 272: 1601–1607.
Cherel, Y. & K. A. Hobson, 2007. Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Marine Ecology Progress Series 329: 281–287.
Cherel, Y., K. A. Hobson & H. Weimerskirch, 2005. Using stable isotopes to study resource acquisition and allocation in Procellariiform seabirds. Oecologia 145: 533–540.
Cherel, Y., V. Ridoux, J. Spitz & P. Richard, 2009. Stable isotopes document the trophic structure of a deep-sea cephalopod assemblage including giant octopod and giant squid. Biology Letters 5: 364–367.
Crespo, B. G., O. Espinoza-González, I. G. Teixeira, C. G. Castro & F. G. Figueiras, 2012. Structure of the microbial plankton community in the NW Iberian margin at the end of the upwelling season. Journal of Marine Systems 95: 50–60.
Dalsgaard, J., M. St John, G. Kattner, D. Müller-Navarra & W. Hagen, 2003. Fatty acid trophic markers in the pelagic marine environment. Advances in marine biology 46: 225–340.
Darnaude, A. M., C. Salen-Picard, N. V. C. Polunin & M. L. Harmelin-Vivien, 2004. Trophodynamic linkage between river runoff and coastal fishery yield elucidated by stable isotope data in the Gulf of Lions (NW Mediterranean). Oecologia 138: 325–332.
Farías, A., J. C. Navarro, V. Cerna, S. Pino & I. Uriarte, 2011. Effect of broodstock diet on the fecundity and biochemical composition of eggs of the Patagonian red octopus (Enteroctopus megalocyathus Gould 1852). Ciencias Marinas 37: 11–21.
García-Garrido, S., I. Hachero-Cruzado, D. Garrido, C. Rosas & P. Domingues, 2010. Lipid composition of the mantle and digestive gland of Octopus vulgaris juveniles (Cuvier, 1797) exposed to prolonged starvation. Aquaculture International 18: 1223–1241.
García-Lafuente, J., J. Delgado, F. Criado-Aldeanueva, M. Bruno, J. Del Río & J. Miguel Vargas, 2006. Water mass circulation on the continental shelf of the Gulf of Cádiz. Deep Sea Research Part II: Topical Studies in Oceanography 53: 1182–1197.
Guerra, A., 1975. Determinación de las diferentes fases del desarollo sexual de Octopus vulgaris Lamarck, mediante uníndice de madurez. Investigacion. Pesquera 39: 397–416.
Hobson, K. A. & Y. Cherel, 2006. Isotopic reconstruction of marine food webs using cephalopod beaks: new insight from captively raised Sepia officinalis. Canadian Journal of Zoology 84: 766–770.
Houston, A. I., P. A. Stephens, I. L. Boyd, K. C. Harding & J. M. McNamara, 2006. Capital or income breeding? A theoretical model of female reproductive strategies. Behavioral Ecology 18: 241–250.
Huang, T., C. Chen, V. Wefler, A. Raftery, 1961. A stable reagent for the Liebermann-Buchardt reaction. Application to rapid serum cholesterol determination. Analytical Chemistry 33: 1405–1407
Jackson, G. D., P. Bustamante, Y. Cherel, E. A. Fulton, E. P. M. Grist, C. H. Jackson, P. D. Nichols, H. Pethybridge, K. Phillips, R. D. Ward & J. C. Xavier, 2007. Applying new tools to cephalopod trophic dynamics and ecology: perspectives from the Southern Ocean Cephalopod Workshop, February 2–3, 2006. Reviews in Fish Biology and Fisheries 17: 79–99.
Kanazawa, A., 2002. Sterols in marine invertebrates. Fisheries Science 67: 997–1007.
Katsanevakis, S. & G. Verripoulos, 2004. Den ecology of Octopus vulgaris Cuvier, 1797, on soft sediment: availability and types of shelter. Scientia Marina 68: 147–157.
Lee, R. F., W. Hagen & G. Kattner, 2006. Lipid storage in marine zooplankton. Marine Ecology Progress Series 307: 273–306.
Lepage, G & C. Roy, 1986. Direct transesterification of all classes of lipids in a one step reaction. Journal of Lipid Research 27: 114–120
Lourenço, S., A. Moreno, L. Narciso, Á. Gonzalez & J. Pereira, 2012. Seasonal trends of the reproductive cycle of Octopus vulgaris in two environmentally distinct coastal areas. Fisheries Research 127–128: 116–124.
Moltschaniwskyj, N. & D. Johnston, 2006. Evidence that lipid can be digested by the dumping squid Euprymna tasmanica, but is not stored in the digestive gland. Marine Biology 149: 565–572.
Navarro, G. & J. Ruiz, 2006. Spatial and temporal variability of phytoplankton in the Gulf of Cádiz through remote sensing images. Deep Sea Research Part II: Topical Studies in Oceanography 53: 1241–1260.
Navarro, J. C. & R. Villanueva, 2000. Lipid and fatty acid composition of early stages of cephalopods: an approach to their lipid requirements. Aquaculture 183: 161–177.
Nixon, M., 1985. Capture of prey, diet and feeding of Sepia officinalis and Octopus vulgaris (Mollusca: Cephalopoda) from hatchling to adult. Vie et Milieu 35: 255–261.
Phillips, K. L., G. D. Jackson & P. D. Nichols, 2001. Predation on myctophids by the squid Moroteuthis ingens around Macquarie and Heard Islands: stomach contents and fatty acid analyses. Marine Ecology Progress Series 215: 179–189.
Phillips, K. L., P. D. Nichols & G. D. Jackson, 2003. Dietary variation of the squid Moroteuthis ingens at four sites in the Southern Ocean: stomach contents, lipid and fatty acid profiles. Journal of the Marine Biological Association of the UK 83: 523–534.
Piché, J., S. J. Iverson, F. A. Parrish & R. Dollar, 2010. Characterization of forage fish and invertebrates in the Northwestern Hawaiian Islands using fatty acid signatures: species and ecological groups. Marine Ecology Progress Series 418: 1–15.
Post, D., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718.
Post, D. M., C. A. Layman, D. A. Arrington, G. Takimoto, J. Quattrochi & C. G. Montaña, 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotopes analyses. Oecologia 152: 179–189.
Quetglas, A., F. Ordines & M. Valls, 2011. What drives seasonal fluctuations of body condition in a semelparous income breeder octopus? Acta Oecologica 37: 476–483.
Quinn, G. & M. Keough, 2002. Experimental Design and Data Analysis for Biologists. Cambridge University Press.
Raheja, R., C. Kaur, A. Sing & A. Bhatia, 1973. New colorimetric method for the quantitative determination of phospholipids without acid digestion. Journal of Lipid Research 14: 695–697
Relvas, P., E. D. Barton, J. Dubert, P. B. Oliveira, Á. Peliz, J. C. B. Da Silva & A. M. P. Santos, 2007. Physical oceanography of the western Iberia ecosystem: latest views and challenges. Progress in Oceanography 74: 149–173.
Rosa, R., P. R. Costa & M. L. Nunes, 2004a. Effect of sexual maturation on the tissue biochemical composition of Octopus vulgaris and O. defilippi (Mollusca: Cephalopoda). Marine Biology 14: 563–574.
Rosa, R., A. M. Marques, M. L. Nunes, N. Bandarra & C. Sousa Reis, 2004b. Spatial-temporal changes in dimethyl acetal (octadecanal) levels of Octopus vulgaris (Mollusca, Cephalopoda): relation to feeding ecology. Scientia Marina 68: 227–236.
Semmens, J., 1998. An examination of the role of the digestive glands of two loliginid squids, with respect to lipid: storage or excretion? Proceedings of the Royal Society B: Biological Sciences 265: 1685–1690.
Semmens, J., G. Pecl, R. Villanueva, D. Jouffre, I. Sobrino, I. Sobrino, J. B. Wood & P. R. Rigby, 2004. Understanding octopus growth: patterns, variability and physiology. Marine & Freshwater Research 55: 367–377.
Shulman, G. E. & R. M. Love, 2006. The biochemical ecology of marine fishes. Advances in Marine Biology 36: 351 pp.
Sieiro, M. P., S. P. Aubourg & F. Rocha, 2006. Seasonal study of the lipid composition in different tissues of the common octopus (Octopus vulgaris). European Journal of Lipid Science and Technology 108: 479–487.
Smith, C. D., 2003. Diet of Octopus vulgaris in False Bay, South Africa. Marine Biology 143: 1127–1133.
Smith, J. M., G. J. Pierce, A. F. Zuur, H. Martins, M. C. Martins, F. Porteiro & F. Porteiro, 2011. Patterns of investiment in reproductive and somatic tissues in the loliginid squid Loligo forbesii and Loligo vulgaris in Iberian and Azorean waters. Hydrobiologia 670: 201–221
Stowasser, G., 2004. Squid and their prey: insights from fatty acid and stable isotope analysis. Thesis presented for the degree of doctor of Philosophy at the University of Aberdeen. University of Aberdeen. Aberdeen.
Tocher, D. R., 2010. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquaculture Research 41: 717–732.
Vander Zanden, M. J. & J. B. Rasmussen, 2001. Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. Limnology and Oceanography 46: 2061–2066.
Villanueva, R. & M. D. Norman, 2008. Biology of the planktonic stages of benthic octopuses. Oceanography and Marine Biology: An Annual Review 46: 105–202.
Zar, J., 1999. Biostatistical Analysis, 4th ed. Prentice Hall, Upper Saddle River.
Zuur, A. F., E. N. Ieno & G. M. Smith, 2007. Analysing Ecological Data. Springer, New York.
Acknowledgments
The authors wish to express their acknowledgment to the anonymous reviewers for their comments, which greatly improved the final version of this paper. The authors also want to thank to António Pedro Mendonça for assistance with the biological sampling and to Cristina Vidal for teaching and supervising the biochemical analyses. Data collection and analytical analysis were conducted under the Data Collection Framework (DCF/DG FISH (EC Regulation No. 665/2008 of the 14 July 2008)) and LARECO project (CTM2011-25929), supported partially by FEDER funds. The first author is supported by a PhD fellowship granted by the Portuguese Foundation for Science and Technology (FCT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Erica A. G. Vidal, Mike Vecchione & Sigurd von Boletzky / Cephalopod Life History, Ecology and Evolution
Rights and permissions
About this article
Cite this article
Lourenço, S., Narciso, L., Gonzalez, Á.F. et al. Does the trophic habitat influence the biochemical quality of the gonad of Octopus vulgaris? Stable isotopes and lipid class contents as bio-indicators of different life-cycle strategies. Hydrobiologia 725, 33–46 (2014). https://doi.org/10.1007/s10750-013-1717-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1717-0