Abstract
Sarcoidosis is a multisystem granulomatous disorder that can potentially involve any organ. Cardiac involvement in sarcoidosis has been reported in up to 25% of patients based on autopsy and imaging studies. The gold standard for diagnosing cardiac sarcoidosis is endomyocardial biopsy demonstrating non-caseating granulomas; however, this technique lacks sensitivity due to the patchy nature of myocardial involvement. This, along with the non-specific clinical presentation, renders the diagnosis of cardiac sarcoidosis extremely challenging. Difficulties in obtaining histopathologic diagnosis and the advances in imaging modalities have led to a paradigm shift toward non-invasive imaging in the diagnosis of cardiac sarcoidosis. Advances in cardiac imaging modalities have also allowed unprecedented insights into the prevalence and natural history of cardiac sarcoidosis. This review discusses the role of non-invasive imaging for diagnosis, risk stratification, and monitoring the response to therapies in cardiac sarcoidosis. Echocardiography remains the first-line modality due to widespread availability and affordability. Cardiac magnetic resonance imaging (CMR) can be used to study cardiac structure, function, and most importantly tissue characterization to detect inflammation and fibrosis. Fluoro-deoxy glucose positron emission tomography (FDG PET) is the gold standard for non-invasive detection of cardiac inflammation, and it offers the unique ability to assess response to therapeutic interventions. Hybrid imaging is a promising technique that allows us to combine the unique strengths of CMR and FDG PET. Understanding the advantages and disadvantages of each of these imaging modalities is crucial in order to tailor the diagnostic algorithm and utilize the most appropriate modality for each patient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a multisystem granulomatous inflammatory disorder of unknown etiology. It was first described by Sir Jonathon Hutchinson in 1878 [1]. Advances in diagnostic modalities have resulted in increased recognition and prevalence of this condition. The diagnosis, however, is elusive and remains challenging to this date. In this review, we discuss the role of various imaging modalities in the evaluation of cardiac sarcoidosis.
Pathogenesis and histopathology
The exact cause of sarcoidosis remains unknown. Environmental antigens and genetic predisposition have been shown to contribute to disease development [2, 3]. Although studies suggest an association between certain genotypes and cardiac sarcoidosis, there are no available genetic markers to identify individuals with a predisposition for cardiac involvement in sarcoidosis [4, 5].
Understanding the histopathologic features of cardiac sarcoidosis allows for the appropriate diagnostic tests to be utilized for evaluation. Cardiac sarcoidosis predominantly involves the myocardium, with the most common sites being interventricular septum, followed by the left ventricular free wall, papillary muscles, and right ventricle [6]. Histopathologic studies have shown the following patterns in sarcoidosis [7]:
-
1.
Edema and lymphocytic infiltration: Interstitial edema can be detected on cardiac magnetic resonance imaging (CMR) with late gadolinium enhancement (LGE) and T2 imaging. Lymphocytic infiltration can be identified using fluorodeoxyglucose positron emission tomography (FDG-PET) to detect increased glucose uptake by metabolically active leucocytes.
-
2.
Granulomatous inflammation: Non-caseating granulomas are the histopathologic hallmark of sarcoidosis. CMR can identify areas of fibrosis associated with granulomas, whereas FDG-PET can identify areas of inflammation due to increased glucose uptake in activated macrophages.
-
3.
Replacement fibrosis: This can be identified as an area of perfusion defect on single positron emission tomography (SPECT) or PET or abnormal LGE on CMR.
Epidemiology
Sarcoidosis is a multisystem disorder with diverse manifestations, and therefore, patients with sarcoidosis may present to different sub-specialty clinics depending on the end-organ involvement. The majority of patients with sarcoidosis have pulmonary involvement; other organs that may be involved are the heart, skin, joints, eyes, liver, spleen, and kidneys [8]. Clinically manifest cardiac sarcoidosis has been reported in about 2–5% of patients with systemic sarcoidosis [6, 9]. However, autopsy studies show that the prevalence of cardiac involvement in sarcoidosis is as high as 25% [10,11,12]. Isolated cardiac sarcoidosis, defined as cardiac involvement with no preexisting diagnosis of systemic sarcoidosis has been reported in one- third of all presentations of cardiac sarcoidosis [13, 14]. Early diagnosis of cardiac involvement is important for risk stratification and initiation of targeted therapies.
The reported prevalence of cardiac sarcoidosis from imaging studies aligns closely with data from autopsy studies [15, 16]. Therefore, advances in imaging have allowed early detection of clinically silent cardiac involvement, resulting in increasing prevalence of cardiac sarcoidosis over the years [17].
Clinical features
From a clinical standpoint, the key sequelae of cardiac sarcoidosis are as follows: (1) heart failure, (2) conduction abnormalities, (3) ventricular arrhythmias, and (4) sudden cardiac death. [13, 18].
Congestive heart failure in cardiac sarcoidosis may be due to involvement of the left ventricle, right ventricle, or both. Left ventricular ejection fraction (LVEF), left ventricular cavity size, and New York Heart Association functional class are independent predictors of mortality [19]. In a case series of 16 patients undergoing orthotopic heart transplantation for cardiac sarcoidosis, the total QRS voltage on 12-lead EKG was found to be low, despite increased weight of the heart [20]. Patients presenting with heart failure as the first clinical manifestation of cardiac sarcoidosis have poor outcomes, likely due to advanced disease [17].
Conduction abnormalities may be secondary to granulomatous inflammation and fibrosis involving the sinoatrial node, atrio-ventricular node, or His-Purkinje system and their corresponding vasculature. According to the 2014 Heart Rhythm Society (HRS) Consensus statement, unexplained Mobitz II or third-degree atrioventricular block in young patients aged less than 60 years should raise suspicion for cardiac sarcoidosis, and these patients should undergo chest computed tomography as well as advanced cardiac imaging for further evaluation [18].
Supraventricular tachyarrhythmias in cardiac sarcoidosis may be due to diastolic dysfunction, increased left atrial pressure, pulmonary hypertension, or atrial granulomatous fibrosis. Patients with atrial LGE on CMR are three times more likely to develop atrial arrhythmias than those without [21].
Ventricular tachyarrhythmia is the most feared complication of cardiac sarcoidosis with scar-related re-entry being the most common underlying mechanism [22, 23]. Coleman et al. conducted a meta-analysis of 10 studies involving 760 patients with known or suspected cardiac sarcoidosis who underwent CMR. They found that amongst patients with preserved LVEF (> 50%), presence of LGE on CMR predicted death or ventricular arrhythmia with an odds ratio of 19.43 (CI 7.62 – 49.56); this association was not present amongst those with reduced LVEF [24]. Similarly, active inflammation detected by increased FDG uptake on cardiac PET scan identifies patients at higher risk for death and ventricular arrhythmias [25]. Therefore, advanced cardiac imaging modalities such as CMR and PET offer incremental prognostic utility above and beyond assessment of LVEF, and are especially useful for risk stratification in patients with preserved left ventricular systolic function.
Diagnosis
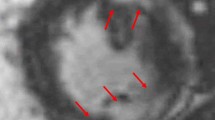
The gold standard for diagnosis of cardiac sarcoidosis is an endomyocardial biopsy (EMB) showing non-caseating granulomas with fibrosis, few eosinophils, and little myocyte necrosis after the exclusion of other causes (Fig. 1) [26]. However, patients with advanced stages of the disease may have non-specific scar pattern. In a study of 25 explanted hearts from patients undergoing heart transplant for advanced heart failure due to cardiac sarcoidosis, 36% were found to have no granulomas in the explanted heart [27]. Additionally, due to the focal and patchy involvement of granulomas with predilection to the mid-myocardial and epicardial layers rather than the endocardium, EMB has poor sensitivity at 20–30% [28, 29]. Due to the difficulty in obtaining a histopathologic diagnosis and with the advances in imaging modalities over the past two decades, there is a paradigm shift towards non-invasive imaging in the diagnostic criteria of cardiac sarcoidosis.
In 1993, the first diagnostic criteria for cardiac sarcoidosis were published by the Japanese Ministry of Health and Welfare (JMHW), and subsequently updated in 2006 [30]. Given the new developments in cardiac imaging, the Japanese society acknowledged the shortcomings of the older guidelines and released an update in 2017 [31]. In the update, criteria for diagnosing isolated cardiac sarcoidosis were created, the use of 18F-FDG was added, and the role of CMR was upgraded from minor to major in the diagnostic criteria (Table 1).
Meanwhile, in 2014, the HRS released an expert Consensus Statement on the Diagnosis and Management of cardiac sarcoidosis arrhythmia [18]. In this statement, apart from confirming the diagnosis via a biopsy, a “probable diagnosis” (defined as > 50% likelihood) of cardiac sarcoidosis can be established based on a histological diagnosis of extra-cardiac sarcoidosis along with typical clinical, imaging, or electrocardiographic findings (Table 2).
There is a paucity of data regarding screening for cardiac sarcoidosis in patients with proven extra-cardiac sarcoidosis. Mehta et al. evaluated 62 patients diagnosed with sarcoidosis and found a prevalence of CS at 39%. Expectedly, patients with cardiac sarcoidosis had more cardiac symptoms than those without (46% vs 5%, respectively) and were more likely to have abnormal Holter monitoring findings (50% vs 3%, respectively) and trans-thoracic echocardiogram (TTE) findings (25% vs 5%, respectively). Interestingly, the degree of pulmonary impairment did not predict cardiac sarcoidosis [32]. In another analysis, combining a TTE and an electrocardiogram (EKG) had a sensitivity of only 32% while the combination of TTE and ambulatory EKG had a sensitivity of 63% [33]. The current advances in CMR and nuclear imaging techniques increase the sensitivity for detection of cardiac sarcoidosis. Their use is restricted, however, due to high cost, limited expertise, and availability.
For patients with biopsy-proven extra-cardiac sarcoidosis, the HRS committee recommends obtaining a cardiac history and an EKG (class I), and screening for cardiac involvement with a TTE (class IIa). Advanced cardiac imaging (CMR or FDG-PET) can be done in patients with signs or symptoms suggestive of cardiac sarcoidosis. However, their routine use in asymptomatic patients is not recommended (class III).
Echocardiography
Echocardiography represents the first line cardiac imaging modality due to wide availability and affordability. However, it has limited sensitivity and specificity for the detection of cardiac sarcoidosis. As mentioned earlier, screening by TTE in asymptomatic patients with extra-cardiac sarcoidosis is recommended by the HRS but not by ATS. Echocardiographic findings are highly variable in cardiac sarcoidosis and are difficult to distinguish from other types of cardiomyopathy.
Cardiac sarcoidosis has a higher predilection for involvement of the left ventricle [34]. Therefore, commonly noted echocardiographic findings include left ventricular dysfunction, global hypokinesis, and left ventricular dilatation. Edema and infiltration in the ventricular wall can cause mild wall thickening simulating left ventricular hypertrophy or resembling hypertrophic cardiomyopathy [35]. Granulomatous inflammation and scar formation in the ventricular septum or the free wall can cause increased ventricular wall echogenicity and ventricular wall thinning leading to ventricular aneurysms, most commonly in the inferolateral wall. The patchy nature of sarcoid infiltration of the heart may result in non-coronary regional wall motion abnormalities. Due to chronic inflammation within the myocardium, different grades of diastolic dysfunction can occur. Papillary muscle dysfunction leading to mitral and tricuspid regurgitation has been reported [36, 37].
Right ventricular involvement is usually secondary to pulmonary hypertension in the setting of left ventricular dysfunction or pulmonary sarcoidosis. Primary right ventricular involvement is less common but can result in focal or global dysfunction [38]. Rarely, it can present in an echocardiographic picture similar to that of arrhythmogenic right ventricular cardiomyopathy [39]. Pericardial involvement may lead to pericardial effusions and rarely cardiac tamponade or constrictive pericarditis [40].
Strain imaging has proven to be beneficial in amyloid heart disease, hypertrophic cardiomyopathy, and chemotherapy-related cardiotoxicity prompting investigators to study its role in cardiac sarcoidosis [41]. Murtagh et al. showed that cardiac sarcoidosis was associated with significantly reduced global longitudinal strain in the presence of preserved LVEF [42]. Moreover, a retrospective study that included 117 patients with extra-cardiac sarcoidosis showed that reduced global longitudinal strain was strongly associated with adverse future heart failure‐related hospitalizations, need for device therapy, arrhythmias, and all-cause mortality [43]. Although strain imaging has the potential of solidifying its role in cardiac sarcoidosis detection, presently the data is very limited. Hence, prospective studies comparing strain to other imaging modalities are needed.
Diagnostic accuracy
Echocardiography has poor sensitivity for detection of early cardiac sarcoidosis. In a retrospective study of 62 patients with sarcoidosis, Mehta et al. reported that echocardiographic abnormalities were noted only in 25% of patients who had CMR or FDG-PET evidence of cardiac sarcoidosis [32]. Therefore, a normal appearing echocardiogram is insufficient to rule out cardiac sarcoidosis and advanced imaging modalities must be considered if clinical suspicion is high.
CMR
Cardiac magnetic resonance imaging (CMR) is an important diagnostic tool in the evaluation of non-ischemic cardiomyopathy, allowing assessment of cardiac structure, function, and tissue characteristics [44]. Scan acquisition protocols for patients with suspected cardiac sarcoidosis include low resolution localizer images, cine imaging in multiple planes, T2-weighted imaging, and LGE [37, 45]. T1 imaging is used to assess ventricular hypertrophy, wall thinning, and wall function; early T2 imaging helps identify edema whereas late T2 imaging is used for assessment of fibrosis and scarring (Fig. 2).
Detecting cardiac sarcoidosis by CMR relies on identifying the presence of LGE along the ventricular wall in patterns that do not follow a coronary distribution [37, 45, 46]. The most commonly involved myocardial segments are the basal and mid-ventricular septum, and left ventricular lateral wall. The mid-myocardial and epicardial layers are frequently affected with relative sparing of the sub-endocardial tissue. These have been associated with worsening arrythmia is sarcoid patients [47]. Increased myocardial signal intensity on T2-weighted imaging suggests increased water content, i.e., edema. However, this technique is limited by frequent artifacts and poor signal to noise ratio, and its use has not been validated in cardiac sarcoidosis [48].
Diagnostic accuracy
In a multicenter study, Smedema et al. evaluated 58 patients with biopsy-proven pulmonary sarcoidosis and compared diagnostic accuracy of CMR using JMHW criteria as the gold standard. They reported a sensitivity of 100% and specificity of 78%, with overall accuracy of 83% [46]. Patel et al. studied 81 consecutive patients with histologically proven extra-cardiac sarcoidosis and found that CMR was more than twice as sensitive for detection of cardiac involvement compared to JMHW criteria. They also found that the presence of LGE portended poor outcomes (9 times higher rate of adverse events and 11.5 times higher rate of cardiac death) compared to those without evidence of delayed enhancement [45].
Role of CMR in the evaluation of cardiac sarcoidosis
The unique advantages of CMR in non-invasive evaluation of cardiac sarcoidosis include:
-
1.
High sensitivity for detection of inflammation and fibrosis
-
2.
Ability to guide endomyocardial biopsy to increase diagnostic yield
-
3.
Lack of ionizing radiation.
However, there are some limitations for this technique which include:
-
1.
Renal insufficiency precludes the use of gadolinium
-
2.
Artifact from cardiac implantable electronic devices such as pacemakers and automatic implantable cardioverter defibrillators may limit interpretability.
-
3.
LGE findings are not specific for cardiac sarcoidosis and it may be challenging to differentiate from myocarditis, and other inflammatory cardiomyopathies.
-
4.
Inability to differentiate active disease from chronic fibrosis. This limits the application of CMR in assessing response to therapy.
Radionuclide imaging
Radionuclide imaging with gallium-citrate SPECT and FDG-PET can be used to detect myocardial inflammation and perfusion defects. Due to lower sensitivity and poor spatial resolution, gallium citrate SPECT has limited utility and should be used only if FDG-PET is not available [49, 50]. FDG-PET has emerged as a promising modality to assess the extent of myocardial involvement as well as systemic sarcoidosis [51].
Image interpretation involves analysis of FDG uptake as well as perfusion images to detect resting defects. FDG is an analog of glucose that can be used to identify tissue with high rate of glucose metabolism. The accumulation of FDG in activated macrophages is the rationale for detection of active granulomas by FDG-PET imaging [52]. Perfusion defects in patients with cardiac sarcoidosis represent segments with inflammation (compression of microvasculature due to edema) or fibrosis.
Patient dietary preparation is the most crucial aspect of the protocol and is required to suppress physiologic myocardial glucose uptake and optimize image quality. This entails a no-carbohydrate, high-fat diet for about 18 to 24 h prior to the test followed by an overnight fast [53]. The concomitant administration of 50 IU/Kg of intravenous heparin (which induces lipolysis) can also be utilized and improves the interpretability of FDG-PET scans for detection of inflammation [54].
Image interpretation
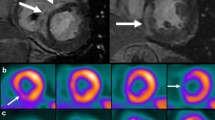
With adequate myocardial glucose suppression and in the absence of inflammation, FDG is seen in the left ventricular blood pool (which appears brighter than the myocardium). Based on FDG uptake, the images are reviewed for the following possible metabolic patterns: (a) no FDG uptake (b) diffuse FDG uptake, (c) focal FDG uptake, (d) focal on diffuse FDG uptake. Myocardial perfusion imaging and FDG uptake images are then aligned and interpreted simultaneously for the following possible patterns (Fig. 3) [15, 37, 55]:
-
a)
Normal perfusion and no FDG uptake: this pattern represents normal myocardium.
-
b)
Normal perfusion and increased FDG uptake: this pattern is seen in segments with early disease, i.e., inflammatory activity.
-
c)
Reduced perfusion and increased FDG uptake: this pattern represents presence of active inflammation and scarring.
-
d)
Reduced perfusion and no FDG uptake: the perfusion defect may be due to scarring from sarcoidosis or other etiologies. Here, lack of FDG uptake is interpreted as lack of active inflammation, but does not rule out the possibility of cardiac sarcoidosis.
Rest rubidium and FDG images pretherapy show normal resting perfusion but patchy FDG uptake in the basal antero-septum and inferior wall segments consistent with an inflammatory cardiac process. Repeat images after 4 months of therapy with corticosteroid show normal resting perfusion and normal blood pooling of FDG consistent with resolution of inflammation
Diagnostic accuracy
A meta-analysis of seven studies assessing accuracy of FDG PET scan for diagnosis of cardiac sarcoidosis with JMHW criteria as the gold standard, showed pooled sensitivity of 89% and specificity of 78%. It is important to keep in mind that the low specificity of FDG-PET in this scenario may reflect on the poor sensitivity of JMHW criteria [15].
Role of FDG-PET in evaluation of cardiac sarcoidosis
FDG-PET is the gold standard for non-invasive evaluation of myocardial inflammation. It has applications ranging from detection of early active disease, monitoring ongoing inflammation and response to treatment, and evaluation of extra-cardiac involvement with full-body imaging [56]. It is also the preferred modality in patients with cardiovascular implantable electronic devices such as permanent pacemakers and implantable cardioverter defibrillators due to concern regarding artifact with CMR. Some limitations of FDG-PET scan include:
-
1.
Use of ionizing radiation
-
2.
Need for meticulous patient preparation to optimize image quality. Unfortunately, 10–15% of these tests remain non-diagnostic due to incomplete suppression of physiologic FDG uptake despite careful preparation [57].
Follow-up imaging to monitor therapy
Radionuclide imaging with serial FDG-PET scans is the best available imaging modality to monitor response to immunosuppressive therapy. Osborne et al. studied 23 consecutive patients undergoing serial PET scans during treatment for cardiac sarcoidosis. They found that a reduction in extent or degree of FDG uptake was associated with an increase in LVEF. Interestingly, they divided the study cohort into responders and non-responders based on the change in the FDG uptake over the follow up period. Patients who had a decrease in FDG uptake were defined as responders, whereas those who had increase or no change in uptake were defined as non-responders. Responders were found to have a significant increase in mean LVEF (8.6% ± 5.2%) whereas non-responders had a decrease in mean LVEF (5.5% ± 3.4%) [58]. In a retrospective study of 28 patients with cardiac sarcoidosis undergoing corticosteroid sparing maintenance therapy (defined as methotrexate and/or adalimumab), patients underwent serial FDG-PET scans at 6-month intervals. FDG uptake resolution was noted in 60% of patients who received methotrexate vs 63% of patients who received adalimumab containing regimens [59]. Therefore, serial FDG-PET imaging represents a unique opportunity to non-invasively monitor the extent and intensity of cardiac inflammation, and assess therapeutic efficacy. The ideal time intervals for serial imaging are not known. There is a need for interventional randomized clinical trials of imaging guided titration of immunosuppressive therapy.
Hybrid imaging
CMR and FDG-PET CT represent complementary imaging modalities in the evaluation of cardiac sarcoidosis. While CMR is superior in terms of morphological and functional assessment as well as tissue characterization, it cannot distinguish active and chronic disease. On the other hand, FDG-PET CT is unique in its ability to detect active myocardial inflammation. Hybrid CMR/PET imaging allows us to combine the strengths of both techniques. This can be accomplished either by co-registration of separately acquired scans or in a single hybrid PET-CMR scanner [60]. A prospective study of 25 patients with clinically suspected active cardiac sarcoidosis showed an area under the curve of 0.98 on receiver operating characteristic analysis for detection of active cardiac sarcoidosis [61]. In a retrospective study of 107 consecutive patients referred for evaluation of known or suspected cardiac sarcoidosis, addition of PET data to CMR findings led to 45% of patients being reclassified as having a higher or lower likelihood of cardiac sarcoidosis. Of these, most (80%) were correctly reclassified when compared with the final diagnosis [62].
Diagnostic algorithm
The two specific clinical scenarios in which diagnostic work-up for cardiac sarcoidosis is typically pursued are as follows: (1) screening for cardiac involvement in patients with biopsy proven extra-cardiac sarcoidosis and (2) evaluation for cardiac sarcoidosis in patients with specific cardiac presentations. Although echocardiographic findings are neither sensitive, nor specific for cardiac sarcoidosis, they may provide useful clues that raise clinical suspicion and prompt the appropriate further diagnostic workup. Since CMR has a high negative predictive value to exclude cardiac sarcoidosis, and does not involve use of ionizing radiation, it is the preferred advanced imaging modality for screening. In patients with a high pre-test likelihood of cardiac sarcoidosis, or those who have cardiovascular implantable electronic devices, it may be reasonable to proceed directly to FDG-PET imaging. We present here a simplified diagnostic algorithm in patients with suspected cardiac sarcoidosis (Fig. 4).
Conclusions
Non-invasive imaging plays a key role in the diagnosis, risk stratification, and monitoring of response to therapeutic interventions in cardiac sarcoidosis. It has enhanced our understanding of the pathophysiology and natural history of this intriguing disease. The use of advanced imaging modalities to guide biopsy offers an exciting avenue to enhance the yield and sensitivity of EMB. Further studies are needed to define the optimal screening protocols for identifying cardiac involvement in patients with known extra-cardiac sarcoidosis. There is also a need for studies to explore the use of advanced imaging modalities for patient selection, choice of immunosuppressive agent, and dose titration of therapy in cardiac sarcoidosis.
Abbreviations
- ATS:
-
American Thoracic Society
- CMR:
-
Cardiac magnetic resonance imaging
- EKG:
-
Electrocardiogram
- EMB:
-
Endomyocardial biopsy
- FDG:
-
Fluoro-deoxy glucose
- HRS:
-
Heart Rhythm Society
- JMHW:
-
Japanese Ministry of Health and Welfare
- LGE:
-
Late gadolinium enhancement
- LVEF:
-
Left ventricular ejection fraction
- MPI:
-
Myocardial perfusion imaging
- PET:
-
Positron emission tomography
- SPECT:
-
Single positron emission tomography
- TTE:
-
Trans-thoracic echocardiogram
References
James DG, Sharma OP (2002) From Hutchinson to now: a historical glimpse. Curr Opin Pulm Med 8(5):416–423. https://doi.org/10.1097/00063198-200209000-00013
Iannuzzi MC, Rybicki BA (2007) Genetics of sarcoidosis: candidate genes and genome scans. Proc Am Thorac Soc 4(1):108–116. https://doi.org/10.1513/pats.200607-141JG
Newman LS, Rose CS, Bresnitz EA et al (2004) A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med 170(12):1324–1330. https://doi.org/10.1164/rccm.200402-249OC
Naruse TK, Matsuzawa Y, Ota M et al (2000) HLA-DQB1*0601 is primarily associated with the susceptibility to cardiac sarcoidosis. Tissue Antigens 56(1):52–57. https://doi.org/10.1034/j.1399-0039.2000.560107.x
Takashige N, Naruse TK, Matsumori A et al (1999) Genetic polymorphisms at the tumour necrosis factor loci (TNFA and TNFB) in cardiac sarcoidosis. Tissue Antigens 54(2):191–193. https://doi.org/10.1034/j.1399-0039.1999.540211.x
Silverman KJ, Hutchins GM, Bulkley BH (1978) Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 58(6):1204–1211. https://doi.org/10.1161/01.cir.58.6.1204
Iwai K, Takemura T, Kitaichi M, Kawabata Y, Matsui Y (1993) Pathological studies on sarcoidosis autopsy. II. Early change, mode of progression and death pattern. Acta Pathol Jpn 43(7–8):377–85. https://doi.org/10.1111/j.1440-1827.1993.tb01149.x
Ungprasert P, Carmona EM, Utz JP, Ryu JH, Crowson CS, Matteson EL (2016) Epidemiology of Sarcoidosis 1946–2013: a population-based study. Mayo Clin Proc 91(2):183–188. https://doi.org/10.1016/j.mayocp.2015.10.024
Baughman RP, Teirstein AS, Judson MA et al (2001) Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 164(10 Pt 1):1885–1889. https://doi.org/10.1164/ajrccm.164.10.2104046
Iwai K, Tachibana T, Takemura T, Matsui Y, Kitaichi M, Kawabata Y (1993) Pathological studies on sarcoidosis autopsy. I. epidemiological features of 320 cases in Japan. Acta Pathol Jpn 43(7–8):372–376. https://doi.org/10.1111/j.1440-1827.1993.tb01148.x
Perry A, Vuitch F (1995) Causes of death in patients with sarcoidosis. a morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med 119(2):167–172
Lynch JP 3rd, Hwang J, Bradfield J, Fishbein M, Shivkumar K, Tung R (2014) Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med 35(3):372–390. https://doi.org/10.1055/s-0034-1376889
Birnie DH, Nery PB, Ha AC, Beanlands RS (2016) Cardiac Sarcoidosis. J Am Coll Cardiol 68(4):411–421. https://doi.org/10.1016/j.jacc.2016.03.605
Kandolin R, Lehtonen J, Graner M et al (2011) Diagnosing isolated cardiac sarcoidosis. J Intern Med 270(5):461–468. https://doi.org/10.1111/j.1365-2796.2011.02396.x
Youssef G, Leung E, Mylonas I et al (2012) The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med 53(2):241–248. https://doi.org/10.2967/jnumed.111.090662
Greulich S, Deluigi CC, Gloekler S et al (2013) CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 6(4):501–511. https://doi.org/10.1016/j.jcmg.2012.10.021
Kandolin R, Lehtonen J, Airaksinen J et al (2015) Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation 131(7):624–632. https://doi.org/10.1161/circulationaha.114.011522
Birnie DH, Sauer WH, Bogun F et al (2014) HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 11(7):1305–1323. https://doi.org/10.1016/j.hrthm.2014.03.043
Yazaki Y, Isobe M, Hiroe M et al (2001) Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 88(9):1006–1010. https://doi.org/10.1016/s0002-9149(01)01978-6
Roberts WC, Becker TM, Hall SA (2018) Usefulness of total 12-lead QRS voltage as a clue to diagnosis of patients with cardiac sarcoidosis severe enough to warrant orthotopic heart transplant. JAMA Cardiol 3(1):64–68. https://doi.org/10.1001/jamacardio.2017.4172
Cain MA, Metzl MD, Patel AR et al (2014) Cardiac sarcoidosis detected by late gadolinium enhancement and prevalence of atrial arrhythmias. Am J Cardiol 113(9):1556–1560. https://doi.org/10.1016/j.amjcard.2014.01.434
Okada DR, Smith J, Derakhshan A et al (2018) Ventricular arrhythmias in cardiac sarcoidosis. Circulation 138(12):1253–1264. https://doi.org/10.1161/circulationaha.118.034687
Naruse Y, Sekiguchi Y, Nogami A et al (2014) Systematic treatment approach to ventricular tachycardia in cardiac sarcoidosis. Circ Arrhythm Electrophysiol 7(3):407–413. https://doi.org/10.1161/circep.113.000734
Coleman GC, Shaw PW, Balfour PC Jr et al (2017) Prognostic value of myocardial scarring on CMR in patients with cardiac sarcoidosis. JACC Cardiovasc Imaging 10(4):411–420. https://doi.org/10.1016/j.jcmg.2016.05.009
Blankstein R, Osborne M, Naya M et al (2014) Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 63(4):329–336. https://doi.org/10.1016/j.jacc.2013.09.022
Litovsky SH, Burke AP, Virmani R (1996) Giant cell myocarditis: an entity distinct from sarcoidosis characterized by multiphasic myocyte destruction by cytotoxic T cells and histiocytic giant cells. Mod Pathol: an official journal of the United States and Canadian Academy of Pathology, Inc. 9(12):1126–34
Fathima S, Roberts WC (2019) Comparison of clinical and morphologic findings in patients with cardiac sarcoidosis severe enough to warrant heart transplantation in those with -vs- those without non-caseating granulomas in the explanted heart (burnt-out sarcoid). Am J Cardiol 124(4):599–603. https://doi.org/10.1016/j.amjcard.2019.05.020
Ardehali H, Howard DL, Hariri A et al (2005) A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J 150(3):459–463. https://doi.org/10.1016/j.ahj.2004.10.006
Sekiguchi M, Hiroe M, Take M, Hirosawa K (1980) Clinical and histopathological profile of sarcoidosis of the heart and acute idiopathic myocarditis. concepts through a study employing endomyocardial biopsy. II. Myocarditis. Jpn Circ J 44(4):264–73. https://doi.org/10.1253/jcj.44.264
Hiraga H, Yuwai K, Hiroe M (2007) Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord 27:89–102
Terasaki F, Yoshinaga K (2017) New guidelines for diagnosis of cardiac sarcoidosis in Japan. Ann Nucl Cardiol 3(1):42–45. https://doi.org/10.17996/anc.17-00042
Mehta D, Lubitz SA, Frankel Z et al (2008) Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest 133(6):1426–1435. https://doi.org/10.1378/chest.07-2784
Crouser ED (2020) Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline 201(8):e26–e51. https://doi.org/10.1164/rccm.202002-0251ST
Bargout R, Kelly RF (2004) Sarcoid heart disease: clinical course and treatment. Int J Cardiol 97(2):173–182. https://doi.org/10.1016/j.ijcard.2003.07.024
Nureki S, Miyazaki E, Nishio S et al (2014) Interventricular septal thickening as an early manifestation of cardiac sarcoidosis. Int Heart J 55(2):181–183. https://doi.org/10.1536/ihj.13-243
Kurmann R, Mankad SV, Mankad R (2018) Echocardiography in sarcoidosis. Curr Cardiol Rep 20(11):118. https://doi.org/10.1007/s11886-018-1065-9
Slart RH, Glaudemans AW, Lancellotti P, Hyafil F, Blankstein R, Schwartz RG, & Masci PG (2017) A joint procedural position statement on imaging in cardiac sarcoidosis: from the Cardiovascular and Inflammation & Infection Committees of the European Association of Nuclear Medicine, the European Association of Cardiovascular Imaging, and the American Society of Nuclear Cardiology. Eur Heart J Cardiovasc Imaging 18(10):1073–1089. https://doi.org/10.1093/ehjci/jex146
Patel MB, Mor-Avi V, Murtagh G et al (2016) Right heart involvement in patients with sarcoidosis. Echocardiography (Mount Kisco, NY) 33(5):734–741. https://doi.org/10.1111/echo.13163
Vasaiwala SC, Finn C, Delpriore J et al (2009) Prospective study of cardiac sarcoid mimicking arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol 20(5):473–476. https://doi.org/10.1111/j.1540-8167.2008.01351.x
Aggarwal NR, Snipelisky D, Young PM, Gersh BJ, Cooper LT, Chareonthaitawee P (2015) Advances in imaging for diagnosis and management of cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging 16(9):949–958. https://doi.org/10.1093/ehjci/jev142
Saleh Y, Abdelkarim O, Herzallah K, Abela GS (2020) Anthracycline-induced cardiotoxicity: mechanisms of action, incidence, risk factors, prevention, and treatment. Heart Fail Rev. https://doi.org/10.1007/s10741-020-09968-2
Murtagh G, Laffin LJ, Patel KV et al (2016) Improved detection of myocardial damage in sarcoidosis using longitudinal strain in patients with preserved left ventricular ejection fraction. Echocardiography (Mount Kisco, NY) 33(9):1344–1352. https://doi.org/10.1111/echo.13281
Felekos I, Aggeli C, Gialafos E et al (2018) Global longitudinal strain and long-term outcomes in asymptomatic extracardiac sarcoid patients with no apparent cardiovascular disease. Echocardiography (Mount Kisco, NY) 35(6):804–808. https://doi.org/10.1111/echo.13846
Al-Mallah MH, Shareef MN (2011) The role of cardiac magnetic resonance imaging in the assessment of non-ischemic cardiomyopathy. Heart Fail Rev 16(4):369–380. https://doi.org/10.1007/s10741-010-9221-3
Patel MR, Cawley PJ, Heitner JF et al (2009) Detection of myocardial damage in patients with sarcoidosis. Circulation 120(20):1969–1977. https://doi.org/10.1161/circulationaha.109.851352
Smedema JP, Snoep G, van Kroonenburgh MP et al (2005) Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 45(10):1683–1690. https://doi.org/10.1016/j.jacc.2005.01.047
Crawford T, Mueller G, Sarsam S et al (2014) Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol 7(6):1109–1115. https://doi.org/10.1161/circep.113.000156
Aljizeeri A, Sulaiman A, Alhulaimi N, Alsaileek A, Al-Mallah MH (2017) Cardiac magnetic resonance imaging in heart failure: where the alphabet begins! Heart Fail Rev 22(4):385–399. https://doi.org/10.1007/s10741-017-9609-4
Nishiyama Y, Yamamoto Y, Fukunaga K et al (2006) Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med 47(10):1571–1576
Yamagishi H, Shirai N, Takagi M et al (2003) Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med 44(7):1030–1036
El-Tallawi KC, Parikh R, Nabi F, Maclayton PI, Trachtenberg BH, Al-Mallah M (2020) A positive Tc-99m PYP scan in a patient with cardiac sarcoidosis. J Nucl Cardiol. https://doi.org/10.1007/s12350-020-02158-5
Treglia G, Annunziata S, Sobic-Saranovic D, Bertagna F, Caldarella C, Giovanella L (2014) The role of 18F-FDG-PET and PET/CT in patients with sarcoidosis: an updated evidence-based review. Acad Radiol 21(5):675–684. https://doi.org/10.1016/j.acra.2014.01.008
Dorbala S, Di Carli MF, Delbeke D et al (2013) SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med 54(8):1485–1507. https://doi.org/10.2967/jnumed.112.105155
Scholtens AM, Verberne HJ, Budde RP, Lam MG (2016) Additional heparin preadministration improves cardiac glucose metabolism suppression over low-carbohydrate diet alone in 18F-FDG PET imaging. J Nucl Med 57(4):568–573. https://doi.org/10.2967/jnumed.115.166884
Okumura W, Iwasaki T, Toyama T et al (2004) Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med 45(12):1989–1998
Khalaf S, Al-Mallah MH (2020) Fluorodeoxyglucose applications in cardiac PET: viability, inflammation, infection, and beyond. Methodist Debakey Cardiovasc J 16(2):122–129. https://doi.org/10.14797/mdcj-16-2-122
Osborne MT, Hulten EA, Murthy VL et al (2017) Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol 24(1):86–99. https://doi.org/10.1007/s12350-016-0502-7
Osborne MT, Hulten EA, Singh A et al (2014) Reduction in 18 F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol 21(1):166–174. https://doi.org/10.1007/s12350-013-9828-6
Rosenthal DG, Parwani P, Murray TO et al (2019) Long-term corticosteroid-sparing immunosuppression for cardiac sarcoidosis. J Am Heart Assoc 8(18):e010952. https://doi.org/10.1161/jaha.118.010952
Nekolla SG, Martinez-Moeller A, Saraste A (2009) PET and MRI in cardiac imaging: from validation studies to integrated applications. Eur J Nucl Med Mol Imaging 36(Suppl 1):S121–S130. https://doi.org/10.1007/s00259-008-0980-1
Dweck MR, Abgral R, Trivieri MG et al (2018) Hybrid magnetic resonance imaging and positron emission tomography with fluorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging 11(1):94–107. https://doi.org/10.1016/j.jcmg.2017.02.021
Vita T, Okada DR, Veillet-Chowdhury M et al (2018) Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging 11(1):e007030. https://doi.org/10.1161/circimaging.117.007030
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Agrawal, T., Saleh, Y., Sukkari, M.H. et al. Diagnosis of cardiac sarcoidosis: a primer for non-imagers. Heart Fail Rev 27, 1223–1233 (2022). https://doi.org/10.1007/s10741-021-10126-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-021-10126-5