Abstract
Ancient sister lakes are considered to be ancient lakes lying in close geographic proximity, sharing a related origin and significant time of co-existence, usually having hydrological connection as well as a balanced degree of faunal overlap and distinctness. A paradigm for studying sister lake relationships are the ancient lakes Ohrid and Prespa in the Balkans, which are characterized by high degrees of endemicity. Three general patterns of endemic species can be distinguished for these lakes: (1) taxa that are endemic to either lake, with no close relatives in the respective sister lake, (2) closely related but distinct endemic taxa in both lakes (sister species) and (3) shared endemic taxa occurring in both lakes. In the present paper, two endemic freshwater pulmonate gastropod species, Radix relicta (Lake Ohrid) and R. pinteri (Lake Prespa), are used to study the evolution of presumed sister species based on biogeographical and comparative DNA data from world-wide Radix taxa. Phylogenetic, phylogeographical and parametric bootstrap analyses all suggest a sister group relationship of R. relicta and R. pinteri (pattern 2 of endemic diversity). Sister to these two taxa is the widespread R. ampla, which does not occur in the vicinity of lakes Ohrid and Prespa. The southern feeder spring complexes of Lake Ohrid are inhabited by another lineage (Radix sp. 1), which resembles Radix relicta in morphology/anatomy. For Lake Prespa, the widespread R. auricularia was reported in addition to the endemic R. pinteri. Comparative phylogenetic data favour a western Adriatic zoogeographical affinity of lakes Ohrid and Prespa over an Aegean-Anatolian faunal connection. The status of lakes Ohrid and Prespa as sister lakes is evaluated in the light of current knowledge on gastropod speciation and endemism in these hotspots of biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ancient lakes are usually seen as compact and isolated geographic entities that have undergone unique histories in a certain hydrological setting (e.g. Gorthner, 1994). It is clear that over geological time scales, these lakes do not remain constant; rather, some of these lakes became repeatedly subdivided and reconnected in the past (e.g. Lake Tanganyika, Coulter, 1991). Even some extant lakes show distinct subdivision like the two parts of Lake Titicaca (Lago Grande and Lago Pequeno). Such conditions have been suggested to trigger allopatric speciation in ancient lakes (e.g. Martens, 1997). Ancient sister lakes, such as the Malili Lake system on the Indonesian island Sulawesi (e.g. Rintelen & Glaubrecht, 2005, 2006), are here considered to be ancient lakes lying in close geographic proximity, sharing a related origin and significant time of co-existence, usually having hydrological connection as well as a balanced degree of faunal overlap and distinctness. A paradigm for ancient sister lakes is the European lake group called Dessaretes located on the Balkan Peninsula. Originally, the Dessaretes consisted of Lake Ohrid (Macedonia, Albania), Lake Prespa (Macedonia, Greece, Albania), Lake Mikri Prespa (Greece, Albania), and Lake Maliq (Albania). The latter was drained in the middle of the last century (Denèfle et al., 2000). Some workers even argued that the Dessarete basin was filled in with water and all of these lakes were interconnected during evolutionary time (reviewed in Albrecht & Wilke, 2008).
Today, Lake Ohrid (693 m a.s.l.) constitutes the oldest lake in Europe, with an estimated age ranging from 2 or 3 my (Stanković, 1960) to 10 my (Spirkovski et al., 2001), and a high degree of endemism (Albrecht & Wilke, 2008). It has a surface area of 358 km² and a maximum depth of 289 m (Matzinger et al., 2007). Lake Prespa, located at an altitude of 849 m a.s.l., is the highest large lake in the Balkans. It covers 254 km² (Matzinger et al., 2006) and has a maximum depth of 58 m (Albrecht & Wilke, 2008). Lake Mikri Prespa (approx. 853 m a.s.l.) is 53 km² in size and 8.4 m deep (Zacharias et al., 2002). The latter lake is directly connected to Lake Prespa and its water level is regulated through the outflow to Lake Prespa. No reliable data is available for age and origin of the two Prespa lakes, though many workers consider Lake Prespa to have a similar age as Lake Ohrid.
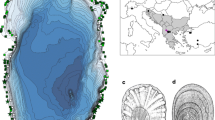
While the sister lake status of Lake Prespa and Lake Mikri Prespa is obvious, it is, on first sight, less clear for Lake Ohrid and Lake Prespa. Though they are less than 10 km apart, a high mountain chain (Galičica Mountains) separates the two basins (Fig. 1).
Subterranean karstic channels, however, are known to connect lakes Ohrid and Prespa (Amataj et al., 2007), and according to Matzinger et al. (2006), Lake Prespa contributes approx. 20% to the water balance of Lake Ohrid. Although the age of Lake Prespa remains unknown, they presumably co-existed for several hundreds of thousand years (Radoman, 1985). In fact, some workers even suggest that the fauna of Lake Prespa is older than that of Lake Ohrid, at least according to fish endemism data (e.g. Karaman, 1971). While fish endemism in both lakes has attracted many researchers, the lakes are arguably most famous for their extraordinary degree of mollusc endemism, which has been studied for more than 100 years (e.g. Sturany, 1894; Polinski, 1932; Hadžišče, 1956; Radoman, 1985; Burch & Hadžišče, 1974). There are three general patterns of endemic species that can be distinguished for lakes Ohrid and Prespa:
-
1)
taxa that are endemic to either lake with no close relatives in the respective sister lake,
-
2)
closely related but distinct endemic taxa in both lakes (sister species) and
-
3)
shared endemic taxa occurring in both lakes.
Of particular interest for evolutionary biologists is the presence of sister species in ancient lakes, as these taxa cannot only help unravelling processes of speciation in space in time; they can also help to establish hypotheses about the hydrological or even geological histories of the respective lakes (e.g. Wilke et al., 2007). Although sister species are often postulated for related water bodies, they are sometimes difficult to identify, particularly when dealing with inconspicuous taxa. In fact, robust data showing sister species relationships in molluscs of lakes Ohrid and Prespa are still sparse, for example, some species of the genus Pisidium (Schultheiß et al., 2008).
A candidate group for studying sister species in these lakes is the pulmonate family Lymnaeidae. The group is best known for some of its members acting as an intermediate host for trematodiases (Bargues et al., 2001). Many representatives are widespread (Remigio, 2002) or even invasive (Cowie, 2001), and narrow-range endemism is unusual in these gastropods. However, for lakes Ohrid and Prespa, one endemic lymnaeid species each has been described. In Lake Ohrid, Radix relicta Polinski, 1929, occurs in depths up to 30 m. This relatively large species (up to 3 cm in length) has a special physiological adaptation to life in greater depths (Kaiser, 1959). Its pneumostome is completely closed, tissues are less vascularised, and respiration takes places exclusively through the body surface. From Lake Prespa, Radix pinteri Schütt, 1974, is believed to be a ‘relictary’ Pontic species (Schütt, 2006). It lives on hard substrata and is characterized by its expanded last whorl and extraordinarily large aperture. While the two species can clearly be distinguished by their shell morphology, there are only relatively few diagnostic anatomical characters separating the two taxa. They include, among others, a cylindrical versus sacciform bursa copulatrix in R. relicta and R. pinteri, respectively (Fig. 2).
Shell morphology and anatomy of Radix relicta (1) and Radix pinteri (2) with shell, mantle pigmentation, male copulatory organ and female genital organs. Abbreviations: bc = bursa copulatrix, bd = bursa duct, cp = corpus pyriforme, m = protractor and retractor muscle, pht = phallotheca, pr = prostate, prp = praeputium, pvd = provaginal duct, v = vagina, vd = vas deferens
Several family-level molecular phylogenies for the Lymnaeidae have been published. These studies include nuclear 18S rDNA (Bargues & Mas-Coma, 1997), ITS2 (Bargues et al., 2001) and mitochondrial 16S rDNA data (Remigio & Blair, 1997; Remigio, 2002). The specific composition and relationships within the genus (or subgenus) Radix, however, remain unknown or confused. Even the monophyly of Radix was recently doubted (Remigio, 2002). Some attempts have been made to clarify the phylogenetics of western and northwestern European Radix populations (Bargues et al., 2001; Pfenninger et al., 2006). Molecular data, however, are almost entirely absent for Balkan taxa in general and for the ancient lake taxa in particular.
Given that only two endemic Radix taxa are known for lakes Ohrid and Prespa, with one taxon occurring in each lake, we here use biogeographical and ecological data, as well as DNA sequences from the mitochondrial cytochrome oxidase c subunit I (COI) gene to:
-
a)
develop a phylogenetic and biogeographical framework for Balkan Radix spp. and discuss their mode of diversification in European ancient lakes,
-
b)
test the sister species relationship of Lake Ohrid Radix relicta and Lake Prespa R. pinteri and study their degree of differentiation as well as their putative relictary status and
-
c)
discuss the status of lakes Ohrid and Prespa as sister lakes.
Materials and methods
Materials
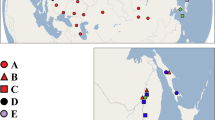
Materials from the Dessarete Lakes and other water bodies of the Balkan Peninsula, including ponds, springs, artificial lakes, streams and rivers, were mainly collected during several field trips carried out between May 2003 and September 2005 (Fig. 2). In addition to the Balkan samples, Radix populations from Germany, Austria, Corsica and Asia Minor were included in the analyses. Individuals were obtained by hand collecting from hard substrata in shallow waters or from stones and rocks lifted from depths to 5 m by snorkelling. Deeper parts of the littoral and sublittoral to 60 m were sampled using a dredge from small boats or from the research vessel of the Hydrobiological Institute in Ohrid (HBI). Locality information, collecting details and GenBank accession numbers are provided in Appendix 1. All materials are deposited at the permanent DNA and tissue collection of the University of Giessen, Systematics and Biodiversity Group (UGSB).
Several outgroup taxa of the family Lymnaeidae representing all European genera with their respective type species were included in the phylogenetic analyses to account for the potential paraphyly of Radix. Representatives of the family Physidae, Haitia acuta and Physa fontinalis, were used as additional outgroups. As primary outgroup for rooting the trees, Planorbarius corneus (family Planorbidae) was used.
DNA isolation and sequencing
The method described in Wilke et al. (2006) was used for isolating DNA from individual snails. The primers for amplifying a fragment of the COI gene with a target length of 655 base pairs (excluding 51 bp primer sequence) were LCO1490 and HCO2198, as described by Folmer et al. (1994). Sequences (forward and reverse) were determined using the LI-COR (Lincoln, NE) DNA sequencer Long ReadIR 4200 and the Thermo Sequenase Fluorescent Labeled Primer Cycle Sequencing kit (Amersham Pharmacia Biotech, Piscataway, NJ). The protein-coding COI sequences were aligned unambiguously by eye using BioEdit 7.0.4.1 (Hall, 1999).
The first base pairs (bp) behind the 3′ end of each primer were difficult to read. We therefore uniformly cut these parts, leaving an up to 600 bp-long overlapping fragment for the COI gene. Sequences of the non-Radix taxa were derived using the methodology described in Albrecht et al. (2004, 2007). All sequences are available at GenBank (see Appendix 1).
Phylogenetic analyses
Two commonly employed approaches were chosen for phylogenetic reconstructions, Bayesian inference (BI) and maximum parsimony (MP). Prior to the phylogenetic analyses, the computer program Modeltest 3.6 (Posada & Crandall, 1998) was used to find the optimal model of DNA substitution based on the Akaike information criterion. For the COI dataset, the K81uf + I + G model was selected with base frequencies of A = 0.3023, C = 0.1204, G = 0.1328 and T = 0.4445; a rate matrix of [A − C] = 1.0000, [A − G] = 9.6093, [A − T] = 2.7121, [C − G] = 2.7121, [C − T] = 9.6093, [G − T] = 1.000; a proportion of invariable sites of 0.3225; and a gamma distribution shape parameter of 0.7060.
BI was performed utilizing MrBayes 3.0b4 (Huelsenbeck & Ronquist, 2001). For a general understanding of phylogenetic relationships within the genus Radix, BI analysis of 40 ingroup specimens and four lymnaeid as well as three non-lymnaeid outgroup species was performed based on the best-fit model. During a preliminary run, the log-likelihoods started at around −8,600 and quickly converged on a stable value of about −4,900 after approximately 6,500 generations. The final run was then carried out with four chains (one cold, three heated) and 1,000,000 sampled generations with the current tree saved at intervals of ten generations. A 50% majority rule tree was constructed from all sampled trees with the first 2,000 trees (= 20,000 generations) ignored as burn in.
The MP analysis was conducted using PAUP* v. 4.0b10 (Swofford, 2002) with branch-and-bound search, 500 random-addition-sequence replications and TBR branch swapping. Statistical robustness was assessed by non-parametric bootstrapping (Felsenstein, 1985) with 500 replications.
Parametric bootstrapping
For testing the sister species relationship of Lake Ohrid Radix relicta and Lake Prespa R. pinteri (i.e. the monophyly of the two taxa), we used the parametric bootstrapping approach (Hillis et al., 1996; Huelsenbeck & Crandall, 1997). First, we conducted maximum likelihood (ML) searches in PAUP under the topological constraint of non-monophyly (null hypothesis) and the best-fit model of sequence evolution. The resulting tree was, together with the aligned sequences, imported into Seq-Gen 1.3.1. (Rambaut & Grassly, 1997) to generate 100 random datasets based on the model suggested by Modeltest. We then analyzed in PAUP the ratios of the likelihood differences in tree lengths between the constrained and unconstrained trees for each of the 100 replicates. The frequency of likelihood ratios of the simulated datasets then was plotted and compared to the likelihood-ratio (constrained vs. unconstrained topologies) of the original dataset. Finally, we estimated how likely it is that this difference could have been observed randomly, that is, whether the null hypothesis can be rejected.
Phylogeographical analysis
We utilized a statistical parsimony (SP) network approach to demonstrate phylogeographical relationships among the putative Radix sister species from lakes Ohrid and Prespa. The SP network was generated in TCS 1.18 (Clement et al., 2000) and a reduced dataset (14 sequences, 365 bp long).
Genetic distances within and between R. relicta and R. pinteri were calculated using MEGA 3.1 (Kumar et al., 2004) by employing the Kimura-2-Parameter model. Standard errors were calculated with 500 bootstrap replicates and a random seed.
Results
Phylogenetic and phylogeographical relationships
The phylogenetic analyses of the mitochondrial COI dataset resulted in a mostly well-supported phylogeny comprising ten major Radix clades (Fig. 3; MP consensus tree with CI of 0.866). The topologies derived from both analyses were compatible. The major clades correspond to six recognized Radix species and four lineages for which most likely no names are available and that are here numbered consecutively for convenience. The endemic Radix relicta (Lake Ohrid) and R. pinteri (Lake Prespa) together form a well-supported monophylum (100% bootstrap support [BS], 0.99 Bayesian posterior probabilities [BPP]) with Radix ampla being its sister species. However, their respective relationships could not be resolved. An unnamed lineage (Radix sp. 1) is endemic to the feeder springs south of Lake Ohrid and clustered as sister taxon to the clade R. relicta + R. pinteri + R. ampla. These four species form a well-supported monophylum (100% BS, 0.96 BPP). A species from the Turkish Burdur Province (Radix sp. 3) was the sister species to the latter clade. The remaining species in the dataset form a second major clade, containing, among others, the widespread Radix labiata. This species was represented by populations from Corsica, Greece, Turkey, Montenegro and Albania, and the Lake Ohrid inflow stream Lubanište and the feeder spring complex Sv. Naum. The sister clade to R. labiata comprises a possibly undescribed species from Lake Trichonis (Greece), Radix natalensis from Lake Malawi (Malawi) and Radix auricularia (Germany, Croatia, Montenegro [Lake Skutari] and many parts of Lake Prespa).
Maximum parsimony majority-rule consensus tree of Radix spp. based on 600 nucleotide positions of the COI gene. The tree was rooted with planorbid and physid outgroups. Additionally, all European lymnaeid genera are represented by their respective type species in the dataset. Maximum parsimony bootstrap values (>70%) are provided above and Bayesian posterior probabilities (>0.80) below the branches. For explanations of species assignments, see text. New species-level clades are consecutively numbered for convenience. Endemics of Lake Ohrid (including its southern feeder springs) and Lake Prespa are highlighted in grey boxes
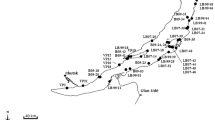
Given the inability of phylogenetic methods used here to fully resolve the closely related sister species R. relicta and R. pinteri, a SP network of 14 specimens was generated (Fig. 4). The network analysis resulted in a total of 11 haplotypes and a clear separation of the two species. Acknowledging that only three specimens of R. pinteri could be included in the analysis, it is obvious that genetic diversity is more pronounced in R. relicta. The SP network suggested one R. relicta haplotype occurring in the spring complex Sv. Naum and the eastern shore of the lake as having the highest probability of being the ancestral haplotype of the analysed specimens. The average K2P diversity within R. relicta was 0.009 (±0.004) and within R. pinteri 0.005 (±0.003), whereas the divergence between R. relicta and R. pinteri was calculated to be 0.024 (±0.008).
Testing the sister species null hypothesis
As the phylogenetic analyses suggest R. relicta and R. pinteri to form a monophyletic group, but could not resolve the relationship between the two species, we tested the null hypothesis that these taxa are not monophyletic using parametric bootstrapping.
From the 100 sampled trees from the simulated datasets, all had differences in log likelihood units of ≤5.1. As the observed difference in the original dataset was 34.0 log likelihood units, the null hypothesis is rejected at P < 0.01, and the alternative hypothesis—monophyly of R. relicta and R. pinteri—can be accepted.
Distribution and ecology
Radix relicta is not restricted to Lake Ohrid but occurs in the Drim System at least upstream to the dam Lake Globočičko (approximately 15 km N of Lake Ohrid). In the lake proper of Lake Ohrid, no other Radix species than R. relicta was found in depths from 0 m to 35 m. It lives mainly on hard substrata (e.g. rocks, Dreissena bundles) or on algae of the Chara belt.
Radix labiata was recorded in one stream approximately 0.5 km SE of Lake Ohrid, as well as in the feeder spring complex Sv. Naum. These springs and the sister complexes in Albania (Zagorican and Tushemist) are inhabited by another lineage (Radix sp. 1), which resembles Radix relicta in morphology/anatomy.
For Lake Prespa, two species of Radix have been recorded so far. The endemic Radix pinteri was found from the littoral to depths of 6 m on muddy substrata and on rocks. This species has not yet been recorded from Lake Mikri Prespa. The second species, Radix auricularia, was mainly found near the surface, sometimes even in amphibious habitats. It also occurs in Lake Mikri Prespa (see Fig. 2).
Discussion
Radix diversity and biogeography in the Balkans
Our study revealed distinct patterns of Radix diversity and distribution on the Balkans. There are widespread species contrasted with endemic taxa. Among the widespread species, we found the common Radix auricularia to occur in the Balkans at least in lakes Skutari and Prespa, as well as in one Dalmatian river. Interestingly, a species with similar morphology (Radix sp. 2; Albrecht & Glöer, unpublished data) so far occurs exclusively in Lake Trichonis (Greece). Hubendick’s (1951) superspecies concept of ‘Radix auricularia’ becomes less convincing given the new data indicate the existence of distinct phylogenetic units within ‘R. auricularia’. Another widespread species is Radix labiata with occurrences throughout the Balkans and southwest into Turkey (Aglasun). Radix labiata is a characteristic species of springs and small rivers. Bargues et al. (2001) also mentioned this taxon from Turkey as well as an undetermined Radix species, indicating the need for more comprehensive sampling in Asia Minor.
Concerning the more restricted Radix species on the Balkan Peninsula, it is an unexpected outcome of this study to find two geographically localized clades (Radix sp. 1 from the Lake Ohrid feeder springs and Radix sp. 2 from Lake Trichonis) besides the well-known Ohrid and Prespa endemic species. The latter, R. relicta and R. pinteri, are closely related to another widespread species with comparably low genetic diversity, R. ampla. Such a relationship was already suggested by Polinski (1932). Radix ampla itself was neither found in lakes Ohrid and Prespa nor in their surroundings. The nearest confirmed records are from Nikšić (Montenegro) as well as from Romania (Glöer & Sîrbu, 2006). This pattern of occurrence of endemics in lakes Ohrid and Prespa in combination with rather long geographical distances to the next known locality of the respective widespread sister species was also found for some other taxa. A good example is the endemic pea clam species studied by Schultheiß et al. (2008). The absence of R. balthica in the investigated waters of the Balkans is remarkable, despite a long list of published records for the area. This can probably be attributed to a persistent taxonomic confusion of European Radix spp. in general (discussed by Bargues et al., 2001) and a mere adoption of a central European taxonomic framework for the Balkan species in particular. Such a complex situation was already demonstrated for other gastropod groups on the Balkans such as the Bithyniidae (Glöer et al., 2007). Given our results, we would expect to find more distinct lineages on the Balkans, a tendency already shown for northwestern Europe (Pfenninger et al., 2006).
Sister species Radix relicta and R. pinteri
The phylogenetic relationship between these two endemic species from lakes Ohrid and Prespa is close as shown in the phylogenetic analysis (Fig. 3). The fact that these sister taxa are not fully resolved is, however, not surprising. Part of the problem is the high diversity within these presumably old populations, which may obscure species-level divergences (Albrecht et al., 2006, discuss a similar example in Lake Ohrid Ancylus), as well as the fact that most phylogenetic packages using traditional tree-building approaches may perform poorly at and below the species level (Posada & Crandall, 2001). Thus, the dedicated phylogeographical network analysis performed here (Fig. 4) can clearly separate the two sister species. Moreover, parametric bootstrapping rejected the null hypothesis of non-monophyly of R. relicta and R. pinteri and the two species can be assigned to pattern 2 of endemicity discussed in the Introduction.
As mentioned before, Lake Ohrid Radix relicta is adapted to live in greater depths. Although adaptation to greater depth and bathymetric segregation is a common feature of the Ohrid gastropod fauna (Radoman, 1985), it is not at all common for lymnaeids. Interestingly, Lake Prespa R. pinteri was also found down to 6 m. It has been suggested that R. pinteri occurs on soft substrata (Schütt, 1974) and that therefore ecological constraints would favour its extraordinary shell shape. We could, however, also regularly find it on stones and rocks. If, indeed, ecophenotypical modulation is responsible for the shell shape pattern seen in R. pinteri, other factors should be considered as well, such as wave action (at least during certain times of the year). The existence of fossil lymnaeids with shell forms having enlarged apertures and expanded last whorls led Schütt (1974, 2006) to conclude that both species represent ‘relictary’ species. The phylogenetic results of our study and the close relationship to Radix ampla raise doubts as to their ancientness. It has to be noted that R. ampla has not been considered a distinct species for a long time (e.g. Jackiewicz, 1998). Our mitochondrial data supports findings based on nuclear sequences, suggesting specific distinctness of R. ampla as previously proposed (Falkner, 1990). Schütt (1974) doubted a close relationship of the endemic Radix with amploid forms and could not address the question of the relationship to R. auricularia. We here showed that Radix auricularia is a distant lineage.
Among the most obvious adaptive zones in the Ohrid basin are spring habitats both within the actual lake or adjacent to it. The most important spring complexes are the southern feeder springs at Sv. Naum (Macedonia) and the less well-studied neighbouring spring complex Zagorican/Tushemist (Albania). These springs have long been recognized as a habitat for endemic species that have close relatives in the actual lake (e.g. Radoman, 1985). We found a distinct Radix lineage (Radix sp. 1) in both springs systems that morphologically resembles Radix relicta. Its specific character had already been suspected based on an immunotaxonomic study (Burch & Hadžišče, 1974). Other examples of closely related species in the feeder spring systems and the actual lake proper include Carinogyraulus spp. (Hubendick & Radoman, 1959; Gorthner & Meier-Brook, 1985) and Ancylus spp. (Albrecht et al., 2006). However, in contrast to Carinogyraulus and Ancylus, Radix did not radiate in Lake Ohrid (a pattern also found in Valvata spp.; Hauswald et al., 2008). Nonetheless, the statistical parsimony network (Fig. 4) shows substructuring of R. relicta throughout the lake as well as in surrounding springs and rivers that could lead to diversification. Although, it is not clear from the available data whether populations of R. relicta in the Drim River (i.e. the outflow of Lake Ohrid) and adjacent waters in the northern Ohrid Basin recently dispersed there or whether they constitute remnant populations from times when all these waters belonged to the former, larger lake and thus were sublacustrine. The pond at the Šum Spring, however, is artificial, and R. relicta might therefore have been introduced there recently (possibly with eggs for the salmon hatchery that uses the pond).
Considering the diversity within R. relicta and the divergence between Lake Ohrid R. relicta and Lake Prespa R. pinteri, the genetic differentiation in the COI gene between the two taxa is with ≤5 mutational steps (Fig. 4) or ≤1.7% K2P distance relatively low, yet distinct. Acknowledging that detailed molecular clock analyses are beyond the scope of the paper and that the limited data set presented here (i.e. missing data) and the likely presence of ancestral polymorphism might result in an overestimation of divergence times, the most recent common ancestor of the two species would be ≤0.75 My old (Albrecht et al., unpublished data).
Sister lakes Ohrid and Prespa
Given the close phylogenetic relationship of endemic taxa from Lake Ohrid and Lake Prespa found in the present study as well as by other workers, it is worth discussing the putative sister lake status of these lakes. According to our understanding of ancient sister lakes, they have to lie in close geographic proximity. This is undoubtedly the case with extant lakes Prespa and Ohrid, although the altitudinal difference is considerable, approximately 156 m. As members of the Dessarete Lake group, both lakes likely share a related origin as graben systems that formed during the Uppermost Meotioan-Pontian (Dumurdzanov et al., 2004), and therefore, share a significant time of co-existence. The hydrological connection criterion is easily applicable for lakes Prespa and Ohrid due to their karstic underground connection (Cvijic, 1911), as demonstrated by modern trace methods (Amataj et al., 2007). As far as a balanced degree of faunal overlap and distinctness is concerned, there are only few examples of species that occur both in lakes Prespa and Ohrid (Radoman, 1985). One case would be Dreissena presbensis (Albrecht et al., 2007). However, a pattern similar to that in Radix was found in the genus Pisidium, of which the respective sister species live in lakes Prespa and Ohrid (Schultheiß et al., 2008). Future phylogenetic analyses of other zoobenthic taxa will unravel the degree of recent faunal overlap between lakes Ohrid and Prespa and give a more complete picture of the biogeographic history of these lakes. Preliminary data for several hydrobiid groups (e.g. Wilke et al., 2007) already challenge Radoman’s (1985) hypothesis of a partial Aegean–Anatolian faunal connection of Lake Prespa. Rather, a western Adriatic zoogeographical affinity (also assumed by Radoman, 1985) appears more likely and supports our sister lake hypothesis for lakes Ohrid and Prespa. As demonstrated here for Radix, these sister lakes provide prime models for studying in situ evolutionary processes.
References
Albrecht, C., R. Schultheiß, T. Kevrekidis, B. Streit & T. Wilke, 2007. Invaders or endemics? Molecular phylogenetics, biogeography and systematics of Dreissena in the Balkans. Freshwater Biology 52: 1525–1536.
Albrecht, C., S. Trajanovski, K. Kuhn, B. Streit & T. Wilke, 2006. Rapid evolution of an ancient lake species flock: freshwater limpets (Gastropoda: Ancylidae) in the Balkan lake Ohrid. Organisms Diversity & Evolution 6: 294–307.
Albrecht, C. & T. Wilke, 2008. Lake Ohrid: biodiversity and evolution. Hydrobiologia. doi:10.1007/s10750-008-9558-y.
Albrecht, C., T. Wilke, K. Kuhn & B. Streit, 2004. Convergent evolution of shell shape in freshwater limpets. The African genus Burnupia. Zoological Journal of the Linnean Society 140: 577–588.
Amataj, S., T. Anovski, R. Benischke, R. Eftimi, L. L. Gourcy, L. Kola, I. Leontiadis, E. Micevski, A. Stamos & J. Zoto, 2007. Tracer methods used to verify the hypothesis of Cvijic about the underground connection between Prespa and Ohrid Lake. Environmental Geology 51: 749–753.
Bargues, M. D. & S. Mas-Coma, 1997. Phylogenetic analysis of lymnaeid snails based on 18S rDNA sequences. Molecular Biology and Evolution 14: 569–577.
Bargues, M. D., M. Vigo, P. Horak, J. Dvorak, R. A. Patzner, J. P. Pointier, M. Jackiewicz, C. Meier-Brook & S. Mas-Coma, 2001. European Lymnaeidae (Mollusca: Gastropoda), intermediate hosts of trematodiases, based on nuclear ribosomal DNA ITS-2 sequences. Infection. Genetics and Evolution 1: 85–107.
Burch, J. B. & S. Hadžišče, 1974. Immunological relationships of two species of Lymnaeidae (Mollusca: Basommatophora) from Macedonia, Yugoslavia. Malacological Review 7: 25–32.
Clement, M., D. Posada & K. Crandall, 2000. TCS. A computer program to estimate gene genealogies. Molecular Ecology 9: 1657–1660.
Coulter, G. W. (ed.), 1991. Lake Tanganyika and Its Life. Oxford University Press, New York.
Cowie, R. H., 2001. Invertebrate invasions on Pacific Islands and the replacement of unique native faunas: a synthesis of the land and freshwater snails. Biological Invasions 3: 119–136.
Cvijic, J., 1911. Fundamentals of geography and geology of Macedonia and Old Serbia. Book III, Serbian Academy of Sciences, Special Edition, Beograd (in Serbian).
Denèfle, M., A.-M. Lézine, E. Fouache & J.-J. Dufaure, 2000. A 12, 000-year pollen record from Lake Maliq, Albania. Quaternary Research 54: 423–432.
Dumurdzanov, N., T. Serofimovski & B. C. Burchfield, 2004. Evolution of the Neogene-Pleistocene Basins of Macedonia. Geological Society of America Digital Map and Chart Series 1: 1–20.
Falkner, G., 1990. Binnenmollusken. In Fechter, R. & G. Falkner (eds), Weichtiere. Europäische Meeres- und Binnenmollusken. Mosaik Verlag, München.
Felsenstein, J., 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791.
Folmer, O., M. Black, W. Hoeh, R. Lutz & R. Vrijenhoek, 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology & Biotechnology 3: 294–299.
Glöer, P., C. Albrecht & T. Wilke, 2007. Enigmatic distribution pattern of the Bithyniidae in the Balkan Region (Gastropoda: Rissooidea). Mollusca 25: 13–22.
Glöer, P. & I. Sîrbu, 2006. Freshwater molluscs species, new fort he Romanian fauna. Heldia 6: 207–216.
Gorthner, A., 1994. What is an ancient lake? Archiv für Hydrobiologie 44: 97–100.
Gorthner, A. & C. Meier-Brook, 1985. The Steinheim Basin as a paleo-ancient lake. In Bayer, U. & A. Seilacher (eds), Sedimentary and Evolutionary Cycles. Springer, Berlin, Heidelberg, New York, Tokyo: 322–334.
Hadžišče, S., 1956. III. Beitrag zur Kenntnis der Gastropodenfauna des Ohridsees. Beschreibung der bis jetzt unbekannten Schnecken und Beispiele der Speciation bei den Gastropoden des Ohridsees. Recueil des Traveaux, Station Hydrobiologique. Ohrid 4: 57–107.
Hall, T. A., 1999. BioEdit. A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98.
Hauswald, A.-K., C. Albrecht & T. Wilke, 2008. Testing two contrasting evolutionary patterns in ancient lakes: species flock vs. species scatter in valvatid gastropods of Lake Ohrid. Hydrobiologia. doi:10.1007/s10750-008-9556-0.
Hillis, D. M., B. K. Mable & C. Moritz, 1996. Applications of molecular systematics. In Hillis, D. M. C. Moritz & B. K. Mable (eds), Molecular Systematics, 2 edn. Sinauer Associates Inc., Sunderland, MA: 515–543.
Hubendick, B., 1951. Recent Lymnaeidae. Their variation, morphology, taxonomy, nomenclature, and distribution. Kunglinska Svenska Vetenskapsacedemiens Handlingar, Ser. 4 3: 1–223.
Hubendick, B. & P. Radoman, 1959. Studies on the Gyraulus species of Lake Ochrid. Morphology. Archiv för Zoologi 12: 223–243.
Huelsenbeck, J. P. & K. A. Crandall, 1997. Phylogeny estimation and hypothesis testing using maximum likelihood. Annual Review of Ecology and Systematics 28: 437–466.
Huelsenbeck, J. P. & F. Ronquist, 2001. MRBAYES. Bayesian inference of phylogeny. Bioinformatics 17: 754–755.
Jackiewicz, M., 1998. European species of the family Lymnaeidae (Gastropoda: Pulmonata: Basommatophora). Genus 9: 1–93.
Karaman, M., 1971. Zoogeographische Verhältnisse des Prespa- und Ohridsees. Izdanija 4: 1–21.
Kaiser, P., 1959. Über die Atmung von Radix relicta Pol. aus dem Ohrid-See. Recueil des travaux Ohrid 35: 1–5.
Kumar, S., K. Tamura & M. Nei, 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence. Alignment Briefings in Bioinformatics 5: 150–163.
Martens, K., 1997. Speciation in ancient lakes (review). Trends in Ecology and Evolution 12: 177–182.
Matzinger, A., M. Jordanoski, E. Veljanoska-Sarafiloska, M. Sturm, B. Müller & A. Wüest, 2006. Is Lake Prespa jeopardizing the ecosystem of ancient Lake Ohrid? Hydrobiologia 553: 89–109.
Matzinger, A., M. Schmid, E. Veljanoska-Sarafiloska, S. Patceva, D. Guseska, B. Wagner, B. Müller, M. Sturm & A. Wüest, 2007. Eutrophication of ancient Lake Ohrid: global warming amplifies detrimental effects of increased nutrient inputs. Limnology and Oceanography 52: 338–353.
Pfenninger, M., M. Cordellier & B. Streit, 2006. Comparing the efficacy of morphologic and DNA-based taxonomy in the freshwater gastropod genus Radix (Basommatophora, Pulmonata). BMC Evolutionary Biology 6: 100.
Polinski, W., 1932. Die reliktäre Gastropodenfauna des Ohrida-Sees. Zoologische Jahrbücher Abteilung Systematik 62: 611–666.
Posada, D. & K. A. Crandall, 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818.
Posada, D. & K. A. Crandall, 2001. Selecting the best-fit model of nucleotide substitution. Systematic Biology 50: 580–601.
Radoman, P., 1985. Hydrobioidea, a superfamily Prosobranchia (Gastropoda), II. Origin, zoogeography, evolution in the Balkans and Asia Minor. Monographs Institute of Zoology 1, Beograd.
Rambaut, A. & N. C. Grassly, 1997. Seq-Gen: an application for the Monte Carlo simulation of DNA sequence evolution along phylogenetic trees, Version 1.1. Computer Applications in the Biosciences 13: 235–238.
Remigio, E. A., 2002. Molecular phylogenetic relationships in the aquatic snail genus Lymnaea, the intermediate host of the causative agent of fascioliasis: insights from broader taxon sampling. Parasitology Research 88: 687–696.
Remigio, E. A. & D. Blair, 1997. Molecular systematics of the freshwater snail family Lymnaeidae (Pulmonata: Basommatophora) utilising mitochondrial ribosomal DNA sequences. Journal of Molluscan Studies 63: 173–185.
von Rintelen, T. & M. Glaubrecht, 2005. Anatomy of an adaptive radiation: a unique reproductive strategy in the endemic freshwater gastropod Tylomelania (Cerithioidea: Pachychilidae) on Sulawesi, Indonesia, and its biogeographic implications. Biological Journal of the Linnean Society 85: 513–542.
von Rintelen, T. & M. Glaubrecht, 2006. Rapid evolution of sessility in an endemic species flock of the freshwater bivalve Corbicula from ancient lakes on Sulawesi, Indonesia. Biology Letters 2: 73–77.
Schultheiß, R., C. Albrecht, U. Bößneck & T. Wilke, 2008. The neglected side of speciation in ancient lakes: phylogeography of an inconspicuous mollusk taxon in lakes Ohrid and Prespa. Hydrobiologia. doi:10.1007/s10750-008-9553-3.
Schütt, H., 1974. Zwei neue reliktäre Süßwassermollusken der Dinariden. Annalen des Naturhistorischen Museums in Wien 78: 473–480.
Schütt, H., 2006. Die Entrollung des Gewindes bei Lymnaeiden (Eine Literaturübersicht). Mitteilungen der deutschen malakozoologischen Gesellschaft 75: 5–7.
Spirkovski, Z., O. Avramovski & A. Kodzoman, 2001. Watershed management in the Lake Ohrid region of Albania and Macedonia. Lakes & Reservoirs: Research and Management 6: 237–242.
Stanković, S., 1960. The Balkan Lake Ohrid and Its Living World. Monographiae Biologicae Vol. IX, Uitgeverij Dr. W. Junk, Den Haag.
Sturany, R., 1894. Zur Molluskenfauna der europäischen Türkei. Annalen des kaiserlich königlichen Naturhistorischen Hofmuseums 9: 369–394.
Swofford, D. L., 2002. PAUP. Phylogenetic analysis using parsimony (and other methods). Version 410b. Sinauer Associates, Sunderland, Mass.
Wilke, T., C. Albrecht, V. V. Anistratenko, S. K. Sahin & Z. Yildirim, 2007. Testing biogeographical hypotheses in space and time: faunal relationships of the putative ancient lake Egirdir in Asia Minor. Journal of Biogeography 34: 1807–1821.
Wilke, T., G. M. Davis, Q. Dongchuan & R. C. Spear, 2006. Extreme mitochondrial sequence diversity in the intermediate schistosomiasis host Oncomelania hupensis robertsoni: another case of ancestral polymorphism? Malacologia 48: 143–157.
Zacharias, I., I. Bertachas, N. Skoulikidis & T. Koussouris, 2002. Greek lakes: limnological overview. Lakes & Reservoirs: Research and Management 7: 55–62.
Acknowledgements
We are very grateful to the colleagues of the Hydrobiological Institute Ohrid for their immense support. S. Trajanovski, B. Budzakoska and S. Trajanovska provided valuable information and took part in some field trips. D. Georgiev and B. Streit generously supported our field work. T. Kevrekidis and A. Mogias perfectly organized field work in Greece. Z. Yildirim and S. Koşal Şahin sent material from Turkey, whereas Z. Fehér provided Albanian material, M. Zettler material from Corsica, R. Patzner from Wallersee, and V. Pešić from Montenegro and Serbia. The comments of two anonymous reviewers enhanced an earlier version of the manuscript. We wish to express our deepest gratitude to all of them.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: T. Wilke, R. Väinölä & F. Riedel
Patterns and Processes of Speciation in Ancient Lakes: Proceedings of the Fourth Symposium on Speciation in Ancient Lakes, Berlin, Germany, September 4–8, 2006
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Albrecht, C., Wolff, C., Glöer, P. et al. Concurrent evolution of ancient sister lakes and sister species: the freshwater gastropod genus Radix in lakes Ohrid and Prespa. Hydrobiologia 615, 157–167 (2008). https://doi.org/10.1007/s10750-008-9555-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9555-1