Abstract
Lipid composition of the mantle and digestive gland of Octopus vulgaris that were not fed for 27 days were determined. Every 3 days, three octopuses were killed and samples of the mantle and the digestive gland (DG) were taken, in order to determine total lipids as well as lipid classes and fatty acids. Composition in total lipids (TL) for the mantle was similar until day 21, then decreased and remained similar until the end of the experiment. Composition in total lipids for the DG decreased significantly after 3 days, then remained similar until day 21, and then decreased until the end of the experiment. As for the lipid classes, in the DG the main components were triglycerides and sterol esters. Sterol esters suffered strong reductions after 10 days of starvation, while triglycerides remained similar until day 21 and then decreased until the end of the experiment. Cholesterol decreased gradually throughout the experimental period. For polar lipids, phosphatidylcholine and phosphatidylethanolamine increased during the first 3 days and then decreased throughout the experiment. In the mantle, the only neutral classes that decrease were triacylglycerols and sterol esters, while no polar lipid classes decreased in this organ. It was noticeable the decrease in almost all fatty acids in the DG after 3 days of starvation, while in the mantle there were no differences in fatty acid concentrations during the experiment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Octopus vulgaris is one of the most promising species for aquaculture diversification. It presents high growth rates (Aguado-Giménez and García-García 2003; Iglesias et al. 2006, 2007) and market price (FAO 2008), associated with a great demand throughout many regions of the world. The common octopus has a planktonic larval stage termed as paralarva (Young and Harman 1988) which lasts for 33–40 days when reared at a mean temperature of 24.7°C (Itami et al. 1963) prior to the benthic juvenile stage. The fattening of O. vulgaris is currently done in Galicia, North Spain (Iglesias et al. 1997), but with low profitability due to the dependence on juveniles captured from the wild and the absence of an adequate artificial diet (Domingues et al. 2009). These are two of the major bottlenecks for the commercial aquaculture of this species, which are enhanced by the high mortality in the paralarvae stage of the octopus culture cycle (Iglesias et al. 2007) and the impossibility of obtaining benthic juveniles on a commercial scale in captivity (Cerezo-Valverde et al. 2008).
Research on cephalopod nutrition started in the early 80s (Boucaud-Camou and Boucher-Rodoni 1983; Boucaud-Camou et al. 1985; DeRusha et al. 1989). One of the first studied species was cuttlefish (Sepia officinalis). Dry or moist pellets (Castro 1990; Lee et al. 1991; Castro et al. 1993; Castro and Lee 1994) or surimi, a fish myofibrillar protein concentrate (Castro et al. 1993; Castro and Lee 1994; Domingues 1999; Domingues et al. 2005), was initially used with poor results (Castro et al. 1993; Castro and Lee 1994; Domingues et al. 2005, 2006). In the past 3 years, a greater effort has been made to develop artificial diets in several cephalopods, especially in O. vulgaris (Cerezo-Valverde et al. 2008; Quintana et al. 2008) and Octopus maya (Domingues et al. 2007; Rosas et al. 2007).
Compared to fish, there is little knowledge on energetic physiology of cephalopods, such as food-storage reserves during starvation periods. Tait (1986) reported that the mantle and the digestive gland of O. vulgaris were the organs that suffered higher variations in biochemical composition after long-term starvation. Early research indicated that several cephalopod species use small amounts of lipids and/or carbohydrates during starvation (Mommsen and Hochachka 1981; O’Dor et al. 1984; Boucher-Rodoni and Mangold 1988; Segawa and Hanlon 1988; Boucher-Rodoni 1989) and have predominant amino acid metabolism (Ballantyne et al. 1981; Lee 1994). Nevertheless, the highest concentrations of lipid in cephalopods are consistently found in the digestive gland (Boucaud-Camou and Yim 1980; Mangold 1983; Moltschaniwskyj and Johnston 2006), which is an organ actively involved in lipid digestion and storage (Rosa et al. 2005). There is evidence that this organ is used for short-term lipid storage (Boucher-Rodoni et al. 1987; Fluckiger et al. 2008), and it is considered to be a good indicator of nutritional status (García et al. 2009). Furthermore, the importance of lipids in cephalopod metabolism has been demonstrated, both in early stages of development (Navarro and Villanueva 2000, 2003; Villanueva et al. 2002) or juveniles and adults (Domingues et al. 2003; Almansa et al. 2006; Ferreira et al. 2009; García et al. 2009).
Because of this, mantle and DG total lipid composition, lipid classes and fatty acids (FA) of up to 27-day starving octopuses were determined during this study in order to determine how they are kept, transformed and used by the animal in both organs under starvation conditions and determine the ability to use them as nutritional reserves. This study could provide information about essential lipid classes and FA for O. vulgaris in order to understand their importance for octopus metabolism and nutrition and help to develop efficient prepared diets for the fattening of this species.
Materials and methods
Capture, experimental design and sampling protocol
Octopuses were captured using artisanal bottom trawl nets in coastal waters of Huelva (South Spain) and brought to our research facility (Center IFAPA Agua del Pino, Cartaya, Spain). Animals were acclimated during 2 weeks in a flow-through system composed of 9 concrete tanks, with 4500 l of water each (3 m × 1 m, water depth of 1.5 m), and fed frozen squid (Loligo gahi) with a daily ration of 5% of body weight per day (BW d−1) (wet weight of food/wet weight of the animal) provided twice a day at 09:00 h and 15:00 h.
After this period, a total of 30 immature octopuses (mean weight ± SD = 1618.3 ± 175.5 g) were randomly taken from the acclimation tanks and individually placed in a flow-through seawater system composed of 30 cylindrical tanks of 140 l each, and water flow of 30 l h−1. No significant differences (P > 0.05) were found in initial total weights for the 30 octopuses. Water temperature was of 20 ± 1°C, salinity varied between 36 ± 1 ppt and pH was of 7.9 ± 0.1. Water flow was adjusted in all tanks to maintain oxygen concentration close to saturation levels. The natural photoperiod in South Spain was used for the experiment (14:10 h).
Every 3 days, three octopuses were killed. The first three octopuses to be killed were used as the control treatment since they were fed with squid before the start of the experiment and they were never starved. This was considered to be day 0 of the experiment. No food was provided to the rest of octopuses during the next 27 days.
Total weight (gram wet weight, g WW) and DG weight (g WW) were recorded for every animal in every sampling period. Octopuses were weighed alive in order to calculate initial and final total weight. In every sampling period, DG weight was recorded after octopuses were killed, after being anesthetized on ice for 2 min. Data obtained were used to calculate:
-
Weight loss (%) = Final Weight × 100 × Initial Weight−1
-
Digestive Gland Index, DGI (%) = DG Weight × 100 × Total Weight−1
Weight loss and DGI were recorded for every sampling period.
Three samples (2 g each) of the mantle and the DG of each animal were taken in order to determine total lipid, lipid classes and FA. Samples were collected and mixed with chloroform/methanol (2:1 volume/volume; v/v) and immediately placed on dry ice and stored at −80°C until lipid analysis.
Finally, moisture content was determined from 500 mg samples of the mantle and the DG using the method of Horwitz (1980).
Biochemical determinations: total lipids, lipid classes and fatty acids
Total lipid was extracted with chloroform/methanol (2:1 v/v) containing 0.01% of butylated hydroxytoluene (BHT) as antioxidant (Christie 1982). The organic solvent was evaporated under a stream of nitrogen and the lipid content determined gravimetrically.
Lipid classes were separated by one-dimensional double development high-performance thin-layer chromatography (HPTLC) using methyl acetate/isopropanol/chloroform/methanol/0.25% (weight/volume; w/v) KCl (25:25:25:10:9 by volume), as the polar solvent system and hexane/diethyl ether/glacial acetic acid (80:20:2 by volume), as the neutral solvent system. Lipid classes were quantified by charring with a copper acetate reagent followed by calibrated scanning densitometry using a CAMAG TLC Scanner 3 dual wavelength flying spot scanner (Olsen and Henderson 1989).
TL extracts were subjected to acid-catalyzed transmethylation for 16 h at 50°C, using 1 ml of toluene and 2 ml of 1% sulfuric acid (v/v) in methanol. The resulting fatty acid methyl esters (FAME) were purified by thin-layer chromatography (TLC) and visualized with iodine in chloroform/methanol (2:1 v/v) 98% (v/v) containing 0.01% BHT (Christie 1982). Prior to transmethylation, nonadecanoic acid (19:0) was added to TL as internal standard. FAME were separated and quantified by using a Shimadzu GC-2010 gas chromatograph (GC) equipped with a flame ionization detector (250°C) and a fused silica capillary column RTX-WAXTM (10 m × 0.1 mm I.D.). Helium was used as carrier gas, and the oven initial temperature was 150°C, followed by an increase at a rate of 90°C min−1 to a final temperature of 250°C for 3 min. Individual FAME were identified by reference to authentic standards and to a well-characterized fish oil (FAME Mix C4–C24 and Menhaden Oil, SUPELCO, USA).
Total lipid content, neutral lipids (NL), polar lipids (PL) and lipid classes from the mantle and the DG of starving O. vulgaris for 27 days were calculated in % dry weight (% DW−1). Fatty acids of total lipid from the mantle and DG of starving octopuses for 27 days was calculated in micrograms of fatty acid per milligram of tissue, mantle or DG and dry weight (μg FA mg DW−1).
BHT, potassium chloride, potassium bicarbonate and iodine were supplied by Sigma Chemical Co. (St. Louis, MO). TLC (20 × 20 cm × 0.25 mm), and plates were purchased from Macherey–Nagel (Düren, Germany). Flat-bottom chamber for 10 × 10 cm plates (with stainless steel lid) and HPTLC (10 × 10 cm × 0.2 mm) plates, pre-coated with silica gel (without fluorescent indicator), were purchased from Merck (Darmstadt, Germany). All organic solvents for GC used were of reagent grade and were purchased from Panreac (Barcelona, Spain).
Statistics
Results are presented as means of triplicates ± SD. All data were checked for normal distribution with the one-sample Kolmogorov–Smirnoff test as well as for homogeneity of the variances with the Levene’s test and, when necessary, arcsin transformation was performed. To all data expressed as percentage, arcsin transformation (Fowler et al. 2002) was applied directly. Changes on DGI through days of starvation were analyzed using partial correlation technique. When a normal distribution and/or homogeneity of the variances were not achieved, data were subjected to a Kruskall–Wallis nonparametric one-way ANOVA test based on rank transformation (Zar 1999). In all statistical tests used, P < 0.05 was considered statistically different. Differences in lipid composition of the mantle and DG between the sampling days were analyzed with one-way ANOVA’s. When differences were found, a Tukey multiple comparison test was performed.
Also, polinomic grade 3 regressions (y = a + bx + cx2 + dx3; Zar 1999) were used to study total weight loss, variations in water content of both mantle and DG, and the DGI.
Results
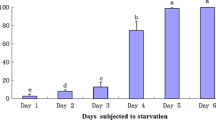
A proportional reduction in total weight was recorded of O. vulgaris according to starvation time. At the end of the experiment and 27-day starvation O. vulgaris lost 35% of total weight (P < 0.05; Fig. 1).
At the end of the experiment, mortality was of 10%, and the three octopuses died after 16, 20 and 23 days of starvation.
Table 1 shows weight of octopus and DG in gram wet weight (g WW), weight loss of octopus and DG in percentage (%), and DGI (%) during the experiment. After 27 days of starvation, octopuses had lost over 35% of their total weight, and 85% of the DG weight. The DGI showed significant changes after 3 days of starvation (Table 1), decreasing consistently during the 27 days of the starvation period.
Moisture of octopus mantle was similar (~80% WW) throughout the experiment (Table 2; Fig. 2), while moisture of the DG (Table 3) increased from the start (57.7% WW), being higher (P < 0.05) after 27 days without eating (76.3% WW). A polinomic grade 3 model of that increment was obtained both for the muscle and the DG moisture changes according to fasting condition (Fig. 3). Muscle and DG moisture changed suddenly at the beginning of the experiment; afterwards, a period of moisture stability was observed between days 10 to 17. After that period, fasting condition provoked a reduction in muscle moisture proportion, while an increment in DG moisture proportion was observed (Fig. 3).
TL from the mantle was different (P < 0.05) after 21 days of starvation and decreased from 5.45% DW at the start to 3.45% DW (P < 0.05) at the end of the experiment (Table 2). Total neutral lipids (TNL) in the mantle decreased gradually and were consistently significantly lower (P < 0.05) after 17 days of starvation. Total polar lipids (TPL) in the mantle only decreased after 21 days of starvation, but after 27 days they were not significantly lower (P > 0.05) than the control (Table 2). No lipid classes were significantly (P > 0.05) lower in the mantle after 27 days of starvation, compared to the control (Table 2).
TL from the DG decreased consistently from 36.5% DW to 9.4% DW, after 27 days of starvation but was only significantly different (P < 0.05) at 27 days of starvation (Table 3). TNL from the DG were only lower compared to the control after 27 days, while TPL were similar (P > 0.05) throughout the experiment, with the exception at the third day of starvation, where an important increase in TPL and all lipid classes occurred (Table 3). For the NL, only triacylglycerols (TG) and sterol esters (SE) were lower after 27 days compared to the control (Table 3). It is interesting to note that when not considering the first sampling period, TG maintained its proportion at the beginning, while after the 21st day it dropped. On the contrary, there was a drop on SE from the start (Table 3).
Generally, the FA in the mantle remained stable throughout the 27 days of starvation (Table 4). Composition was only lower for the 17th day of starvation for pentadecanoic acid (15:0), palmitoleic acid (16:1 n-7), docosapentaenoic acid (22:5 n-3) and docosahexanoic acid (DHA, 22:6 n-3), PUFA, and n-3 and n-3 HUFA. The arachidonic acid (ARA, 20:4 n-6), eicosapentanoic acid (EPA, 20:5 n-3) and DHA concentrations, as well as the DHA/EPA ratio remained unchanged throughout the experiment (Table 4).
For the DG, the majority of FA remained stable until 17 days of starvation (Table 5). After that, concentrations of almost all FA decreased or remained stable. Saturates, monoenes and PUFA decreased consistently after 17 days. Only two FA (22:1 n-9 and 22:5 n-3) decreased significantly (P < 0.05) after only 3 days of starvation (Table 5). The ARA concentration remained unchanged throughout the experiment, while DHA decreased gradually but was only significantly different (P < 0.05) at 27 days of starvation, while EPA concentrations decreased consistently after 17 days of starvation. The n-3 HUFA, n-9 and DHA/ARA ratio decreased gradually but were only significantly different (P < 0.05) at 21 days of starvation. The DHA/EPA ratio remained unchanged during the 27 days (Table 5).
Discussion
Major changes happened in the DG where the weight loss reached 85% at 27 days of starvation, being significant (P < 0.05) even only after 3 days (34%). Starvation period of 27 days provoked a loss of body mass between 28 to 45% of the initial weight, suggesting that animals were using muscle for their metabolic needs.
The DGI decreased consistently during the 27 days of the starvation period (from 7.7 to 1.4%) and showed significant changes even at the first sampling period, only after 3 days of starvation (from 7.7 to 4.1%) (Table 1). Castro et al. (1992) also reported a decrease in the DGI from 7.5% to 4.1% after 53 days of starvation in S. officinalis, although this decline was not as pronounced as the one obtained here. Moreover, the weight of the DG was also considerably reduced and the moisture content increased.
Lipid composition of cephalopods in the mantle is close to 2% (Lee 1994). Both polar and neutral lipid fraction seemed to be equal in mantle content throughout the fasting (≈50% each) (Almansa et al. 2006). Several studies (Sinanoglou and Miniadis-Meimaroglou 1998, 2000; Navarro and Villanueva 2000; Almansa et al. 2006; Ferreira et al. 2009) have shown that mantle of cephalopods is rich in phospholipids, especially phosphatidylcholine (PC), phosphatidylethanolamine (PE) and cholesterol (CHO). Data from the present study confirm this profile for O. vulgaris. However, it is interesting to note the high content of mantle SE at the beginning of the experiment. In general, the mantle lipid composition remained stable, and only SE content showed differences (P < 0.05) after 10 days of starvation. This reduction in SE could be related to their use as energy source from the mantle of O. vulgaris.
The most abundant fatty acids in the mantle of O. vulgaris in this study were palmitic acid (16:0), DHA (22:6 n-3) and EPA (20:5 n-3), similarly to FA composition of the DG. The same fact is reported for mantle of S. officinalis by Ferreira et al. (2009) and for the mantle of many other cephalopod species by other authors (Sinanoglou and Miniadis-Meimaroglou 1998; Miliou et al. 2006; Zlatanos et al. 2006).
No important differences in FA and/or FA series were found in the mantle after 27 days of starvation. In this sense, it is interesting to notice that DHA/EPA ratios for this experiment (~1.5), even after 27 days of starvation, are close to the ones reported for the mantle of O. vulgaris by Miliou et al. (2006), between 1.5 and 1.9, Zlatanos et al. (2006) of 1.5, and by Sinanoglou and Miniadis-Meimaroglou (1998) of 1.5. Even when O. vulgaris (García et al. 2009) and S. officinalis (Ferreira et al. 2009) were in poor nutritional conditions, due to the use of inadequate diets, the ratio DHA/EPA in the mantle was maintained.
In general terms, mantle of cephalopods is characterized by no significant variations, despite the lipid composition of the diet, food ratios or maturation cycle (Moreno et al. 1998; Sinanoglou and Miniadis-Meimaroglou 1998; Moltschaniwskyj and Jackson 2000; Almansa et al. 2006; Ferreira et al. 2009).
DG of cephalopods has high lipid content and lipase activity (Moltschaniwskyj and Johnston 2006) and plays an important role in lipid digestion and metabolism, enzyme secretion, digestion and absorption (Semmens et al. 1995). There is evidence that the DG could be used for short-term lipid storage in some cephalopods (Castro et al. 1992; Fluckiger et al. 2008).
Several studies (Fluckiger et al. 2008; Ferreira et al. 2009) have shown that the European cuttlefish DG is rich in PL (especially PC and PE according to Ferreira et al. (2009)) and NL, especially TG and SE (and in CHO by Ferreira et al. (2009)). The present work confirms that O. vulgaris DG displays a similar NL and PL pattern (Table 3). Also, in underfed conditions for S. officinalis (Ferreira et al. 2009) or starvation (present work), the levels of NL, specifically TG and SE, decreased significantly (P < 0.05).
Total lipids and lipid classes, excepting SE, showed no significant variations in the DG during the first 10 days, and composition of LC in % of total lipids was also maintained during this period. All this suggests that there was a regulated initial stage of lipid mobilization during the first 10 days of starvation, and compensatory mechanisms could be acting to maintain the physiological functions of the digestive gland, reflecting the adaptation of O. vulgaris to tolerate short-term starvation periods. After this period of time, lipids in the DG were more actively used.
The lipid decrease detected in this study was due to a decrease in NL fractions, mainly TG and SE. This suggests that SE and TG could be involved in lipid mobilization for energy purposes. It is known that TG may have a dual application: a metabolic, where it can be used as source for ATP production through oxidation, and/or structural, where it can be a source of FA for PL biosynthesis (Sargent et al. 1995). In this sense, it is interesting to note that all PL classes increased significantly after 3 days of starvation in the DG of O. vulgaris and TG decreased significantly during the same period. This peak could be associated to a possible biosynthesis of polar lipids from FA resultant from the energetic metabolism of TG during the early stages of starvation or to the genetic variability associated to living organism. Castro et al. (1992) also observed a strong variation in lipid classes from cuttlefish starved for 2 days and indicates as one of the possible explanations for this fact the high degree of variability among individual cephalopods (Clarke et al. 1989). The existence of synthesis or transport of lipids from other tissues other than the mantle and subsequent deposition in the gland during the early stages of starvation has been reported for cephalopods (Heras and Pollero 1989, 1990).
On the other hand, PL concentrations in the DG remained unchanged throughout the remaining 27 days of starvation. The more stable characteristic of polar lipids, since they are structural parts of membranes, could explain their unchanged concentrations in the DG throughout the 27 days of starvation.
The most abundant fatty acids in the DG of O. vulgaris in this study were DHA (22:6 n-3), palmitic acid (16:0) and EPA (20:5 n-3). These were also the three FA that were mostly mobilized during the starvation period and should be considered the most important FA in the DG of O. vulgaris. The same fact is reported for O. vulgaris (García et al. 2009) and S. officinalis (Ferreira et al. 2009).
Monoenes are a common energy substrate in marine species (Sargent et al. 1995). During this study, monoenes were used during starvation by octopuses, and a preferential use of saturates and monoenes (mainly) was detected after 21 days of starvation (P < 0.05). This can be expected because of the bigger energetic yield obtained through oxidation of monoenes and saturated fractions, the fact that many PUFA are essential and because of the higher levels of PUFA in the structural lipids. As in the present experiment, the DG of cuttlefish fed artificial diets (and in poor nutritional condition) showed lower contents of monoenes, saturates and PUFA, compared to natural diets (Ferreira et al. 2009). This was also reported for the squid Loligo vulgaris and O. vulgaris (Zlatanos et al. 2006), and the use of monoenes from the DG during long-term starvation periods in S. officinalis were also reported by Castro et al. (1992).
Like in other marine species, fatty acids of the n-6 series are minor components of TL composition, in contrast with those of the n-3 series (Sargent et al. 1995). However, it is noteworthy that no significant differences in levels of ARA in DG were detected in the present study, although differences were detected in other essential fatty acids as EPA and DHA; hence, the gradual decrease in the EPA/ARA ratio and DHA/ARA ratio observed. This could be due either to the maintenance of this FA or a possible ARA biosynthesis. In this sense, results on the fatty acid profile of O vulgaris paralarvae (Navarro and Villanueva 2000; Miliou et al. 2006) showed that ARA, 18:2 n-6 and other n-6 intermediate metabolites present surprizingly high values for a marine species. From these and other findings in other marine molluscs (Uki et al. 1986; Dunstan et al. 1996; Durazo-Beltrán et al. 2003), it is suggested that 20:4 n-6 might not be essential since ability for enzymatic bioconversion of C18 precursors to 20:4 n-6 could be present in this species. Moreover, Almansa et al. (2006) and Miliou et al. (2006) suggested a n-6 HUFA metabolism in cephalopods.
ARA is the primary precursor of eicosanoids in mammals and fish, competing with EPA in this role and is released from membrane phospholipids in response to several stimuli during “arachidonic acid cascade” (Miliou et al. 2006). Eicosanoids have a wide range of physiological actions, for example, in blood clotting, the immune response, the inflammatory response, cardiovascular tone, renal function, neural function and reproduction (Tocher 2003). Therefore, the scarce differences observed in ARA content during the present study could be associated with the important metabolic functions that this fatty acid has, and/or with the maintenance of cell membrane structure and function, as has been suggested by Miliou et al. (2006). All this suggests that ARA is an important fatty acid to be considered in O. vulgaris physiology.
No differences were detected in DHA/EPA ratio in the DG during the 27 days of fasting, suggesting that there was no preferential use in the DG of either during fasting or that a balance was tried to be maintained while using both. Similarly, Navarro and Villanueva (2003) reported the decrease in PUFA and particularly DHA in O. vulgaris paralarvae fed inadequate diets. On the contrary, Ferreira et al. (2009) reported different ratios in the DG of S. officinalis, which were much lower (<0.5), and in this case with significant differences between animals fed crustaceans (between 0.2 and 0.5) and artificial diets, which promote negative growth and poor condition (between 0.9 and 1.0). This author also reported higher ratios when feeding sardine (1.2). Fluckiger et al. (2008) reported lower ratios DHA/EPA in the DG of S. officinalis, compared to the present study, when feeding crustaceans (between 0.3 and 0.6) and higher ratios when feeding fish (between 1.5 and 1.8). Castro et al. (1992) also reported lower ratios DHA/EPA in the DG of this cephalopod species (<0.4). These findings may indicate that different routes of utilization of EPA and DHA between these two cephalopod species indicating that dietary requirements and diet formulation may require different approaches for both species.
DHA plays a multifunctional role in cell membrane physiology (Almansa et al. 2003; Horrocks and Farooqui 2004). On the other hand, EPA is one of the most important eicosanoids precursors, which are implicated in numerous physiological processes (Sargent et al. 1995). The importance of EPA and DHA for cephalopod juvenile nutrition, in this case S. officinalis, was also reported by Perrin et al. (2004).
In conclusion, mantle lipid stability should be beneficial form the consumer point of view. Also, results indicate that the DG could be used as an indicator of the nutritional status of the animal. In this case, levels of SE could be used to determine situations of short-term starvation or underfeeding, while low levels of TG and SE could indicate large periods of starvation or inadequate feeding. Although levels of ARA are low in the DG, this appears to be an important FA in O. vulgaris lipid metabolism. Results also suggest a possible different metabolic route of the use of DHA and EPA in the DG between cephalopods, as O. vulgaris studied here, and S. officinalis. Similar Studies regarding protein and amino acid utilization in the mantle and DG during starvation (due to their important in cephalopod metabolism) should also be conducted, to better comprehend cephalopod general metabolism.
References
Aguado-Giménez F, García-García B (2003) Growth and food intake models in Octopus vulgaris Cuvier (1797): influence of body weight, temperature, sex and diet. Aquacult Int 10:361–377
Almansa E, Sánchez JJ, Cozzi S, Rodríguez C, Díaz M (2003) Temperature–activity relationship for the intestinal Na+-K+-ATPase of Sparus aurata. A role for the phospholipid microenvironment? J Comp Physiol B 173:231–237
Almansa E, Domingues P, Sykes A, Tejera N, Lorenzo A, Andrade J (2006) The effects of feeding with shrimp or fish fry on growth and mantle lipid composition of juvenile and adult cuttlefish (Sepia officinalis). Aquaculture 256:403–413
Ballantyne JS, Hochachka PW, Mommsen TP (1981) Studies on the metabolism of the migratory squid, Loligo opalescens: enzymes of tissues and heart mitochondria. Mar Biol Lett 2:75–85
Boucaud-Camou E, Boucher-Rodoni R (1983) Feeding and digestion in cephalopods. Mollusca Physiol 5(2):149–187
Boucaud-Camou E, Yim M (1980) Fine structure and function of the digestive cell of Sepia officinalis (Mollusca: Cephalopoda). J Zool 191:89–105
Boucaud-Camou E, Yim M, Tresgot A (1985) Feeding and digestion of young Sepia officinalis L. during post-hatching development. Vie Milieu 35:263–266
Boucher-Rodoni R (1989) Consommation d’oxygéne et excrétion ammoniacale de Nautilus macrophalus. C r hebd Séanc Acad Sci Paris 309:173–179
Boucher-Rodoni R, Mangold K (1988) Comparative aspects of ammonia excretion in cephalopods. Malacologia 29:145–151
Boucher-Rodoni R, Boucaud-Camou E, Mangold K (1987) Feeding and digestion. In: Boyle P (ed) Cephalopod life cycles. Academic Press, London, pp 85–108
Castro BG (1990) Can Sepia officinalis L. be reared on artificial food? Mar Behav Physiol 19:35–38
Castro BG, Lee PG (1994) The effects of semi-purified diets on growth and condition of Sepia officinalis L (Mollusca: Cephalopoda). Comp Biochem Physiol 109:1007–1016
Castro BG, Garrido JL, Sotelo CG (1992) Changes in composition of digestive gland and mantle muscle of the cuttlefish Sepia officinalis during starvation. Mar Biol 114:11–20
Castro B, DiMarco FP, DeRusha RH, Lee PG (1993) The effects of surimi and pelleted diets on the laboratory survival, growth, and feeding rate of the cuttlefish Sepia officinalis L. J Exp Mar Biol Ecol 170:241–252
Cerezo-Valverde J, Hernández M, Aguado-Giménez F, García-García B (2008) Growth, feed efficiency and condition of common octopus (Octopus vulgaris) fed on two formulated moist diets. Aquaculture 275:266–273
Christie WW (1982) Lipids analysis, 2nd edn. Pergamon Press, Oxford
Clarke A, Rodhouse PG, Holmes LJ, Pascoe PL (1989) Growth rate and nucleic acid ratio in cultured cuttlefish Sepia officinalis (Mollusca: Cephalopoda). J Exp Mar Biol Ecol 133:229–240
DeRusha RH, Forsythe JW, DiMarco FP, Hanlon RT (1989) Alternative diets for maintaining and rearing cephalopods in captivity. Lab Ani Sci 39(4):306–312
Domingues P (1999) Development of alternative diets for the mass culture of the European cuttlefish Sepia officinalis. PhD thesis, University of the Algarve, Portugal, 95 p
Domingues P, Poirier R, Dickel L, Almansa E, Sykes A, Andrade JP (2003) Effects of culture density and live prey on growth and survival of juvenile cuttlefish, Sepia officinalis. Aquacult Int 11:225–242
Domingues PM, DiMarco FP, Andrade JP, Lee PG (2005) The effects of diets with amino acid supplementation on the survival, growth and body composition of the cuttlefish Sepia officinalis. Aquacult Int 13:423–440
Domingues P, Bettencourt V, Guerra A (2006) Growth of Sepia officinalis in captivity and in nature. Vie Millieu 56:109–120
Domingues P, López N, Muñoz J, Maldonado T, Gaxiola G, Rosas C (2007) Effects of an artificial diet on growth and survival of the Yucatan octopus, Octopus maya. Aquac Nutr 13:273–280
Domingues P, García S, Hachero-Cruzado I, López N, Rosas C (2009) The use of alternative prey (crayfish, Procambarus clarkii, and hake, Merlucius gayi) to culture Octopus vulgaris (Cuvier, 1797). Aquacult Int. doi:10.1007/s10499-009-9259-1
Dunstan GA, Baillie HJ, Barrett SM, Volkman JK (1996) Effect of diet on the lipid composition of wild and cultured abalone. Aquacult Abalone Cult 140:115–127
Durazo-Beltrán E, D’Abramo LR, Toro-Vazquez JF, Vasquez-Peláez C, Viana MT (2003) Effect of triacylglycerols in formulated diets on growth and fatty acid composition in tissue of green abalone (Haliotis fulgens). Aquaculture 224:257–270
FAO (2008) The state of the world fisheries, aquaculture 2008. SOFIA, Rome, Italy 218 p
Ferreira A, Marquez L, Andrade J, Lorenzo A, Domingues P (2009) The use of alternative diets to culture juvenile cuttlefish, Sepia officinalis: effects on growth and lipid composition. Aquac Nutr. doi:10.1111/j.1365-2095.2009.00661.x
Fluckiger M, Jackson GD, Nichols P, Virtue P, Daw A, Wotherspoon S (2008) An experimental study of the effect of diet on the fatty acid profiles of the European Cuttlefish (Sepia officinalis). Mar Biol 154:363–372
Fowler J, Cohen L, Jarvis P (2002) Practical statistics for field biology, 2nd edn. Wiley, West Sussex, England 259 pp
García S, Domingues P, Navarro JC, Hachero-Cruzado I, Garrido D, Rosas C (2009) Growth, partial energy balance, mantle and digestive gland lipid composition of Octopus vulgaris (Cuvier, 1797) fed with to artificial diets. Aquac Nutr. doi:10.1111/j.1365-2095.2009.00746.x
Heras H, Pollero RJ (1989) Blood lipids of the small octopus Octopus tehuelchus (Mollusca: Cephalopoda) at different stages of sexual maturation. Comp Biochem Physiol 92 A:571–575
Heras H, Pollero RJ (1990) Occurrence of plasma lipoproteins in octopods. Partial characterization and interorgan transport of lipids. J Exp Mar Biol Ecol 140:29–38
Horrocks LA, Farooqui AA (2004) Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fat Acids 70:361–372
Horwitz W (1980) Methods of analysis, 13th edn. Association of Official Analytical Chemists, Washington DC
Iglesias J, Sánchez FJ, Otero JJ (1997) Primeras experiencias sobre el cultivo integral del pulpo (Octopus vulgaris Cuvier) en el IEO. In: Costa J, Abellán E, García B, Ortega A, Zamora S (eds), Actas del VI C Nacional de Acuicultura. Cartagena 1997. ISBN: 84-491-0323-1, 221–226 pp
Iglesias J, Fuentes L, Sánchez J, Otero JJ, Moxica C, Lago MJ (2006) First feeding of Octopus vulgaris Cuvier, 1797 paralarvae using Artemia: effect of prey size, prey density and feeding frequency. Aquaculture 261:817–822
Iglesias J, Sánchez FJ, Bersano JGF, Carrasco JF, Dhont J, Fuentes L, Linares F, Muñoz JL, Okumura S, Roo J, van der Meeren T, Vidal EAG, Villanueva R (2007) Rearing of Octopus vulgaris paralarvae: present status, bottlenecks and trends. Aquaculture 266:1–15
Itami K, Izawa Y, Maeda S, Nakai K (1963) Notes on the laboratory culture of the octopus larvae. Bull Jap Soc Sci Fish 29:514–520
Lee PG (1994) Nutrition of cephalopods: fuelling the system. Mar Freshw Behav Physiol 25:35–51
Lee PG, Forsythe JW, DiMarco FP, DeRusha R, Hanlon RT (1991) Initial palatability and growth trials on pelleted diets for cephalopods. Bull Mar Sci 49:362–372
Mangold K (1983) Food, feeding and growth in cephalopods. Mem Natl Mus Vic 44:81–93
Miliou H, Fintikaki M, Tzitzinakis M, Kountouris T, Verriopoulos G (2006) Fatty acid composition of the octopus, Octopus vulgaris, in relation to rearing temperature and body weight. Aquaculture 256:311–322
Moltschaniwskyj NA, Jackson GD (2000) Growth and tissue composition as a function of feeding history in juvenile cephalopods. J Exp Mar Biol Ecol 253:229–241
Moltschaniwskyj NA, Johnston D (2006) Evidence that lipid can be digested by the dumpling squid Euprymna tasmanica, but is not stored in the digestive gland. Mar Biol 149:565–572
Mommsen TP, Hochachka PW (1981) Respiratory and enzymatic properties of squid heart mitochondria. Eur J Biochem 120:345–350
Moreno JEA, Moreno VJ, Ricci L, Roldán M, Gerpe M (1998) Variations in the biochemical composition of the squid Illex argentinus from the South Atlantic Ocean. Comp Biochem Physiol 119B:631–637
Navarro JC, Villanueva R (2000) Lipid and fatty acid composition of early stage of cephalopods: an approach to the lipid requirements. Aquaculture 183:161–177
Navarro JC, Villanueva R (2003) The fatty acid composition of Octopus vulgaris paralarvae reared with live and inert food: deviation from their natural profile. Aquaculture 219:613–631
O’Dor RK, Boucher-Rodoni R, Wells MJ, Wells J (1984) Nutrient absorption, storage and remobilization in Octopus vulgaris. Mar Behav Pysiol 11:239–258
Olsen RE, Henderson RJ (1989) The rapid analysis of neutral and polar marine lipids using double-development HPTLC and scanning densitometry. J Exp Mar Biol Ecol 129(2):189–197
Perrin A, Le Bihan E, Koueta N (2004) Experimental study of enriched frozen diet on digestive enzymes and growth of juvenile cuttlefish Sepia officinalis L. (Mollusca Cephalopoda). J Exp Mar Biol Ecol 311:267–285
Quintana D, Domingues P, García S (2008) Effects of two artificial wet diets agglutinated with gelatin on feed and growth performance of common octopus (Octopus vulgaris) sub-adults. Aquaculture 280:161–164
Rosa R, Pereira J, Nunes ML (2005) Biochemical composition of cephalopods with different life strategies, with special reference to giant squid, Architeuthis sp. Mar Biol 146:739–751
Rosas C, Cuzon G, Pascual C, Gaxiola G, López N, Maldonado T, Domingues P (2007) Energy balance of Octopus maya fed crab and artificial diet. Mar Biol 152:371–378
Sargent JR, Bell JG, Henderson RJ, Tocher DR (1995) Origins and functions of n-3 polyunsaturated fatty acids in marine organisms. In: Ceve G, Paltauf F (eds) Phospholipids: characterization, metabolism and novel biochemical applications. American Oil Chemical Society Press, Champaign, IL, pp 248–259
Segawa S, Hanlon TR (1988) Oxygen consumption and ammonia excretion rates in Octopus maya, Loligo forbesi and lolliguncula brevis (Mollusca: Cephalopoda). Mar Behav Physiol 13:389–400
Semmens JM, Moltschaniwskyj NA, Alexander CG (1995) Effect of feeding on the digestive gland of the tropical sepioid Idiosepius pygmaeus. J Mar Biol Ass UK 75:885–897
Sinanoglou VJ, Miniadis-Meimaroglou S (1998) Fatty acid of neutral and polar lipids of (edible) Mediterranean cephalopods. Food Res Int 31:467–473
Sinanoglou VJ, Miniadis-Meimaroglou S (2000) Phospholipids in Mediterranean cephalopods. J Biosci 55:245–255
Tait RW (1986) Aspects physiologiques de la sénescence post reproductive chez Octopus vulgaris. Ph D Thése L’Université Paris VI
Tocher DR (2003) Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci 11:107–184
Uki N, Sugiura M, Watanabe T (1986) Requirement of essential fatty acids in the abalone Haliotis discus hannai. Bull Jpn Sot Sci Fish 52:1013–1023 (in Japanese, with English abstract)
Villanueva R, Koueta N, Riba J, Boucaud-Camou E (2002) Growth and proteolytic activity of Octopus vulgaris paralarvae with different food rations during first feeding, using Artemia nauplii and compound diets. Aquaculture 205:269–286
Young RE, Harman RF (1988) “Larva”, “paralarvae” and “subadult” in cephalopod terminology. Malacologia 29:201–207
Zar JH (1999) In: Ryu T (ed) Biostatistical analysis, 4th edition. Prentice-Hall Inc, Upper Saddle River, NJ, 663 p
Zlatanos S, Laskaridis K, Feist C, Sagredos A (2006) Proximate composition, fatty acid analysis and protein digestibility-corrected amino acid score of three Mediterranean cephalopods. Mol Nutr Food Res 50:967–970
Acknowledgments
The authors wish to thank the “Plan Nacional de pulpo”—JACUMAR—Project “Engorde de pulpo, Octopus vulgaris.” 2007/2009, for the funding for this research. Sandra García-Garrido wishes to thank the “Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria” (INIA) for the Pre-doctoral grant no 47 (BOE no 308 26/12/2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García-Garrido, S., Hachero-Cruzado, I., Garrido, D. et al. Lipid composition of the mantle and digestive gland of Octopus vulgaris juveniles (Cuvier, 1797) exposed to prolonged starvation. Aquacult Int 18, 1223–1241 (2010). https://doi.org/10.1007/s10499-010-9335-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-010-9335-6