Abstract
Examples from fishless aquatic habitats show that competition among zooplankton for resources instigates rapid exclusion of competitively inferior species in the absence of fish predation, and leads to resource monopolization by the superior competitor. This may be a single species or a few clones with large body size: a cladoceran such as Daphnia pulicaria, or a branchiopod such as Artemia franciscana, each building its population to a density far higher than those found in habitats with fish. The example of zooplankton from two different fish-free habitats demonstrates the overpowering force of fish predation by highlighting the consequences of its absence. Released from the mortality caused by predation, a population of a superior competitor remains at a density equal to the carrying capacity of its habitat, in a steady state with its food resources, consisting of small green flagellate algae, which are successful in compensating high loss rates due to grazing, by fast growth. In such a situation, the high filtering rate of Daphnia or Artemia reduces resources to levels that are sufficient for assimilation to cover the costs of respiration (threshold food concentration) in adults but not in juveniles. This implies long periods of persistence of adults refraining from producing live young, because production of instantly hatching eggs would be maladaptive. Severe competition for limiting resources imposes a strong selective pressure for postponing reproduction or for producing resting eggs until food levels have increased. Offspring can only survive when born in a short time window between such an increase in food levels and its subsequent decline resulting from population growth and intense grazing by juveniles. Such zooplanktons become not only a single-species community, but also form a single cohort with a long-lifespan population. The observations support the notion that diversity may be sustained only where predation keeps densities of coexisting species at levels much below the carrying capacity, as suggested by Hutchinson 50 years ago.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The year 1959 was the centenary of the publication of the first edition of Darwin’s ‘On the origin of species by means of natural selection’ and the 150th anniversary of his birth. Perhaps there was something in the air that year because this was also a time of inspiration and excitement that accelerated our quest to understand the reasons why biotic diversity is so great in some habitats yet reduced in others.
One of these inspirations was the ‘Homage to Santa Rosalia or why are there so many kinds of animals’ by Hutchinson (1959). This essay articulated most of the contemporary ideas of that time on the importance of food chain interactions and diversity of plants as both substrate and food resource as the key reasons for the ‘extraordinary diversity of the terrestrial fauna’. This was soon complemented by Hutchinson’s original notion of high diversity resulting from ‘non-equilibrium conditions’ outlined in another of his famous papers on ‘The paradox of the plankton’ (Hutchinson, 1961). This concept of frequent environmental changes altering the competitive abilities of coexisting species opened the way to what was later known as the ‘intermediate disturbance hypothesis’ (Connell, 1978). Both of Hutchinson papers supported the notion of animal diversity reflecting the diversity of their plant resources, allowing for fine niche partitioning. These earlier papers focused on competition, which at the time, was thought to be the primary factor structuring ecological communities.
However, another source of inspiration that year was an article on cichlids of Lake Nyasa/Malawi by Fryer (1959a) which supported the completely different concept that diversity within a food web may be sustained by predation rather than from the bottom-up processes of competition. Seeking reasons for the stable coexistence of many cichlid species with overlapping food niches in the same benthic habitat, Fryer suggested that this may occur due to the activity of predators by ‘retarding the growth of populations of non-predatory species’, hence ‘helping to prevent competition between them for the available food’. In a second article, he contemplated three particular reasons why predation by piscivores may not only sustain coexistence of different species, but could also assist the speciation of non-predatory species (Fryer, 1959b).
Unaware of Fryer’s hypothesis, Hutchinson (inspired by a conversation with MacArthur) articulated the same notion in his paper on ‘The paradox of the plankton’ (Hutchinson, 1961). He asserted that ‘if one of the two species is limited by a predator, while the other is either not so limited or is fed on by a different predator, co-existence of the two species may in some cases be possible’. This idea was later expanded by Hutchinson’s students and colleagues. Slobodkin (1963) showed that the Lotka-Voltera model of inter-specific competition would preclude the exclusion of inferior species if supplemented with high predation-induced mortality in the population of each competitor. This concept opened the way to the mechanistic theory of competition of Tilman (1982), with the outcome of competition strongly modified by a population’s ability to cope with high loss rates by compensating for high mortality with equally high reproduction. Rosenzweig & MacArthur (1963) suggested that the risk of individual prey to predators is reduced at low population density: that is, below a level equal to the number of prey being able to find a refuge. They argued that prey can persist at densities below those where predators switch to alternate resources or migrate in search of locations with more abundant resources. Paine (1966) demonstrated that experimental removal of a ‘keystone species’ (Paine, 1969), the starfish Pisaster sp., a top predator in the intertidal zone, led to a community of reduced diversity, because the resources became monopolized by a superior competitor (a species of mussel). In a more recent paper, Paine (2002) reached a similar conclusion for plant diversity in the low intertidal zone, which increased when the superior competitor, annual kelp (Alaria marginata), was heavily grazed, thereby permitting competitively inferior perennial species to grow to high abundance. Additional early work documented how the risk of predation can cause herbivores to seek refuge and thus forage ineffectively (Stein & Magnuson, 1976; Lima 1985, 1998). Consequently, either direct predation or changes in prey behavior may increase stocks of primary producers, and reduce competitive exclusion of grazers.
Interestingly, the ‘top-down’ hypothesis has not been successfully applied to explain the high diversity of phytoplankton. Did Hutchinson have some hint that high mortalities in algal populations resulting from strong grazing pressure by filter-feeding zooplankton would lead to a single algal species monopolizing resources rather than to the coexistence of many taxa by preventing resource competition between them, as is the case in our lakes? Nor has the ‘top-down’ explanation been explored with regard to zooplankton diversity, even though the impact of fish predation on zooplankton size distribution has been known since it was first reported by Hrbáček et al. (1961, 1962), and was used as a cornerstone of the size-efficiency hypothesis of Brooks & Dodson (1965, Fig. 1A, B). On the contrary, the role of predation in sustaining the stable coexistence of closely related species has long been considered a hypothesis that is difficult to prove (Chesson, 2000; Chase et al., 2002), and frequently questioned by numerous examples of diversity reduced by predation (e.g. Spiller & Schoener, 1998; Almany & Webster, 2004). Moreover, the high diversity of zooplankton has often been attributed to other reasons, such as resource partitioning, disturbance and density fluctuations (Weider, 1992; Huisman & Weissing, 1999; Chesson, 2000; Abrams & Holt, 2002; Nelson et al., 2005).
Size distribution of a zooplankton community of diverse species composition in Crystal Lake (USA) sampled in 1964 (A) and 1942 (B). Large-bodied zooplankton, superior in competition for resources, were present in 1942 (B) but absent in 1964 (A) due to their inferiority in evading predation by a visually oriented planktivorous fish. The change in size distribution, which occurred between 1942 and 1964, followed the establishment of a landlocked population of alewife (Alosa pseudoharengus) in the lake (A and B adapted from Fig. 4 in Brooks & Dodson, 1965). Judging from the size distribution of the zooplankton community of Lake Czarny (C)—a lake remaining fishless for millennia—the shift in body size would have been more severe in Crystal Lake if all species of fish had been absent in 1942
This ‘top-down’ explanation in relation to zooplankton diversity was eventually suggested by Gliwicz (2001) and supported by experimental evidence showing that stable population density proportions of large- and small-bodied Daphnia species are fixed by size-selective and density-dependent predation by planktivorous fish (Gliwicz & Wrzosek, 2008). The population density level of each species is inversely related to its specific body size, hence the reaction distance from which it can be seen by a foraging fish and the threshold density level at which it is excluded from the fish’s diet.
This explanation also fits an earlier suggestion (Gliwicz, 2002) that only the rates of change of different parameters describing a zooplankton community (the rate of individual body growth, rate of reproduction, population growth rate) are controlled from the bottom-up by resource limitation. In contrast, the state variables (biomass, individual body size, population density) are controlled from the top down, and fixed at a species-specific level by predation. The different nature of the bottom-up and the top-down impacts becomes more apparent when the zooplankton community and the population ecology of an individual are examined in habitats where top-down impacts by planktivorous fish are precluded. These impacts of fish predation are often precluded in large eutrophic and mesotrophic lakes when fish are busy spawning for a limited time, producing a short-lived spring clear water phase by allowing s large-bodied Daphnia to increase in numbers with smaller cladoceran species competitively excluded (Lampert, 1988). Sarnelle (1993) showed that this type of competitive exclusion by large-bodied Daphnia may last for several weeks if the abundance of planktivorous fish is greatly reduced by a fish kill in the preceding winter. Spring clear water phases in large lakes are always terminated by summer when fish find their way to the sites with abundant Daphnia prey. Extensive periods of a clear water “phase” only occur in habitats that are free of fish. Such habitats are, however, always extreme because of one reason or another—the extremity often being the cause for the absence of fish. Here, we use two examples of fish-free lake ecosystems to show that in the absence of fish predation, the size distributions of zooplankton populations shift towards larger individuals (Fig. 1C) and the species diversity is reduced. The abiotic environmental conditions in these two ecosystems could also contribute to the low species diversity. Regardless of the cause of the low diversity, we show that one or a few large-bodied filter-feeding zooplankton species monopolize resources and hold them at extremely low levels that merely allow for slow growth of the most efficient individuals. In this situation, all efforts of an individual become focused on competition for resources and the need to choose the right time for reproduction to allow for the survival of its offspring.
Materials and methods
To gain further insight into the most fundamental features of zooplankton from habitats free of fish, we reexamine our data from two fishless habitats that are distinctly different in their biological, chemical, and morphological characteristics: Lake Czarny in the Tatra mountains, Poland (Gliwicz, 1986; Gliwicz et al., 2001; Slusarczyk, 2009) and Great Salt Lake, Utah, USA (Wurtsbaugh & Gliwicz, 2001; Gliwicz, 2003). We also present unpublished results of experiments designed to explain some peculiarities of reproduction in zooplankton from fish-free habitats.
The lakes
Lake Czarny (LC, Czarny Staw pod Rysami, 49° 11′ 18″ N, 20° 4′ 34″ E) is located just above the timberline at an elevation of 1581 m above sea level in one of the largest valleys in the Tatra ridge. It is a classic example of a glacial cirque lake or tarn with a regular circular shape, an area of 21 ha and a maximum depth of 76 m. It is ultraoligotrophic, with Secchi disc transparency ranging from 10–24 m, and supports low densities of phytoplankton composed of small flagellate Chlorophyta, representing extremely low levels of food for filter-feeding zooplankton. Unlike the neighboring downstream Lake Morskie Oko (at an elevation 1395 m), fish are absent from Lake Czarny [the two lakes are compared in Gliwicz et al. (2001) and Gliwicz (2003)]. The outflow that cascades over a moraine edge down to Morskie Oko is impenetrable to the salmonid fish that have been present in the neighboring lake for millennia. In contrast to the diverse zooplankton community of Morskie Oko, that of Lake Czarny is very simple, being comprised of Daphnia, a single predacious copepod Cyclops abyssorum tatricus (Kozminski), and low densities of the rotifer Asplanchna priodonta Gosse, which appears for a short period in summer.
Great Salt Lake (GSL, 112° 30′W, 42° N), located at an elevation of 1280 m, is another rare example of an aquatic habitat that lacks fish. It is a eutrophic terminal lake, a remnant of the former freshwater Lake Bonneville which covered 49,000 km2 of the Great Basin of western North America 15,000 years ago. The lake’s southern basin (Gilbert Bay), separated from an even more saline northern basin (Gunnison Bay) by a railway causeway, covered an area of 2626 km2 during the study, and had respective mean and maximum depths of 4.9 and 9.5 m and varying salinity within the range of 130–160 g l−1. High salinity levels ensure that this portion of the lake is completely free of fish. This lake has a very simple food web with a plankton community consisting primarily of the flagellated green phytoplankter Dunaliella viridis (Teodoresco), that usually constitutes over 95% of the phytoplankton at any one time, although over 50 phytoplankton taxa have been identified (G. Belovsky, personal communication). D. viridis is the mayor food source for the single zooplankter, Artemia franciscana Kellog, a brine shrimp (Montague et al., 1982; Wurtsbaugh, 1995). We also analyzed plankton in Farmington Bay of the Great Salt Lake. This shallow 260 km2 bay receives considerable river inflow and thus has salinities varying from <10–90 g l−1, and consequently it has a more diverse plankton assemblage including invertebrate predators. It also receives excessive nutrient loading and is hypereutrophic.
Field data
The zooplankton communities of the two lakes were sampled in 1996–1998 (LC, at one station) and 1994–1995 (GSL, at 12 stations), respectively, by vertical hauls from depths of 45 and 3–9 m to the surface using 44- and 30-cm diameter conical plankton nets with 200- and 153-μm mesh (no other rotifer species were revealed from tube samples for phytoplankton counts in the GSL and parallel hauls with 50-μm mesh nets in LC). The samples were preserved in 4% sugar-formaldehyde which prevented the loss of eggs from Daphnia brood cavities in LC. Phytoplankton and microzooplankton samples were collected from each lake using a tube sampler and preserved with either Lugol’s iodine solution (LC) or sugar-formalin (GSL). These samples indicated moderate abundances of ciliated protozoans, but no rotifers. The vertical profiles of temperature in the lakes were assessed with thermistors. The dry weight of GSL Artemia was measured by weighing individual specimens that had been killed in formalin, rinsed in distilled water and dried overnight at 60°C.
Experimental test of the impact of food level on reproductive performance
Live Daphnia and Artemia from each lake were transferred to the laboratory in natural lake water held at a temperature close to that of the lake and placed in the experimental systems subsequently used to assess patterns of reproduction at different food levels.
Daphnia collected from LC in May, from under the ice cover, were grown at a temperature of 6°C (2°C higher than in LC) in a flow-through system (Stich & Lampert, 1984), to minimize food level fluctuations, for 18 days until 70% of the animals growing at the highest food level had laid eggs into their brood cavities. Food was provided by a constant flow of filtered lake water carrying suspensions of the green algae Scenedesmus obliquus. Each 250 ml chamber contained 20 animals and there were three replicate chambers for each of three food levels of 0.015, 0.05, and 0.15 mg POC (particulate organic carbon) l−1: the lowest level corresponding to that observed in the lake throughout the winter until May (Fig. 2). During daily inspections, egg-bearing females were removed from the system and the number of eggs per clutch counted.
Seasonal changes in the mean water column density of three subsequent cohorts of LC Daphnia (thick lines showing means and SE from three vertical hauls) and POC (dotted line) in Lake Czarny [according to Gliwicz et al. (2001) and Slusarczyk (2009)]. The two coexisting Daphnia morphs are not discriminated here, but their densities can be found in Slusarczyk, (2009). The POC measurements used for 1996-97 were assumed to be the same as those of the following year, 1997–1998
Artemia were grown at 20°C (0–5°C lower than GSL in June–September) for 50 days as batch cultures in 36 glass beakers filled with 100 ml filtered lake water supplemented with the green algae D. viridis as food. Each beaker contained one female and one male in coupled pair. Two food levels were employed, fluctuating within the ranges of 0.1–1.0 and 10–20 μg chlorophyll a l−1 (18 and 18 beakers with each), with the lower level corresponding to the natural lake situation throughout the summer and fall. Every day, each Artemia pair was transferred to a new beaker containing fresh medium, while the offspring—both the naupli from the ovoviviparous eggs and cysts—were counted to assess the clutch size. Each of the 36 couples produced at least a single clutch of eggs, but in the low food level many females died on the day that they produced their first clutch.
Results
The datasets from both the Lake Czarny (LC) and Great Salt Lake (GSL) experiments revealed that in the absence of fish, a single species of large-bodied filter-feeding entomostracan monopolized resources (Figs. 2, 3). Food resources in each of the lakes persisted at an extremely low level throughout the summer in spite of the dramatic difference in fertility. Chlorophyll a was undetectable (<1 μg l−1) in LC even during the June peak of POC following the spring overturn (Fig. 2). Chlorophyll was not much higher in GSL (Fig. 3), although the low level in this lake resulted almost entirely from the high feeding rate of Artemia, which are capable of filtering the entire lake volume more than once a day. The severe food limitation persisting in the two habitats (an obvious reason for the population density at the carrying capacity level) resulted in the dominance of a single-cohort generation in both LC Daphnia and GSL Artemia throughout the summer, with younger individuals being gradually eliminated by starvation, and the majority of older individuals refraining from producing immediately hatching eggs (Figs. 4, 5).
Seasonal changes in the mean water column density of A. franciscana (thick lines showing means and SE from 5–12 stations) and epilimnetic chlorophyll a levels (dotted line) in the Great Salt Lake [according to Wurtsbaugh & Gliwicz (2001)]
Seasonal change in the body size distribution and fecundity of LC Daphnia shown as the density of each discrete size class on each of the 12 sampling dates, from 13 March 1996 to 6 January 1997. The proportions of egg-bearing (light shaded) and ephippia-bearing (dark shaded) females are indicated. The two coexisting morphs are not discriminated here, but their size distributions can be found in Slusarczyk (2009). Two discrete cohorts clearly coexisted in the lake from 16 May to 16 October 1996. The earlier generation of adults survived from the summer of 1995, and the new 1996 generation hatched from ephippia (starting a new population of the ‘transparent’ morph) or from instantly developing eggs [starting the new cohort of the ‘orange’ morph, from Gliwicz (2003)]
Seasonal changes in the size distribution and fecundity of GSL Artemia shown as the density of each discrete size class on each of the 10 sampling dates, from 2 June to 14 November 1994. The proportions of egg-bearing females (light shaded) and females with cysts in their egg sacks (dark shaded) are indicated [from Gliwicz (2003)]
The Lake Czarny Daphnia
One-year of data on LC zooplankton (Gliwicz et al., 2001) revealed that the large-bodied Daphnia, the sole filter-feeding herbivore monopolizing resources in the absence of fish, co-exist with cyclopoid copepods, Cyclops abyssorum tatricus, and, sporadically ,with the uncommon predatory rotifer Asplanchna priodonta. In contrast to the scarce small-bodied Daphnia, which reproduce year-round in the fish-containing downstream lake, the LC Daphnia persisted as a single cohort of individuals born or hatched from ephippia during a short summer period when food was most abundant (Fig. 2). The LC Daphnia born in summer were able to over-winter, either as ephippia or in the form of active adults that refrained from reproduction until the following year, when they produced eggs at an age of almost 1-year-old (Fig. 4). The new-year generation was initiated from both ephippial eggs and eggs released by the over-wintering adults. Only a small fraction of the adult population was recruited from the second new-year generation arising from eggs released by a few new generation females. In each of the two generations, reproductive effort was restricted to a short time window when food levels were sufficiently high (Fig. 2) to allow juvenile growth and predation by Cyclops was low enough to permit adequate survival of eggs and neonates. No immediately hatching eggs were produced outside this reproductive period despite the fact that the body lipid levels of the adult Daphnia were as high as at the time of summer reproduction (Gliwicz et al., 2001; Slusarczyk, 2009), suggesting a deliberate halt to reproduction and its postponement until the following summer.
The two LC Daphnia color morphs that were considered to be D. pulicaria Forbes in our earlier study (Gliwicz et al., 2001), were recently shown to represent distinct lineages, with the ‘orange’ morph related to an eastern Nearctic clade of D. pulicaria, and the ‘transparent’ morph related to a European clade of the tenebrosa group (Slusarczyk, 2009). Thus, the previous notion of a single Daphnia species monopolizing resources had to be replaced by a new notion of the two large-bodied Daphnia sub-species coexisting partitioning the scarce resources by adopting dramatically different life histories. Slusarczyk (2009) has shown that while the ‘transparent’ morph was found to complete its life cycle within a single season by investing its resources into diapausing eggs that would hatch the following summer, the ‘orange’ morph remained active throughout the winter, postponing its reproduction until the next-year peak in food abundance, when the newborn had the best chance of surviving and growing to maturity. The gradual shift in size distribution and size-specific fecundity depicted in Fig. 4 has been separately demonstrated for each of the two morphs by Slusarczyk (2009), revealing similarity between the ‘transparent’ LC Daphnia morph and the GSL Artemia (see below).
The ‘orange’ LC Daphnia morph, brought to the laboratory in May and grown in the flow-through system at three different food levels, exhibited the ability to break the pause in reproduction that normally extended to 10 months in the lake (Fig. 6). This restarting of reproduction was due to the presence of higher food levels. In the lowest food level of 0.015 mg POC l−1 (similar to the lake throughout the winter), no eggs were produced. However, at the intermediate food level of 0.05 mg POCl l−1, the first Daphnia produced an egg in just 2 days, and the group of 60 attained a mean clutch size of 1.8 ± 0.9 SD, with 22 of the females releasing eggs. At the highest food level of 0.15 mg POC l−1 the eggs were not produced until the 8th day, but the 42 producing females in this group of 60 attained a mean clutch size of 3.7 ± 1.8 SD).
Clutch size and the time required to produce the first clutch of eggs in ‘orange’ morph LC Daphnia brought into the laboratory in May 2004 and grown in a flow-through system at three different food levels: 0.015 mg POC l−1 (no eggs produced), 0.050 mg POC l−1 (empty circles) and 0.150 mg POC L−1 (filled circles)
The Great Salt Lake Artemia
In the GSL Artemia was the sole zooplankter found across the entire GSL southern basin (Gilbert Bay) from March to December (Fig. 3). After Artemia disappeared in December, chlorophyll increased from ca. 1–25 μg l−1, and an elongate ciliate (measuring 80 × 19 μm) became abundant and persisted throughout the winter (details in Wurtsbaugh & Gliwicz, 2001). Interestingly, whenever grazing by Artemia was prevented in GSL water samples, chlorophyll a levels rose to high levels. When lake water with chlorophyll levels below 0.5 μg Chl l−1 was brought into the laboratory and Artemia removed, chlorophyll a increased to 25 μg chl l−1 in 10 days. In these experiments Dunaliella was the dominant or even the exclusive component of the phytoplankton (details in Gliwicz, 2003).
In the lake, however, the density of Dunaliella was extremely low and its biovolume was sometimes less than that of other taxa. An earlier study (Wurtsbaugh, 1992) also demonstrated that low phytoplankton density was the result of high grazing pressure by Artemia. According to Reeve (1963), a single Artemia filters 240 ml d−1 and therefore, at the average population density of four sub-adult and adult individuals per liter, this branchiopod is capable of filtering the entire lake volume once a day. Thus, the Dunaliella population density remains extremely low, as do the densities of other green algae, diatoms and cyanobacteria that are able to reproduce fast enough to compensate for grazing losses. In contrast to D. viridis, which is a typical euplanktonic species, many other taxa are not suspended in the lake water, but live in refuges where grazing losses are lower, among them large singular diatoms such as Nitchia epithemides and Amphora coffeiformis. These refuges are provided by the interiors of the long tubular setae of the Artemia exoskeleton, which form the combs on the filtration appendages. The exoskeleton is shed at each of the 13 or 14 molts necessary for Artemia to attain maturity and large quantities float in the water. The appendages are more resistant to bacterial degradation than other parts of the exoskeleton because of their thick chitinous walls which provide the necessary flexibility to these locomotory and filtration structures. Each has dozens of long tubes with an extensive exterior and interior surface area colonized by different species of algae and cyanobacteria that grow and multiply fast due to the high nutrient levels and light intensity in the GSL (details in Gliwicz, 2003). This diverse algal–cyanobacteria community was found to represent up to 20% of the available food for adult Artemia throughout the summer and fall, when the preferred free-swimming Dunaliella was held at an extremely low density in the entire GSL southern basin (Fig. 3).
The low phytoplankton availability in the GSL during the summer is the probable reason why: (i) the lipid index of individual Artemia was found to gradually decline from June to November (Wurtsbaugh & Gliwicz, 2001), (ii) the survival of juvenile Artemia was much lower than that of full grown adults (Fig. 5), (iii) Artemia switched their mode of reproduction from cyst production to instantly hatching eggs at low food levels in the lake (Fig. 7), and (iv) Artemia body weight was considerably smaller than in the Farmington Bay of the GSL (Fig. 8), where chlorophyll was much higher and Artemia less abundant due to lower salinity that allowed invertebrate predators to become abundant and control Artemia abundance. A similar phenomenon has been reported for the entire southern basin where a temporary decline in its salinity allowed the predaceous insect Trichocorixa verticalis to invade the pelagic region of the lake and change the ecosystem from the overwhelming domination of Artemia to a multi-species zooplankton community (Wurtsbaugh & Berry, 1990; Wurtsbaugh, 1992).
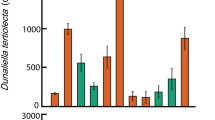
Clutch size and its distribution in time in GSL Artemia brought into the laboratory in July 1994 and grown in a batch culture at two different food levels: 0.5 μg chlorophyll l−1 (low food) imitating the level in the southern bay (A), and 30 μg chlorophyll l−1 (high food) similar to that in the Farmington Bay (B). Note that in Artemia transferred to high food level (B), the production of ovoviviparous eggs (circles) becomes replaced by cysts (triangles) production but only after 10 days of evident adjustment
Length-weight relationships of GSL Artemia from two different habitats: the southern basin offshore station with low food level (<0.5 μg chlorophyll l−1, empty circles) and the Farmington Bay with high food level (20–30 μg chlorophyll l−1, filled circles). The difference between the slopes and the elevations is significant on each of the three datasets at P < 0.0476 for the slopes on 14 August, and P < 0.0001 for the slopes on 15 September and 3 October, and the elevations on all three dates
The algae colonizing discarded exoskeletons are not readily accessible to Artemia juveniles and unavailable to naupli—the exoskeletons are simply too large to be ingested by small naupli (Fig. 9). This may be why Artemia survival was found to be higher in older than younger instars, which is evident from the seasonal change in size distribution shown by the densities of discrete size classes (Fig. 5).
A The residue viewed under a dissecting microscope of a typical plankton sample with a153-μm mesh net from the Great Salt Lake with two Artemia cysts, a day old nauplius (top-right corner), and a grown adult male (bottom-left corner). In the background are multiple shed exoskeletons of Artemia filtering limbs, each with green algae (mainly Dunaliella) colonizing the interior and exterior surface of each seta that can be seen under higher magnification of an inverted microscope (B). The size proportions show that algae colonizing discarded exoskeletons are not accessible to Artemia naupli and not easy to ingest by Artemia adults, yet many adults sampled from the lake had their intestines filled with densely packed exoskeletons with most algae digested, but some surviving the gut passage alive
From June onwards the larger juveniles (>5 mm body length) grew slowly by about 1 mm per month (Fig. 5). The increase in body length in the main cohort of Artemia juveniles stopped altogether in August, when the majority of animals attained maturity and their reproduction became mainly oviparous, with 96–98% producing clutches of cysts. Small numbers of naupli continued to be produced in mid-summer and up until September, but survival of these was apparently minimal and few grew beyond sizes of 2–3 mm (Fig. 5).Thus, there was no measureable recruitment into the early juvenile stages (3–5 mm) until the last adults died in December.
It is possible that recruitment of nauplii was decreased during the low food period due to a phenomenon that females can withhold eggs in the egg sacks and not release them into the lake water. This phenomenon was observed in the laboratory experiments (Gliwicz et al., 1995). The eggs were retained in the brood sacs as long as two conditions remained unfulfilled:
-
(1)
A new clutch of eggs has to be produced in the ovaries. Below a threshold food concentration, Artemia females are prevented from allocating sufficient resources to reproduction, which results in a long inter-brood interval as the clutches of eggs are withheld in the brood sacs. Even at the raised temperature of 25°C, many ovigerous females failed to release eggs from their brood sacs until they eventually died after 10 to 16 days.
-
(2)
A male has to be available to fertilize the next clutch of eggs. This was found to be the case with both ovoviviparous and oviparous eggs. On several occasions, in experiments to assess inter-brood intervals, two females were accidentally kept without a male. In this situation, both individuals retained the eggs in their brood sacs for up to 14 days, until the mistake was recognized.
A switch in reproduction from cysts to instantly developing eggs was observed in the GSL Artemia when transferred from low to high food level. A dramatic difference in selecting the mode of reproduction was also apparent between mating couples grown at the different food levels: the low food level (0.5 μg chlorophyll a l−1) imitated the situation in the southern bay, while the high food level (30 μg chlorophyll a l−1) was similar to that found in the Farmington Bay (Fig. 7). At the low food levels, only 1 of the 13 broods produced was ovoviviparious, with the bulk of the reproduction going into cysts, and brood size were relatively small—15.6 (mean ± 7.3 1SD) eggs female−1 day−1. In contrast, after the 10th day in the high food level treatment, 85% of the broods were ovoviviparous, and mean brood size was 53.6 (mean, ±24.8 1 SD) eggs female−1 day−1. Note, however, that there were frequent shifts between ovoviviparity and cyst production even within single mating pairs (Fig. 7).
Discussion
Habitats free of fish—a single lesson from two different lakes
The analysis of two very different systems lacking fish predators, and few invertebrate predators shows how a dominant herbivore can monopolize food resources and minimize the growth of other plankton—even their own offspring. In the Great Salt Lake, the dominance of Artemia and the low zooplankton diversity is largely driven by the fact that few invertebrates have evolved to tolerate salinities above 10% (Williams et al., 1990). Nevertheless, the importance of predation in structuring the Great Salt Lake community was demonstrated during extreme wet years when the salinity was reduced to <6%, and the invertebrate predator, T. verticalis, invaded the open waters of the lake and greatly reduced the abundance of Artemia. Without the dominant herbivore, phytoplankton levels rose markedly, and the community became more diverse with large populations of rotifers, two copepods and some Artemia (Wurtsbaugh, 1992).
The phenomenon of withholding eggs in the ovaries or in brood sacs by Artemia females may also be associated with the ability of females to assess whether food levels are above a threshold concentration sufficient to allow naupli and neonates to survive and grow. This Artemia reproductive behavior would thus resemble that of LC ‘orange’ Daphnia which refrain from reproducing until the next-year time window where the food level has increased to meet the demands of the offspring. Although such a possibility cannot be excluded, this phenomenon would most probably play a different role in Artemia. Our experimental Artemia females shifted between producing ovoviviparous eggs and cysts every second, third or fourth clutch. Even at very low food levels, there was never a complete switch to cyst production. Instead, the inter-brood interval was extended or females refrained from releasing eggs altogether (Gliwicz et al., 1995). In the very low summer food levels in the GSL, the fate of most ovoviviparous eggs was death, but cysts fared better. Despite the fact that massive numbers of cysts are harvested for the aquaculture industry from the lake surface (1,800 tons dry weight annual average in 1990–1996; Wurtsbaugh & Gliwicz, 2001), many would be left intact until the high spring temperatures and high food levels allow them to hatch and initiate the new generation.
GSL Artemia naupli can survive, grow in length and even molt to produce the second or third instars on maternal reserves only. However, at food levels below the threshold concentration required for assimilation to equal respiration, they cannot increase body mass. The threshold food concentration for Artemia juveniles is presumably higher than that for adults, as is the case in other filter-feeding herbivores such as Daphnia (Gliwicz, 1990; Kreutzer & Lampert, 1999). Food limitation is also likely to be more severe for juveniles because the diverse algal–cyanobacteria community colonizing Artemia exoskeletons is not accessible to them. This inability of naupli and juveniles to survive competition with adults was confirmed in our laboratory and in in situ experiments (Gliwicz et al., 1995); the naupli lost weight and died as 15-day-old juveniles at the length that they had hatched at. The only reasonable explanation for the production of some clutches of ovoviviparous eggs in their natural habitat is that the lake’s spatio-temporal complexity (with shallow bays and estuaries of small rivers in which food levels may periodically be higher than offshore) affords latecomers some chance of survival.
The LC Daphnia juveniles are probably unable to survive the long periods of low food during the winter. The experiments with the ‘orange’ morph of LC Daphnia brought into the laboratory in May showed that females are able to assess the chance of juvenile survival in the lake and do not reproduce until they receive strong and persistent information indicating higher food levels. Furthermore, most appeared to wait for another couple of days to make sure that any food increase was not a short-term phenomenon (Fig. 6). Otherwise, they seem willing to postpone reproduction until the time window in July when both higher food levels (Fig. 2) and a lowered risk of falling prey to Cyclops (Gliwicz et al., 2001) increase the chances of survival and growth of their offspring. Both factors may work in tandem, complementing and reinforcing one another, with individual fitness stemming from a trade-off in terms of selecting the right time for reproduction.
The July time window for the reproduction of the ‘orange’ LC Daphnia morph also represents a high food window for the ‘transparent’ LC Daphnia morph to hatch from ephippia. At present, we do not know whether this morph could be induced to switch its mode of reproduction from producing resting eggs to oviparity if they were taken from the lake in October, when the last females produce their final eggs of the year (Fig. 4). Throughout the time the two morphs coexist in the lake, the ‘transparent’ LC Daphnia has remained significantly smaller than the ‘orange’ LC Daphnia morph (Slusarczyk, 2009). This smaller size may be the reason for its competitive inferiority and the slightly higher food threshold concentration needed for growth and reproduction, as predicted by the size-efficiency hypothesis (Brooks & Dodson, 1965; Gliwicz, 1990). Thus, it may also explain why the ‘transparent’ LC Daphnia does not remain active in wait for the June peak in food abundance. Instead, in competition with the superior ‘orange’ morph for scarce food resources, it produces diapausing eggs to successfully survive the long, cold and hungry winter.
Even without the synchrony of reproduction observed in the ‘transparent’ LC Daphnia, the pattern of the growth and survival for an Artemia cohort is nearly the same (Figs. 4, 5). However, in Artemia, the outcome is not exclusively the product of inter-clonal and intraspecific competition for resources, but also reflects a more complex mode of obligatory sexual reproduction. A male Artemia grasps the female using its powerful claws, renewing its permanent grip after the molt to stay with the same sexual partner for the next stage duration. This avoids competition with co-occurring males that might otherwise inseminate a clutch of eggs ready to be released to the egg sac (‘post-insemination mate association’). This behavior, where time is invested to prevent the partner from re-mating, is common in insects and mites (Alcock, 1994); an increased chance of paternity is preferred to the possibility of fertilizing another female that lacks a mate.
The time window for reproductive success is clearly different in GSL Artemia and LC Daphnia. In GSL, early spring, when the primary producers have recovered from the previous year’s grazing pressure, is the only time of high food levels and appropriate temperatures for growth. Therefore, this is the time of mass hatching from diapausing cysts and of intense reproduction in Artemia (Wurtsbaugh & Gliwicz, 2001). In 1995, most of the first-cohort Artemia were already adult by 5 May, with 90% of the females bearing large clutches of ovoviviparous eggs: 170 (±12) eggs per clutch (mean ± SE) or 77 eggs l−1. However, at the next sampling (5 June), the density of juveniles was only 7 ind. l−1, or a tenth of the expected value based on egg production. Therefore, 90% of the second generation hatching from ovoviviparous eggs had died, evidently because food levels had declined from 25 μg chl a l−1 on 10 May to <1 μg chl a l−1. Most of the survivors may have been juveniles that had hatched early enough to enjoy high food levels, so that the population mainly consisted of the offspring of the first members of the new generation arising from cysts that had over-wintered at locations where spring began earlier. Other reproductive patterns have been noted in the GSL in different years, with nauplii production and growth into juvenile stages occurring in the summer of some years (G. Belovsky, personal communication).
The results of hatching experiments with both Daphnia and Artemia showed that the adult females are physiologically ready and, in spite of food shortages, have accumulated sufficient maternal resources to reproduce. These experiments also showed that the temporary abstention from reproduction of immediately hatching eggs is an important life-history decision in both species when the chance of survival has been reduced due to the sub-threshold food levels available to the newborn. With the perspective that food resources would be held at this low level by adults until they die or until the spring overturn makes food more abundant, the females either have to wait, or to produce resting stages (ephippia or cysts). The former strategy is employed by the ‘orange’ LC Daphnia lineage, while the latter by the ‘transparent’ LC Daphnia and the GSL Artemia.
In contrast to the ‘orange’ LC Daphnia that over-winter in temperatures close to 4°C, the GSL Artemia cannot survive winter temperatures that can fall to −1°C throughout this lake. As a result, the population ceases to exist in December, and restarts again the following spring with the hatching of cysts. The newborn juveniles enjoy high food levels which allow high rates of growth and reproduction and at this point, the GSL Artemia represent a perfect example of a typical time-limited population (Schoener, 1973). For a time-limited population at high food levels, a slightly higher temperature is more important than the absolute food level. With increases in temperature and growth of individuals, the population soon becomes resource limited again, when its density and biomass return to the carrying capacity level. A. franciscana transported to warmer climates can over-winter as adults (e.g., Wear & Haslett, 1987), thus following a similar strategy to that used by the ‘orange’ LC Daphnia.
In comparison to the ‘orange’ LC Daphnia, the ‘transparent’ LC Daphnia is likely to be more demanding with regard to food levels. Although the individual threshold food concentration of adults of the two lineages has not been estimated, the ‘transparent’ Daphnia were found to be persistently smaller than the ‘orange’ ones when their sizes were compared throughout the entire time of their coexistence in the lake (see Fig. 3b in Slusarczyk, 2009).
In conclusion, from the study of both the LC Daphnia and the GSL Artemia it is apparent that in the absence of fish predation, the zooplankton community is substituted by a single herbivore that monopolizes resources. Interspecific competition is replaced by even harsher intraspecific competition, which leads to synchronous life histories, resulting in the competitive superiority of adults over juveniles. In addition, the Artemia example shows that the same population may be time-limited in spring, but resource-limited in summer.
Habitats free of fish—highlighting the importance of fish predation
Each of the two lakes of our study represents an extreme habitat. The Lake Czarny is a typical alpine lake, cold and infertile. The Great Salt Lake is highly fertile but hypersaline. However, less extreme habitats are seldom free of fish because humans stock nearly every available water hole, and no less extreme habitats could be located to be used as examples of lakes free of fish. There are other examples, but they are either equally extreme, and just as remote, or they are examples of small, temporary or manipulated systems, many of them reviewed by Gliwicz (2003). There are examples of a single large-bodied Daphnia in isolated arctic or high-elevation ultraoligotrophic lakes of Europe, Equatorial Africa and Asia, in ponds of Norwegian highlands (Daphnia umbra of Larsson & Wathne, 2006) and Italian Alps (D. longispina of Cammarano & Manca, 1997), tarns of Mount Elgon and Mount Kenya at 3475 to 4330 m ASL (Daphnia dolichocephala Sars of Löffler, 1968), in the Pamir (Rylov, 1930), Hindukush (Rühe, 1915) and Tibetan Himalaya (Daphnia tibetana of Hutchinson, 1937 and Manca et al., 1994). There is also an example of a highly eutrophic Bohemian fishpond that, by mistake, was left unstocked for the entire season. Its otherwise diverse zooplankton was rapidly replaced by a single-species, a large-bodied Daphnia pulicaria, that were surviving on low food levels of flagellated green algae which were suppressed by heavy grazing from 60–80 Daphnia l−1. The Daphnia were unable to reproduce for 100 days, until the mistake was detected and the pond was stocked with carp again (details in Fott et al., 1974, 1980; Gliwicz, 2003).
The importance of fish predation in shaping the structure of zooplankton communities has been clear since it was first reported by Hrbáček et al. (1961, 1962), and subsequently used as a keystone of the size-efficiency hypothesis of Brooks & Dodson (1965). It is evident that the increased impact of fish predation causes zooplankton size distribution to shift considerably towards small-bodied species (Fig. 1). It might also be anticipated that increased fish predation should keep different zooplankton species at densities well below the carrying capacity level to allow stable coexistence, as was the case with different cichlid species in Lake Malawi (Fryer, 1959a, 1959b) and sedentary invertebrates on the rocky shore of Washington’s Pacific coast (Paine, 1966).
However, the outcome when fish are completely absent, thus allowing competition that is not restricted by mortality induced by predation, is often ignored or unknown. Only by consideration of the zooplankton communities in habitats free of fish, such as Lake Czarny or Great Salt Lake, is it possible to grasp the real role of fish predation in shaping zooplankton community composition and the age structure of each component species. Only then can some comprehension be gained of the real world where fish predation fosters the coexistence of many zooplankton species in spite of the high overlap in their diets and hence niche dimensions. Furthermore, only then does it become clear why (i) the densities of coexisting zooplankton species are similar from one lake to another, with small-bodied species always more abundant than large-bodied ones, and (ii) the proportions of large and small-bodied species are similar across habitats comprising a wide productivity spectrum, with each species at a density fixed by fish predation at the species-specific level where it becomes included in a fish’s diet (Gliwicz & Wrzosek, 2008).
This simple world of fish-free habitats is unknown to most limnologists and absent from contemporary textbooks. Current knowledge of aquatic systems and our understanding of diversity offshore are based on observations of habitats that have contained fish for millennia. Aquatic habitats that are free of fish are rare and marginal. Although they may provide important forage for birds, they are considered a waste by fishery people. They may also seem uninteresting to limnologists as well, for they lack complex food webs and the multitude of intriguing interactions that occur between the many coexisting species in a typical marine or freshwater habitat. They also lack the challenging magic of the Hutchinson’s ‘paradox of the plankton’. There is, however, one aspect of the limnology of fish-free habitats that makes understanding them more important. This does not relate to the habitats themselves, but rather lies in the chance they offer to grasp the overpowering force of fish predation by illustrating the consequences of its absence. Besides this powerful lesson, the example of zooplankton from two different fish-free habitats discussed here also strengthens the argument that diversity may be sustained only where predation keeps densities of coexisting species at levels below the carrying capacity, as was pondered by Hutchinson 50 years ago. It shows that different species coexist because each is maintained at a low species-specific density level, which is inversely related to body size and irrespective of food level, because greater recruitment at higher food is instantly compensated for by raised mortality resulting from the response of fish to increased prey abundance.
References
Abrams, P. A. & R. D. Holt, 2002. The impact of consumer resource cycles on the coexistence of competing consumers. Theoretical Population Biology 62: 281–295.
Alcock, J., 1994. Postinsemination associations between males and females in insects: the mate-guarding hypothesis. Annual Review of Entomology 39: 1–21.
Almany, G. R. & M. S. Webster, 2004. Odd species out as predators reduce diversity of coral-reef fishes. Ecology 65: 2933–2937.
Brooks, J. L. & S. I. Dodson, 1965. Predation, body size and composition of plankton. Science 150: 28–35.
Cammarano, P. & M. Manca, 1997. Studies on zooplankton in two acidified high mountain lakes in the Alps. Hydrobiologia 356: 97–109.
Chase, J. M., P. A. Abrams, J. P. Grover, S. Diehl, P. Chesson, R. D. Holt, S. A. Richards, R. M. Nisbet & T. J. Case, 2002. The interactions between predation and competition: a review and synthesis. Ecology Letters 5: 302–315.
Chesson, P., 2000. Mechanisms of maintenance of species diversity. Annual Revue of Ecology and Systematics 31: 343–366.
Connell, J. H., 1978. Diversity in tropical rain forests and coral reefs. Science 199: 1302–1310.
Fott, J., V. Korinek, M. Prazakova, B. Vondrus & K. Forejt, 1974. Seasonal development of phytoplankton in fish ponds. Internationale Revue der gesamten Hydrobiologie 59: 629–641.
Fott, J., B. Desortova & J. Hrbacek, 1980. A comparison of the growth of flagellates under heavy grazing stress with a continuous culture. In Continuous cultivation of microorganisms. Proceedings of the 7th Symposium, Prague: 395–401.
Fryer, G., 1959a. The trophic interrelationships and ecology of some littoral communities of Lake Nyasa and a discussion on the evolution of a group of rock-frequenting Cichlidae. Proceedings of the Zoological Society of London 132: 153–281.
Fryer, G., 1959b. Some aspects of evolution in Lake Nyasa. Evolution 13: 440–451.
Gliwicz, Z. M., 1986. Predation and the evolution of vertical migration in zooplankton. Nature 320: 746–748.
Gliwicz, Z. M., 1990. Food thresholds and body size in cladocerans. Nature 343: 638–640.
Gliwicz, Z. M., 2001. Species-specific population-density thresholds in cladocerans? Hydrobiologia 442: 291–300.
Gliwicz, Z. M., 2002. On the different nature of top-down and bottom-up effects. Freshwater Biology 47: 2296–2312.
Gliwicz, Z. M., 2003. Between hazards of starvation and risk of predation: the ecology of offshore animals. International Ecology Institute, Oldendorf/Luhe: 379 pp.
Gliwicz, Z. M. & D. Wrzosek, 2008. Predation-mediated coexistence of large- and small-bodied Daphnia at different food levels. The American Naturalist 172: 358–374.
Gliwicz, Z. M., W. A. Wurtsbaugh & A. Ward, 1995. Brine shrimp ecology in the Great Salt Lake, Utah. June 1994–May 1995 performance report to the Utah Division of Wildlife Resources, Salt Lake City, Utah: 83 pp.
Gliwicz, Z. M., M. Slusarczyk & A. Slusarczyk, 2001. Life-history synchronization in a long-lifespan single-cohort Daphnia population of an alpine lake free of fish. Oecologia 128: 368–378.
Hrbáček, J., 1962. Species composition and the amount of zooplankton in relation to the fish stock. Rozpravy ceskosloveske Akademie Véd Rada Matematicko-Prirodovedecka 72: 1–114.
Hrbáček, J., M. Dvorakova, V. Korinek & L. Prochazkova, 1961. Demonstration of the effect of the fish stock on the species composition of zooplankton and intensity of metabolism of whole plankton association. Verhandlungen der Internationalen Vereinigung fűr Theoretische und Angewandte Limnologie 14: 192–195.
Huisman, J. & F. J. Weissing, 1999. Biodiversity of plankton by species oscillations and chaos. Nature 402: 407–410.
Hutchinson, G. E., 1937. Limnological studies in Indian Tibet. Internationale Revue der gesamten Hydrobiologie 35: 124–177.
Hutchinson, G. E., 1959. Homage to Santa Rosalia or why are there so many kinds of animals? The American Naturalist 93: 145–159.
Hutchinson, G. E., 1961. The paradox of the plankton. The American Naturalist 95: 137–146.
Kreutzer, C. & W. Lampert, 1999. Exploitative competition in differently sized Daphnia species: a mechanistic explanation. Ecology 80: 2348–2357.
Lampert, W., 1988. The relationship between zooplankton biomass and grazing. A review. Limnologica 19: 11–20.
Larsson, P. & I. Wathne, 2006. Swim or rest during winter—what is best for an alpine daphnid? Archiv fűr Hydrobiologie 167: 265–280.
Lima, S. L., 1985. Maximizing feeding efficiency and minimizing time exposed to predators: a trade-off in black-capped chickadee. Oecologia 66: 60–67.
Lima, S. L., 1998. Stress and decision making under the risk of predation: developments from behavioral, reproductive, and ecological perspectives. In Møller, A. P., M. Milinski & P. J. B. Slater (eds), Stress and Behavior. Advances in the Study of Behavior, Vol. 27. Academic Press, San Diego: 215–290.
Löffler, H., 1968. Die Crustaceenfauna der Binnengewässer ostafrikanischer Hochberge. Hochgebirgsforschung 1(8): 107–170.
Manca, M., P. Cammarano & T. Spagnulo, 1994. Notes on Cladocera and Copepoda from high altitude lakes in the Mount Everest Region (Nepal). Hydrobiologia 287: 225–231.
Montague, C. L., W. R. Fey & D. M. Gillespie, 1982. A causal hypothesis explaining predator-prey dynamics in Great Salt Lake, Utah. Ecological Modeling 17: 243–270.
Nelson, W. A., E. McCauley & F. J. Wrona, 2005. Stage-structured cycles promote genetic diversity in a predator-prey system of Daphnia and algae. Nature 433: 413–417.
Paine, R. T., 1966. Food web complexity and species diversity. The American Naturalist 100: 65–75.
Paine, R. T., 1969. A note on trophic complexity and community stability. The American Naturalist 103: 91–93.
Paine, R. T., 2002. Trophic control of production in a rocky intertidal community. Science 296: 736–739.
Reeve, M. R., 1963. The filter-feeding in Artemia. I. In pure cultures of plant cells. Journal of Experimental Biology 40: 195–205.
Rosenzweig, M. L. & R. H. MacArthur, 1963. Graphical representation and stability conditions of predator-prey interactions. The American Naturalist 47: 209–223.
Rühe, F. E., 1915. Die Süsswassercrustaceen der deutschen Südpolarexpedition 1901–1903 mit Aussschluss der Ostracoden. Deutsche Südpolar-Expedition 1901–1903. Zoologie 16(8).
Rylov, V. M. 1930. Cladocera et Copepoda. In: Abhandlungen der Pamir. Expedition 1928. Leningrad.
Sarnelle, O., 1993. Herbivore effects on phytoplankton succession in a eutrophic lake. Ecological Monographs 63: 129–149.
Schoener, T. W., 1973. Population growth regulated by intraspecific competition for energy or time: some simple representations. Theoretical Population Biology 4: 56–84.
Slobodkin, L. B., 1963. Growth and regulation of animal populations. Holt, Rinehart & Winston, New York.
Slusarczyk, M., 2009. Extended lifespan traded for diapause in Daphnia. Freshwater Biology 54: 2252–2262.
Spiller, D. A. & T. W. Schoener, 1998. Lizards reduce spider species richness by excluding rare species. Ecology 79: 503–516.
Stein, R. A. & J. J. Magnuson, 1976. Behavioral response of crayfish to a fish predator. Ecology 57: 751–761.
Stich, H. B. & W. Lampert, 1984. Growth and reproduction in migrating and non-migrating Daphnia species under simulated food and temperature conditions of diurnal vertical migrations. Oecologia 61: 192–196.
Tilman, D., 1982. Resource competition and community structure. Princeton University Press, Princeton.
Wear, R. G. & S. J. Haslett, 1987. Studies on the biology and ecology of Artemia from Lake Grassmere, New Zealand. In Sorgeloos, P., et al. (eds), Artemia research and its applications. Vol. 3. Ecology, culturing and use in aquaculture. Universa Press, Wetteren, Belgium: 101–133.
Weider, L. J., 1992. Disturbance, competition and the maintenance of clonal diversity of Daphnia pulex. Journal of Evolutionary Biology 5: 505–522.
Williams, W. D., A. J. Boulton & R. G. Taaffe, 1990. Salinity as a determinant of salt lake fauna: a question of scale. Hydrobiologia 197: 257–266.
Wurtsbaugh, W. A., 1992. Food-web modification by an invertebrate predator in the Great Salt Lake (USA). Oecologia 89: 168–175.
Wurtsbaugh, W. A., 1995. Brine shrimp ecology in the Great Salt Lake, Utah. 1995 performance report to the Utah Division of Wildlife Resources, Salt Lake City, Utah.
Wurtsbaugh, W. A. & T. S. Berry, 1990. Cascading effects of decreased salinity on the plankton, chemistry, and physics of the Great Salt Lake (Utah). Canadian Journal of Fisheries and Aquatic Sciences 47: 100–109.
Wurtsbaugh, W. A. & Z. M. Gliwicz, 2001. Limnological control of brine shrimp population dynamics and cysts production in the Great Salt Lake, Utah. Hydrobiologia 466: 119–132.
Acknowledgments
We are grateful to Piotr Maszczyk and Alan Ward for their assistance in the field and laboratory work, Mirek Slusarczyk and John R. Gittins for their instructive comments on the earlier drafts of this manuscript, and the two anonymous reviewers for their very constructive and helpful suggestions. This research was supported by Quinney Visiting Scholarship, Utah State University to Z.M.G., by grant from the Utah Division of Wildlife Resources to W.A.W., and by grants 6P04F01921 and 2P04G01430 from the State Committee for Scientific Research, Poland, to Z.M.G. The paper was written in the refuge offered to Z.M.G. by S. Nandini, S.S.S. Sarma and G. Ortiz at Iztacala Campus of the Universidad National Autonoma de Mexico.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: L. Naselli-Flores & G. Rossetti / Fifty years after the “Homage to Santa Rosalia”: Old and new paradigms on biodiversity in aquatic ecosystems
Rights and permissions
About this article
Cite this article
Gliwicz, Z.M., Wursbaugh, W.A. & Szymanska, E. Absence of predation eliminates coexistence: experience from the fish–zooplankton interface. Hydrobiologia 653, 103–117 (2010). https://doi.org/10.1007/s10750-010-0347-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0347-z