Abstract
Biodiversity patterns in cladoceran communities were investigated in urban waterbodies in relation with residential land use, pond management, and waterbody environments. We evaluated species richness in the pelagic and littoral zones of eighteen waterbodies of a large Canadian city. Gamma diversity (26 species) observed at a small scale in the urban survey was important comparatively to large-scale surveys of lakes. Beta diversity ranged from 1 to 8 species among waterbodies. We tested if littoral species greatly contributed to regional diversity in urban waterbodies. Littoral species (Chydoridae, Ilyocryptidae, Macrothricidae, Polyphemidae) accounted for 58% of the total species pool. We distinguished five cladoceran assemblages associated to different waterbodies (temporary ponds, permanent lakes, and wetlands). Cladoceran communities were more diverse and variable in permanent lakes than in temporary ponds. Changes in cladoceran species assemblages among waterbodies were driven by variations in waterbody size and phosphorus enrichment, macrophyte and algal biomass, urban density, pond management practices, and the presence of potential predators as fish and macroinvertebrates. Our study indicates that both artificial ponds and lakes and natural wetlands are valuable habitats for the conservation of cladoceran biodiversity and rare endemic species in urban regions. Further research on pond management strategies promoting urban aquatic biodiversity should be undertaken.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although small lakes and ponds represent important freshwater systems at a global scale (Downing, 2010), they were, until recently, a neglected component of research in limnology and ecology. Recent research conducted in Europe has shed new light on pond ecosystem structure and function in order to evaluate their role for biodiversity conservation (Céréghino et al., 2008a, b; De Meester & Vyverman, 2010; Miracle et al., 2010; Oertli et al., 2010; De Meester et al., 2011). Most studies conducted in agricultural landscapes recognized farm ponds as important habitats for the maintenance of aquatic biodiversity (Oertli et al., 2002; Biggs et al., 2005; De Bie et al., 2008). Despite their small size, farm ponds often constitute biodiversity “hot spots” (Williams et al., 2004; Scheffer et al., 2006) because they can harbor high local species richness (alpha diversity) or rare and endemic species not commonly found in lakes and rivers. They also contribute to regional diversity (beta- and gamma diversity) due to high variation in community composition through time and space. The importance of ponds for sustaining aquatic biodiversity in urban landscapes was less investigated than in rural landscapes, although they should be taken into account for strategies of management and conservation in urban areas. Pond studies in urban region are suitable for understanding the impacts of human development on aquatic ecosystems and detecting the influence of residential land use and waterbody environments on aquatic communities and biodiversity.
In America, most studies have focussed on large-scale surveys of lakes in relatively unperturbed watersheds, such that knowledge of pond ecology is more limited than in Europe. Only a small number of studies have been carried out on ponds and lakes in urban or agricultural areas in the USA (Beaver et al., 1999; Schell et al., 2001; Dodson et al., 2005; Dodson, 2008; Drenner et al., 2009), and Argentina (Frutos & Carnevali, 2008; José de Paggi et al., 2008). In Canada, no attempt has been made to evaluate the potential of urban waterbodies for sustaining aquatic biodiversity, despite more and more of watersheds being rapidly converted to human land uses in urban regions (BiodivCanada, 2011).
Zooplankton has already been used to assess the biodiversity of man-made ponds and shallow lakes (Serrano & Fahd, 2005; Boix et al., 2008; De Bie et al., 2008; Louette et al., 2008; Antón-Pardo & Armengol, 2010; Léon et al., 2010; Sahuquillo & Miracle, 2010). Among crustaceans, cladocerans may be the best indicator for biodiversity assessment in ponds and small lakes because they are easy to identify, play a key role in food webs, and respond to environmental gradients (Jeppesen et al., 2000). Cladocerans can respond both to bottom-up factors, such as changes in water quality and nutrients, algal resources, and aquatic vegetation (Hann & Zrum, 1997; Walseng et al., 2006; Gélinas & Pinel-Alloul, 2008; Peretyatko et al., 2009), and top-down factors induced by fish and macroinvertebrate predators (Gélinas et al., 2007; Boven & Brendonck, 2009; Drenner et al., 2009). They have a wide range of phenotypes that allow them to colonize, survive, and develop differently in temporary and permanent ponds (Boven & Brendonck, 2009; Drenner et al., 2009). Cladocerans can survive drying or freezing. They are also able to rapidly recolonize ponds as a result of efficient recruitment from the dormant propagule bank and the ability to reproduce parthenogenetically (Brendonck & De Meester, 2003). Cladocerans are also key species for pond restoration because large-bodied daphnids are efficient grazers of algae and may enhance water transparency in shallow lakes and ponds (Peretyatko et al., 2009).
In the present study, we used cladocerans as a model to investigate biodiversity patterns among different types of waterbodies (permanent or temporary ponds, small artificial lakes, and wetlands) in a large urban region in Canada. Variations in cladoceran species richness were evaluated within waterbodies, between littoral and pelagic zones, and among waterbodies. We estimated regional species richness (gamma diversity) in the urban area using cumulative species richness curves and tested if littoral species were major contributors to regional diversity in urban ponds, as observed in lakes (Walseng et al., 2006). We also tested the hypothesis that the cladoceran community structure will differ between the different types of waterbodies due to low dispersal in fragmented landscapes (Shurin, 2000; Vanschoenwinkel et al., 2007, 2011). We evaluated the relative influence of three different types of drivers on cladoceran community structure: (i) landscape use and pond management practices; (ii) morphometry, water transparency, aquatic vegetation, and bottom-up forcing by nutrients and algal biomass; and (iii) top-down forcing by fish and macroinvertebrate predators. Finally, the implications of our findings for pond conservation and management are discussed.
Materials and methods
Study sites and environmental variables
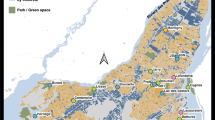
The study was carried out in 18 different waterbodies located throughout the Island of Montreal (Québec, Canada) (45.46–45.69°N; 73.50–73.90°W) (Fig. 1). The Montreal city landscape is dominated by intense residential and industrial activities and supports a high density of human population (1.659 million of inhabitants: http://ville.montreal.qc.ca). Our survey included 15 artificial ponds and small lakes located in municipal parks and residential districts and 3 natural wetlands, including two in large recreation parks (Table 1). These waterbodies represent the diversity of aquatic habitats that can be found in dense urban areas of North American cities. Our sampled waterbodies are surveyed by the water quality monitoring program of the city of Montreal (RSMA: Réseau de suivi du milieu aquatique). Environmental explanatory variables were categorized into three sets: (i) landscape and management, (ii) morphometry and bottom-up, and (iii) top-down variables (Table 1). Ponds and lakes were artificial except Castors, being constructed during the last decades for water retention or recreational purposes, whereas two wetlands (Bizard and Prairies) were formed by a hydrologic network of streams. The sampled waterbodies were evenly distributed in areas of low, medium, and high urban density. Five temporary ponds were emptied each year in autumn and refilled in the spring with municipal water. Three waterbodies were occasionally treated with copper sulfate to control algal blooms. Twelve waterbodies had littoral macrophyte beds, and in three of them, plants were manually removed to decrease macrophyte growth and cover. Waterbody size and trophy were highly variable (Table 1). Surface area ranged from 0.04 to 11.5 ha, perimeter from 0.07 to 2.34 km, and mean depth from 0.3 to 11 m. Secchi water transparency ranged from 0.3 to 3.7 m and, in some shallow ponds, light could reach the bottom. Total chlorophyll biomass varied from 1.1 to 63.9 μg L−1 along a gradient of nutrient enrichment (TP: 8–192 μg L−1). Some eutrophic ponds and wetlands were dominated by abundant populations of cyanobacteria, chlorophytes, and diatoms, whereas pristine artificial ponds supported low populations of chlorophytes and diatoms. Three artificial ponds emptied during winter were fishless, while the others supported diverse communities of littoral fish, mostly planktivorous, including strictly planktivorous sticklebacks in two ponds. The abundance of macroinvertebrates, potential predators of zooplankton in the littoral zone, was also highly variable; the most dominant taxa were the Pleidae, Coenagrionidae, Notonectidae, Veliidae, and Corixidae (Table 1).
Location of the 18 waterbodies sampled in the Island of Montreal in Québec (Canada). Different symbols were attributed to waterbody types: temporary municipal ponds: filled square (Beaubien, Jarry, Lafontaine, Liesse, Pratt); permanent ponds and lakes: filled circle (Angrignon, Battures, Brunante, Castors, Centenaire, Cygnes, Héritage, JBNénuphars, JBalgues, Lacoursière, RMontigny); wetlands: filled triangle (Bizard, Prairies). Site symbols were colored according to the cluster groups (see Fig. 5)
Data collection and field sampling
Data on the origin, urban density, management practices, morphometry, and water quality of each waterbody were obtained from the division of environment of the City of Montréal. Values for the surface and the perimeter of each waterbody were obtained by overlaying a polygon over the waterbody on aerial photographs in Google Earth. Water quality monitoring was done at seven sampling dates during summer 2010 (every 2–3 weeks from May to October). For this study, we only used total phosphorus (TP) concentration measured in July and October as an index of nutrient enrichment and waterbody trophic status.
Field sampling was carried out once between July 7th and 23rd, 2010. We collected triplicate samples in the pelagic and littoral zones, resulting in a total of six samples per waterbody. This sampling scheme enabled us to test within- and among-site variation in species richness and community structure when aquatic vegetation and communities should be the most diverse and abundant. Site locations were determined for each waterbody using a portable GPS (Magellan RoadMate 1470) and recorded in decimal degrees. Depth and water transparency were measured with a Secchi disk at three pelagic points within each waterbody and averaged. At each sampling site, we determined the biomass of total chlorophyll (μg L−1) and of four spectral groups of algae (Green, Blue-green cyanobacteria, Diatoms, Cryptophyta-picocyanobacteria) using a Fluoroprobe probe (Beutler et al., 2002; Pinel-Alloul et al., 2008). At each sampling site, zooplankton was sampled by filtering pond water into a plankton net (20 × 20 cm square opening, 53-μm mesh size) (Filion et al., 1993). A 3-l plastic bucket was dipped at arm’s length (1-m) until a total volume of 30 l was obtained. Zooplankton was preserved in buffered 4% formalin after narcotization in carbonated water. To avoid dispersal of zooplankton organisms among waterbodies, the plankton net was carefully rinsed and dried between sampling trips. A week after field sampling, organisms were removed from the formaldehyde solution, by filtering samples on a 50-μm mesh net, and transferred in 75% ethanol solution. Organisms were concentrated into 25-ml scintillation vials and stained with a rose Bengal solution. Benthic macroinvertebrates were sampled in the littoral zone at a depth of 1 m with a kick net (46 × 23 cm opening, 500-μm mesh size net) that was pushed up to 2 cm in the sediments and dragged over a distance of 1.5 m. In the pelagic zone, macroinvertebrates were sampled in the sediment with an Ekman sampler. Collected macroinvertebrates were screened into 1-mm and 500-μm metal sieves. Both size fractions were pooled in a large bucket and preserved in 75% ethanol solution. Macroinvertebrates were also stained with rose Bengal after sampling to facilitate sorting. The presence (and coarse identification) of fish was based on actual capture during kick netting for macroinvertebrates, visual observations, and information gained from managers.
Zooplankton and macroinvertebrate analyses
In the laboratory, a fixed proportion (25%) of each zooplankton sample was taken with a large opening pipette, transferred to a Ward rotative cell (Ward, 1955), and analyzed under a Leica Wild M3B stereomicroscope. All cladocerans were counted and identified to species or taxa groups using appropriate keys (Edmonston, 1959; Ward & Whipple, 1959; Amoros, 1984; Hebert, 1995). Counts of cladoceran species were expressed as numbers of individuals per liter accounting for subsampling fractionation during microscopic analysis and the total volume of water filtered during field sampling.
Samples of macroinvertebrates were sieved in their entirety in the laboratory on a 500-μm mesh sieve to eliminate small detritus and organic matter. Macroinvertebrates were sorted and counted under a dissecting microscope. All insect larvae were identified to the family level using the work of Merritt & Cummins (1996) and Smith (2001). Total abundances of large insect larvae, known as potential predators of zooplankton (Chaoboridae, Corixidae, Notonectidae, Gerridae, Veliidae, Pleidae, Haliplidae, Dytiscidae, Coenogradidae, Aeshnidae, Libellulinae), were recorded.
Data analyses
For the present study, biodiversity was expressed in terms of species richness, which has become the common currency of biodiversity assessments (Gaston & Spicer, 2004). We measured diversity at three different levels: (i) sample (alpha) diversity as the species richness in a sample collected at each littoral or pelagic site, (ii) local (beta) diversity as the total species richness observed in all pelagic and littoral sites within one waterbody, and (iii) regional (gamma) diversity as the species richness cumulated in all waterbodies within the urban region (Indermuehle et al., 2004). To test the species-area hypothesis (Dodson, 1992), we established the species accumulation curves in the pelagic and littoral zones, and in the whole waterbody, by generating 1,000 random site combinations varying in size from 2 to 17 waterbodies and computing for each combination the mean and standard deviation of species richness (Pinto-Coelho et al., 2005).
As vegetated littoral zones are suitable refuges for large phytophilous cladocerans, they may present higher species richness and therefore be important contributors to biodiversity (Walseng et al., 2006). We evaluated the effects of zone (pelagic or littoral), waterbody (n = 18), as well as their interaction on cladoceran species richness with a mixed-model Analysis of Variance (model I ANOVA). Both pelagic/littoral zone and waterbody were considered as fixed factors. The residuals of the model were extracted in order to test them for normality and homogeneity of variance. We also evaluated how much species found in the littoral zone contributed to the total pool of species compared to the pelagic zone and compared species accumulation curves in pelagic and littoral zones.
K-means clustering analysis (Legendre & Legendre, 1998) was performed on the species abundance data tables at fine (108 sites × 26 cladoceran species) and coarse (18 waterbodies × 26 cladoceran species) levels to evaluate spatial heterogeneity in cladoceran assemblages. Prior to analysis, species data were transformed using the Hellinger method (Legendre & Gallagher, 2001). This analysis consisted of dividing the dataset into k groups using the Calinski–Harabasz criterion (Calinski & Harabasz, 1974) which produced the most parsimonious community dendrogram in relation to waterbody types. K-means clustering was run for 10,000 iterations. We compared cladoceran abundances and community composition among cluster groups.
To reduce the number of explanatory variables in the cladoceran-environment model, we performed a PCA analysis on quantitative environmental variables to seek for the most discriminant environmental factors (Appendix S1). Then, a redundancy analysis (RDA) was performed to determine the significance of the environmental factors in each set of variables (landscape–management, morphometry–bottom-up, top-down factors, Table 1) for explaining variation in cladoceran assemblages among sampled sites and waterbodies. Before computing the RDA, species densities were transformed using the Hellinger pre-transformation (Legendre & Gallagher, 2001). Given that TP concentrations were measured at only one point within each waterbody, we attributed the same value to all samples (replicates in littoral and pelagic zones) for every waterbody. Chlorophyll concentrations (total and each phytoplankton group) and water transparency were measured at the three pelagic sampling points and the average value was attributed at each pelagic site. In two very shallow ponds (Pratt, Beaubien), there was no distinction between pelagic and littoral zones. Thus, the same values were attributed to each zone. Qualitative variables were used as dummy variables and attributed at all sites within a given waterbody. Quantitative variables were transformed (Loge or 4th root) in order to obtain a normal distribution and to reduce its asymmetry (Legendre & Legendre, 1998). The adjusted R 2 value of the full RDA model was corrected using the Ezekeil’s correction (Peres-Neto et al., 2006). To determine the most parsimonious cladoceran-environment model, we used a forward selection procedure with double-stopping criterion (Blanchet et al., 2008). Finally, variation partitioning (Borcard et al., 1992) was applied to evaluate the relative importance of each set of environmental variables in structuring cladoceran community (Appendix S2). The significance of each individual fraction was tested using permutation tests (p-perm = 0.0001 after 9,999 permutations). All statistical analyses were performed in R version 2.1.2.2 (R Development Core Team, 2010).
Results
Cladoceran diversity patterns
We found a total of 26 cladoceran taxa (gamma diversity) in the 18 waterbodies sampled in the urban region of Montreal, but the majority occurred only sporadically (Table 2). The list included 11 Chydoridae, eight Daphniidae, the group of Bosminidae, one Ilyocryptidae, two Macrothricidae, one Polyphemidae, and two Sididae. Mean frequency of species occurrence was 41%. However, only five taxa (Alona spp., Chydorus spp., Bosminidae, Diaphnanosoma brachyurum, Scapholeberis rammneri) were found in more than 10 waterbodies, with occurrence frequency higher than 55%. The cladoceran community showed a high level of endemism as 14 of the 26 species were recorded in less than 5 waterbodies. The Chydoridae were mainly found in the littoral zones, and they contributed strongly to the total pool of species, representing 42% of species occurrence in all waterbodies. The Macrothrycidae, Ilyocryptidae, Polyphemidae, five Chydoridae (Acroperus harpae, Alona affinis, Disparalona hamata, Graptoleberis testudinaria, Leydigia quadrangularis), and one Sididae (Sida crystallina) were strictly found in littoral sites, in low occurrence and abundance. The Daphniidae accounted for 30% of the total pool of species. Strictly pelagic Daphnia species were rare and represented only by D. galeata. The Bosminidae, most of the Daphniidae species (Ceriodaphnia dubia, C. reticulata, Daphnia ambigua, D. pulex, Scapholeberis rammneri, Simocephalus serrulatus, S. vetulus), and one Sididae (Diaphanosoma brachyurum) ranged across littoral and pelagic sites.
Local species richness (beta diversity) was low with only four species per waterbody on average, and it ranged from 1 to 8 species among waterbodies. Median values varied from 1 to 6.5 species per waterbody (Fig. 2a). Sample species richness (alpha diversity) was highly variable (0–12 species). Contrary to our hypothesis, the ANOVA analysis indicated that variation in cladoceran species richness was greater between littoral and pelagic zones within each waterbody than among waterbodies (Table 3). Local sources of variation between the pelagic and littoral zones within waterbodies accounted for 87% of the total variation, whereas regional sources of variation among waterbodies represented only 8.5% of the total variation. However, this pattern of variation varied greatly between waterbodies and was not observed in each waterbody, as indicated by the significant interaction term. Species richness varied widely within several waterbodies (Lafontaine, Cygnes, Bizard, Brunante, Angrignon, Castors, Battures, JBNénuphars, JBAlgues) due to higher cladoceran biodiversity in the littoral zone than in the pelagic zone (Fig. 2b). In contrast, in some small temporary ponds (Beaubien, Pratt, Liesse, Jarry), in two lakes (Centenaire, Lacoursière), and in one wetland (Prairies), no or very little changes in species richness were observed among the littoral and pelagic zones (Fig. 2b).
Local species richness in the 18 sampled waterbodies (a) and species richness in littoral (yellow) and pelagic (blue) zones within each waterbody (b). In total, six samples were taken in each waterbody (a), with three samples in each zone (b). Waterbodies were ordered accordingly to cluster groups (see Fig. 5). The band represents the median and the boxplot margins indicate the first and third quartiles
The accumulation curve of species did not reach an asymptote for the overall biodiversity sampled in the urban region (Fig. 3a), probably because of the high number of species showing very low frequency of occurrence (Table 2). The cumulative curve showed that the small waterbodies do not have a predominant contribution to total cladoceran diversity, probably because the smallest ponds were less rich in species; they were the most artificial (concrete surface, water emptying, and sediment removal every year). Accumulation curves constructed for the littoral and the pelagic zones (Fig. 3b) clearly demonstrated the strong contribution of the littoral species to total cladoceran diversity. On average, the littoral zone supported 2 times more cladoceran species than the pelagic zone.
Cladoceran abundance and community structure
Cladoceran density was highly variable among species (Table 2) and waterbodies (Fig. 4a). The mean density was 41 Ind. L−1 across the urban region and varied from 0.53 to 211 Ind. L−1 among waterbodies. Median values ranged from 0.13 to 203 Ind. L−1. Most of the waterbodies (13/18) supported small cladoceran populations, with less than 50 Ind. L−1. However, 5 waterbodies (Beaubien, Lafontaine, Centenaire, Jarry, Battures) showed a higher density of cladocerans.
Cladoceran abundance (loge(x + 1) transformed) (a) and composition (b) in the 18 sampled waterbodies. The band represents the median and the boxplot margins indicate the first and third quartiles. CHYD Chydoridae; DAPH Daphniidae; BOSM Bosminidae; SIDI Sididae; OTHER Ilyocryptidae, Macrothricidae, Polyphemidae
Community composition was also highly variable among waterbodies (Fig. 4b). It reflected the spatial heterogeneity in cladoceran species assemblages across the urban region, as shown by the clustering analysis (Fig. 5). We discriminated 5 different groups in relation to the types of waterbodies when using local assemblages with littoral and pelagic sites pooled together. These groups represent the most parsimonious dendrogram, each of them having different cladoceran diversities (Fig. 2a) and community compositions (Fig. 4b) associated to different types of waterbodies. Clustering analysis performed using 108 samples sites gave similar groups, although littoral and pelagic sites dissociated in the most diverse waterbodies (data not shown). Group 1 included two temporary shallow ponds located in municipal parks (Beaubien, Lafontaine) which supported relatively diverse (4–5 species) and abundant (69–210 Ind. L−1) cladoceran populations, dominated (86–98%) by the Daphniidae species (Ceriodaphnia reticulata, Daphnia pulex, Scapholeberis rammneri). Group 2 was composed of two very small temporary ponds also located in municipal parks (Pratt, Liesse), containing quite diverse (3–5 species) but less abundant cladoceran populations (5–14 Ind. L−1), also dominated (89–95%) by the Daphniidae species (Ceriodaphnia dubia, Scapholeberis rammneri). Group 3 included five artificial permanent lakes, one temporary pond (Jarry), and one retention reservoir (RMontigny) located in dense residential areas. They formed two subgroups of low (two species) or medium (4–6 species) diversity with distinct species assemblages. Lacoursière, Centenaire, and Jarry were dominated by the Bosminidae; Centenaire and Jarry were the most similar, supporting dense populations (56–131 Ind. L−1); and Lacoursière was distinct due to low density of cladocerans (2 Ind. L−1) and higher abundance of Chydoridae. In the second subgroup, Cygnes, Heritage, and RMontigny showed low cladoceran density (1–8 Ind. L−1) and were dominated by the Chydoridae. RMontigny supported the less diverse and abundant cladoceran populations. Group 4 represented the cladoceran community of the two large wetlands located in natural recreation parks (Bizard, Prairies) that were poor in species (1–3), of low density (1–2 Ind. L−1), and dominated by the Sididae (43–57%); littoral species such as the Macrothricidae and Ilyothrycidae also had higher occurrence frequency in this group. Group 5 included two subgroups of permanent lakes located in areas of lower urban density and in the Botanical Garden. Brunante and Angrignon were included in one subgroup which was more diverse (4–8 species); JBNénuphards and JBalgues were similar in composition, well diverse (5–6 species), and showed intermediate densities (19–32 Ind. L−1); Castor and Battures were both dominated by the Sididae Diaphanosoma brachyurum associated to Bosminidae or Chydoridae.
Cluster groups (dendrogram) of the cladoceran assemblages in the 18 waterbodies. Parsimonious clustering was made at 1.3 height to discriminate cladoceran assemblages according to the different types of waterbodies: G1 and G2 temporary municipal ponds; G3 and G5 mostly permanent ponds and lakes; G4 natural wetlands
Relationships with environmental factors
PCA axes I and II accounted for 45% of the total variation exhibited by the three sets of quantitative environmental variables (Appendix S1). Axis I represented a gradient of waterbody size (surface, perimeter, depth) and algal biomass (Chl, green algae), while axis II was associated with macroinvertebrate density and TP. Diatoms had high loadings on both axes. The RDA model retained 23 explanatory variables with the forward selection procedure among the three types of environmental factors (Table 4). It explained 62% of variation in cladoceran assemblages among and within waterbodies. The presence/absence of fish was the first driver of cladoceran assemblages, followed by the dominance of blue-green/cyanobacteria algae, the abundance of macroinvertebrates (Notonectidae, Chaoboridae, Libellulidae), the level of urban density, and the management practice of emptying in temporary ponds. Axis 1 of the RDA ordination accounted for 21.46% of the explained variation (Fig. 6a). It reflected the differences between the impoverished cladoceran communities of the temporary small ponds emptied once a year, containing chaoborid invertebrate predators but no fish (on the positive side), and the more diverse cladoceran community found in permanent ponds and lakes of large size in area of medium urban density, that had littoral submerged macrophytes and had fish but few chaoboridae. Axis 2 accounted for 15.14% of the total explained variation (Fig. 6a). It reflected a gradient in total chlorophyll and TP concentrations, macrophytes cover, higher biomass of blue-green algae and diatoms, and higher abundance of macroinvertebrates (Notonectidae, Libellulidae).
Triplot of the RDA ordination showing the first 15 explanatory variables (a), the most discriminant cladoceran taxa (b), and the sampled sites in the 18 waterbodies (c): colors were assigned to sampling sites as in cluster groups (see Fig. 5): G1 red open squares, G2 blue squares, G3 green lozenges, G4 yellow triangles, and G5 orange open triangles. See Table 2 for species codes
Cladoceran species distribution was related to environmental gradients. Along the first axis, the Bosminidae were more abundant in the permanent lakes, while Daphniidae were found in the temporary ponds and lakes (Fig. 6b). In the lakes inhabited by littoral planktivorous fish, the small Bosminidae (BOSM) formed very large populations (Centenaire, Battures, Jarry). In the temporary waterbodies, the practices of water emptying and filling at fall and spring created new habitats. This, along with the absence of fish and macrophytes, enabled the successful colonization of different pioneer species that established large populations [Ceriodaphnia reticulata (CERE) in Beaubien, Scapholeberis rammneri (SCRA) in Pratt, Daphnia pulex (DAPU) in Lafontaine, Simocephalus vetulus (SIVE) in Liesse]. Along the second axis, high abundance of Diaphanosoma brachyurum (DIBR) was also observed in the permanent lakes enriched in TP, chlorophyll, and algae (diatoms and blue-green algae), where the macrophytes enabled important populations of macroinvertebrates to develop.
Variation partitioning revealed that the morphometry–bottom-up variables explained about a quarter (26%) of the variation in cladoceran community assemblages (Appendix S2). Landscape and management variables as well as top-down variables accounted for 10–11% of the explained variation. The amount of variation shared between two sets of explanatory variables ranged from less than 1 to 5%. About 6% of the variation in the cladoceran community was shared among all three sets of explanatory factors.
Discussion
It should be noted that this study is based on a single sampling campaign and that seasonal variation was not covered during summer 2010. This may lead to an underestimation of cladoceran species richness in these aquatic habitats subjected to important anthropogenic disturbances. However, recent studies indicate that there are no significant changes in cladoceran species richness during summer (Boven & Brendonck, 2009) or among years (Louette et al., 2008) in temporary or new created ponds. In fact, our short-term survey gave estimates of local and total species richness in urban ponds that are comparable to estimates from European and American large-scale or long-term surveys of land and pond zooplankton. The regional pool of 26 cladoceran species recorded across our small urban region is important comparatively to global species richness of cladocerans reported over large continental scales. Walseng et al. (2006) found a total of 77 littoral and pelagic cladoceran species in 2,466 lakes distributed over the entire mainland of Norway. On average, 33 cladoceran species were recorded in the pelagic zone of 1,891 Norwegian lakes (Hessen et al., 2007) and in 1,665 Canadian lakes (Pinel-Alloul et al., 2013). A total of 88 species of cladocerans were found in reservoirs, lakes, and small waterbodies across the Iberian Peninsula (Alonso, 1991). Moreover, the total species richness found in our study is comparable with that reported in other studies conducted in temperate lakes and shallow ponds in agricultural landscapes of North America and Europe. Drenner et al. (2009) found a total of 28 taxa of crustacean zooplankton in 38 ponds in the Texas grassland, Dodson et al. (2005) 25 cladoceran taxa in shallow lakes within Southeastern Wisconsin, Boven & Brendonck (2009) 31 cladoceran species in 18 temporary freshwater ponds in Hungary, Léon et al. (2010) 27 cladoceran species in 120 farm ponds in southern Spain, and José de Paggi et al. (2008) and Frutos & Carnevali (2008), between 13 and 16 species in urban ponds in Argentina. In a large survey on various types of waterbodies (ditches, pools, wheel tracks, streams, ponds, lakes) in agricultural landscapes of Flanders, De Bie et al. (2008) found a total of 53 cladoceran species, including around 15–20 species in ponds and lakes as in our study. Finally, in a long-term survey (1978–1996), Rusak et al. (2002) reported a total of 33 cladoceran taxa (including 12 Daphnia species) in 22 unmanipulated north-temperate lakes.
Cladoceran taxa recorded in our study showed a wide range in occurrence frequency (5–83%), and most of the taxa occurred at low frequency as observed in other ponds (Drenner et al., 2009) and small lakes (Dodson et al., 2005; Walseng et al., 2006). Cladoceran communities in our urban waterbodies were dominated by the Chydoridae (Alona spp., Chydorus spp., Pleuroxus denticulatus, P. procurvus), the Daphniidae (Ceriodaphnia dubia, Scapholeberis rammeri, Simocephalus serrulatus), the Bosminidae, and the Sididae (Diaphanosoma brachyurum). This assemblage is typical of northern shallow lakes and ponds (Hann & Zrum, 1997; Dodson et al., 2005; Louette et al., 2008; Drenner et al., 2009). As expected, a higher number of littoral species were found in permanent lakes compared to temporary ponds. The dominance of littoral species in permanent lakes reflects the higher diversity of niches in vegetated littoral habitats compared to temporary waterbodies without vegetation (Alonso, 1991; Walseng et al., 2006; Šorf & Devetter, 2011). Most of the taxa of Chydoridae, Ilyotrichidae, Macrothricidae, and Polyphemidae, as well as one species of Sididae (Sida crystallina), were considered as strictly littoral/benthic species associated to macrophytes and sediments (Walseng et al., 2006), whereas the Daphniidae, Bosminidae, and one species of Sididae (Diaphanosoma brachyurum) ranged freely among the pelagic and the littoral habitats. Some daphnid taxa (Ceriodiaphnia, Simocephalus) were found more often in temporary ponds where they can depend of the presence of aquatic vegetation (Hann & Zrum, 1997; Drenner et al., 2009), whereas others (Daphnia, Bosmina, Diaphanosoma) occurred mostly in permanent lakes or in ponds with long hydroperiods (Walseng et al., 2006; Boven & Brendonck, 2009).
According to the species-area curve developed by Dodson (1992) for crustacean zooplankton in North American lakes, we should expected to find 4–9 species in ponds within the range of surface areas observed in our study (0.04–11.52 ha). Indeed, local species richness of cladocerans (beta diversity) in our sampled waterbodies ranged from 1 to 8 species, which is similar to that observed in Texan ponds of similar size (Drenner et al., 2009, pp. 2–10 crustacean species), in small and shallow lakes (surface area <1 ha, depth <1 m) in southeastern Wisconsin (Dodson et al., 2005, pp. 5–9 crustacean taxa; Dodson, 2008, pp. 3–10 crustacean species), in small lakes and ponds in Flanders (De Bie et al., 2008, pp. 4–6 cladoceran species), and in temporary ponds in Hungary (Boven & Brendonck, 2009, pp. 7–8 cladoceran species). Our study on urban ponds did not support the species-area model predicting an increase in zooplankton species richness with lake area (Dodson, 1992; Dodson et al., 2000). The cumulative species richness curve did not reach an asymptote when waterbodies were ranked by increasing size, indicating that many waterbodies may be unsaturated with species. Furthermore, we did not find any significant relationships between cladoceran species richness and size of urban waterbodies (surface area, perimeter or depth: data not shown), in accordance with other lake studies (Walseng et al., 2006; Hessen et al., 2007) and ponds (Drenner et al., 2009).
Our survey revealed a high variation in cladoceran community composition within waterbodies (among littoral and pelagic zones) in permanent lakes, resulting in high variation in alpha diversity. The most heterogeneous and diverse systems (Angrignon, JBNenuphars) that were highly covered by macrophytes, showed threefold higher species richness in the littoral sites than in the pelagic sites. In contrast, in the small temporary ponds without macrophytes and fish (Liesse, Pratt, Beaubien, Lafontaine), the cladoceran community was species poor and very homogeneous. These results point out to the fact that temporary ponds tend to support poorer species assemblages than permanent lakes, as observed in other studies (Drenner et al., 2009). In our urban region, cladoceran assemblages differed clearly among the different types of waterbodies, and several species showed pronounced affinity with one or a few specific waterbody types. Pelagic zooplankton species, like the Bosminidae or the Sididae Diaphanosoma brachyurum, were clearly more associated with deep and permanent lakes. These species, common in natural lakes in Quebec (Pinel-Alloul et al., 1990), have poor dispersal and establishment capacities and are poorly represented in newly created or temporary ponds (Louette et al., 2008). In contrast, small temporary ponds, emptied every year at fall, contained a few species with good dispersal and establishment capabilities, like Daphnia pulex, Ceriodaphnia reticulata, Simocephalus vetulus, and Scapholeberis rammneri. These observations support other reports on rapid colonization of newly created aquatic habitats by cladocerans (Cáseres & Soluk, 2002; Cohen & Shurin, 2003; Louette et al., 2008). They also suggest that cladoceran communities in temporary and permanent urban ponds differ both in composition and size structure, as reported in agricultural ponds (Drenner et al., 2009) and Mediterranean temporary ponds (Alonso, 1991). In addition to previous reports, our study suggests that in a highly fragmented urban area, dispersal and colonization processes (Vanschoenwinkel et al., 2007, 2011) are more limited and priority effect more important than in agricultural open landscapes or in experimental artificial pools. Indeed, cladoceran species richness was very low (<3) in our small temporary ponds, and only one species developed important populations.
Variation in cladoceran community in our urban waterbodies was related to management practices and residential land use, the presence of potential predators as fish and macroinvertebrates, nutrient enrichment, and algal resources. Our RDA model is consistent with previous models that report pond drying and fish as key ecological factors that shape cladoceran communities in small lakes (Welborn et al., 1996; Iglesias et al., 2011) and ponds (Drenner et al., 2009; Gliwicz et al., 2010). Our study showed strong evidence of the effects of the presence of aquatic vegetation, in association with increasing nutrient and algae enrichment, and higher abundance of macroinvertebrate predators on cladoceran community structure. However, it is difficult to disentangle the effects of each type of environmental factors because explanatory variables tend to be intercorrelated. The association between management practices (water emptying, macrophytes removal, chemical treatment) and the absence of fish and macrophytes in municipal ponds shaped profoundly cladoceran communities by decreasing species richness and by simplifying community structure. Other studies have already argued that hydroperiod (length of inundation period) and fish predation are the most important community-structuring factors in temporary pools and wetlands (Jenkins et al., 2003; Drenner et al., 2009; Vanschoenwinkel et al., 2009; Gliwicz et al., 2010). On the opposite side, the presence of macrophytes in permanent lakes and wetlands created the conditions for more diverse cladoceran communities as reported by Peretyatko et al. (2009) and Šorf & Devetter (2011). Finally, our study gave additional support for increasing influence of macroinvertebrate predators on cladoceran community in urban ponds as observed in temporary freshwater pools (Boven & Brendonck, 2009). However, as the abundance of macroinvertebrates (Notonectidae, Libellulidae) was correlated both with TP and chlorophyll concentrations, and the presence of macrophytes, it remained difficult to relate changes in the cladoceran communities to any of these specific bottom-up or top-down variables. Within and among waterbodies, variation in the cladoceran communities in our study may result from complex interactions between indirect effects of management practices and urban land use and direct trophic interactions within the aquatic food webs.
Although caution is needed when generalizing patterns observed in our short-term survey for the conservation and management of urban aquatic habitats, our study indicates that in order to conserve the cladoceran diversity in urban waterbodies, it is necessary to protect aquatic habitats of different types, including temporary and permanent habitats, as both of them are valuable by supporting either rare endemic species or higher biodiversity. Disturbance of urban waterbodies by negative management practices (water emptying, removing of aquatic vegetation and sediment, and chemical treatment) should be avoided to enable saturation of available niches by rich and diverse cladoceran species. Further research must assess determinants of pond ecosystem structure and function and their responses to environmental and anthropogenic disturbances in order to promote conservation of aquatic biodiversity in urban areas.
References
Alonso, M., 1991. Review of Iberian Cladocera with remarks on ecology and biogeography. Hydrobiologia 225: 37–43.
Amoros, C., 1984. Introduction pratique à la systématique des eaux continentales françaises. 5. Crustacés Cladocères. Association Française de Limnologie, Paris.
Antón-Pardo, M. & X. Armengol, 2010. Zooplankton community from restored peridunal ponds in the Mediterranean region (L’Albufera Natural Park, Valencia, Spain). Limnetica 29: 133–144.
Beaver, J. R., A. M. Miller-Lemke & J. K. Acton, 1999. Midsummer zooplankton assemblages in four types of wetlands in the Upper Midwest USA. Hydrobiologia 380: 209–220.
Beutler, M. K., H. Wiltshire, B. Meyer, C. Moldaenke, C. Lüring, M. Meyerhöfer, U. P. Hansen & H. Dau, 2002. A fluorometric method for the differentiation of algal population in vivo and in situ. Photosynthetic Research 72: 39–53.
Biggs, J., P. Williams, P. Whitfield, P. Nicolet & A. Weatherby, 2005. 15 years of pond assessment in Britain: results of lessons learned from the work of pond conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 693–714.
BiodivCanada, 2011. Milieux humides. http://www.biodivcanada.ca/.
Blanchet, F. G., P. Legendre & D. Borcard, 2008. Forward selection of explanatory variables. Ecology 89: 2623–2632.
Boix, D., S. Gascón, J. Sala, A. Badosa, S. Brucet, R. López-Flores, M. Martinoy, J. Gifre & X. D. Quintana, 2008. Patterns of composition and species richness of crustaceans and aquatic insects along environmental gradients in Mediterranean water bodies. Hydrobiologia 597: 53–69.
Borcard, D., P. Legendre & P. Drapeau, 1992. Partialling out the spatial component of ecological variation. Ecology 73: 1045–1055.
Boven, L. & L. Brendonck, 2009. Impact of hydroperiod on seasonal dynamics in temporary pool cladoceran communities. Fundamental and Applied Limnology – Archiv fűr Hydrobiologie 174(2): 147–157.
Brendonck, L. & L. De Meester, 2003. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491: 65–84.
Calinski, T. & J. Harabasz, 1974. A dendrite method for cluster analysis. Communications in Statistics 3: 1–27.
Cáseres, C. E. & D. A. Soluk, 2002. Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia 131: 402–408.
Céréghino, R., J. Biggs, B. Oertli & S. Declerck, 2008a. The ecology of European ponds: defining the characteristics of a neglected freshwater habitat. Hydrobiologia 597: 19–27.
Céréghino, R., A. Ruggiero, P. Marty & S. Angélibert, 2008b. Biodiversity and distribution patterns of freshwater invertebrates in farm ponds of a southwestern French agricultural landscape. Hydrobiologia 597: 43–51.
Cohen, G. M. & J. B. Shurin, 2003. Scale dependence and mechanisms of dispersal in freshwater zooplankton. Oikos 103: 603–617.
De Bie, T., S. Declerck, K. Martens, L. de Meester & L. Brendonck, 2008. A comparative analysis of cladoceran communities from different water body types: patterns in community composition and diversity. Hydrobiologia 597: 19–27.
De Meester, L. & W. Vyverman, 2010. BIOPOOL – EuroDIVERSITY – Connectivity, dispersal and priority effects of drivers of biodiversity and ecosystem function in pond and pool communities. http://biobel.biodiversity.be/project/show/3555.
De Meester, L., P. Kestemont, K. Martens & W. Vyverman, 2011. PONDSCAPE: Towards a sustainable management of pond diversity at the landscape level. http://www.pondscape.be/index.html.
Dodson, S., 1992. Predicting crustacean zooplankton species richness. Limnology and Oceanography 37: 848–856.
Dodson, S., 2008. Biodiversity in southern Wisconsin storm-water retention ponds: correlations with watershed cover and productivity. Lake and Reservoir Management 24: 370–380.
Dodson, S. I., S. E. Arnott & K. L. Cottingham, 2000. The relationship in lake community between primary productivity and species richness. Ecology 81: 2662–2679.
Dodson, S. I., R. A. Lillie & S. Will-Wolf, 2005. Land use, water chemistry, aquatic vegetation, and zooplankton community structure of shallow lakes. Ecological Applications 15: 1191–1198.
Downing, J. A., 2010. Emerging global role of small lakes and ponds: little things mean a lot. Limnetica 29(1): 9–24.
Drenner, S. M., S. I. Dodson, R. W. Drenner & J. E. Pinder III, 2009. Crustacean zooplankton community structure in temporary and permanent grassland ponds. Hydrobiologia 632: 225–233.
Edmonston, W. T., 1959. Fresh-Water Biology. Wiley, New York.
Filion, J.-M., P. Chain & M. Futter, 1993. Cantilevering vertical tow nets to reduce tow-line-induced zooplankton avoidance. Journal of Plankton Research 15: 581–587.
Frutos, S. M. & R. Carnevali, 2008. Zoo-heleoplankton structure in three artificial ponds of North-eastern Argentina. International Journal of Tropical Biology 56: 1135–1147.
Gaston, K. J. & J. I. Spicer, 2004. Biodiversity: An Introduction. Blackwell, Oxford.
Gélinas, M. & B. Pinel-Alloul, 2008. Relating crustacean zooplankton community structure to residential development and land-cover disturbance near Canadian Shield lakes. Canadian Journal of Fisheries and Aquatic Sciences 65: 2689–2702.
Gélinas, M., B. Pinel-Alloul & M. Slusarczyk, 2007. Formation of inducible cyclomorphosis defences in response to YOY fish and invertebrate predation by two Daphnia species co-existing in Lake Brome (Québec, Canada). Hydrobiologia 594: 175–185.
Gliwicz, Z. M., W. A. Wursbaugh & E. Szymanska, 2010. Absence of predation eliminates coexistence: experience from the fish-zooplankton interface. Hydrobiologia 653: 103–117.
Hann, B. J. & L. Zrum, 1997. Littoral microcrustaceans (Cladocera, Copepods) in a prairie coastal wetland: seasonal abundance and community structure. Hydrobiologia 357: 37–52.
Hebert, P. D. N., 1995. The Daphnia of North America: An Illustrated Fauna. CD-ROM. University of Guelph, Ontario.
Hessen, D. O., V. Bakkestuen & B. Walseng, 2007. Energy input and zooplankton species richness. Ecography 30: 749–758.
Iglesias, C., N. Mazzeo, M. Meerhoff, G. Lacerot, J. M. Clemente, F. Scasso, C. Kruk, G. Goyenola, J. García-Alonso, S. L. Amsinck, J. C. Paggi, S. José de Paggi & E. Jeppesen, 2011. High predation is of key importance for dominance of small bodied zooplankton in warm shallow lakes: evidence from lakes, fish enclosures and surface sediments. Hydrobiologia 667: 133–147.
Indermuehle, N., B. Oertli, N. Menetrey & L. Sager, 2004. An overview of methods potentially suitable for pond biodiversity assessment. Archives des Sciences 57: 131–140.
Jenkins, D. G., S. Grissom & K. Miller, 2003. Consequences of prairie wetland drainage for crustacean biodiversity and metapopulations. Conservation Biology 17: 158–167.
Jeppesen, E., J. P. Jensen, M. Sondergaard, T. Lauridsen & F. Landkildehus, 2000. Trophic structure, species richness and biodiversity in Danish lakes: changes along a phosphorus gradient. Freshwater Biology 45: 201–218.
José de Paggi, S., J. Paggi, P. Collins, J. Collins & G. Bernal, 2008. Water quality and zooplankton composition in a receiving pond of the stormwater runoff from an urban catchment. Journal of Environmental Biology 29: 693–700.
Legendre, P. & E. D. Gallagher, 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129: 271–280.
Legendre, P. & L. Legendre, 1998. Numerical Ecology. Elsevier, Amsterdam.
Léon, D., P. Peñalver, J. Caras, M. Juan, F. Fuentes, I. Gallego & J. Toja, 2010. Zooplankton richness in farm ponds of Andalusia (southern Spain). A comparison with natural wetlands. Hydrobiologia 29: 153–162.
Louette, G., L. De Meester & S. Declerck, 2008. Assembly of zooplankton communities in newly created ponds. Freshwater Biology 53: 2309–2320.
Merritt, R. W. & K. W. Cummins (eds), 1996. Introduction to the Aquatic Insects of North America, 3rd ed. Kendall/Hunt Publishing Company, Dubuque, Iowa.
Miracle, M. R., B. Oertli, R. Céréghino & A. Hull, 2010. Pond conservation from science to practice: 3rd European Pond Workshop. Limnetica 29(1): 1–181.
Oertli, B., D. A. Joye, E. Castella, R. Juge, D. Cambin & J.-B. Lachavanne, 2002. Does size matter? The relationship between pond area and biodiversity. Biological conservation 104: 59–70.
Oertli, B., R. Céréghino, J. Biggs, S. Declerck, A. Hull, & M. R. Miracle, 2010. Pond conservation in Europe. Developments in Hydrobiology, Vol. 210. Springer, Dordrecht.
Peres-Neto, P. R., P. Legendre, S. Dray & D. Borcard, 2006. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87: 2614–2625.
Peretyatko, A., S. Tessier, S. De Backer & L. Triest, 2009. Restoration potential of biomanipulation for eutrophic peri-urban ponds: the role of zooplankton size and submerged macrophyte cover. Hydrobiologia 634: 125–135.
Pinel-Alloul, B., G. Méthot, G. Verreault & Y. Vigneault, 1990. Zooplankton species associations in Quebec lakes: variation with abiotic factors, including natural and anthropogenic acidification. Canadian Journal of Fisheries and Aquatic Sciences 47: 110–121.
Pinto-Coelho, R., B. Pinel-Alloul, G. Méthot & K. Havens, 2005. Relationships of crustacean zooplankton with latitude and trophic gradients in lakes and reservoirs of temperate and tropical regions. Canadian Journal of Fisheries and Aquatic Sciences 62: 348–361.
Pinel-Alloul, B., M. Gélinas & A. Ghadouani, 2008. Development and persistence of deep chlorophyll maxima in oligotrophic lakes over the summer season. Verhandlungen des Internationalen Verein Limnologie 30(3): 409–415.
Pinel-Alloul, B., A. André, P. Legendre, J. Cardille, K. Patalas & A. Salki, 2013. Broad-scale geographic patterns of diversity and community structure of pelagic crustacean zooplankton in Canadian lakes. Global Ecology and Biogeography. doi:10.1111/geb.12041.
R Development Core Team, 2010 R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
RSMA. Réseau de suivi du milieu aquatique de la ville de Montréal: http://ville.montreal.qc.ca/portal/page?_pageid=7237,75317908&_dad=portal&_schema=PORTAL).
Rusak, J. A., N. D. Yan, K. M. Somers, K. L. Cottingham, F. Micheli, S. R. Carpenter, T. M. Frost, M. J. Paterson & D. J. McQueen, 2002. Temporal, spatial, and taxonomic patterns of crustacean zooplankton variability in unmanipulated north-temperate lakes. Limnology and Oceanography 47: 613–625.
Sahuquillo, M. & M. R. Miracle, 2010. Crustacean and rotifer seasonality in a Meditteranean temporary pond with high diversity (Lavajo de Abajo de Sinarcas, Eastern Spain). Limnetica 29: 75–92.
Scheffer, M., G. J. van Geest, K. Zimmer, E. Jeppesen, M. Sondergaard, M. G. Butler, M. Hanson, S. Declerck & L. De Meester, 2006. Small habitat size and isolation promote species richness: second-order effects on biodiversity in shallow lakes and ponds. Oikos 112: 227–231.
Schell, J. M., C. J. Santos-Flores, P. E. Allen, B. M. Hunker, S. Kloehn, A. Michelson, R. A. Lillie & S. I. Dodson, 2001. Physical-chemical influences on vernal zooplankton community structure in small lakes and wetlands of Wisconsin, U.S.A. Hydrobiologia 445: 37–50.
Serrano, L. & K. Fahd, 2005. Zooplankton communities across a hydroperiod gradient of temporary ponds in the Donna National Park (SW Spain). Wetlands 25: 101–111.
Shurin, J., 2000. Dispersal limitation, invasion resistance, and the structure of pond zooplankton communities. Ecology 81: 3074–3086.
Smith, D. G., 2001. Pennak’s Freshwater Invertebrates of the United States: Porifera to Crustacea, 4th edn. Wiley, New York.
Šorf, M. & M. Devetter, 2011. Coupling of seasonal variations in the zooplankton community within the limnetic and littoral zones of a shallow pond. Annales de Limnologie – International Journal of Limnology 47: 259–268.
Vanschoenwinkel, B., C. De Vries, M. Seaman & L. Brendonck, 2007. The role of metacommunity processes in shaping invertebrate rock pool communities along a dispersal gradient. Oikos 116: 1255–1266.
Vanschoenwinkel, B., A. Hulsmans, E. De Roeck, C. De Vries, M. Seaman & L. Brendock, 2009. Community structure in temporary freshwater pools: disentangling the effects of habitat size and hydroregime. Freshwater Biology 54: 1487–1500.
Vanschoenwinkel, B., J. Mergeay, T. Pinceel, A. Waterkeyn, H. Vandewaerde, M. Seaman & L. Brendonck, 2011. Long distance dispersal of zooplankton endemic to isolated mountaintops – an example of an ecological process operating on an evolutionary time scale. PLoS ONE 6: 1–10.
Walseng, B., D. O. Hessen, G. Halvorsen & A. K. Schartau, 2006. Major contribution of littoral crustaceans to zooplankton species richness in lakes. Limnology and Oceanography 51: 2600–2606.
Ward, J., 1955. A description of new zooplankton counter. Quarterly Journal of Microscopical Science 96: 371–373.
Ward, H. & G. Whipple, 1959. Freshwater Biology. Wiley, New York: 1248 pp.
Welborn, G. A., D. K. E. Skelly & E. E. Werner, 1996. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics 27: 337–363.
Williams, P., M. Whitfield, J. Biggs, S. Bray, G. Fox, P. Nicolet & D. Sear, 2004. Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biological Conservation 15: 329–341.
Acknowledgments
El-Amine Minouni, Ph.D. candidate, is a student member of the CSBQ (Centre de la Science de la Biodiversité) and the GRIL (Groupe de Recherche Interuniversitaire en Limnologie et en Environnement Aquatique) research networks. We thank all students and research fellows who participated in field sampling (Adrien André, Anne-Hélène Lejeune, Joseph Nzieleu Tchapgnouo, Lama Aldamman, Ginette Méthot) or laboratory analyses (Louise Cloutier, Maryse Robert, Thomas McGran, Nicolas Dedieu). We acknowledge the collaboration of Guy Deschamps, Sylvie Comtois, Claude Juteau, and Denis Fournier from the City of Montreal for logistic support and access to the sites and for supplying land use and water quality data. We thank A. Patoine of the University of Moncton who kindly improved and corrected the English of the manuscript and Ariane Denis-Blanchard who drew Fig. 1 using GIS tools. The research was funded by the CRSNG (Conseil de Recherche en Sciences Naturelles et en Génie) and the FQRNT (Fonds de Recherche du Québec—Nature et Technologies).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Marina Manca & Piet Spaak / Cladocera: Proceedings of the 9th International Symposium on Cladocera
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pinel-Alloul, B., Mimouni, EA. Are cladoceran diversity and community structure linked to spatial heterogeneity in urban landscapes and pond environments?. Hydrobiologia 715, 195–212 (2013). https://doi.org/10.1007/s10750-013-1484-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1484-y