Abstract
Whilst biological traits of river macroinvertebrates show unimodal responses to geographic changes in habitat conditions in Europe, we still do not know whether spatial turnover of species result in distinct combinations of biological traits for pond macroinvertebrates. Here, we used data on the occurrence of 204 macroinvertebrate taxa in 120 ponds from four biogeographic regions of Europe, to compare their biological traits. The Mediterranean, Atlantic, Alpine, and Continental regions have specific climate, vegetation and geology. Only two taxa were exclusively found in the Alpine and Continental regions, while 28 and 34 taxa were exclusively recorded in the Atlantic and Mediterranean regions, respectively. Invertebrates in the Mediterranean region allocated much energy to reproduction and resistance forms. Most Mediterranean invertebrate species had narrow thermal ranges. In Continental areas, invertebrates allocated lesser energy to reproduction and dispersal, and organisms were short lived with high diversity of feeding groups. These characteristics suggest higher resilience. The main difference between ponds in the Alpine and Atlantic regions was their elevation. Alpine conditions necessitate specific adaptations related to rapid temperature fluctuations, and low nutrient concentrations. Even if our samples did not cover the full range of pond conditions across Europe, our analyses suggest that changes in community composition have important impacts on pond ecosystem functions. Consistent information on a larger set of ponds across Europe would be much needed, but their low accessibility (unpublished data and/or not disclosed by authors) remains problematic. There is still, therefore, a pressing need for the incorporation of high quality data sets into a standardized database so that they can be further analyzed in an integrated European-wide manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ponds can be defined as small standing freshwater bodies about 1 m2 to a few hectares in surface area, and from a few centimetres to a few metres in depth (Oertli et al., 2005). Recent studies have shown that ponds in Europe are just as important as rivers and lakes in supporting a wide range of aquatic biodiversity (Williams et al., 2004; Davies et al., 2008). Information collected at sites across Europe which represent a geographical distribution of biodiversity found in the Atlantic, Central and Mediterranean regions, illustrated that all types of freshwater systems (rivers, lakes, and ponds) support unique species not found in other water body types (Céréghino et al., 2008a), but that collectively, ponds host more species, and more rare species, than streams, rivers or lakes (Williams et al., 2004). Until recently, ponds have been mostly ignored by freshwater biologists or regarded as smaller versions of larger lakes, and much research aimed at assessing the biodiversity that ponds support (Oertli et al., 2010). Ponds are threatened by human activity (agriculture, industry, and urbanization), though these ecosystems have obvious ecological functions (Chase & Ryberg, 2004; Hansson et al., 2005) and recognized social and economic uses (Chapman et al., 2001). During the twentieth century, wetland losses reached 40–90% in a number of northwestern European countries (Hull, 1997). As a result of large-scale habitat loss, many species are also endangered, thus motivating studies aiming at understanding fundamental patterns of pond communities from local to regional scales (Oertli et al., 2010).
Both scientific studies and regional surveys of ponds have provided large volumes of site-specific data (e.g. Pond Action, 1994; Boix et al., 2001; Sahuquillo et al., 2007; Oertli et al., 2008) from which spatial patterns of biological communities can be explored. However, at a regional-continental scale, use of biological traits (e.g. body size, feeding habits, and life-history) is likely to be more useful than species lists in patterning community organization where different geographic regions are covered, though biologists are traditionally wedded to the use of such lists (Cayrou et al., 2000). Traits reflect environmental conditions and may be shared among many species (Southwood, 1988; Statzner et al., 2001; Santoul et al., 2005); species occurrence may have a strong stochastic element. Traits may give greater insight into habitat change (Dolédec et al., 1999; Statzner et al., 2004) and their determination requires much less taxonomic expertise (Dolédec et al., 1998, 2000), so that it can be utilized where limited information is available, and/or for animal groups where taxonomic knowledge is limited.
The aim of this study was to compare the biological traits of macroinvertebrate communities among four biogeographic regions of Europe (Mediterranean, Atlantic, Alpine, and Continental) defined by characteristic vegetation, climate, and geology by the European Environment Agency (EEA, 2002). To date, most of our current understanding of invertebrate traits in ponds has come from local-scale studies, where traits were examined in relation to environmental variables such as hydroperiod (Gascon et al., 2008), vegetation cover (Céréghino et al., 2008b, c), or pond successional stage (Ruhí et al., 2009). Although these studies showed that changes in species composition in relation to physical–chemical conditions can affect community functions in ponds, we still do not know whether large-scale changes in taxonomic compositions result in changes in community functions. Here, we examined whether spatial turnover in invertebrate taxa from Northwestern to Southwestern Europe result in distinct compositions of biological traits for pond macroinvertebrates. To this end, data on the macroinvertebrate communities of 120 ponds were collected from the published literature (occurrence matrix for 204 taxa), then biological traits were described using a fuzzy-coding method (species–traits matrix). A simultaneous analysis of two matrices performed through a co-inertia analysis (CoA) was used to investigate the spatial distribution of species–trait combinations (Chevenet et al., 1994). The results were analyzed with reference to geographic differences in environmental conditions, but also highlighted current methodological gaps and future needs.
Methods
Data selection
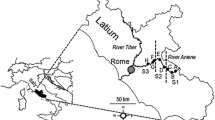
Previous studies provided the database on invertebrate communities (see Fig. 1). We assembled data to generate a presence/absence matrix for 204 taxa in 120 ponds (see Appendix 1 for list of taxa—Supplementary material). Since our aim was to compare traits among regions, ponds were not expected to be evenly distributed within the various regions. Instead, earlier studies provided lists of taxa originating from four biogeographic regions (Mediterranean, Atlantic, Alpine, and Continental), and three main gradients: latitude (North–South, from UK to southern Spain), elevation (0 → 2,600 m a.s.l), and hydroperiod (permanent or temporary). Biogeographic regions were defined and characterized for climate, geology, and vegetation in EEA (2002). Criteria for selection were the rating of the data based on the description of the sampling protocols (intensive sweeping of a hand net through the various habitat types, replicate samples from the various habitats), and at least two sampling periods at distinct seasons, to take into account invertebrate seasonality. The distribution of selected sites is shown in Fig. 1. Species lists per site were compiled as the sum of recorded species among the two periods. If a data source provided a longer time series of invertebrate occurrences, we selected periods that had data being representative for one annual cycle. Finally, taxa identifications had to allow aggregation at the genus (most insect taxa, Mollusca) or family (Oligochaeta) level, which corresponded to the lowest systematic units in the trait database. Given that we wished to analyse occurrence (i.e. qualitative) data, our criteria for data selection (mostly based on sampling efforts in space and time) suggest that we could be confident that the authors of selected studies did well at describing their pond invertebrate communities, and, subsequently, that the data assembled for the present study provided a reliable presence–absence matrix.
Distribution of sampling sites across the various biogeographic regions of Western Europe. 1. Oxford, UK (15 ponds, Pond Action, 1994); 2. De Maten Nature Reserve, Belgium (6 ponds, Van de Meutter et al., 2005); 3–4. Switzerland (11 ponds, Oertli et al., 2000); 5–6. Macun Cirque, Switzerland (25 ponds, Oertli et al., 2008); 7. Causses du Quercy, France (13 ponds, unpublished data); 8. Astarac region, France (15 ponds, Céréghino et al., 2008c); 9. Vaucluse, France (5 ponds, unpublished data); 10. Camargue, France (3 ponds, unpublished data); 11. Rome area, Italy (15 ponds, Bazzanti et al., 1996; Della Bella et al., 2005); 12. North-eastern Spain (3 ponds, Ruhí et al., 2009); 13. Leon area, Spain (3 ponds, Garcia-Criado & Trigal, 2005); 14. Valencia area, Spain (6 ponds, Sahuquillo et al., 2007)
Species traits were obtained from Tachet et al. (2000) (Appendix 2—Supplementary material). These traits were adult body size, life-cycle duration, number of cycles per year, aquatic stage, reproduction mode, dispersal mode, resistance forms, respiration mode, locomotion, food, feeding habits, substrate, trophic status, salinity, temperature tolerance, saprobity, and pH tolerance. Each trait was divided into categories, and categories for each biological trait were ordinal (e.g. <2.5 mm, 2.5–5 mm, 5–10 mm, etc., for adult body size) or nominal (e.g. egg, larva, nymph, adult, for aquatic stage) (see Appendix 2—Supplementary material). Species–trait information was then structured using a fuzzy-coding technique (Chevenet et al., 1994): scores ranged from ‘0’, indicating ‘no affinity’ to ‘5’, indicating ‘high affinity’ for a given species–trait category. This technique allowed us to take into account ontogenetic niche shifts and within-taxon variability in traits. For instance, a genus that mostly contains predators and a few scapers would be given an affinity of 3 for the feeding category predator, and a 1 for the category scraper. A predator that feeds on benthic algae during its early instars would be assigned the same coding. This procedure allowed us to build the ‘species–traits matrix’.
Data analysis
First, correspondence analysis (CA) was used to ordinate the ponds according to species presence/absence data, thus summarizing the variability of invertebrate communities, and providing insights for the discussion of the subsequent species–traits analysis. The significance of the axes was determined at P < 0.05 by testing the eigenvalues of the inertia matrix. Second, the species–traits matrix was studied by a ‘Fuzzy Correspondence Analysis’ (FCA) (Chevenet et al., 1994). Finally, a simultaneous analysis of the two matrices (species distributions and species–traits matrices) was performed using CoA (Dolédec & Chessel, 1994). This analysis studies co-structure by maximizing covariance between faunistic and biological traits ordination scores in the first two analyses (Dray et al., 2003). The aim of the CoA is to visualize spatial variations in the combinations of biological traits of pond invertebrates. A Permutation test (Dolédec & Chessel, 1994) was used to check the significance of the resulting correlation between the two sets of data resulting from the two kind of analysis (CA and FCA). We carried out 500 co-inertia analyses of the taxonomic and biological traits data sets after random permutation of their rows. We measured the correlation between the two tables using the RV-coefficient, a multidimensional equivalent of the ordinary correlation coefficient between two variables (Robert & Escoufier, 1976, Dolédec et al., 2006). The test was significant when the observed value was in a class containing only few random values among the 500 possible. The objective is to determine the common structure between the two sets of data, and then to interpret differences in pond ecosystems in terms of combinations of biological traits of their aquatic communities. Mann–Whitney tests were employed to test significant differences in pond distribution in the CA and in the CoA according to biogeographic regions and to hydroperiod, by using the coordinates of samples on the most relevant axis. These analyses were performed with R software.
Results
Taxa distribution
The Atlantic and Mediterranean regions had the highest taxon richness (145 and 160 taxa, respectively). Thirty and 53 taxa were found to occur in the Alpine and Continental regions. When a CA was carried out on the invertebrate matrix (presence/absence) (Fig. 1), the first two axes contributed 19.6 and 13.4% to the overall variance, respectively. Axis 2 showed a geographical gradient of ponds, which roughly corresponded to the North (top) to South (bottom) distribution of the ponds, i.e. to a latitudinal gradient of underlying variables probably related to climatic and geologic conditions. The scatterplot of the CA could be divided into three subsets along axis 2 when sampling sites were more specifically grouped according to their biogeographic origin (Fig. 2a), i.e. Mediterranean, Atlantic and Alpine, and Continental. There was also a gradient of hydroperiod (from permanent to temporary ponds) from Northern (top area of the scatterplot) to Southern regions (bottom) (Fig. 2a) (Mann–Whitney test on samples’ coordinates along axis 2, P < 0.01 for ponds grouped by region and by hydroperiod). Whilst only two taxa were exclusively found in the Alpine (the coleopteran Limnius) and Continental (the odonate Epitheca bimaculata) regions, 28 and 34 taxa were exclusively recorded in the Atlantic and Mediterranean regions respectively (Fig. 2b). Among these invertebrates, the commonest ‘Atlantic’ taxa were mostly trichopterans (Phryganea, Anabolia, Glyphotaelius pellucidus, and Trianodes), but also the amphipod Crangonyx, the heteropteran Callicorixa, the gastropod Armiger crista, or the turbellarian Dendrocoelum lacteum. Mediterranean ponds hosted the more distinct fauna. The most common taxa were Anostraca fairy shrimps, the tadpole shrimps Triops cancriformis and Lepidurus apus, the coleopteran Cybister lateralimarginalis, the backswimmer Anisops sardea, or the gastropod Physella. We assume that axis 1 displayed a gradient of local conditions, because ponds from Central and Southern Europe were distributed along the ordinate. Therefore, the gradient analysis performed through CA basically portrayed the gradual biogeographic changes in the compositional structure of invertebrate communities across Western Europe, with respect to variables acting at broad scales (axis 2) and local scales (axis 1).
Correspondance analysis (CA) of invertebrate taxa and ponds (axes 1 and 2). a Distribution of ponds according to biogeographic regions (left panel) and to hydroperiod (P = permanent ponds, T = temporary ponds) and b distribution of taxa on the first two axes of the CA (numbers correspond to taxa, see Appendix 1—Supplementary material)
Spatial patterns of invertebrate traits
A permutation test indicated that the co-inertia between the species distribution and species–trait matrices was highly significant (RV = 0.54; P < 0.001). Axes 1 and 2 expressed 24 and 22% of the overall variance, respectively. Thus, the CoA (Fig. 3a, b) of species’ distributions and their biological traits made it possible to derive some spatial patterns of species–trait combinations across Europe. Axis 2 showed a biogeographic gradient. As in the CA, the scatterplot of the CoA could be divided into three main subsets along axis 2 when sampling sites were grouped according to their biogeographic origin (Fig. 3a), i.e. Mediterranean, Atlantic and Alpine, and Continental (Mann–Whitney tests on pond coordinates along axis 2, P < 0.01). We assume that axis 1 displayed a gradient of local conditions, because ponds from each of the three above mentioned subsets displayed taxonomic variations along the abscissa. Specifically, the grouping of ponds according to their permanent or temporary nature suggested a gradient of hydroperiod along axis 1, from temporary (left) to permanent (right) (Mann–Whitney tests on pond coordinates along axis 1, P < 0.01). Mediterranean ponds (top area of the scatterplot), either permanent or temporary, had a higher proportion of interstitial or attached organisms, feeding by sediment absorption, consuming bacteria or fine organic detritus (e.g. Oligochaeta) (Fig. 3b). Most species used asexual reproduction, and had cocoons as resistance form. Mediterranean pond macroinvertebrates were mostly small-sized (<2.5 mm), but the largest species were also found to occur in these communities (>80 mm, e.g. Hirudo medicinalis, the threatened medicinal leech, a species listed in Annex II of the Habitats Directive and in the IUCN Red List). Finally, it is worth noting that there was a higher proportion of stenothermic (narrow thermal range) invertebrates in the Mediterranean region. At the opposite of the scatterplot (right bottom corner), Continental ponds were characterized by invertebrates with short life cycles (<1 year), and a higher proportion of rather large (20–80 mm) predatory species (e.g. the odonate Coenagrionidae) that prey on other macroinvertebrates, or even on small vertebrates. Finally, there was no clear distinction between Atlantic and Alpine ponds (all permanent). Compared with the above mentioned fauna, Atlantic and Alpine pond invertebrates were mostly medium-sized detritivores or herbivores (2.5–20 mm), with a higher proportion of grazers and filterers.
Co-inertia analysis results: a left panel: ordination of the 120 ponds on the first two axes of the CoA (Axes 1 and 2 contributed to 24 and 22% of the overall variance, respectively); left panel: grouping of samples according to biogeographic regions; right panel: grouping of samples according to hydroperiod (P = permanent, T = temporary). Ellipses contain 90% of the ponds for each region, thus summarizing the distribution of the data. b Distribution of species–trait categories on the first two axes (see Appendix for coding of categories—Supplementary material). Categories are positioned at the weighted average of their species
Discussion
Whilst the biological trait profiles of river invertebrates in Europe (Statzner et al., 2004) show rather unimodal responses, pond invertebrates exhibit a higher diversity of ecological strategies, and biological traits combinations from Northern to Southern areas are more different than in rivers. Presumably, this is owing to the fact that ponds display a broader range of physical and chemical characteristics (i.e. a broader range of niche opportunities) than streams, rivers or lakes, whereas the larger water bodies tend to have similar more physical and chemical properties across regions (Davies et al., 2008). This idea is strengthened by observations made in very small aquatic habitats with a simple structure like phytotelmata (plant-held waters such as tree-holes). In these containers, niche opportunities are rather consistent, and invertebrate species traits remain similar despite geographical changes in taxonomic composition (see e.g. Kitching, 2000). In ponds, it thus appeared that changes in community composition across Europe have a stronger impact on community functions than in rivers. This observation also suggests that the macroinvertebrates found in the various regions are phylogenetically more distant in ponds than in rivers (e.g. high proportions of crustaceans in the Mediterranean, high proportions of trichopterans in the Atlantic).
Most of our study ponds in the Mediterranean Region are extreme sites, characterized by an altered salinity due to their vicinity with the sea, and/or high evaporation rate related to arid climate and fluctuating hydrology. If life-cycle characteristics and organism size are associated with latitude and climatic conditions (Johansson, 2003), then a dominance of organisms with short life-span and/or asexual reproduction and small size was expected in Mediterranean climate areas, as these traits promote survivorship in unstable conditions (Bonada et al., 2007). The biological traits of invertebrates in the Mediterranean Region suggested that species allocated much energy to reproduction and resistance (asexual reproduction was dominant, formation of cocoons). These characteristics, and others such as feeding habits based on fine particulates and microorganisms, suggest that populations are selected by unstable habitats or by habitats fluctuating in an unpredictable way (Begon et al., 1996). The higher proportion of stenothermic species (species with narrow thermal ranges) in the Mediterranean region is probably the major highlight of this study, as this probably makes some Mediterranean pond invertebrates more susceptible to extinction in a context of climate change (i.e. species that cannot migrate northward if habitat becomes unsuitable). Throughout Europe and elsewhere, environmental policies aiming at preserving the biological diversity of terrestrial and/or aquatic ecosystems heavily rely on action plans for the delineation of zones of ecological interest (which concentrate rare and/or threatened species, or which have patrimonial values), and environmental managers ask for explicit schemes such as distribution patterns that allow them to identify areas at greater ecological risk. It is worth noting that the Mediterranean region has the highest taxonomic richness for pond invertebrates (160 out of 204 European taxa), and that 20% of these taxa were exclusive to this region. With high proportions of stenothermic invertebrates, threats associated with climate change clearly align with zones of ecological interest, and this should add new impetus to calls for freshwater conservation policies in the area. The traits of pond invertebrates in Continental areas suggested that lesser energy was allocated to reproduction and dispersal, whereas habitat occupancy and resource use was favoured by larger body size, short life cycles (thus enabling many temporally segregated populations to utilize small ecosystems), and a higher diversity of feeding groups. These characteristics suggest higher resilience, and the occurrence of strong competitors. Those populations are believed to be selected by more stable and structured habitats allowing interspecific competition and/or resource partitioning through the spatial and temporal segregations of species (Begon et al., 1996), a description which fits better with Continental ponds. Finally, despite similarities in their biological traits, invertebrate communities of the Alpine and Atlantic regions showed large differences in terms of number of taxa (30 and 145 taxa, respectively). Alpine ponds may represent harsh environments that are physically stressful (rapid and wide daily temperature fluctuations, intense UV, strong wind, abundant precipitation, and low nutrient concentrations), so that a smaller proportion of potential colonizers could become established.
Within-region variability in traits composition was chiefly apparent along axis 1, and was probably related to the peculiarities of the sub-areas from which the data were collected. In the southernmost areas, the annual hydrologic cycle is an important variable differentiating pond communities (Della Bella et al., 2005). From ephemeral pools to semi-permanent and permanent ponds, periodic drying is a major constraint to invertebrates (Jeffries, 1994; Schneider & Frost, 1996; Williams, 1996). Hydrologic cycles govern the biological traits combinations of communities by increasing habitat complexity through macrophyte cover, water chemistry, and substrate size. For instance, greater habitat complexity increases the number of available microhabitats for those taxa which utilize floating and submersed macrophytes as food, substrate, or refuge from predators. Such a causal relationship, already reported by Bazzanti et al. (2009), could explain the increase in shredder, scrapers, and leaf piercer at the right side of the CoA scatterplot. However, these observations call for finer analyses that should consider each region separately, to decipher the role of microclimatic, landscape, and pond habitat variables in selecting sets of species from the regional pool. Ideally, these analyses should be based on quantitative macroinvertebrate data from a large number of evenly distributed ponds, and on environmental variables recorded in a standardized way. Therefore, a posteriori analyses of pre-existing datasets (this study) are not recommended at the regional scale, where fieldwork can be more easily designed to ensure homogeneity and replication in the data. Finally, although our data covered 120 ponds, these were derived (rather unevenly) from relatively small sampling areas. As a result, taxa lists were quite similar within each sampling area, which implies limited representation for each region. For instance, it is likely that differences in traits decrease from the core of each region to its interface with other regions.
A key objective of the current research on small water bodies is to develop the knowledge base and tools to enable integrated monitoring and assessment for the conservation of freshwater biodiversity (EPCN, 2007). Exploration of fundamental ecological patterns is needed, to cover the range of habitats found along broad geographical, altitudinal, and environmental gradients. These analyses, in addition to giving a vital understanding of large-scale patterns, are also intended to reveal gaps in existing knowledge. Obviously, our analysis of invertebrate traits did not cover the full range of pond conditions across Europe. The very first and probably more problematic gap is the lack of accessible and curated data. We must unfortunately acknowledge that only a very small fraction of the existing information on pond biodiversity is made available to researchers and practitioners. Although we could gather information on about one hundred ponds supposed to represent various biogeographic regions, we are still unable to meet our attempts to obtain information on a much larger set of ponds that would be evenly distributed across Europe. These data certainly exist, but the heterogeneity of the existing formats (e.g. data per sample units, aggregated data) and low accessibility (e.g. unpublished data not found with literature databases, not disclosed by authors) remain a problematic issues. There is still, therefore, a pressing need for the incorporation of high quality national data sets into a standardized database so that they can be further analyzed in an integrated European-wide manner.
References
Bazzanti, M., S. Baldoni & M. Seminara, 1996. Invertebrate macrofauna of a temporary pond in Central Italy: composition, community parameters and temporal succession. Archiv für Hydrobiologie 137: 77–94.
Bazzanti, M., V. Della Bella & F. Grezzi, 2009. Functional characteristics of macroinvertebrate communities in Mediterranean ponds (Central Italy): influence of water permanence and mesohabitat type. Annales de Limnologie—International Journal of Limnology 45: 29–39.
Begon, M., J. L. Harper & C. R. Towsend, 1996. Ecology, 3rd ed. Blackwell Science, Oxford.
Boix, D., J. Sala & R. Moreno-Amich, 2001. The faunal composition of Espolla pond (NE Iberian peninsula): the neglected biodiversity of temporary waters. Wetlands 21: 577–592.
Bonada, N., M. Rieradevall & N. Prat, 2007. Macroinvertebrate community structure and biological traits related to flow permanence in a mediterranean river network. Hydrobiologia 589: 91–106.
Cayrou, J., A. Compin, N. Giani & R. Céréghino, 2000. Species associations in lotic macroinvertebrates and their use for river typology. Example of the Adour-Garonne drainage basin (France). Annales de Limnologie—International Journal of Limnology 36: 189–202.
Céréghino, R., J. Biggs, S. Declerck & B. Oertli, 2008a. The ecology of European ponds: defining the characteristics of a neglected freshwater habitat. Hydrobiologia 597: 1–6.
Céréghino, R., A. Ruggiero, P. Marty & S. Angélibert, 2008b. Influence of vegetation cover on the biological traits of pond invertebrate communities. Annales de Limnologie—International Journal of Limnology 44: 267–274.
Céréghino, R., A. Ruggiero, P. Marty & S. Angélibert, 2008c. Biodiversity and distribution patterns of freshwater invertebrates in farm ponds of a southwestern French agricultural landscape. Hydrobiologia 597: 43–51.
Chapman, L. J., J. Balirwa, F. W. B. Bugenyi, C. Chapman & T. L. Crisman, 2001. Wetlands of East-Africa: biodiversity, exploitation and policy perspectives. In Gopal, B., W. J. Junk & J. A. Davis (eds), Biodiversity in Wetlands: Assessment Function and Conservation, Vol. 2. Backhuys Publishers, Leiden, The Netherlands: 101–131.
Chase, J. M. & W. A. Ryberg, 2004. Connectivity, scale-dependence, and the productivity–diversity relationship. Ecology Letters 7: 676–683.
Chevenet, F., S. Dolédec & D. Chessel, 1994. A fuzzy coding approach for the analysis of long-term ecological data. Freshwater Biology 31: 295–309.
Davies, B., J. Biggs, P. Williams, M. Whitfield, P. Nicolet, D. Sear, S. Bray & S. Maund, 2008. Comparative biodiversity of aquatic habitats in the European agricultural landscape. Agriculture Ecosystems and Environment 125: 1–8.
Della Bella, V., M. Bazzanti & F. Chiarotti, 2005. Macroinvertebrate diversity and conservation status of Mediterranean ponds in Italy: water permanence and mesohabitat influence. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 583–600.
Dolédec, S. & D. Chessel, 1994. Co-inertia analysis: an alternative method for studying species–environmental relationships. Freshwater Biology 31: 277–294.
Dolédec, S., B. Statzner & V. Frainay, 1998. Accurate description of functional community structure: identifying stream invertebrates to species-level? Bulletin of the North American Benthological Society 15: 154–155.
Dolédec, S., B. Statzner & M. Bournaud, 1999. Species traits for future biomonitoring across ecoregions: patterns along a human-impacted river. Freshwater Biology 42: 737–758.
Dolédec, S., J. M. Olivier & B. Statzner, 2000. Accurate description of the abundance of taxa and their biological traits in stream invertebrate communities: effects of taxonomic and spatial resolution. Archiv für Hydrobiologie 148: 25–43.
Dolédec, S., N. Phillips, M. R. Scarsbrook & R. H. Riley, 2006. Comparison of structural and functional approaches to determining landuse effects on grassland stream invertebrate communities. Journal of the North American Benthological Society 25: 44–60.
Dray, S., D. Chessel & J. Thioulouse, 2003. Co-inertia analysis and the linking of ecological data tables. Ecology 84: 3078–3089.
EEA (European Environment Agency), 2002. Europe’s biodiversity—biogeographical regions and seas. Report No. 1. Free download at http://www.eea.europa.eu/publications/report_2002_0524_154909.
EPCN (European Pond Conservation Network), 2007. Developing the pond manifesto. Annales de Limnologie—International Journal of Limnology 43: 221–232.
Garcia-Criado, F. & C. Trigal, 2005. Comparison of several techniques for sampling macroinvertebrates in different habitats of a North Iberian pond. Hydrobiologia 545: 103–115.
Gascon, S., D. Boix, J. Sala & X. D. Quintana, 2008. Relation between macroinvertebrate life strategies and habitat traits in Mediterranean salt marsh ponds (Emporda wetlands, NE Iberian Peninsula). Hydrobiologia 597: 71–83.
Hansson, L.-A., C. Brönmark, P. A. Nilsson & K. Åbjörnsson, 2005. Conflicting demands on wetland ecosystem services: nutrient retention, biodiversity or both? Freshwater Biology 50: 705–714.
Hull, A., 1997. The pond life project: a model for conservation and sustainability. In Boothby, J. (ed.), British Pond Landscape, Proceedings from the UK Conference of the Pond Life Project. Pond Life Project, Liverpool: 101–109.
Jeffries, M., 1994. Invertebrate communities and turnover in wetlands ponds affected by drought. Freshwater Biology 32: 603–612.
Johansson, F., 2003. Latitudinal shifts in body size of Enallagma cyathigerum (Odonata). Journal of Biogeography 30: 29–34.
Kitching, R. L., 2000. Food webs and container habitats: the natural history and ecology of phytotelmata. Cambridge University Press, UK.
Oertli, B., D. Auderset-Joye, E. Castella, R. Juge & J. B. Lachavanne, 2000. Diversité biologique et typologie écologique des étangs et petits lacs de Suisse. Univ. Genève, Laboratoire d’Ecologie et de Biologie Aquatique. Report to project 753-BA-1113: 340 pp.
Oertli, B., J. Biggs, R. Céréghino, P. Grillas, P. Joly & J. B. Lachavanne, 2005. Conservation and monitoring of pond biodiversity: introduction. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 535–540.
Oertli, B., N. Indermuehle, S. Angélibert, H. Hinden & A. Stoll, 2008. Macroinvertebrate assemblages in 25 high alpine ponds of the Swiss National Park (Cirque of Macun) and relation to environmental variables. Hydrobiologia 597: 29–41.
Oertli, B., R. Céréghino, J. Biggs, S. Declerck, A. Hull & M. R. Miracle, 2010. Pond Conservation in Europe, Developments in Hydrobiology 210. Springer, Berlin: 385 pp.
Pond Action, 1994. The Oxfordshire Pond Survey. A Report to the World Wide Fund for Nature (WWF-UK). Oxford Brookes University, Oxford.
Robert, P. & Y. Escoufier, 1976. A unifying tool for linear multivariate statistical methods: the RV-coefficient. Journal of Applied Statistics 25: 257–265.
Ruhí, A., D. Boix, J. Sala, S. Gascón & X. D. Quintana, 2009. Spatial and temporal patterns of pioneer macrofauna in recently created ponds: taxonomic and functional approaches. Hydrobiologia 634: 137–151.
Sahuquillo, M., J. M. Poquet, J. Rueda & M. R. Miracle, 2007. Macroinvertebrate communities in sediment and plants in coastal Mediterranean water bodies (Central Iberian Peninsula). Annales de Limnologie—International Journal of Limnology 43: 117–130.
Santoul, F., J. Cayrou, S. Mastrorillo & R. Céréghino, 2005. Spatial patterns of the biological traits of freshwater fish communities in S.W. France. Journal of Fish Biology 66: 301–314.
Schneider, D. W. & T. M. Frost, 1996. Habitat duration and community structure in temporary ponds. Journal of the North American Benthological Society 15: 64–86.
Southwood, T. R. E., 1988. Tactics, strategies and templets. Oikos 52: 3–18.
Statzner, B., A. G. Hildrew & V. H. Resh, 2001. Species traits and environmental constraints: entomological research and the history of ecological theory. Annual Review of Entomology 46: 291–316.
Statzner, B., S. Dolédec & B. Hugueny, 2004. Biological trait composition of European stream invertebrate communities: assessing the effects of various trait filter types. Ecography 27: 470–488.
Tachet, H., P. Richoux, M. Bournard & P. Usseglio-Polatera, 2000. Invertébrés d’eau douce. Systématique, biologie, écologie. CNRS Editions, Paris.
Van de Meutter, F., R. Stoks & L. De Meester, 2005. The effect of turbidity state and microhabitat on macroinvertebrate assemblages: a pilot study of six shallow lakes. Hydrobiologia 542: 379–390.
Williams, D. D., 1996. Environmental constraints in temporary freshwaters and their consequences for the insect fauna. Journal of the North American Benthological Society 15: 634–650.
Williams, P., M. Whitfield, J. Biggs, S. Bray, G. Fox, P. Nicolet & D. Sear, 2004. Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biological Conservation 115: 329–341.
Acknowledgments
This work was funded by the MAVA Foundation as part of the Pro-Pond Project. Three anonymous reviewers provided valuable comments on an earlier version of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: D. Boix, B. Oertli, R. Céréghino, T. Kalettka, J. Biggs & A. P. Hull / Pond Research and Management in Europe – Proceedings of the 4th conference of the European Pond Conservation Network (Berlin 2010)
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Céréghino, R., Oertli, B., Bazzanti, M. et al. Biological traits of European pond macroinvertebrates. Hydrobiologia 689, 51–61 (2012). https://doi.org/10.1007/s10750-011-0744-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0744-y