Abstract
Plant requires seventeen essential mineral elements for proper growth and functioning classified as macro and micro-nutrients. Apart from these, cerium (Ce), cobalt (Co), iodine (I), aluminum (Al), selenium (Se), sodium (Na), lanthanum (La), silicon (Si), titanium (Ti), and vanadium (V) are evolving as pivotal bio-stimulants in plant growth and providing stress tolerance. Although, they are not mandatory for all plants directly but when they are supplemented, promote the plant growth positively and simulate multiple abiotic and biotic stresses tolerance. Though, these elements have crucial role in plant growth, still obscurethe uptake, transport and molecular understanding as much of macro and micronutrients. However, in recent years scientists are giving more emphasis to explore their mechanisms associated with enhancing antioxidant defense, stress responsive proteins accumulation, and transcription factors under variety of stresses. Likely, they are also crosstalk with other essential elements and plant growth regulators (PGRs) (salicylic acid, SA; jasmonic acid, JA), which is crucial for signaling network perception and regulate plant growth. Recent technologies developed in the field of nanotechnology assist in the further understanding of their uptake, transport and functions at cellular level andoptimizing their concentrations for better plant growth. Bio-fortification of crops with beneficial elements provides some cues regarding their importance in plant growth and also in human balance nutrition. To considering the importance of these compound, this review aimed to explore the uptake and transport mechanisms of beneficial elements and their function in plant development. Consequently, we pinpoint the crosstalk’s between PGRs and other mineral elements, which advance their crucial role during plant mineral nutrition and growth signaling. At the end, this review focused on the crucial role and mechanisms associated with these elements under multiple abiotic stresses that open exciting avanues in several directions related to crop stress breeding program.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant requires the supply of 17 elements’ indispensable for their growth and survival, known as essential/necessary elements. Based upon the required quantity; they are classified further into macro and micronutrients having > 1000 and < 100 mg kg− 1 dry weight respectively (Arnon and Stout 1939). However, few other elements also deploy an auspicious consequence to specific plant taxa and defined as beneficial elements. They do not fall under classical essentiality criteria for mineral nutrients by Arnon and Stout (1939); however, they are present ubiquitously (Pilon-Smits et al. 2009). Beneficial elements are not vital for survival but boost the plant biomass and yield (Marschner 2012). These beneficial elements (BEs) Al, Ce, Co, I, La, Na, Se, Si, Ti, and V have an indispensable role but still unexplored as macro and micronutrients. (Vatansever et al. 2017). Also, various essential elements can be substituted by beneficial elements for different non-specific metabolic processes, such as Si and C, Se and S, and Co and Ni (Ramirez et al. 2018). They stimulate various growth-promoting pathways and help to alleviate abiotic and biotic stresses (Gómez-Merino and Trejo-Téllez 2018; Val-Torregrosa et al. 2021). Their functions can be predicted based on their localization in specific tissues; for example, higher tissue concentration suggests a structural or osmotic role or low tissue concentration suggests a potential role as enzyme co-factor (Pavlovic et al. 2021).
Recently, awareness about these elements has kept rising as scientists are trying to decipher novel aspects of plant nutrition to optimize plant yield. There are several novel strategies/methologies to mitigate the abiotic and biotic stress such as enhance expression of stress responsive genes through biotechnological tools, development of stress tolerant transgenics, engineering of mineran nutrient transporter, transcription factors and metabolite biosynthesis pathway genes and use of biostimulant such as beneficil elements (Nguyen et al. 2018; Yoon et al. 2020). These compounds had found to effectively minimize reactive oxygen species (ROS), enhance antioxidant defense, stress proteins accumulation, expression of stress responsive transcripts, and transcription factors under unfavourable situations (Imtiaz et al. 2018; Tripathi et al. 2021a; Huang et al. 2021). Likely, they also crosstalk with other essential elements and PGRs for optimizing signaling network and regulate plant processes under diverse environments (Iqbal et al. 2021a; Tripathi et al. 2021a). For instance, recent study on Si uptake and transportation revealed that it activates the IPT7 (isopentenyl transferase), S-adenosyl-L-methionine dependent methyltransferase, 1-aminocyclopropane-1-carboxylate-oxidase genes associated with cytokinin, and ethylene (Khan et al. 2021). This study also reviewved the effect of Si on the expression of diverse PGRs in response to multiple stress conditions and suggested that Si crosstalk with signaling molecules is central mechanism to tolerance plant under unfavorable conditions. Recently, bio-fortification of crops with beneficial elements provides some cues regarding their importance in plant nutrition (Lara et al. 2017; Li et al. 2017). These elements can also be proved instrumental against variety of biotic and abiotic stresses. For example, selenium improves oxidative stress tolerance, Si counters salinity, drought, and induced resistance against pests and pathogens, and Ti reduces the injuries against Xanthomonas (Tripathi et al. 2021a; Wu et al. 2021). Also, some of these elements could modulate plant growth and development and photosynthesis (Piccolo et al. 2021). These elements have crucial importance in reduce chloroplast damage, improve electron transport rate, mesophyll conductance, carboxylation of rubisco, and rubiso activase activity, which ultimately boost the overall photosynthesis rate (Piccolo et al. 2021). Aluminium regulates the flower color and root development and triggers the antioxidative pathways; cerium helps grow shoots in certain species and is involved with catalase as a cofactor (Bojórquez-Quintal et al. 2017). These are also important for agriculture, especially in legume crops for mineralization, solubilization, and nitrogen fixation. For example, cobalt acts in atmospheric nitrogen fixation; similarly, iodine can improve nitrogen use in plants (Farooq et al. 2012; Medrano-Macías et al. 2016).
Further, BEs also involved in different signaling networking, for intance the La modulating Ca-calmodulin pathway (Aldon et al. 2018). Calcium mediated proteins such as Ca-dependent protein kinase (CPK), calcineurin- B like protein (CBL), and CPK-related protein kinase (CRK) initiate the downstream phosphorylation signaling, which liked to activation of stress responsive genes and ion channels activity (Saito and Uozumi 2020). Apart from that, some beneficial elements such as V improve plant secondary metabolism (Hanus-Fajerska et al.2021). A recent report summarized the integration of Si and secondary metabolites and concluded that Si amolierate the abiotic and biotic stress condition through the accumulation of secondary metabolites such as polyphenol oxidase (PPO), terpense, polyamines etc. (Ahanger et al. 2020). For example, in Camellia sinensis addition of 0.006 ppm Co ion act as elicators and promote the production of cinnamic acid up to 11.9% (Sutini et al. 2019). Moreover, other elements like chromium (Cr), silver (Ag), Tungsten (W), and fluorine (Fl) are little explored in plant nutrition and stress biology. Considering the emerging importance of these elements, scientists have investigatedtheir uptake and transport in the plant. Their transport is primarily active co-dependent upon the specific transporter for essential elements (Fricke 2015; Adebayo et al. 2020). However, some of the beneficial elements transported through specific transporter, for example, Lsi1 and Lsi2 efflux transporter, actively transport the silicon (Ma et al. 2007; Yamaji et al. 2008). Further, the similarity between cation elements makes them replaceable for some metabolic pathways and often modulates signaling pathways with their cross-talk through PGRs and other signaling components (Kim et al. 2016; Hosseini et al. 2019).
Therefore, studying the cellular mechanisms of beneficial elements under different abiotic stresses will be of more interest. In the present time researcher primarily concentrated on the finding the uptake and transport mechanism of these element, which are stll less known. Also, the regulatory mechanism of benefical elements are poorly known and nanotechnology and biotechnology approaches help a lot in finding the deep understanding regarding these elements. Morover, the biofortication and transgenic approachesalso used to prepare super food for human health. This review explores the effects of beneficial factors in some model or cultivated plants where considerable modifications as well as their potential use as novel components for agricultural production has been noted. We will further cover their transport and absorption mechanism and cross-talk with plant growth regulators and other essential mineral nutrients inside the plant to understand their signaling network. An effort was made to highlight significant synergistic interactions of individual nutrients at the physiological, molecular, and biochemical levels. Therefore, this review will outline the beneficial elements crucial for global agrifood innovation.
Uptake and transport mechanism of beneficial elements
Silicon uptake and transport
Si is rarely present in free form and combinedly present as silicate and oxides. Silicon exists in soil solution mainly as Si(OH)4. It is primarily deposited in the cell walls, endoplasmic reticulum, and intercellular spaces as hydrated, amorphous silica (SiO2.nH2O) (Gunes et al. 2007). Based on the Si accumulation capacity, plants are divided as Si accumulator, non-accumulator and intermediate (Ma et al. 2001). Since the apoplastic pathway is hindered by the Casparian strips in rice roots, the symplastic pathway therefore, facilitates the Si translocation (Hodson and Evans 2020). First Si transporter gene identified in rice was Lsi1 (low silicon 1) (Ma et al. 2004). Lsi1 is a major Si influx passive transporter that belongs to group III Nodulin 26- like intrinsic protein (NIP3), an aquaporin subfamily at distal side of exodermis and endodermis of root cells (Deshmukh et al. 2020). Lsi2 is an anion efflux transporter and involved in the active transport of Si out of the cell to vascular tissues through the generating proton gradient (Mitani-Ueno and Ma 2021). Lsi6 is also a Si influx transporter and unloads Si from xylem into shoot parenchyma cells and different aerial plant parts (Pontigo et al. 2015). In nutshell, the Lsi1, uptakes monosilicic acid (Si) from solution to root exodermis cells which enters into the root cortical cells through active transport of Lsi2 and then through apoplastic movements, reaches to endodermis through aerenchyma. Thus, Lsi1 and Lsi2 load the Si into root xylem and Lsi6 facilitates the transport of Si to the shoot portion (Yamaji et al. 2015).
Selenium uptake and transport
Selenium exits as selenide (Se2−), selenite (SeO32−) and selenate (SeO42−). Selenium is essential for animals and humans because of its role as cofactor in glutathione peroxidase. Many species of genera Astragalus, Xylorrhiza and Stanteyea are typical selenium- accumulators. Se uptake primarily depends upon the availability of Se (Chauhan et al. 2019). Plants prefer to uptake Se as Selenate (SeO2 − 4) or Selenite (SeO2 − 3). The transporter proteins for Se uptake in plants are present in root cell membranes. The high-affinity sulfate transporters AtSULTR1;1 and AtSULTR1;2, involved in Se uptake in A. thaliana (El Mehdawi et al. 2018). Once selenite influx into root cells by AtSULTR1;2, it further gets transported to leaves via xylem using low-affinity SULTR2;1 transporter at roots and leaf vascular tissues (Hawkesford 2003). At this stage, selenate enters into the reductive sulfur assimilation pathway and is assimilated into selenocysteine and selenomethionine (Schiavon et al. 2015). The uptake mechanism of selenite involved phosphate transporters such as Oryza sativa phosphate transporter 2(OsPT2); OsPT8 (Song et al. 2017). Zhao et al. (2010) reported that apart from phosphate transporters, silicon influx transporter (OsNIP2;1) was also involved in selenite uptake in rice.
Sodium uptake and transport
Brownell (1965) established the fact that sodium is an essential element for the halophyte Artriplex vesicaria.and some of the C4 species e.g. Chenopodiaceae, Amaranthaceae, and Cyperaceae. In these species, Na is vital for regenerating phosphoenol pyruvate, the substrate for the first carboxylation in the C4 pathway. A high K+/ Na+ ratio maintained across the plasma membrane (PM) for K+ influx in the cells. However, under saline conditions, the passive influx of Na+ into the plant cells is more favoured (Gupta et al. 2002). Na+ efflux is facilitated by a Na+/ H+ antiporter (SOS1). Na+ influx mostly occurs through ion channels such as nonselective cation channels (NSCC) in the roots for Na+ entry and another one is high affinity K+ transporter HKT (Apse and Blumwald 2007). Salt overly sensitive (SOS1) is Na+/ H+ antiporter upregulated under salt stress in which Na+ is export from cytosol to apolpast against import of H+ (Quan et al. 2017). The SOS1 is regulated by protein phosphorylation SOS2–SOS3 kinase complex, facilitated by high concentration of Ca2+ in cell (Quintero et al. 2002). SOS2 also regulates NHX activity (Fig. 1). SOS2 is a serine/threonine protein kinase (CIPK24) of the family SnRK3/CIPK. SOS3 is a myristoylated calcium-binding protein (CBL4) belonging to the recovering like SCaBPs/CBLs family (Sanchez-Barrena et al. 2007).
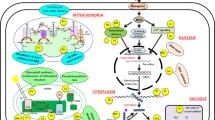
Highlights the mechanism of beneficial elements transport in plants. The transport system of plants are includes the influx and uptake of ion through the selective ion channels such as HKT, HAK etc. which present of root membrnaes. The second categories of transporters involve the efflux of toxic ion through the efflux transporters such as IREG, MATE etc. These channels help in reducing the toxic concentration of these ions. Third categories of transporters involve the transporter which help in loading of ion in xylem tissue and present at root-shhot junction. After the ion transport in xylem ions reached to cell and target tissues where they transported through the specific channels and finally excess ions sequester in vacoular tissue to maintain ion homeostasis. *DMT; divalent metal transporter, ZIP; zinc regulated transporter, HMA; heavy metal transporting P-type ATPase, NHX; Na+/H+ antiporter, NIP; nodulin 26-like intrinsic proteins, VALT; vacoular Al transporter; IREG and IRT; iron regulted transporter, FPN; ferroprotein, SULTR; sulfate transporter, MATE; multidrug and toxic compound extrusion transporter, ALMT; Al activated malate transporter, SOS; salt overly sensitive transporter, ABC; ATP binding cassette transporter, PT; phosphate transporter; NRAT; nitrate transporter, HKT and HAK; high affinity K transporter, GLR; glutamate receptor transporter, CNGC; cyclic nucleotied gated channel, NSCC; non selective cation channels.
Aluminium uptake and transport
Under normal soil conditions, Aluminium (Al) exists in the form of Aluminium phosphate, silicate, oxides, and sulfide. Under acidic pH conditions (below 5.5), aluminium salt dissociates and converted into free aluminium ion (Al3+) in the soil (Kochian et al. 2004). Xia et al. (2010) identified a PM-localized Al3+ transporter, OsNRAT1 (NRAMP Al transporter 1) in rice. The uptake of Al3+ in the cytoplasm activates the organic anion permease on the PM, which releases organic acids like citrate, oxalate, and malate. These organic anions chelate with the soil Al3+ and provide tolerance to Al toxicity. Aluminium activated transporters (ALMTs) and multidrug and toxic compound extrusion (MATE) transporters are specifically involved in the release of malate and citrate respectively in the soil which is involved in Al3+ extrusion process (Kochian et al. 2015). NIP1;2 (NOD26-like intrinsic protein 1–2) and VALT1 (Vacuolar Al transporter 1) transporter genes are involved in the Al3+ tolerance mechanism through sequestration into the vacuole (Negishi et al. 2012; Wang et al. 2017).
Other beneficial uptake and transport
Other than the above beneficial elements Co, I, La, Ti and Ce are also uptake and transported to shoot and leaves. For example, Co is not very abundant in plants, and taken up either as Co2+ or as an organic complex. Organic-bound fraction is the important source of Co in soils. Roots absorb Co through cortical cells in the xylem and operated by both active and passive diffusion (Kaur et al. 2016). Co uptake and transported through the transporters Nhif, NiCoTs, ZIP, CbiMnQO, and FPN (Komeda et al. 1997; Morrisey et al. 2009; Bao et al. 2017). The important beneficial elements transporters and their functions are highlighted in Table 1. However, there are limited study on I, La, Ti, V and Ce transporters from soil to stem and depth studies are going to trace their transporters and uptake mechanism. The important transporters involved in the beneficial elements influx, efflux, translocation and vacoular sequestration in plants are highlighted in Fig. 1.
Beneficial elements crosstalk with plant growth regulators and other nutrients
Silicon (Si) is a potent element interacted with number of phytohormones and plant nutrients (Chaiwong et al. 2020). Si increases auxin content and basipetal auxin translocation by improving PIN (PIN-FORMED) transporters (Moradtalab et al. 2018). Recent study conducted in poinsettia using the 75mgL− 1 Si and showed that it alleviated the inhibitory effect of NPA (napthylpthamic acid; inhibitor of auxin transport) and promote root growth (Hu et al. 2020). Likewise, Tripathi et al. (2021b) found the involvement of Si in adventitious root formation under As stress associate with interaction of IAA (indole acetic acid) and nitric oxide (NO). Si mediated reduction of senescence and promotion of zeatinin meristematic regions under chilling stress (Moradtalab et al. 2018; Souri et al. 2020) and regulation of IPT (isopentyl transferase) genes under salinity stress in tomato (Gou et al. 2022). The deficiency of magnesium and its subsequent effect on plant morphology is alleviated by Si through cytokinin triggering (Hosseini et al. 2019). Silicon regulates the proline and cytokinin content in cucumber and confer salinity stress tolerance through the expression of CKX (cytokinin oxidase/ dehydrogenases) and IPT genes (Zhu et al. 2020). The secondary metabolites are accumulated at different vegetative and reproductive stages through Si mediated suppression of DELLA protein and thereby augmenting the effect of gibberellins along with synthesis of active form gibberellins (Moradtalab et al. 2018). Furthermore, reports of precursor GA (GA1, GA20) synthesis via Si mediated regulation of HSPs and antioxidant genes (for SOD, CAT, GADPH etc.) under drought and/or salinity are evident (Al Murad et al. 2020; Khan et al. 2020a; Kim et al. 2016; Souri et al. 2020). Preliminary ABA assay in different horticultural crops showed temporal increase in ABA content with respect to exogenous Si application through Si mediated up-regulation of ABA biosynthetic genes under salinity condition (Al Murad et al. 2020). Conversely, Si helps in decreasing the ABA content by regulating transcription factors (for genes viz. PYL1, PYL4 and PYR8) under drought stress by by maintaining stomatal conductance and gaseous exchange in plants (Moradtalab et al. 2018; Khan et al. 2019a, 2020b). Synergistic effect of Si and brassinosteroids helps the plants in maintaining high proline and antioxidant content which ensures plants’ resistance to abiotic stresses (viz. salt, HM stress) through intact membrane system (Siddiqui et al. 2018; Hussain et al. 2019; Maghsoudi et al. 2019). Such synergistic effect (between ABA and Si) is also reported by Gurmani et al. (2013) that produced better yield through improved photosynthesis along with a reduced ratio between Na+ and K+. Silicon application augments the endogenous SA production (through accumulation of PAL enzyme) under HM stress (Maghsoudi et al. 2019a; Souri et al. 2020). Another hormone JA reported to be more effective (imparting more genetic stability) during the onset of abiotic stress when applied along with silicon compared to their individual application (Nascimento et al. 2019). When exposed to environmental stress, both SA and Si elevated genes regulating antioxidant, rhizosphere acidification and Si absorption, and SA biosynthesis. ROS scavenging by ascorbate-glutathione pathway is possible through crosstalk of nitric oxide and silicon which further diminishes the effect of HM stress (Singh et al. 2020). Silicon takes part in reducing the competition between polyamines and ethylene biosynthesis through inhibition of ethylene precursor molecule (ACC) thus providing an additional support towards polyamine dominancy for salinity tolerance (Yin et al. 2019).
Interaction of Se with auxin hormone could be one of the reasons through which plant maintain high cellular water content through modified root architecture and Se-ethylene interplay a crucial role during post-harvest life of cut flowers (Costa et al. 2020). Experiment conducted using the Se and Zn nanoparticles (NPs) in B. napus under salinity showed improved in seed germination by reduction of ABA (CYP707A1, 3 and 4) and eleavation of GA related genes (GA20ox and GA3ox) (El-Badri et al. 2021). Titanium at higher concentration acts as stressor and the application of GA improves the wheat growth at all the doses of TiO2 nanoparticles, showing the antagonistic interaction (Alharby et al. 2021). The study conducted using the 24-epibrassinolide and TiNPs in Pleioblastus pygmaeus provide improvemnts under Cu and Cd toxicity by enhancing antioxidant activity (85%), pigments (43%), and non-enzymatic antioxidant activities (47%) (Emamverdian et al. 2022). Similarly, application of TiNPs in Arabidopsis promted the root growth by inducing the PIN2 auxin transporter (Wei et al. 2020). Application of Co under unfavourable conditions increased ABA and ET hormones, which were linked to enhance plant resistance, promote abscission and reduce water losses in Phasoleus vulgaris (Schautmann and Wenzel 2002). A recent study conducted in switch grass reveals that application of La and ABA co-regulates the chlorophyll production and act as antagonist during seedling development (He et al. 2020). Similarly, the seed primed with La improve maize grain yield by improving photosynthetic attributes, reducing antioxidant defense and alter the level of ABA (Cui et al. 2019). There are also some evidences that showed that presence of Al induces the contents of chlorophyll, carotenoids, sugars, proline, PGRs such as ABA, auxin, CKs and secondary metabolites, which regulates the plant growth positively (Moreno-Alvarado et al. 2017). Study suggested that application of tricontanol (TRIA), Ce and La induced the early bolting in Arabidopsis through the regulation of cytokinin levels (He and Loh 2002). Thus, the beneficial element crosstalk’s with plant growth regulators and at low dose positively regulates the plant growth. The crostalks of beneficial element with important PGRs in regulation to plant growth and development are highlighted in Fig. 2.
Highlights the crosstalks between beneficial elements and plant growth regulators (PGRs) in regulation of different processes in plants. This figure highlights the crosstalk of different beneficial element such as La, Se, Si, Al, Na, Ce, Co, Ti, I and V with PAs, JAs, ABA, CK, GA, ET and auxin. This crostalk regulates the different processes and signaling network during different plant growth stages. *ABA; abscisic acid, GA; gibberellic acid, JA; jasmonic acid, NO; nitric oxide, CK; cytokinin, ET; ethylene, IPT; isopentenyl transferase, PAs; polyamines, CKX; cytokinin dehydrogenase, PGRs; plant growth regulators, SAMS; s-adenosyl methionine synthetase, SA; salicylic acid, NECD; 9- cis -epoxycarotenoid dioxygenase, CYP; cytochrome P450 monooxygenases, PYR; PYRABACTIN RESISTANCE1, PYL; PYR1-LIKE, ZEP; zeaxanthin epoxidase, GSNOR; S-nitrosoglutathione reductase, GLB; beta-galactosidase, PIN; PIN-FORMED, ACS; 1-Aminocyclopropane-1-carboxylic acid synthase.
Beneficial element Se act as antagonist of As toxicity through expression of As transporters and regulatory elements (Chauhan et al. 2020). Beneficial element Al increases P use efficiency and alleviates H+ and Fe toxicity under acidic condition and their interaction affect plant growth and development (Matsumoto and Yamaya 1986). Antagonistic reduction of Fe and Mn uptake by Al is strongly believed to be the mechanism that Al alleviates Fe and Mn toxicity (Ghasemi-Fasaeiet al. 2005). Ce along with being beneficial element, regulate the mechanism of other elements in plants such as decrease nitrogen assimilation and Mo absorption and interfere with Fe absorption in soil (Peralta-videa et al. 2014; Ramírez et al. 2018). For nitrogen fixation Co play vital role as constitute of cobalamin and alleviates N absorption (Chmielowska-Bak et al. 2014). Iodine Interfere in plant metabolism by showing synergistic effect with Cu, Fe, Mn. (Schlorke et al. 2016). Iodine also competes with Se for absorption site (Smolenet al. 2016). Lanthanum frequently has been found in conjunction with Ce and other elements from this family. At low dose of La, it promotes uptake of essential elements Fe, K, Ca, Mg and higher La concentration inhibits Ca, Cd and Fe absorption (Agathokleous et al. 2018). Ti helps in alleviating Cd toxicity in plants. Silicon alleviates As, B and Cd toxicity (Rizwan et al. 2019). Under stress conditions Si can be seen to enhance tolerance to multiple biotic and abiotic stresses (Kleiber 2018). Vanadium lower dose promotes P accumulation and vice-versa, also reduce Zn accumulation. Appropriately beneficial elements play major role to improve plant resistance to abiotic and biotic stresses at their low levels. But, the effective ranges for the beneficial elements is somewhat narrow and higher dose can be detrimental to plant metabolism. The crosstalks of beneficial elements with other important macro, micro-nutirents and heavy metals are highlighted in Table 2; Fig. 3.
highlights the crosstalks of beneficial elements with other elements (macro + micro + heavy metals). Different color indicates the relationship between them [red (antagonistic), Green (synergestic), Turquoise (dual effect)]. Some beneficial elements showed the dual effect means at low concentration acts as synergistic and opposite when the concentration is high. These figure can help in the use of beneficial elements in understanding the response of other metals and the effects on nutrient resource
Physiological, biochemical and molecular understanding of beneficial element for abiotic stress tolerance
Beneficial elements have dynamic role in regulating several crucial processes and biochemical reactions in plant during abiotic stresses, which are highlighted in Table 3; Fig. 4 and discussed thoroughly.
Highlight the systematic way of response and mechanism of beneficial elements for abiotic stress tolerances. * ZIP; zinc regulated transporter, NHX; Na+/H+ antiporter, NIP; nodulin 26-like intrinsic proteins, FPN; ferroprotein, SULTR; sulfate transporter, ALMT; Al activated malate transporter, ABC; ATP binding cassette transporter, CNGC; cyclic nucleotied gated channel.
Role of silicon in mitigating abiotic stress
In the last two decades, various researches have been accomplished in different crops and revealed the role of Si in managing unfavourable situations (Jinger et al. 2017; 2020c). However, meagre studies have been done so far in relation to modus of operandi of Si in abiotic stress mitigation (Jinger et al. 2018b; 2020d). Application of silicon fertilizer (soluble silicate or basic slag) in soil can enhance 10–25% yield of many crops including sugarcane, rice, sunflower and sugar beet. The role of Si in multiple abiotic stress tolerance are highlighted in Fig. 5.
Si in mitigating temperature stress
Elevated/high temperature (HT) leads to water losses from the plant due to increased transpiration. Thakral et al. (2021) reported that it might be due to breakdown of lipid and proteins of the cell membrane caused malfunctional changes in the cell membrane due to HT. Maghsoudi et al. (2016) showed that during HT, Si improved thermal stability and cell integrity of PM by reducing leakage of electrolytes in wheat. Application of Si in rice imparts its conglomeration in the vacant spaces within the cellulose micelle of epidermal cells, where it leads to formation of silica-cellulose membrane, that arrest the transpiration during HT stress. The combined foliar application of 24-epibrassinolide and Si improved the root length (37.25), shoot length (35%), leaf area (25.93%), photosynthetic rate (15.25%), stomatal conductance (10.32%), and antioxidant enzymes, which enhance HT tolerance abaility in wheat crop (Hussain et al. 2019). HT in wheat crop damage the ultrastructure of leaf such and cell organelle such as chloroplast and nucleus, which were restored whencrop are subjected to Si and SiNPs treatments They also reported the enhanced expression of aquaporins genes (PIP1; PM intrinsic protein and NIP2; noduline-26-like intrinsic protein), which maintain the cell water potential during heat stress (Younis et al. 2020). The similar kind of result was obtained in tomato crop, where the application of Si upregulated the expression of heat shock factors related genes (HsfA1a-b, HsfA2-3, and HsfA7), ABA (NECD) and SA related gene (R1b1, PR-P2, and PAL) under heat stress in tomato crop (Khan et al. 2020a&b).
Similarly, organo-silicon compounds protect the rice plants from frost injury (Loginov et al. 2011). Si fertilization protect the wheat plant from freezing stress by increasing antioxidant defence activity, membrane permeability, water retention in leaf tissues and by lowering lipid peroxidation (Liang et al. 2008). Current investigation reported that low temperature stress in aloevera and bamboo was suppressed by application of Si through increased antioxidant activities (Azarfam et al. 2020). In maize crop Si restored the micronutrient content (Zn and Mn), hormonal homeostasis and antioxidant defence activities as chilling stress subjected at early growth phase (Moradtalab et al. 2018). Transcriptomics analysis of grafted cucumber under low temperature stress Si improved the phenylpropanoid metabolism, MYB 44 (MYB domain protein), fatty acid metabolism, and antioxidant enzymes related transcript, which further related to chilling stress tolerance (Luan et al. 2022).
Role of Si in mitigating moisture stress
Physiological processes are hindered when plants face flooding and drought conditions owing to excess yield of ROS, which diminished the growth and development phenomenon in plants. Si application improves the oxidative defence system of the plants growing under flooded condition by increasing their resistance to flooding (Sayed and Gadallah 2014). Si fertilization minimised the oxidative damage in barley, when it was cultivated in the saturated soil. Recent studies reported that Si enhances antioxidant defence, root characters and plastid anatomy of rice when grown in waterlogged conditions (Pan et al. 2021). Si has potential role in mitigating drought stress (DS) conditions (Jinger et al. 2020a). In wheat, Si supplementation imparts drought resistance by alleviating the oxidative damage and improving the root architecture, biochemical and antioxidants defense such as expression of antioxidant enzymes, AsA-GSH pool and glyoxalase system (Hasanuzzamanet al. 2018; Zahoor et al. 2020). Drought stress mitigation in rice linked to Si application, which reduced theproline content. Similarly, Si application in maize improved and maintained the turgidity of the plant facing drought by decreasing transpiration (Mauad et al. 2016). Si application increased the root water uptake through the root endodermal silicification, suberization, and Casparian band development, which ultimately affect the root hydraulic conductance and water retention under DS (Fleck et al. 2015; Soukup et al. 2017). Moreover, the Si improved the expression of aquaporins transcripts (PIP1;6, PIP2;2, and PIP2;6) in sorghum crop in response to DS, which ultimately contributed in improvement of root hydraulic movement and uptake capacity (Chen et al. 2018; Wang et al. 2021). Likely, Si improved the light energy absorption and transformation through the stability of photosystem I (PS-I), PS-II, and chlorophyll protein complexes under DS (Wang et al. 2019).
Si application increased the lodging resistance and lignin deposition in rice crop (Dorairaj et al. 2020). Similarly, Jinger et al. (2020b) reported less than 1% lodging or practically no lodging in aerobic rice treated with calcium silicate at 120 kg Si/ha along with 90 kg P2O5/ha and 100% lodging in untreated (control) field. A recent study conducted in rice under submergence stress reveals that the submergence reduced the root and shoot biomass, photosynthesis attributes, and damage the chlorophyll ultrastructure, which is restored when plants subjected to Si treatment (Pan et al. 2021). A sufficient supply of silicon permits rice plants to keep the leaves erect, uncurved and thus improves the assimilatory system, antioxidant defense, and osmolytes under submergence stress (Iqbal et al. 2021b).
Role of Si in mitigating salinity stress
Salinity is one of the major abiotic stresses causing ion toxicity (Na+ and Cl−) in the plant system. Si employs various processes for supressing salinity stress in the plant. Reduction in ion toxicity and improvement in water retention by decreasing transpiration, regulation of biosynthesis of phyto-hormones, depletion in osmotic stress, and modification of gene articulation are important Si-regulated tolerance mechanisms under salinity (Zargar et al. 2019).) Si improved the K+/Na+ ratio and increases relative water content, water use efficiency, photosynthetic efficiency by decreasing transpiration rate and ultimately provide salinity tolerance (Mateos-Naranjo et al. 2013). Si effect on the H+-ATPases and H+-PPase activity of PM and tonoplast, respectively, which eventually leads to compartmentalization of the salts in the vacuoles (Liang et al. 2006). Si supplementation minimizes the oxidative loss by improving antioxidant enzymes activity (superoxide dismutase, peroxidase, glutathione and ascorbate peroxidase) (Li et al. 2015). Si fertilization improves the salt resistance by regulating the levels of JA, ABA, proline, GA, and glycine betaine (Koentjoro et al. 2021). Recently, Muneer and Jeong (2015) reported the application of Si in tomato plant grown under salt stress activated the genes controlling salinity stress (DREB-1, DREB-2 and DREB-3). The transcriptomic analysis of si application under salinity stress revealed the upregulationof ABA biosynthesis genes (NECD1, &4), Na transporter (SOS1&2), and down regulate the NHX1, which ulmately regulate the oxidative stress and Na/K+ ratio (Kim et al. 2014; Bosnic et al. 2018). The protemics analysis under salinity stress in Rosa hybrida revealed the upregulation of proteins related to photosynthesis (22%), stress/redox equilibrium (12%), ion binding (13%), transcription/translation (20%), and ubiquitination (8%), when subjected to Si supplementation (Soundararajan et al. 2017).
Role of Si in mitigating heavy metal toxicity
Si ameliorated the toxicity of heavy metals (HMs) through the activation of numerous mechanisms like, immobilization, compartmentalization, co-precipitation, chelation, antioxidant stimulation, and alteration in gene expression (Zargar et al. 2019). Supplementation of Si decreases toxicity of Al, B, Cd, Mn, and Zn (Bhat et al. 2019). Immobilization of HMs through Si application in soil is the simplest and widely accepted mechanism. It occurs owing to changes in soil reaction and evolution of metal in soil by genesis of silicate nexus. Gu et al. (2011) observed that Si increased soil pH and decreased the phyto-availability of HMs to a great extent in rice. It also reduced the translocation of Zn from root to shoot and accelerated the attachment of Zn with cellulose of plant wall, thus reducing Zn content within above ground parts (Yamaji et al. 2008). Gu et al. (2011) reported co-precipitation mechanism, where Si and Cd make insoluble compounds in the culm of paddy which decreased HMs content in the shoot portion. Heavy metal detoxification by Si also involves the chelation of HMs with flavonoid-phenolic or organic acids. Application of Si in maize led to chelation of Si with catechin and quercetin (phenolic compounds) which ultimately reduced the Al toxicity (Wang et al. 2004). HMs toxicity detoxification through Si also involved the activation of antioxidant defense. Application of Si in cucumber reduced Mn toxicity by reducing lipid peroxidation and increasing activity of antioxidants like SOD, APX, and GSH (enzymatic); and ascorbate and glutathione (non-enzymatic) (Shi et al. 2005). Recently, the role of Si in amelioration of HMs toxicity through the modulation of secondary metabolites production are well known. For instance, Si application modulated the expression of phenol and lignins in Barley (Vaga et al. 2019), flavonic and phenolic compounds in cucumber (Maksimavic et al. 2007), and tetrapreonoid and carotenoids in maize (Paula et al. 2015) under Al, Mn and Zn toxicity respectively. Consequently, Si allivated the toxicity of HMs through the downregulation of Nramp5 (natural resistance-associated macrophage protein 5), HMA2&3(The P1B-type heavy metal ATPase 2/3), NRT2 (nitrate transporter protein), AMT1 (ammonium transporter protein), PHT2 (phosphate transporter protein), and KAT 1 (potassium transporter protein), which help in sequestrationof HMs and improve the transport of essential nutrients (vaculik et al. 2020).
Titanium (Ti)
Ti playes a pivotal role for improving the growth of plants due to promoting the uptake of nutrients, enhancing chlorophyll content and photosynthetic activity, and improving stress tolerance capacity (Buettner et al. 2012; Lyu et al. 2017). The seedlings of dust indicator plant named as Tapertip hawksbeard showed 11-fold increment in Ti while exposed to contaminated soil (Cook et al. 2009). Ti shows both synergistic as well as antagonistic properties depending upon the amount of various elements present in the surroundings (Bacilieri et al. 2017). Tytanit or Mg-Titanit is used for commercial use and these were found to work as bio stimulant. Hussain et al. (2021) reported that the phosphorous uptake in shade grown potato is enhanced with foliar application of Ti; significant improvement is also noticed in various plant growth and biomass attributes even in high and low phosphorus containing soils. They further indicated with 500 mg L− 1 of Ti application was capable to enhance the rate of photosynthesis by 45% under shade grown and phosphorous stressed soybean crop. Its foliar application either alone or in combination with Mg to potato, winter wheat and spring barley enhanced the availability of nitrogen under N deficient condition (Tlustoš et al. 2005). Ti @≥ 10 mg/kg concentrations was found toxic in oats (Avena sativa L. cv. Zlaťák) but addition of Mg was found to play an ameliorating role (Kužel et al.2007). It was further shown that when the soil is with nutritional optimum then Ti plays significant role in equalizing the plant nutrient content, particularly in Fluvisol, which are deficient in Mg, Fe, Zn, and Mn. Hence, Ti help to regulate plant growth during nutritional stress (Wadas and Kalinowski 2019). Further, tytanit application significantly increased hybrid alfalfa and red clover (Medicago x variaT. Martin and Trifoliumpratense L) yields, without improving the nutritional value of the plants (Sosnowski et al. 2020). TiO2-NP treatment in Zea mays L. moderates the H2O2 imposed stress effects by improving the proline content (1.23 fold), inducing the activity of SOD enzyme (1.42 fold) of antioxidative cascade, up regulating the activity of guaiacol peroxidase (91.07 fold) and down regulating CAT activity; this delineates how TiO2-NP application modulates the oxidative stress in C4 plants (Sarkar et al. 2021). Use of n-TiO2 in combination with SNP enhanced SOD, CAT and APX activities with decreased MDA and H2O2 in barley under salinity; proposing it a promising approach for alleviating the salinity stress in barley (Karami and Sepheri 2018). Likewise, The Ti-NP showed the beneficial effect under cold stress in chickpea, heat stress in tomato, drought in wheat, and Cd toxicity in soybean (Qi et al. 2013; Jaberzadeh et al. 2013; Mohammadi et al. 2014; Singh and Lee 2016). In wheat crop application of TiNPs and calcium phosphate improved sedling root, and shoot length, fresh and dry biomass by 33, 53, 48 and 44% respectively under DS (Mustafa et al. 2021). They also found that the DS tolerance in wheat plant primarily through the enhanced activities of SOD, POX and CAT by 83, 74 and 81% respectively, which linked to reduce oxidative damage. The application of TiNPs under As toxicity upregulated the expression of GSH1 (glutathione), PCs (phytochelatins), and ABC1 genes in rice, which ameliotae the HMs toxicity symptom and reduce oxidative stress (Kiany et al. 2022).
Cobalt (Co)
The essentiality of cobaltin legume N2 fixation and in root nodules of non-legumes (e.g. alder). Cobalt is known to play a pivotal role during overall plant growth, depending on its overall concentration in the root rhizosphere and in plants. Co is beneficial for plant growth at a low concentration; high concentration causes the detrimental effects (Gad 2012). Co is necessary for the outward appearance of plants like development of plant buds-leaf discs, plant stem coleoptiles etc. Beside this, cobalt is also important for various chemical and biological reactions essential in plants. In legumes Co, participated in pigments formation, activate enzymes needed for symbiotic N fixation (Farooq et al. 2012). Now it’s been well well-known that Rhizobium and several other N2 fixing microorganism have a necessary necessity of cobalt. The coenzyme cobalamine (Vitamin B12 and its derivatives) maily has Co (III) as the metal component. Propionate also serves as the energy source for plant growth (Gupta and Gupta 2018). Cobalt has a positive effect under DS in higher plants (Pilon-Smits et al. 2009; Gad et al. 2018; Akeel and Jahan 2020; Brengi et al. 2021). Co improves leaf resiatance to dehydration and cytoplasmic pressure, decreases the wilting coefficient hence increasing drought resistance (Schautmann, and Wenzel 2002). Priming of soybean seeds with cobalt solutions reduce the drastic effects of DS during seedling growth (Blaylock et al. 2000). Cobalt level has a positive effect on plant growth, leaf water status, ABA as well as xylem and phloem tissues being increased especially under water deficit conditions. Treatment of 7.5ppm Co had a major effect on tomato growth, yield, and fruit quality (Gad 2005). Application of Co @ 3 mg/kg soil improved leaf water content in tomato and potato leaves. Further it is also seen that application of Co improved water absorption capacity and increased water use efficiency hence saving 20% of irrigation water in beans. Co application also reduced transpiration rate, increased water absorption capacity, promotive effect on improved water potential and abscisic acid, auxin and gibberellin as well as xylem and phloem under water-limited conditions (Anter and Gad 2001; Gad et al. 2018).
Sodium (na)
Globally, sodium (Na+) salt is dominant in the saline soils and sixth most plentiful element found on earth (Rodríguez-Rosales et al. 2009). Na+ toxicity not only causes physiological drought by lowering soil solution osmotic potential but also makes nutritional disorder (Khan et al. 2019). Na+ can be a beneficial nutrient for those plants where potassium is deficient. Under salt stress, osmatic adjustment can be achieved by accumulation of various osmolytes i.e. organic solutes (proline, sugar, glycine betaine etc.) and inorganic ions (Na+, K+, Ca+ and Cl−). Partitioning of sodium in the vacuole is considered as a phenomenon of osmotic adjustment which reduces water potential and thus increases drought resistance (Cui et al. 2019). Na+ require less energy for osmotic regulation in plants so the use of Na+ as osmolyte is more economic and efficient (Chen et al. 2009). Sodium efflux protect the plants against salinity stress, under salt stress accumulation of Na+ in plant cell is determined by the ion exchange action of Na+ efflux and influx. Under adverse conditions, excess light energy is generated which decreases the activities of PSII & PSI resulting in decreases rate of photosynthetic and accumulation of large amount ROS (Hui-Hui et al. 2020). Na+ accumulation can be utilized not only for osmotic adjustment but also to reduce the photosynthesis inhibition via increased non-photochemical quenching caused by excess light energy (Xu et al. 2018). The higher concentration of Na+ in leaf increased leaf succulence, decreased cell solute potential, swelled leaf organ, enhanced water uptake and down-regulated stomatal density and stomatal aperture (Xi et al. 2018).
Lanthanum (La)
Lanthanum (La), a metal element which is a part of the rare earth element (REE) family. La has been commonly used in agriculture, where it showed beneficial effects on physio-biochemical attributes of plants at lowered concentrations (Hu et al. 2002). La is known to cause hormesis, a dose-dependent actions characterised by low-dose beneficial and high-dose inhibitory. In wheat, treated with La at 0.5–25 mg/L resulted in reduction of primary root elongation, and shoot biomass, which were associated to impaired nutrient homeostatus (Hu et al. 2002). At doses greater than 0.2 mM in solution, La inhibited maize and mungbean growth, root function, and nutritional status (Diatloff et al. 2008). In horseradish, La promoted cell growth and expansion at 30–35mM LaCl3, but at 80mM caused damage to cell growth (Wang et al. 2014). In soybean 5–10mM La improved overall plant performance through improved nutrient homeostatis, whereas at 20–160mM concentration significantly affected photosynthetic rate and biomass attributes (de Oliveira et al. 2015). Application of lanthanum nitrate (1 mM) in tomato was non-responsive under drought stress and seems to be the result of unbalanced cell metabolism due to high dose of lanthanum which cause hormensis (Ippolito et al. 2011).
La has been studied at the seedling stage in few crops to find out its ability to alleviate heavy metal stress. For example, La amolierated the Cd toxicity in Phaseolus vulgaris and maize, which were due to increase in photosynthetic improvement, decrease MDA content, and induced antioxidant potential (Huang and Zhou 2006). Also, LaCl3 application in maize crop improved tolerance to Cr stress through the upregulation of antioxidant activities, regulation of AsA- GSH cycle and improved chloroplast functions (Dai et al. 2017; Dai and Shan 2019). Furthermore, Exogenous application of La (III) of 20 mg/L in rice ameliorated Cu toxicity stress in rice through reduction in oxidative stress, improved chlorophyll content and promoted overall growth. Also, the concentrations of Cu (II) in rice leaves, stem and roots tissue were reduced with the use of La (III) via improving the ultrastructure of mesophyll cells (Zhong and Cheng 2020). Under salt stress, La3+ application (0.1mM) alleviate salinity induced osmotic stress in Saussurea involucrata as evident with the increase in leaf water potential, increased soluble proteins and proline content, improved total chlorophyll and carotenoids and decreased MDA content with elevated antioxidant machinery such as SOD, APX, CAT, and GR (Xu et al. 2007). Also, tomato plants treated with LaCl3 regulates AsA-GSH cycle in chloroplast and alleviates salt induced damages (Huang and Shan 2018). A recent study revealed that the crosstalk of La and Ca reduced the accumulation of Cd in plant and ameliorate the Cd toxicity through the downregulation of Nramp5 transporter gene (Yang et al. 2021).
Aluminum (Al)
Aluminum is the 3rd prevalent element accounting for around 8.1% of its mass (Bojorquez-Quintalet al. 2017). A growing body of research supports involvement of Al roles in enhancing enhancing phosphorus efficiency, amelioration of Mn and Fe toxicity under acidic environments and managing plant growth (Muhammad, et al. 2019). Al also gives abiotic stress tolerance to plants by activating stress-related genes, as well as drawing PGPRs to roots through producing root exudates (Wang et al. 2015).
Al has been shown to affect a variety of intercellular functions by altering the cell wall properties and affecting transport of molecules across the PM (Singh et al. 2017). In M. malabathricum, Q. serrata and tea increased the P uptake and also root growth by higher P availability (Tomioka et al. 2005). Furthermore, the presence of Al affects nitrate, potassium, and magnesium absorption and transport, as well as root growth. Phosphorus tolerant genotypes exuded more malate from the root tips, implying that the increased phosphorus is due to increased organic acid secretion in the roots, which was aided by Al treatment. Citrate secretion was enhanced by Al and substantially reduced by P deficit, according to several publications. SbMATE expression-controlled citrate secretion, improved P nutrition, and raised Al toxicity tolerance, according to several reports (Richardson et al. 2011).
In acidic soil, Al decreases expansion of leaf, stomatal movement, and photosynthetic rate, by reducing P availability (Lyu et al. 2020). Similarly, Al stress reduces the ability of hyper accumulators to extract phytochemicals from contaminated soils (Zhou et al. 2020). Al stress disrupt soil rhizobia by reducing the efficacy of nodulation and N-fixation in legume species, by altering hormone levels and impair root growth (Kopittke et al. 2016; Jaiswal et al. 2018). The maintenance of DNA integrity, higher uptake of critical nutritional elements, and restricted uptake of HMs in root cells linked to Al-induced growth in tea plants (Sun et al. 2020). Following that, flavin monooxygenase-like proteins (YUCCA) were discovered to function downstream of TAA1 and regulate auxin concentrations in the trasition zone of the root apex in response to Al stress (Lvet al. 2019). Furthermore, under Al stress modulating auxin signalling in barley, miR393 plays a critical role in root growth suppression (Bai et al. 2017). Transcription factor (TF) sensitive to proton rhizotoxicity 1 (STOP1) involved in Al tolerance, and have crucial role in the Al signaling pathway. AtSTOP1, a zinc finger TF regulates the expression of downstream-STOP1 Al-resistance genes and its SUMOylation control Al resistance (Fanget al. 2020). To summarise, Al increases plant growth at low concentrations, enhances nutrient uptake, has a favourable influence on metabolism, and protects plants from abiotic and biotic stressors.
Iodine (I)
Iodine is neither macro nor micro nutrient, however, it has critical role in production of antioxidants adaption for new environment and improvement of plant performance (Medrano et al. 2021). Further, the exogenous application of iodine in plants shows the beneficial effects like promotion of growth, enhancement in the production of antioxidants and increase in the tolerance to abiotic stress (Gonzaliet al. 2017). Several studies confirmed that iodine can act as pro oxidant which induces the antioxidant synthesis in tomato, lettuce and pepper etc. after application of iodate and iodide. These chemicals were found to increase both enzymatic (SOD and APX) and non-enzymatic (glutathione, and anthocyanins) antioxidants to impart tolerance to abiotic stresses (Medrano et al. 2021). Use of iodine or its products to promote plant growth and tolerance to stresses in plants has not been well explored. Thus, there is need to enhanceuse these products in agriculture which can contribute towards bio-fortification of crops (Gonzaliet al. 2017). KI used as a desiccant for screening of DS tolerance rice varieties at flowering stage (Kumar et al. (2012). Furthermore, at grain filling stage, it induces the effect of terminal drought by chemical desiccant (Kordenaeej et al. 2013).
Leyva et al. (2011) demonstrated the occurrence of salinity tolerance (100 mM of NaCl) with application of iodate (20–80 μm) in lettuce which results in increment in biomass and level of soluble sugars and decline in the Na+ and Cl− ions along with enhanced antioxidant activity. Soybean seed treated with of IO3 (20-80 μm) showed the tolerance against 100 mM of cadmium chloride and heat stress. This study also confirms the finding that the exogenous application of iodine can be beneficial for stress tolerance in plants (Gupta and Rita 2016). Seed treatment with dry dressing of iodine and calcium carbonate in soybean and sunflower resulted in better germination, seedling growth and low level of membrane damage under high temperature and humidity (Deynéeand Mukherjee 1984). Application of iodine based product (KIO3) in strawberry did not interfere under normal situations but under salt stress it improved the yield and fruit quality due to increased activities of antioxidants in both leaves and fruits (Medranoet al.2021). The bioinformatics study shown that the iodinated protein in shoots were associated with chloroplast and involves in photosynthesis but in roots associated with various peroxidases. These reportes confirm the iodine as a potent nutrient involves in photosynthesis (Kiferle et al. 2021).
Selenium
Selenium is another beneficial element that is necessary for the health of humans and animal. Meanwhile, a recent study discovered that it is also necessary for plant growth and development. Hamilton (2004) asserts that Se small concentrations essential for normal functioning; moderate for maintaining homeostatic functions and large causing harmful effects. Numerous publications have been published in recent years describing Se as a powerful phytoprotectant capable of mitigating the harmful impacts of a variety of stresses (Hasanuzzaman et al. 2010; Sieprawska et al. 2015). Species of plants cultivated in medium supplemented with selenium have demonstrated increased tolerance to abiotic stress. According to earlier studies, Se can mitigate crop plants’ abiotic stress by protecting chloroplasts, and increase chlorophyll content under stress circumstances (Sattar et al. 2019). Selenoproteins (Se) prevent oxidative stress-induced damage to plants by increasing metabolism, controlling redox reactions, enhancement in water and nutrient uptake under unfavourable situations (Sieprawska et al. 2015). When the production of ROS is out of balance in cells, stress is induced, and this leads to significant physiological disturbances (Lin et al. 2012). Under stress, Se enhances antioxidant enzyme activity and minimized oxidative stress through lipid peroxidation in terms of malondialdehyde concentration, either directly or indirectly (Sattar et al. 2019). Elevated proline is seen as a promising sign of cold (Cechin et al. 2008), metal stress (Zahedi et al. 2011), salinity (Chun et al. 2018), drought stress (Ahmed et al. 2021). As previously reported, Se boosted proline accumulation, improving photosynthetic efficiency, ATP synthesis, and water utilization (Desoky et al. 2021). Proline mitigates the detrimental effects of ROS and increases plant resistance by inhibiting ROS produced under salinity stress (Howlader et al. 2018). Se restored proline and soluble sugar levels while preventing photosynthetic damage by maintaining RWC and protecting Rubisco (Desoky et al. 2021). Se modulating ROS mechanisms under abiotic stress conditions indicates strengthening of antioxidant defense (Ahmed et al. 2018). Wang et al. (2012) demonstrated under salt stress condition, chloroplasts and mitochondria, as well as other cellular organelles, were favorably impacted by Se. When it comes to chlorophyll, chloroplasts, and leaf mesophyll cells, the mitochondrial cristae grew more visible and more abundant. Exogenous Se treatment improved chlorophyll and carotenoid pigments and improved stomatal conductance and photosynthesis (Elkelish et al. 2019). Salinity disturbs water status in the soil which reduce the water uptake from roots to shoots (Ahanger et al. 2017). The results suggest that Se treatment boosted RWC through the deposition of sugars under stress situations. It is possible that Se administration increases the Na+/H+ antiporter expression, hence diminishing its harmful effects (Desoky et al. 2021). In metal stress condition, Se ions can prevent such interaction by ‘inactivating’ metals prior they bind to proteins (MeSeO3) and forming SeH groups in proteins (Sieprawska et al. 2015). Beneficial effects of Se on crucial processes under adverse conditions are associated with strengthening of antioxidative machinery and, improved tolerance to oxidative stress. Application of SeNPs with NO stimulated the expression of dehydration response element B1A (DREB1A), PAL (phenylalanine ammonia lyase), hydroxycinnamoyl-CoA quinate transferase (HCT1) increased 29.6, 36.4% and 30-fold respectively in chickory, which ultimately improved the antioxidant defense and secondary metabolism (Abedi et al. 2021).
Cerium (Ce)
Cerium is one of the rare earth element (REE) out of 17 element present (Ramos et al. 2016), accounting for 0.0043% of total mineral. It has role in improvement of germination, height of the plant, root development and biomass weight, productivity, content of the chlorophyll, total sugars, and the nutritional condition of numerous species are among the beneficial impacts (Chen et al. 2015). Aside from promoting N assimilation and PSII activity, the Ce can also stimulate plant functional processes. The effects of Ce on metabolic activities and physiology were dose- and time-dependent, species- and vegetative-stage specific, and dependent on management conditions (Zhao et al. 2012). Under various abiotic stresses, Cerium promotes plant growth by improving photosynthetic activity and antioxidant capacity.Consistent with alterations in antioxidant enzymes and antioxidants, the addition of Ce3+ lowered the elevated levels of H2O2 and MDA in leaves generated by stress treatments (Hong et al. 2017). Different findings revealed that Ce3+ might enhance antioxidant potential and protect the plant membrane system from oxidative stress-induced damage produced by a variety of stress. According to Liang et al. (2006) adding Ce3+ to rape seedlings (Brassica juncea L.) under UVB exposure increased growth, photosynthesis and the antioxidant defence. In plant science and agriculture, CeO2 nanoparticles (Ce-NP) are used to stimulate antioxidant enzyme activity and minimize membrane damage and leakage (Cao et al. 2018). Utilizing Ce-NPs aids in cell structure preservation by acting catalyst for chlorophyll formation and ROS minimizing, as well as preserving chloroplastic structure and PM integrity (Jahani et al. 2019; Jurkow et al. 2020). Under salt stress condition, Moldavian balm plants morphological, physiological and biochemical activity improved by the Ce-NPs exogenous application (Mohammadi et al. 2021). In rice crop poly acrylic acid CeNPs used under salt stress and showed increment in NO production (30.5%) by expression of nia2 (nitrate reductase) and redox signaling (Zhou et al. 2021). Therefore, more studies needs to be focused at molecular level to in-depth study in respect to their action in plant growth and stress tolerances.
Vanadium (V)
Vanadium along with being chemical hard malleable element, it is also an imperative beneficial element. Its mean concentration varies from 20 to 120 mg kg-1 which is almost similar to Zn concentration in earth crust (Baken et al. 2012). As early mentioned that small amount of beneficial elements act as nutrient although only tetravalent form (V+ 4) of vanadium is beneficial to plant growth and development due to its least toxicity, mobility and most dominant in the soil (Tripathi et al. 2018). Tetravalent form (V+ 4) help chlorophyll biosynthesis, nitrogen fixation and also increase potassium uptake and accumulation in the plants (Barker and Pilbeam 2015). Higher level of V cause plant physiological balance disturbance, reduction in biomass growth and ultimately yield production and appropriate concentration of vanadium can enhance plant growth and development (Hanus-Fajerskaet al.2021). Different V solution containing 40 and 80 mg/l had vigorous stem, leaf and root growth, more stem length, higher number of leaves in Chinese green mustard plants. While in tomato plants chlorophyll concentration, leave and flowers number, and stem and root attributes were reduced under 0–20 mg/l NH4VO3. Tomato plants started wilting after 2–3 days nutrient solutions with 40 and 80 mg/l V concentration and showed negative response to root, stem and leaf growth (Badmaevet al. 1999; Vachirapatamaet al. 2011) Another experiment with application of different doses of V show positive effect in maize (Zea mays) production and kernel quality (Mukherjee et al. 2004). In basil (Ocimum basilicum), there is linear relationship in dry biomass increment and increasing V doses up to 0 to 40 mg L-1 (Harland and Harland 1994). Arabidopsis thaliana can tolerate more oxidative stress with oxidovanadium (IV) penetration (Rojek et al. 2019). In some plant species V facilitates electron transportation in light reaction through photosystems I and II and act like redox catalyzer.
It has also been confirmed that V cross talk with certain elements such as P, Zn, Mo and Cu and amolirated the Cu toxicity. Low (47 mg/L) concentration of V promotes P accumulation and enhanced chlorophyll and amino acid production, while higher V concentration cause decrement in P concentration and promote ROS production (García-Jiménez et al. 2018; Imtiaz et al. 2017). Some ionic form of V is structurally similar to phosphate (Pi), which interfering in the enzymatic reaction of like phosphatases, ATPases, and phosphortransferases (Martinet al.1996). Ultimately V used to decrease the harmful effects or prevent transport of toxic trace metals, such as Cu, Hg, and Pb (Akoumianaki-Ioannidouet al. 2016).
Role of beneficial elements against biotic stress
The beneficial elements do not seem to be essential for the vital functions of the plants, but may play ancrucial role during activation of plant defense, thereby protection of plants from different biotic stresses (plant pathogens), insect pests and herbivores (Gómez-Merino, and Trejo-Téllez 2018). Si has inevitable role in plant defense mechanisms against several plant pathogens. Si can activate plant defense by different ways like activating defense related enzymes, antimicrobial compounds (phytoalexins), pathogenesis related proteins (PRPs), and secondary metabolites, regulating different signaling pathways and activation of defense related genes (Ranjan et al. 2021).
The powdery mildew (Erysiphe cichoracearum) infected Arabidopsis plants when treated with Si showed increased production of JA, ET, and SA in leaves, leads to resistance (Bakhat et al. 2018). Similar activation of JA and ET biosynthesis pathway and induction of plant resistance was observed in Si treated rice crop infected with Magnaporthe oryzae (Brunings et al. 2009). A likewise result was observed on spot blotch (Bipolaris sorokiniana) infected wheat plants when treated with Si showed late pathogen entry into epidermal cells and reduced colonization in foliar tissues (Domiciano et al. 2013). Si treated rice plants showed less number of lesions of Pyricularia grisea and Rhizoctonia solani due to increased incubation period and restricted hyphal entry into epidermal cells (Rodrigues et al. 2001; Seebold et al. 2004). Si activated JA and ET induced resistance in response to bacterial disease like, Ralstonia solanacearum in tomato (Chen et al. 2009; Ghareeb et al. 2011).
Selenium treated B. juncea plants showed protection from a fungal pathogen Alternaria brassicicola causing Alternaria blight and the general stem/root pathogen Fusarium sp. by inhibiting the growth of the fungus. (Hanson et al. 2003). Similarly, the application of sodium selenite on tomato plants against Fusarium wilt caused by Fusariumoxysporum f. sp. lycopersici race 3, showed increased production of total protein contents, phenolic compounds and antioxidant potential in susceptible and resistant cultivars. (Companioniet al. 2012). The role of selenium has also been observed in sodium selenite treated tomato plants infected with the gray mold pathogen Botrytis cinerea. The gray mold in tomato caused by the B. cinerea, inhibited spore germination by the 24 mg/L Se linked to increased ROS production (Wu et al. 2016). Titanium (Ti) as a beneficial element against plant pathogens has also been observed. Lyu et al. (2017) showed that nanoparticles nTi increase tolerance to bacteria Xanthomonas perforans. The reduced disease intensification and incidence of leaf blight (Xanthomonas oryzae pv. oryzae) and curvularia leaf spot (Curvularia lunata) were observed with TiO2 (Chao and Choi 2005). Similarly, TiO2 application suppressed brown blotch disease caused by Mycosphaerellacruenta and Cercospora leaf spots caused by Cercosporarosicola in field grown cowpea crops (Owolade and Ogunleti 2008). The beneficial role of TiO2 has been showed in case of Geranium bacterial leaf spot (Xanthomonas hortorum pv. pelargonii) and Poinsettia bacterial leaf spot (Xanthomonas axonopodis pv. poinsettiicola) (Norman and Chen 2011). Furthermore, TiO2 importance was showed in recycled irrigation water for elimination of both fungal and bacterial pathogens (Yao et al. 2007). The concentration of 40 mg/L nTiO2 effectively reduced the spot blotch in wheat caused by Bipolaris sorokiniana (Satti et al. 2021).
Till now, there are very limited research studies on effect of iodine against plant pathogen. However, the possible potential of iodine as an inductor of plant defense against plant pathogens cannot be ruled out. Iodine may activate plant defense through redox potential or changes in the chemical nature of the cuticle (Shaw et al. 2007), which is very essential for the activation of systemic acquired resistance (SAR) (Xia et al. 2009). The iodine induced modifications in the cuticle may alter the interaction of host and pathogens (Gniwotta et al. 2005; Silva-Moreno et al. 2016). Therefore, iodine could be used as beneficial tools against plant pathogens.
There are very little reports on the use of Lanthanum (La) and Cobalt (Co) against plant pathogens. Although, La increases plant biomass and possibly improves plant defense mechanisms against plant pathogens due to increased Si accumulation in stems. (Ilya Fastovets et al. 2017). Similarly, Co at 2 ppm can reduce disease incidence of Fusarium wilt diseases of lentil. (El-Hersh et al. 2011). The above studies on the role of beneficial elements against plant pathogens indicated that it has immense potential to be used as novel tools for the activation of plant defense and controlling plant diseases (Fig. 6).
Highlights the mechanism of biotic stresses through the beneficial elements. The beneficial elements acts as barrier for the pathogens which inhibit the entry into the plant system, secondly they also activated some cell wall related enzymes which also defense against biotic stresses. Beneficial elements also enhanced the secondary metabolites production, which activated the defense system. Moreover, they involved in the production of antioxidant enzymes which minimize the oxidative stress. With this the activation of JA, SA, and ET hormone activated systematic resistance against biotic stresses. Together, all of these changes enhance the plant defense system and provide immunity against biotic stresses
Conclusions
Presented review analysis complied the crucial role of beneficial elements in one frame and concluded that they have potential to improve plant health in sustainable ways. Although, earlier studies considered them as toxic materials as they showed toxicity symptoms on higher concentrations. These elements have stronger potential to crosstalk’s with essential and heavy metals in plant tissues and need of more research focusing on increasing the nutrient use efficiency and alleviation of heavy metals toxicity. Consequently, their strong potential with plant growth regulators, modulates the crucial signaling network and processes. Moreover, their potential application in stress tolerance is excellent, as they are strong activator of antioxidant defense systems. Further, the bio-fortification of agricultural crop with beneficial elements is future need equally as micro-nutrients. Some of previous attempt for beneficial elements biofortification in crop plants are highlighted in Table 4, which showed the extraordinary results in plant growth and development. Therefore, future research program could be more focus on bio-fortification and the molecular studies which opens new avenue in stress physiology and mineral nutrition to secure global food security in near future.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Abbas SM (2012) Effects of low temperature and selenium application on growth and the physiological changes in sorghum seedlings. J Stress Physiol Biochem 8(1):268–286.
Abedi S, Iranbakhsh A, OraghiArdebili Z, Ebadi M (2021) Nitric oxide and selenium nanoparticles confer changes in growth, metabolism, antioxidant machinery, gene expression, and flowering in chicory (Cichoriumintybus L.): potential benefits and risk assessment. Environ Sci Pollut Res 28(3):3136–3148.
Adebayo AH, Yakubu OF, Bakare-Akpata O (2020) Uptake, metabolism and toxicity of selenium in tropical plants. In Importance of selenium in the environment and human health. Intechopen DOI: 10.5772/intechopen.90295.
Agathokleous E, Kitao M, Calabrese EJ, (2018) The rare earth element (REE) lanthanum (La) induces hormesis in plants. Environ Pollut 238:1044–1047.
Ahanger MA, Bhat JA, Siddiqui MH, Rinklebe J, Ahmad P (2020) Integration of silicon and secondary metabolites in plants: a significant association in stress tolerance. J Exp Bot 71(21: 6758–6774.
Ahanger MA, Tomar NS, Tittal M, Argal S, Agarwal RM (2017) Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biol Plants 23:731–744.
Ahmad Z, Anjum S, Skalicky M, Waraich EA, Muhammad STR, Ayub MA, Hossain A, Hassan MM, Brestic M, Sohidul IM, Habib-Ur-Rahman M (2021) Selenium Alleviates the Adverse Effect of Drought in Oilseed Crops Camelina (Camelina sativa L.) and Canola (Brassica napus L.). Mol 26(6):1699.
Ahmad Z, Waraich EA, Akhtar S, Anjum S, Ahmad T, Mahboob W, Hafeez OBA, Tapera T, Labuschagne M, Rizwan M (2018) Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol Plant 40(4):80. Doi: 10.1007/s11738-018-2651-6.
Aihemaiti A, Jiang J, Gao Y, Meng Y, Zou Q, Yang M et al (2019) The effect of vanadium on essential element uptake of Setaria viridis’ seedlings. J Environ Manage 237:399–407.
Akeel A, Jahan A (2020) Role of cobalt in plants: its stress and alleviation. In: Naeem, M., Ansari, A., Gill, S. (Eds.), Contaminants in Agriculture. Springer, Cham, pp 339e357.
Akladious SA (2012) Influence of different soaking times with selenium on growth, metabolic activities of wheat seedlings under low temperature stress. Afr J Biotechnol 11:14792–14804.
Akoumianaki-Ioannidou A, Barouchas PE, Ilia E, Kyramariou A, Moustakas NK (2016) Effect of vanadium on dry matter and nutrient concentration in sweet basil (‘Ocimum basilicum’L.). Aust J Crop Sci 10(2):199–206.
Al Murad M, Khan AL, Muneer S (2020) Silicon in horticultural crops: cross-talk, signaling, and tolerance mechanism under salinity stress. Plants 9(4): 460.
Aldon D, Mbengue M, Mazars C, Galaud JP (2018) Calcium Signalling in Plant Biotic Interactions. International journal of molecular sciences 19(3):665. https:// doi.org/10.3390/ijms19030665.
Alharby HF, Rizwan M, Iftikhar A, Hussaini KM, Rehman MZ, Bamagoos AA, Alharbi BM, Asrar M, Yasmeen T, Ali S (2021) Effect of gibberellic acid and titanium dioxide nanoparticles on growth, antioxidant defense system and mineral nutrient uptake in wheat. Ecotoxicol Environ Saf 221:112436. doi: 10.1016/ j.ecoenv. 2021. 112436.
Altaf MM, Diao XP, Rehman A, Imtiaz M, Shakoor A, Altaf MA, Ghani MU (2020) Effect of Vanadium on Growth, Photosynthesis, Reactive Oxygen Species, Antioxidant Enzymes, and Cell Death of Rice. J Soil Sci Plant Nutr 20:2643–2656.
Anter F, Nadia G (2001) Cobalt absorption in relation to plant water balance. Egypt J soil Sci 41(1–2):111–122
Apse MP, Blumwald E (2007) Na+ transport in plants. FEBS Lett 581(12):2247–54. doi: 10.1016/j.febslet.2007.04.014.
Arnon DI, Stout PR (1939) Experimental methods for the study of the role of copper, manganese, and zinc in the nutrition of higher plants. Am J Bot 26:144–149.
Azarfam S, Nadian H, Moezzi A, Gholami A (2020) Effect of silicon on phytochemical and medicinal properties of aloe vera under cold stress. Appl Ecol Environ Res 18:561–575.
Bacilieri FS, Pereira de Vasconcelos AC, Quintao Lana RM, Mageste JG, Torres JLR (2017) Titanium (Ti) in plant nutrition-A review. Aust J Crop Sci 11(4): 382–386.
Badmaev V, Prakash S, Majeed M (1999) Vanadium: a review of its potential role in the fight against diabetes. J Altern Complement Med 5(3):273–291.
Bai B, Bian H, Zeng Z, Hou N, Shi B, Wang J, Zhu M, Han N (2017) MiR393-mediated auxin signaling regulation is involved in root elongation inhibition in response to toxic aluminum stress in barley. Plant Cell Physiol 58:426–439.
Baken S, Larsson MA, Gustafsson JP, Cubadda F, Smolders E (2012) Ageing of vanadium in soils and consequences for bioavailability. Eur J Soil Sci 63(6):839–847.
Bakhat HF, Bibi N, Zia Z, Abbas S, Hammad HM, Fahad S, Ashraf MR, Shah GM, Rabbani F, Saeed S (2018). Silicon mitigates biotic stresses in crop plants: a review. Crop Protection 104: 21–34.
Bao Z, Qi X, Hong S, Xu K, He F, Zhang M, et al (2017) Structure and mechanism of a group-I cobalt energy coupling factor transporter. Cell Res 27(5):675–687.
Barker AV, Pilbeam DJ (2015) Handbook of plant nutrition. CRC press.
Ben Y, Cheng M, Wang L, Zhou Q, Yang Z, Huang X (2021) Low-dose lanthanum activates endocytosis, aggravating accumulation of lanthanum or/and lead and disrupting homeostasis of essential elements in the leaf cells of four edible plants. Ecotoxicol Environ Saf 221:112429.
Bhat JA, Shivaraj SM, Singh P, Navadagi DB, Tripathi DK, Dash PK, Solanke AU, Sonah H, Deshmukh R (2019) Role of silicon in mitigation of heavy metal stresses in crop plants. Plants 8:71. https://doi:10.3390/plants8030071
Blasco B, Leyva R, Romero L, Ruiz JM (2013) Iodine effects on phenolic metabolism in lettuce plants under salt stress. J Agric Food Chem 61(11):2591–2596.
Blasco B, Ríos JJ, Leyva R, Cervilla LM, Sánchez-Rodríguez E, Rubio-Wilhelmi MM et al (2011) Does iodine biofortification affect oxidative metabolism in lettuce plants?. Biol Trace Elem Res 142(3):831–842.
Blaylock MJ, Huang JW (2000) Phytoextraction of Metals. Phytoremediation of Toxic Metals: Using Plants to Clean Up the Environment. In: Raskin, I., Ensley, B.D. (Eds.), John Wiley and Sons, Toronto pp 303.
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465(1–2):140–151.
Bojórquez-Quintal E, Escalante-Magaña C, Echevarría-Machado I, Martínez-Estévez M (2017) Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front Plant Sci 8:1767. https://doi.org/10.3389/fpls.2017.01767.
Bosnic P, Bosnic D, Jasnic J, Nikolic M (2018) Silicon mediates sodium transport and partitioning in maize under moderate salt stress. Environ Exp Bot 155:681–687.
Brengi SH, Khedr AAEM, Abouelsaad IA (2021) Effect of melatonin or cobalt on growth, yield and physiological responses of cucumber (Cucumis sativus L.) plants under salt stress. J Saudi Soc Agric Sci 21(1): 51–60 https://doi.org/ 10.1016/j.jssas.2021.06.012
Brownell PF (1965) Sodium as an Essential Micronutrient Element for a Higher Plant (Atriplex vesicaria). Plant Physiol 40(3):460.
Brunings AM, Datnoff LE, Ma JF, Mitani N, Nagamura Nagamura Y, Rathinasabapathi B, Kirst M (2009) Differential gene expression of rice in response to silicon and rice blast fungus Magnaporthe oryzae. Ann Appl Biol 155:161–170. doi: 10.1111/j.1744-7348.2009.00347.x
Buettner KM, Collins JM, Valentine AM (2012) Titanium (IV) and vitamin C: aqueous complexes of a bioactive form of Ti (IV). Inorg Chem 51(20):11030–11039.
Cakmak I, Guilherme LRG, Rashid A, Hora KH, Yazici A, Savasli Eet al (2017) Iodine biofortification of wheat, rice and maize through fertilizer strategy. Plant and Soil 418(1):319–335.
Cao F, Wang N, Zhang M, Dai H, Dawood M, Zhang G, Wu F (2013) Comparative study of alleviating effects of GSH, Se and Zn under combined contamination of cadmium and chromium in rice (Oryza sativa). BioMetals 2:297–308.
Cao Z, Rossi L, Stowers C, Zhang W, Lombardini L, Ma X (2018) The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L.) Merr.) under different soil moisture conditions. Environ Sci Pollut Res 25(1):930–939.
Cartes P, Jara AA, Pinilla L, Rosas A, Mora ML (2010) Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann Appl Biol 156(2):297–307.
Cechin I, Corniani N, DeFátima FT, Cataneo AC (2008) Ultraviolet-B and water stress effects on growth, gas exchange and oxidative stress in sunflower plants. Radiat Environ Biophys 47:405–413.
Chaiwong N, Bouain N, Prom-U-Thai C, Rouached H (2020) Interplay between silicon and iron signaling pathways to regulate silicon transporter Lsi1 expression in rice. Front Plant Sci 11:1065.
Chao SHL, Choi HS (2005) Method for Providing EnhancedPhotosynthesis. Jeonju: Korea Research Institute of Chemical Technology, Jeonju, South Korea. Bull 11: 1–34.
Chauhan R, Awasthi S, Indoliya Y, Chauhan AS, Mishra S, Agrawal L et al (2020) Transcriptome and proteome analyses reveal selenium mediated amelioration of arsenic toxicity in rice (Oryza sativa L.). J Hazard Mater 390:122122.
Chauhan R, Awasthi S, Srivastava S, Dwivedi S, Pilon-Smits EA, Dhankher OP, Tripathi RD (2019) Understanding selenium metabolism in plants and its role as a beneficial element. Crit Rev Environ Sci Technol 49(21):1937–1958.
Chen CS, Xie ZX, Liu XJ (2009) Interactive effects of drought and salt stresses on winter wheat seedlings growth and physiological characteristics of stress-resistance. Chin J Appl Ecol 20(4):811–816.
Chen D, Wang S, Yin L, Deng X (2018) How does silicon mediate plant water uptake and loss under water deficiency?. Front Plant Sci 9:281.
Chen J, Zhong Y (2021) Lanthanum (III)-amino Acid Chelate Mitigates Copper (III) Stress in Rice (Oryza Sativa). DOI: https://doi.org/10.21203/rs.3.rs-499369/v1
Chen WJ, Tao Y, Gu YH et al (2001) Effect of lanthanide chloride on photosynthesis and dry matter accumulation in tobacco seedlings. Biol Trace Elem Res 79:169–176. https://doi.org/10.1385/ BTER:79:2:169
Chen Y, Luo Y, Qiu N, Fei H, Sheng L, Wang R et al (2015) Ce3+ induces flavonoids accumulation by regulation of pigments, ions, chlorophyll fluorescence and antioxidant enzymes in suspension cells of Ginkgo biloba L. Plant Cell Tiss Organ Cult 123(2):283–296.
Chen YY, Lin YM, Chao TC, Wang JF, Liu AC, Ho FI et al (2009) Virus-induced gene silencing reveals the involvement of ethylene-, salicylic acid and mitogen-activated protein kinase-related defense pathways in the resistance of tomato to bacterial wilt. Physiol Plant 136:324–335. doi: 10.1111/j.1399-3054.2009.01226.x
Chmielewska-Bak J, Lefèvre I, Lutts S, Kulik A, Deckert J (2014) Effect of cobalt chloride on soybean seedlings subjected to cadmium stress. Acta Societatis Botanicorum Poloniae 83(3):201–207.
Chun SC, Paramasivan M, Chandrasekaran M (2018) Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front Microbiol 9:2525.
Companioni B, Medrano J, Torres JA, Flores A, Rodríguez E, Benavides A (2012) Protective action of sodium selenite against fusarium wilt in tomato:Total protein contents, levels of phenolic compounds and changes in antioxidant potential. Acta Hortic 947(1):321–328. Doi:10.17660/ActaHortic.2012.947.41.
Cook LL, McGonigle TP, Inouye RS (2009) Titanium as an Indicator of Residual Soil on Arid-Land Plants. J Environ Qual 38(1):188–199.
Costa LC, Luz LM, Nascimento VL, Araujo FF, Santos MN, Franca CDF, Silva TP, Fugate KK, Finger FL (2020) Selenium-ethylene interplay in postharvest life of cut flowers. Front Plant Sci 11:2055.
Crusciol CAC, Soratto RP, Castro GSA, Ferrari Neto J, daCosta CHM (2013) Leaf application of silicic acid to upland rice and corn. Embrapa Amapá-Artigo em periódico indexado (ALICE) 34(6):2803–2808. Doi: 10.5433/1679-0359.2013v34n6p2803.
Cui W, Kamran M, Song Q, Zuo B, Jia Z, Han Q (2019) Lanthanum chloride improves maize grain yield by promoting photosynthetic characteristics, antioxidants enzymes and endogenous hormone at reproductive stages. J Rare Earths 37(7):781–790.
Cui YN, Xia ZR, Ma Q, Wang WY, Chai WW, Wang SM (2019) The synergistic effects of sodium and potassium on the xerophyte Apocynum venetum in response to drought stress. Plant Physiol Biochem 135:489–498.
Dai H, Shan C (2019) Effects of lanthanum on the antioxidant capacity of chloroplasts and chlorophyll fluorescence parameters of maize seedlings under chromium stress. Photosynthetica 57(1):27–31.
Dai H, Shan C, Zhao H et al (2017) Lanthanum improves thecadmium tolerance of Zea mays seedlings by the regulationof ascorbate and glutathione metabolism. Biol Plantarum 61:551–556.
de Lima Lessa JH, Araujo AM, Ferreira LA, daSilva Júnior EC, deOliveira C, Corguinha APB et al (2019) Agronomic biofortification of rice (Oryza sativa L.) with selenium and its effect on element distributions in biofortified grains. Plant and Soil 444(1):331–342.
de Oliveira C, Ramos SJ, Siqueira JO, FaquinV, deCastro EM, Amaral, DC et al (2015) Bioaccumulation and effects of lanthanum on growthand mitotic index in soybean plants. Ecotoxicol Environ Saf 122:136–144.
Deshmukh R, Sonah H, Belanger RR (2020) New evidence defining the evolutionary path of aquaporins regulating silicon uptake in land plants. J Exp Bot 71(21):6775–6788.
Desoky EM, Abdel-Rahman M, Mohamed F, Abo EM, Esayed M, Safaa MAArnaout, Mohamed F. Awad, Mohamed F. Ramadan, Seham AI (2021) Physiological and Biochemical Mechanisms of Exogenously Applied Selenium for Alleviating Destructive Impacts Induced by Salinity Stress in Bread Wheat. Agron 11(5):926.
Deynée PG, Mukherjee RK (1984) Iodine treatment of soybean and sunflower seeds for controlling deterioration. Field Crop Res 9:205–213. doi:10.1016/0378-4290(84)90026-1
Diatloff E, SmithFW, Asher CJ (2008) Effects of lanthanum and Cerium on the growth and mineral nutrition of corn and mungbean. Ann Bot 101:971–982. doi: 10.1093/aob/mcn021
D’Imperio M, Renna M, Cardinali A, Buttaro D, Santamaria P, Serio F (2016) Silicon biofortification of leafy vegetables and its bioaccessibility in the edible parts. J Sci Food Agric 96(3):751–756.
Djanaguiraman M, Prasad PV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48(12):999–1007.
Domiciano GP, Rodrigues FA, GuerraAMN, Vale FXR (2013) Infection process of Bipolaris sorokiniana on wheat leaves is affected by silicon. Trop Plant Pathol 38:258–263. doi: 10.1590/S1982-56762013005000006
Dorairaj D, Ismail MR, Sinniah UR, Kar Ban T (2020) Silicon mediated improvement in agronomic traits, physiological parameters and fibre content in Oryza sativa. Acta Physiol Plant 42:38. https://doi.org/10.1007/s11738-020-3024-5
Eitinger T, Suhr J, Moore L, Smith JAC (2005) Secondary transporters for nickel and cobalt ions: theme and variations. Biometals 18(4) 399–405.
El Mehdawi, AF, Jiang Y, Guignardi ZS, Esmat A, Pilon M, Pilon-Smits EA, Schiavon M (2018) Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol 217(1):194–205.
El-Badri AM, Batool M, Wang C, Hashem AM, Tabl KM, Nishawy E, Kuai J, Zhou G, Wang B (2021) Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol Environ Saf 225:112695.
El-Hersh MS AbdEl-Hai KM, Ghanem KM (2011) Efficiency of Molybdenum and Cobalt Elements on the Lentil Pathogen. Plant Pathol 5:102–114.
Elkelish AA, Soliman MH, Alhaithloul HA, El-Esawi MA (2019) Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem 137:144–153.
Emamverdian A, Ding Y, Barker J, Liu G, Hasanuzzaman M, Li Y, Ramakrishnan M, Mokhberdoran F (2022) Co-Application of 24-Epibrassinolide and Titanium Oxide Nanoparticles Promotes Pleioblastuspygmaeus Plant Tolerance to Cu and Cd Toxicity by Increasing Antioxidant Activity and Photosynthetic Capacity and Reducing Heavy Metal Accumulation and Translocation. Antioxidants 11(3):451.
Fang Q, Zhang J, Zhang Y, Fan N, van den Burg HA, Huang CF (2020) Regulation of aluminum-resistance in Arabidopsis involves the SUMOylation of the zinc finger transcription factor STOP1. Plant Cell 32:3921–3938.
Farooq M, Wahid A, Siddique KHM (2012) Micronutrient application through seed treatments-a review. J Soil Sci Plant Nutr 12:125–142.
Fleck AT, Schulze S, Hinrichs M, Specht A, Waßmann F, Schreiber L, Schenk MK (2015) Silicon promotes exodermalCasparian band formation in Si-accumulating and Si-excluding species by forming phenol complexes. PLoSOne 10(9):e0138555.
Fricke W (2015) The significance of water co-transport for sustaining transpirational water flow in plants: a quantitative approach. J Exp Bot 66:731–739. doi: 10.1093/jxb/eru466.
Gad N (2005) Interactive effect of salinity and cobalt on tomato plants II-Some Physiological Parameters As Affected By Cobalt And Salinity. Res J Agric & Biol Sci 1(3): 270–276.
Gad N (2012) Role and importance of cobalt nutrition on groundnut (Arachis hypogaea) production. World Appl Sci J 20:359–367. https://doi.org/10.5829/ idosi.wasj.2012.20.03.2819
Gad N, Abdel-Moez MR, Fekry Ali, ME, Abou-Hussein SD (2018) Increasing salt tolerance in cucumber by using cobalt. Middle East J Sci Res 8:345–354
Ghanati F, Morita A, Yokota H (2005) Effects of aluminum on the growth of tea plant and activation of antioxidant system. Plant and soil 276(1):133–141.
Ghareeb H, Bozsó Z, Ott PG, Repenning C, Stahl F, Wydra K (2011) Transcriptome of silicon-induced resistance against Ralstonia solanacearum in t he silicon non-accumulator tomato implicates priming effect. Physiol Mol Plant Pathol 75:83–89. doi: 10.1016/j.pmpp.2010.11.004.
Ghasemi-Fasaei R, Ronaghi A, Maftoun M, Karimian NA, Soltanpour PN, (2005) Iron‐manganese interaction in chickpea as affected by foliar and soil application of iron in a calcareous soil. Commun Soil Sci Plant Anal 36(13–14):1717–1725.
Gniwotta F, Vogg G, Gartmann V, Carver TLW, Riederer M,Jetter R (2005) What do microbes encounter at the plant surface? Chemical
composition of pea leaf cuticular waxes. Plant Physiol 139:519–530. doi: 10.1104/pp.104.053579
Gómez-Merino FC, Trejo-Téllez LI (2018) The role of beneficial elements in triggering adaptive responses to environmental stressors and improving plant performance. In Biotic and Abiotic Stress Tolerance in Plants, Springer, Singapore pp 137–172.
Gonzali S, Kiferle C, Perata P (2017) Iodine biofortification of crops: Agronomic biofortification, metabolic engineering and iodine bioavailability. Curr Opin Biotechnol 44:16–26.
Gou T, Su Y, Han R, Jia J, Zhu Y, Huo H, Liu H, Gong H (2022) Silicon delays salt stress-induced senescence by increasing cytokinin synthesis in tomato. Sci Hortic 293: 110750.
Gu HH, Qiu H, Tian T, Zhan SS, Chaney RL, Wang SZ, Tang YT, Morel JL, Qiu RL (2011) Mitigation effects of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on multi-metal contaminated acidic soil. Chemosphere 83:1234–1240.
Gunes A, Inal A, Bagci EG, Pilbeam DJ (2007) Silicon-mediated changes of some physiological and enzymatic parameters symptomatic for oxidative stress in spinach and tomato grown in sodic-B toxic soil. Plant and Soil 290(1):103–114.
Guo P, Li Q, Qi YP, Yang LT, Ye X, Chen HH, Chen LS (2017) Sulfur-mediated-alleviation of aluminum-toxicity in Citrus grandis seedlingsInt. J Mol Sci 18(12):2570.
Gupta N, Bajpai M, Majumdar R, Mishra P (2015) Response of iodine on antioxidant levels of Glycine max L. Grown under Cd2+ stress. Adv Biol Res (Rennes) 9:40–48. doi: 10.5829/idosi.abr.2015.9.1.9183
Gupta N, Rita (2016) Effect of iodine on non enzymatic antioxidant levels of glycine max l. Grown Under heavy metaland heat stress. Innovare j sci 4(5):1–3.
Gupta NK, Meena SK, Gupta S, Khandelwal SK (2002) Gas exchange, membrane permeability and ion uptake in two species of Indian jujube differing in salt tolerance. Photosynthetica 40(4):535–539.
Habibi G (2013) Effect of drought stress and selenium spraying on photosynthesis and antioxidant activity of spring barley. Acta Agric Slov 101(1):31–39.
Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Sci Total Environ 326:1–31
Hanin M, Ebel C, Ngom M, Laplaze L, Masmoudi K (2016) New insights on plant salt tolerance mechanisms and their potential use for breeding. Front Plant Sci 7:1787.
Hanson B, Garifullina GF, Lindblom SD, Wangeline A, Ackley A, Kramer K et al (2003) Selenium accumulation protects Brassica juncea from invertebrate herbivory and fungal infection. New Phytol 159:461–469. doi: 10.1046/j.1469-8137.2003.00786.x.
Hanus-Fajerska E, Wiszniewska A, Kamińska I (2021) A Dual Role of Vanadium in Environmental Systems—Beneficial and Detrimental Effects on Terrestrial Plants and Humans. Plants 10(6):1110.
Harland BF, Harden-Williams BA (1994) Is vanadium of human nutritional importance yet?. J Am Diet Assoc 94(8):891–894.
Hasanuzzaman M, Hossain MA, Fujita M (2010) Selenium in higher plants: physiological role, antioxidant metabolizm and abiotic stress tolerance. J Plant Sci 5:354–375.
Hasanuzzaman M, Nahar K, Anee TI, Khan MIR, Fujita M (2018) Silicon-mediated regulation of antioxidant defense and glyoxalase systems confers drought stress tolerance in Brassica napus L. S Afr J Bot 115:50–57.
Hattori T, Inanaga S, Araki H, An P, Morita S, Luxová M, Lux A (2005) Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol Plant 123(4): 459–466.
Hawkesford MJ (2003) Transporter gene families in plants: the sulphate transporter gene family—redundancy or specialization?. Physiol Plant 117(2):155–163.
Hawrylak-NowakB (2013) Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul 70(2):149–157.
Hawrylak-NowakB, Matraszek R, Pogorzelec M (2015) The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol Plant 37(2):41.
He X, You P, Sun Y (2020) Lanthanum and abscisic acid coregulate chlorophyll production of seedling in switchgrass. PloS one 15(5):e0232750. https://doi.org/10.1371/ journal.pone.0232750.
He YW, Loh CS (2002) Induction of early bolting in Arabidopsis thaliana by triacontanol, cerium and lanthanum is correlated with increased endogenous concentration of isopentenyl adenosine (iPAdos). J Exp Bot 53(368):505–512.
Hille R, Nishino T, Bittner F (2011) Molybdenum enzymes in higherorganisms. Coord Chem Rev 255:1179 − 1205.
Hodson MJ, Evans DE (2020) Aluminium–silicon interactions in higher plants: an update. J Exp Bot 71(21):6719–6729.
Hong F, Qu C, Wang L (2017) Cerium Improves Growth of Maize Seedlings via Alleviating Morphological Structure and Oxidative Damages of Leaf under Different Stresses. J Agric Food Chem 65(41):9022–9030.
Hosseini SA, Naseri Rad S, Ali N, Yvin JC (2019) The ameliorative effect of silicon on maize plants grown in Mg-deficient conditions. Int J Mol Sci 20(4):969.
Howladar SMA (2018) novel moringa oleifera leaf extract can mitigate the stress effects of salinity and cadmium in bean (Phaseolus vulgaris L.) plants. Ecotoxicol Environ Saf 100: 69–75.
Hu J, Li Y, Jeong BR (2020) Silicon promotes root development by modulating polar transport of auxin during cutting propagation of poinsettia. In III International Symposium on Germplasm of Ornamentals, 1291:269–276.
Hu X, Ding Z, Chen, Y, Wang X, Dai L (2002) Bioaccumulationof lanthanum and cerium and their effects on the growth of wheat (Triticum aestivum L.) seedlings Chemospere 48:621–629. doi: 10.1016/S0045-6535(02)00109-1.
Huang CF, Yamaji N, Mitani N, Yano M, Nagamura Y, Ma JF (2009) A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell 21: 655–667. doi: 10.1105/tpc.108.064543.
Huang G, Shan C (2018) Lanthanum improves the antioxidant capacity in chloroplast of tomato seedlings through ascorbate-glutathione cycle under salt stress. Sci Hortic 232:264–268.
Huang H, Li M, Rizwan M, Dai Z, Yuan Y, Hossain MM, Cao M, Xiong S, Tu S (2021) Synergistic effect of silicon and selenium on the alleviation of cadmium toxicity in rice plants. J Hazard Mater 401:123393.
Huang X, Zhou Q (2006) Alleviation effect of lanthanum on cadmiumstress in seedling hydroponic culture of kidney bean and corn. J Rare Earth 24:248–252. doi: 10.1016/S1002-0721(06)60103-8.
Huihui Z, Xin L, Yupeng G, Mabo L, Yue W, Meijun A, Yuehui Z, Guanjun L, Nan X, Guangyu S (2020) Physiological and proteomic responses of reactive oxygen species metabolism and antioxidant machinery in mulberry (Morus alba L.) seedling leaves to NaCl and NaHCO3 stress. Ecotoxicol Environ Saf 15(193):110259.
Hussain M, Khan TA, Yusuf M, Fariduddin Q (2019) Silicon-mediated role of 24-epibrassinolide in wheat under high-temperature stress. Environ Sci Pollut Res 26(17):17163–17172.
Hussain S, Shafiq I, Skalicky M, Brestic M, Rastogi A, Mumtaz M, Hussain D, Iqbal N, Raza MA, Guo L,Yang W (2021) Titanium application increases phosphorus uptake through changes in Auxin content and later root formation in soybean. Front Plant Sci 12:743618. doi: 10.3389/fpls.2021.743618.Fastovets I, Kotelnikova A, Olga R, Nikolai S, Elena P(2017)Effects of soil lanthanum on growth and elemental composition of plants. Geophys 19 EGU2017-305-2
Imtiaz M, Ashraf M, Rizwan MS, Nawaz MA, Rizwan M, Mehmood S, Yousaf B, Yuan Y, Ditta A, Mumtaz MA et al (2018) Vanadium toxicity in chickpea (Cicer arietinum L.) grown in red soil: Effects on cell death, ROS and antioxidative systems. Ecotoxicol Environ Saf 158, 139–144.
Imtiaz M, Rizwan MS, Mushtaq MA, Yousaf B, Ashraf M, Ali M, Tu S (2017) Interactive effects of vanadium and phosphorus on their uptake, growth and heat shock proteins in chickpea genotypes under hydroponic conditions. Environ Exp Bot 134:72–81.
Iqbal M, Khan R, Ashfaque F, Chhillar H, Irfan M, Khan NA (2021a) The intricacy of silicon, plant growth regulators and other signaling molecules for abiotic stress tolerance: An entrancing crosstalk between stress alleviators. Plant Physiol Biochem 162:36–47.
Iqbal Z, Sarkhosh A, Balal RM, Rauf S, Khan N, Altaf MA, Camara-Zapata JM, Garcia-Sanchez F, Shahid MA (2021b) Silicon Nanoparticles Mitigate Hypoxia-Induced Oxidative Damage by Improving Antioxidants Activities and Concentration of Osmolytes in Southern Highbush Blueberry Plants. Agronomy 11(11):2143.
Jaberzadeh A, Moaveni P, Moghadam HRT, Zahedi H (2013) Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 41(1):201–207.https://doi.org/10.15835/ nbha4119093.
Jahani S, Saadatmand S, Mahmoodzadeh H, Khavari-Nejad RA (2019) Effect of foliar application of cerium oxide nanoparticles on growth, photosynthetic pigments, electrolyte leakage, compatible osmolytes and antioxidant enzymes activities of Calendula officinalis L. Biologia 74(9):1063–1075.
Jaiswal SK, Naamala J, Dakora FD (2018) Nature and mechanisms of aluminium toxicity, tolerance and amelioration in symbiotic legumes and rhizobia. Biol Fertil Soils 54: 309–318.
Jenkit KS, Wang CY, Mackenzie B, Knutson M (2010) Physiologic implications of metal-ion transport by ZIP14 and ZIP8, Biometals 25:643e655.
Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D (2017) Effect of exogenous selenium supply on photosynthesis, Na + accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci rep 7(1):1–14.
Jinger D, Devi MT, Dhar S, Dass A, Rajanna GA, Upadhaya P, Raj R (2017) Silicon in mitigating biotic stresses in rice (Oryza sativa L.) – a review. Ann Agric Res 38:1–14.
Jinger D, Devi MT, Dhar S, Dass A, Sharma VK, Vijayakumar S, Joshi E, Jatav HS, Singh N (2020c) Silicon application mitigates abiotic stresses in rice: A review. Indian J Agric Sci90:2043–50.
Jinger D, Dhar S, Dass A, Sharma VK, Parihar M, Rana K, Gupta G Jatav HS (2020a) Crop productivity, grain quality, water use efficiency, and soil enzyme activity as influenced by silicon and phosphorus application in aerobic rice (Oryza sativa). Commun Soil Sci Plant Anal 51:2147–2162.
Jinger D, Dhar S, Dass A, Sharma VK, Vijayakumar S, Gupta G (2020b) Influence of residual silicon and phosphorus on growth, productivity, lodging and grain quality of succeeding wheat under rice-wheat cropping system. J Environ Biol 41: 1676–84.
Jinger D, Dhar S, Kaur R (2018b) Crop lodging: its causes and management for sustainable crop production. Indian Farming 68:24–27.
Jinger D, Dhar S, Vijayakumar S, Pande VC, Kakade V, Jat RA, Dinesh D (2020d) Silicon nutrition of graminaceous crops. Indian Farming 70:18–2.
Jurkow R, Sękara A, Pokluda R, Smoleń S, Kalisz A (2020) Biochemical response of oak leaf lettuce seedlings to different concentrations of some metal (oid) oxide nanoparticles. Agronomy 10(7):997.
Karami A, Sepehri A (2018) Nano titanium dioxide and nitric oxide alleviate salt induced changes in seedling growth, physiological and photosynthesis attributes of barley. Zemdirbyste-Agriculture 105(2):123‒132. DOI 10.13080/z-a.2018.105. 016.
Kashif M, Sattar A, Ul-Allah S, Sher A, Ijaz M, Butt M, Qayyum A (2021) Silicon Alleviates Arsenic Toxicity in Maize Seedlings by Regulating Physiological and Antioxidant Defense Mechanisms. Soil Sci Plant Nutr 21(3): 1–9.
Kataoka T, Hayashi N, Yamaya T, Takahashi H (2004) Root-to-shoot transport of sulfate in Arabidopsis. Evidence for the role of SULTR3:5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiol 4:4198–4204.
Kaur S, Kaur N, Siddique KH, Nayyar H (2016). Beneficial elements for agricultural crops and their functional relevance in defense against stresses. Arch Agron Soil Sci 62(7):905–920.
Kaya C, Ashraf M, Al-Huqail AA, Alqahtani MA, Ahmad P (2020) Silicon is dependent on hydrogen sulphide to improve boron toxicity tolerance in pepper plants by regulating the AsA-GSH cycle and glyoxalase system. Chemosphere 257: 127241.
Kaya C, Tuna L, Higgs D (2006) Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. J Plant Nutr 29(8):1469–1480.
Khaliq A, Ali S, Hameed A, Farooq MA, Farid M, Shakoor MB, Rizwan M (2015) Silicon alleviates nickel toxicity in cotton seedlings through enhancing growth, photosynthesis, and suppressing Ni uptake and oxidative stress. Arch Agr Soil Sci 62(5):633–647. DOI: 10.1080/03650340.2015.1073263
Khan A, Bilal S, Khan AL, Imran M, Shahzad R, Al-Harrasi A (2020a) Silicon and gibberellins: synergistic function in harnessing ABA signaling and heat stress tolerance in date palm (Phoenix dactylifera L.). Plants 9(5):620.
Khan A, Khan AL, Imran M, Asaf S, Kim YH, Bilal S (2020b) Silicon-induced thermotolerance in Solanum lycopersicum L. via activation of antioxidant system, heat shock proteins, and endogenous phytohormones. BMC plant biology 20:1–18.
Khan A, Khan AL, Muneer S, Kim YH, Al-Rawahi A, Al-Harrasi A (2019) Silicon and salinity: crosstalk in crop-mediated stress tolerance mechanisms. Front Plant Sci 10:1429.
Khan MIR, Ashfaque F, Chhillar H, Irfan M, Khan NA (2021) The intricacy of silicon, plant growth regulators and other signaling molecules for abiotic stress tolerance: An entrancing crosstalk between stress alleviators. Plant Physiol Biochem 162:36–47.
Kiany T, Pishkar L, Sartipnia N, Iranbakhsh A, Barzin G (2022) Effects of silicon and titanium dioxide nanoparticles on arsenic accumulation, phytochelatin metabolism, and antioxidant system by rice under arsenic toxicity. Environ Sci Pollut Res 1–13.
Kiferle C, Martinelli M, Salzano AM, Gonzali S, Beltrami S, Salvadori PA, Hora K, Holwerda HT, Scaloni A, Perata P (2021) Evidences for a Nutritional Role of Iodine in Plants. Front Plant Sci 12:616868. doi: 10.3389/fpls.2021.616868.
Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y (2007) The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J 50:207e218.
Kim YH, Khan AL, Lee IJ (2016) Silicon: a duo synergy for regulating crop growth and hormonal signaling under abiotic stress conditions. Crit Rev Biotechnol 36(6):1099–1109.
Kim YH, Khan AL, Waqas M, Shim JK, Kim DH, Lee KY, Lee IJ (2014) Silicon application to rice root zone influenced the phytohormonal and antioxidant responses under salinity stress. J Plant Growth Regul 33(2):137–149.
Kleiber T, Krzesiński W, Przygocka-Cyna K, Spiżewski T (2018) Alleviation effect of selenium on manganese stress of plants. Ecol Chem Eng 25(1):143.
Kochian LV, Hoekenga OA, Pineros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55 459–493.
Kochian LV, Piñeros MA, Liu J,Magalhaes JV (2015) Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol 66 571–598.
Koentjoro Y, Sukendah, Purwanto E, Purnomo D (2021) The role of silicon on content of proline, protein and abscisic acid on soybean under drought stress. Earth Environ Sci 637:012086. http://doi.org/10.1088/1755-1315/637/1/012086
Komeda H, Kobayashi M, Shimizu S (1997) A novel transporter involved in cobalt uptake. Proceed Nat Acad Sci 94(1):36–41.
Kopittke PM (2016) Role of phytohormones in aluminium rhizotoxicity. Plant Cell Environ 39:2319–2328.
Kopittke PM, Gianoncelli A, Kourousias G, Green K, McKenna BA (2017) Alleviation of Al toxicity by Si is associated with the formation of Al–Si complexes in root tissues of sorghum. Front Plant Sci 8: 2189.
Kordenaeej A, Nejad AA, Shojaeian AA, Lelley T, Sharafi Y( 2013) Simulating the effect of terminal drought stress by potassium iodide and its use in mapping QTLs for yield and yield components in bread wheat. Int J Agro Plant Prod 4:659–63.
Kužel S, Cigler P, Hrubý M, Vydra J, Pavlíková D, Tlustoš P (2007) The effect of simultaneous magnesium application on the biological effects of titanium. Plant Soil Environ 53(1):16–23.
Lara TS, de Lima Lessa JH, deSouza KRD, Corguinha APB, Martins FAD, Lopes G, Guilherme LRG (2019) Selenium biofortification of wheat grain via foliar application and its effect on plant metabolism. J Food Compost Anal 81:10–18.
Leyva R, Sánchez-Rodríguez E, Ríos JJ, Rubio-Wilhelmi MM, Romero L, Ruiz JM, Blasco B (2011) Beneficial effects of exogenous iodine in lettuce plants subjected to salinity stress. Plant Sci 181(2):195–202.
Leyva R, Sánchez-Rodríguez E, Ríos JJ, Rubio-Wilhelmi MM, Romero L, Ruiz JM et al (2011) Beneficial effects of exogenous iodine in lettuce plants subjected to salinity stress. Plant Sci 181 195–202. doi: 10.1016/j.plantsci.2011.05.007
Li Guo-tai. (2010) Effect of cerium on chilling resistance of cucumber seedlings. Hortic Sci 16:47–48.
Li H, Zhu Y, Hu Y, Han W, Gong H (2015) Beneficial effects of silicon in alleviating salinity stress of tomato seedlings grown under sand culture. Acta Physiol Plant 37:1–9
Li MQ, Hasan MK, Li CX, Ahammed GJ, Xia XJ, Shi K, Zhou YH, Reiter RJ, Yu JQ, Xu MX, Zhou J (2016) Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J Pineal Res 61 (3):291–302.
Li R, Li DW, Liu HP, Hong CL, Song MY, Dai ZXet al (2017) Enhancing iodine content and fruit quality of pepper (Capsicum annuum L.) through biofortification. Sci Hortic 214:165–173.
Liang CJ, Huang XH, Zhou QJ (2006) Effects of cerium on growth and physiological mechanism in plants under enhanced ultraviolet-B radiation. J Environ Sci 18:1147 − 1151.
Liang Y, Chen Q, Liu Q, Zhang W, Ding R (2003) Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J Plant Physiol 160:1157–1164.
Liang Y, Zhang W, Chen Q, Liu Y, Ding R (2006) Effect of exogenous silicon (Si) on H+-ATPase activity, phospholipids and fluidity of plasma membrane in leaves of salt-stressed barley (Hordeum vulgare L.). Environ Exp Bot 57:212–219.
Liang Y, Zhu J, Li Z, Chu G, Ding Y, Zhang J, Sun W (2008) Role of silicon in enhancing resistance to freezing stress in two contrasting winter wheat cultivars. Environ Exp Bot 64:286–294.
Libik-Konieczny M, Kozieradzka-Kiszkurno M, Desel C, MichalecWarzecha Z, Miszalski Z, Konieczny R (2015) The localization of _ NADPH oxidase and reactive oxygen species in in vitro-cultured Mesembryanthemum crystallinum L. hypocotyls discloses their differing roles in rhizogenesis. Protoplasma 252:477–487.
Lin CY, Trinh NN, Lin CW, Huang HJ (2013) Transcriptome analysis of phytohormone, transporters and signaling pathways in response to vanadium stress in rice roots. Plant Physiol Biochem 66 98–104.
Lin L, Zhou W, Dai H, Cao F, Zhang G, Wu F (2012) Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J Hazard Mater 235–236:343–351.
Liu H, Shi Z, Li J, Zhao P, Qin S, Nie Z (2018) The impact of phosphorus supply on selenium uptake during hydroponics experiment of winter Wheat (Triticum aestivum) in China. Front Plant Sci 9:373.
Liu X, Yin L, Deng X, Gong D, Du S, Wang S, Zhang Z (2020) Combined application of silicon and nitric oxide jointly alleviated cadmium accumulation and toxicity in maize. J Hazard Mater 395:122679.
Liu Y, Xu R (2015) The forms and distribution of aluminum adsorbed onto maize and soybean roots. J Soils Sediments 15:491–502.
Loginov SV, Matichenkov VV, Matichenkov IV (2011) Effect of organo-silicon stimulators on the frost resistance of rice. (In) Proceedings of the 5th International Conference on Silicon in Agriculture, Beijing, China, pp 117.
Luan H, Niu C, Nie X, Li Y, Wei M (2022) Transcriptome and Physiological Analysis of Rootstock Types and Silicon Affecting Cold Tolerance of Cucumber Seedlings. Plants 11(3):445.
Lv B, Yan Z, Tian H, Zhang X, Ding Z (2019) Local auxin biosynthesis mediates plant growth and development. Trends Plant Sci 24: 6–9.
Lyu B, Guo X, Gao D, Kou M, Yu Y, Ma J, Chen S, Wang H, Zhang Y, Bao X (2020) Auxin metabolic network regulates the plant response to metalloids stress. J Hazard Mater 405–124250.
Lyu S, Wei X, Chen J et al (2017) Titanium as a beneficial element for crop production. Front Plant Sci 8:597. https://doi.org/10.3389/fpls.2017.00597
Lyu S, Wei X, Chen J, Wang C, Wang X, Pan D (2017) Titanium as a beneficial element for crop production. Front Plant Sci 8:597.
Ma JF, Mitani N, Nagao S, Konishi S, Tamai K, Iwashita T, Yano M (2004) Characterization of the silicon uptake system and molecular mapping of the silicon transporter gene in rice. Plant Physiol 136(2):3284–3289.
Ma JF, Miyake Y, Takahashi E (2001) Silicon as a beneficial element for crop plants. Plant Sci 8:17–39.
Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T et al (2007) An efflux transporter of silicon in rice. Nature 448(7150): 209–212.
Maathuis FJ (2014) Sodium in plants: perception, signalling, and regulation of sodium fluxes. J Exp Bot 65(3):849–858.
Maathuis FJM (2009) Physiological functions of mineral macronutrients. Curr Opin Plant Biol 12:250–258.
Maghsoudi K, Arvin MJ, Ashraf M (2019a) Mitigation of arsenic toxicity in wheat by the exogenously applied salicylic acid, 24-epi-brassinolide and silicon. J Soil Sci Plant Nutr 20(2):577–588.
Maghsoudi K, Emam Y, Pessarakli M (2016) Effect of silicon on photosynthetic gas exchange, photosynthetic pigments, cell membrane stability and relative water content of different wheat cultivars under drought stress conditions. J Plant Nutr 39:1001–1015.
Maghsoudi, K, Emam Y, Ashraf M (2016) Foliar application of silicon at different growth stages alters growth and yield of selected wheat cultivars. J Plant Nutr 39(8): 1194–1203.
Marschner H (2012) Marschner’s mineral nutrition of higher plants. London: Academic press. DOI: https://doi.org/10.1016/C2009-0-63043-9.
Martin A, Berndt H, Lücke B, Meisel M (1996) Reaction pathway of benzonitrile formation during toluene ammoxidation on vanadium phosphate catalysts. Top Catal 3(3–4):377–386.
Mateos-Naranjo E, Andrades-Moreno L, Davy AJ (2013) Silicon alleviates deleterious effects of high salinity on the halophytic grass Spartina densiflora. Plant Physiol Biochem 63:115–121.
Matsumoto H, Yamaya T (1986) Inhibition of potassium uptake and regulation of membrane-associated Mg2+-ATPase activity of pea roots by aluminium. Soil Sci Plant Nutr 32(2):179–188.
Mauad M, Crusciol CAC, Nascente AS, Grassi Filho H, Lima GPP (2016) Effects of silicon and drought stress on biochemical characteristics of leaves of upland rice cultivars. Rev Cienc Agron 47:532–539.
Medrano Macías J. López Caltzontzit MG, Rivas Martínez EN, Narváez Ortiz WA, Benavides Mendoza A, Martínez Lagunes P (2021) Enhancement to Salt Stress Tolerance in Strawberry Plants by Iodine Products Application. Agron 11:602. https://doi.org/10.3390/ agronomy11030602
Medrano-Macías J, Leija-Martínez P, González-Morales S, Juárez-Maldonado A, Benavides-Mendoza A (2016) Use of Iodine to Biofortify and Promote Growth and Stress Tolerance in Crops. Front Plant Sci 7:1146. https://doi.org/10.3389/ fpls.2016.01146.
Mimmo T, Tiziani R, Valentinuzzi F, Lucini L, Nicoletto C, Sambo P et al (2017) Selenium biofortification in Fragaria × ananassa: implications on strawberry fruits quality, content of bioactive health beneficial compounds and metabolomic profile. Front Plant Sci 8:1887.
Mitani-Ueno N, Ma JF (2021) Linking transport system of silicon with its accumulation in different plant species. Soil Sci Plant Nutr 67(1):10–17.
Mohammadi MHZ, Panahirad S, Navai A, Bahrami MK, Kulak M, Gohari G (2021) Cerium oxide nanoparticles (CeO2-NPs) improve growth parameters and antioxidant defense system in Moldavian Balm (Dracocephalum moldavica L.) under salinity stress. Plant Stress 1:100006. https://doi.org/10.1016/j.stress.2021.100006
Mohammadi R, Maali-Amiri R, Mantri NL (2014) Effect of TiO2 nanoparticles on oxidative damage and antioxidant defense systems in chickpea seedlings during cold stress. Russ J Plant Physiol 61(6):768–775.
Montesano FF, DImperio M, Parente A, Cardinali A, Renna M, Serio F (2016) Green bean biofortification for si through soilless cultivation: Plant response and Si bioaccessibility in pods. Sci Rep 6(1):1–9.
Moradtalab N, Weinmann M, Walker F, Höglinger B, Ludewig U, Neumann G (2018) Silicon improves chilling tolerance during early growth of maize by effects on micronutrient homeostasis and hormonal balances. Front Plant Sci 9:420.
Moreno-Alvarado M, García-Morales S, Trejo-Téllez LI, Hidalgo-Contreras JV, Gómez-Merino FC (2017) Aluminum enhances growth and sugar concentration, alters macronutrient status and regulates the expression of NAC transcription factors in rice. Front Plant Sci8:73.
Morrissey J, Baxter IR, Lee J, Li L, Lahner B, Grotz N et al (2009) The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell 21:3326–3338. doi: 10.1105/tpc.109.069401
MP Ippolito, Fasciano C, d’Aquino L, Tommasi F (2011) Responses ofantioxidant systems to lanthanum nitrate treatments in tomato plants during drought stress. Plant Biosyst 145(1):248–252, DOI: 10.1080/11263504.2010.509937
Muhammad N, Zvobgo G, Zhang GP (2019) A review: The beneficial effects and possible mechanisms of aluminum on plant growth in acidic soil. J Integr Agric 18:1518–1528.
Mukherjee B, Patra B, Mahapatra S, Banerjee P, Tiwari A, Chatterjee M (2004) Vanadium—an element of atypical biological significance. Toxicol Lett 150(2):135–143.
Muneer S, Jeong BR (2015) Proteomic analysis of salt-stress responsive proteins in roots of tomato (Lycopersicon esculentum L.) plants towards silicon efficiency. Plant Growth Regul http://doi.org/10.1007/1072 5-015-0045
Mustafa H, Ilyas N, Akhtar N, Raja NI, Zainab T, Shah T, Ahmad A, Ahmad P (2021) Biosynthesis and characterization of titanium dioxide nanoparticles and its effects along with calcium phosphate on physicochemical attributes of wheat under drought stress. Ecotoxicol Environ Saf 223:112519.
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216.
Nagpal NK (2004) Technical Report, Water Quality Guidelines for Cobalt. Water Quality—Standards—British Columbia. Ministry of Water, Land and Air Protection, 6
Nascimento AM, Assis FAD, Moraes JC, Silveira FAD, Pio LAS, Botelho FBS (2019) Silicon and methyl jasmonate in the vegetative development and genetic stability of rice. Acta Sci Agron 41. https://doi.org/10.4025/actasciagron.v41i1.36483.
Nawaz F, Ahmad R, Ashraf MY, Waraich EA, Khan SZ (2015) Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol Environ Saf 113:191–200.
Negishi T, Oshima K, Hattori M, Kanai M, Mano S, Nishimura M, Yoshida K (2012) Tonoplast-and plasma membrane-localized aquaporin-family transporters in blue hydrangea sepals of aluminum hyperaccumulating plant. PLoSONE 7(8):e43189. https://doi.org/10.1371/journal.pone.0043189
Nguyen HC, Lin KH, Ho SL, Chiang CM, Yang CM (2018) Enhancing the abiotic stress tolerance of plants: from chemical treatment to biotechnological approaches. Physiol Plant 164(4):452–466.
Norman DJ, Chen J (2011) Effect of foliar application of titanium dioxide on bacterial blight of geranium and Xanthomonas leaf spot of poinsettia.Hortscience 46:426–428.
Owolade OF, Ogunleti DO (2008) Effects of titanium dioxide on the diseases, development and yield of edible cowpea. J Plant Protect Res 48:
329. –335. doi: 10.2478/v10045-008-0042-5
Pan T, Zhang J, He L, Hafeez A, Ning C, Cai K (2021) Silicon enhances plant resistance of rice against submergence stress. Plants 10:767.
Pang X, Wang DH, Xing XY, Peng A, Zhang FS, Li CJ (2002) Effect of La3+ on the Activities of Antioxidant Enzymes in Wheat Seedlings under Lead Stress in Solution Culture. Chemosphere47:1033–1039.
Pavlovic J, Kostic L, Bosnic P, Kirkby EA, Nikolic M (2021) Interactions of Silicon with Essential and Beneficial Elements in Plants. Front Plant Sci 12:697592. doi:10.3389/fpls.2021.697592.
Pedas P, Husted S (2009) Zinc transport mediated by barley ZIP proteins are induced by low pH. Plant Signal Behav 4:842–845. doi: 10.4161/psb.4.9.9375
Peralta-Videa JR, Hernandez-Viezcas JA, Zhao L, Diaz BC, Ge Y, Priester J H, Holden PA, Gardea-Torresdey JL (2014) Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol Biochem 80:128 − 135.
Piccolo E, Ceccanti C, Guidi L, Landi M (2021) Role of beneficial elements in plants: implications for the photosynthetic process. Photosynthetica 59(2):349–360. doi: 10.32615/ps.2021.032
Pilon-Smits EA, Quinn CF, Tapken W, Malagoli M, Schiavon M (2009) Physiological functions of beneficial elements. Curr Opin Plant Biol 12(3):267–74. doi: 10.1016/j.pbi.2009.04.009.
Pontigo S, Ribera A, Gianfreda L, de la Luz Mora M, Nikolic M, Cartes P (2015) Silicon in vascular plants: uptake, transport and its influence on mineral stress under acidic conditions. Planta 242(1):23–37.
Qi M, Liu Y, Li T (2013) Nano-TiO2 improve the photosynthesis of tomato leaves under mild heat stress. Biol Trace Elem Res 156(1):323–328.
Quan R, Wang J, Yang D, Zhang H, Zhang Z, Huang R (2017) EIN3 and SOS2 synergistically modulate plant salt tolerance. Sci Rep 7: 44637.
Quintero FJ, Ohta M, Shi H, Zhu JK, Pardo JM (2002) Reconstitution in yeast of the Arabidopsis SOS signalling pathway for Na+ homeostasis. Proc Natl Acad Sci USA 99(13):9061–9066.
Ramírez-Olvera SM, Trejo-Téllez LI, García-Morales S, Pérez-Sato JA, Gómez-Merino FC (2018) Cerium enhances germination and shoot growth, and alters mineral nutrient concentration in rice. PLoS One 13(3):e0194691.
Ramos SJ, Dinali GS, Oliveira C, Martins GC, Moreira CG, Siqueira JO et al (2016) Rare Earth Elements in the soil environment. Curr Pollut Rep 2(1):28–50.
Ranjan A, Sinha R, Bala M, Pareek A, Singla-Pareek SL, Singh AK (2021) Silicon-mediated abiotic and biotic stress mitigation in plants: Underlying mechanisms and potential for stress resilient agriculture. Plant Physiol Biochem163: 15–25.
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, Oberson A (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349:121–156.
Rios JJ, Blasco B, Rosales MA, Sanchez-Rodriguez E, Leyva R, Cervilla LM, Romero L, Ruiz JM (2010) Response of nitrogen metabolism in lettuce plants subjected to different doses and forms of selenium. J Sci Food Agric 90(11):1914–1919.
Rizwan M, Ali S, Rehman MZ, Malik S, Adrees M, Qayyum MF, Ahmad P (2019) Effect of foliar applications of silicon and titanium dioxide nanoparticles on growth, oxidative stress, and cadmium accumulation by rice (Oryza sativa). Acta Physiol Plant 41(3):1–12.
Rodrigues FA, Datnoff LE, Korndorfer GH, Seebold KW, Rush MC (2001) Effect of silicon and host resistance on sheath blight development in
rice. Plant Dis 85:27–832. doi: 10.1094/PDIS.2001.85.8.827
Rojek J, Kozieradzka-Kiszkurno M, Kapusta M, Aksmann A, Jacewicz D, Drzeżdżon J, Tesmar A, Żamojć K, Wyrzykowski D, Chmurzyński L (2019) The effect of vanadium (IV) complexes on development of Arabidopsis thaliana subjected to H2O2-induced stress. Funct Plant Biol 46(10):942–961.
Saito S, Uozumi N (2020) Calcium-regulated phosphorylation systems controlling uptake and balance of plant nutrients. Front Plant Sci 11:44.
Sanchez-Barrena MJ, Moreno-P´erez S, Angulo I, Mart´ınez-Ripoll M, Albert A (2007) The complex between SOS3 and SOS2 regulatory domain from Arabidopsis thaliana: cloning, expression, purification, crystallization and preliminary X-ray analysis. Acta cryst Section F 63(7):568–570.
Sarkar B, De AK, Saha I, Ghosh A, Debnath SC, Adak MK (2021) Amelioration with titanium dioxide nanoparticle for regulation of oxidative stress in maize (Zea mays L.). J Microbiol Biotechnol Food Sci 9(2):320–329.
Sattar A, Naveed M, Ali M, Zahir ZA, Nadeem SM, Yaseen M, Meena VS, Farooq M, Singh R, Rahman M, Meena HN (2019) Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl Soil Ecol 133:146–159.
Satti SH, Raja NI, Javed B, Akram A, Mashwani Z-u-R, Ahmad MSet al.(2021) Titanium dioxide nanoparticles elicited agromorphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE 16(2):e0246880. https://doi.org/ 10.1371/journal.pone.0246880
Sayed S, Gadallah M (2014) Effects of silicon on Zea mays plants exposed to water and oxygen deficiency. Russ J Plant Physiol 61:460–466.
Schautmann H, WenzelAA (2002) The accumulation of ABA in plants during wilting under stress condition. J Plant Nutr 3:283
Schiavon M, Pilon M, Malagoli M, Pilon-Smits EA (2015) Exploring the importance of sulfate transporters and ATP sulphurylases for selenium hyperaccumulation—a comparison of Stanleya pinnata and Brassica juncea. Front Plant Sci 6:2. doi: 10.3389/fpls.2015.00002.
Schlorke D, Flemmig J, Birkemeyer C, Arnhold J (2016) Formation of cyanogen iodide by lactoperoxidase. J Inorg Biochem 154:35–41.
Seebold KW, Datnoff LE, Correa-Victoria FJ, Kucharek TA, Snyder GH (2004) Effects of silicon and fungicides on the control of leaf and neck blast in upland rice. Plant Dis 88:253–258. doi: 10.1094/Pdis.2004.88.3.253
Shaw G, Scott LK, Kinnersley RP (2007) Sorption of caesium, iodine and sulphur in solution to the adaxial leaf surface of broad bean (Vicia faba L.).
Environ Exp Bot 59:361–370. doi: 10.1016/j.envexpbot.2006.04.008
Shi P, Huang Z, Chen G (2006) Influence of lanthanum on theaccumulation of trace elements in chloroplasts of cucumber seedling leaves. Biol Trace Elem Res 2:181–188. doi: 10.1385/BTER:109:2:181
Shi Q, Bao Z, Zhu Z, He Y, Qian Q, Yu J (2005) Silicon-mediated alleviation of Mn toxicity in Cucumis sativus in relation to activities of superoxide dismutase and ascorbate peroxidase. Phytochemistry 66:1551–1559.
Shyam R, Aery NC (2012) Effect of cerium on growth, dry matter production, biochemical constituents and enzymatic activities of cowpea plants [Vigna unguiculata (L.) Walp.]. J Soil Sci Plant Nutr 12(1):1–14.
Sieprawska A, Kornaś A, Filek M (2015) Involvement of selenium in protective mechanisms of plants under environmental stress conditions – review. Acta Biol Crac Ser Bot 57:1–12
Silva-Moreno E, Brito-Echeverría J, López M, Ríos J, Balic I, CamposVargas R et al (2016) Effect of cuticular waxes compounds from table grapes on growth, germination and gene expression in Botrytis cinerea. World J Microbiol Biotechnol 32:74. doi: 10.1007/s11274-016-2041-4
Singh AK, Singh A, Singh AK, Shamim MD, Vikram P, Singh S, Chaturvedi G (2012) Application of potassium iodide as a new agent for screening of drought tolerance upland rice genotypes at flowering stage. Plant Knowledge J 1: 25–32. Küpper FC, Carrano CJ et al. (2019) Key aspects of the iodine metabolism in brown algae: A brief critical review. Metallomic 11:756–764.
Singh J, Lee BK (2016) Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): a possible mechanism for the removal of Cd from the contaminated soil. J Environ Manage 170:88–96.
Singh S, Prasad SM, Sharma S, Dubey NK, Ramawat N, Prasad R (2020) Silicon and nitric oxide-mediated mechanisms of cadmium toxicity alleviation in wheat seedlings. Physiol Plant. https://doi.org/10.1111/ppl.13065
Singh S, Tripathi DK, Singh S, Sharma S, Dubey NK, Chauhan DK, Vaculík M (2017) Toxicity of aluminium on various levels of plant cells and organism: A review. Environ Exp Bot 137:177–193.
Singh S, Tripathi DK, Singh S, Sharma S, Dubey NK, Chauhan DK, Vaculik M (2017) Toxicity of aluminium on various levels of plant cells and organism: a review. Environ Exp Bot 137:177–193.
Smolen S, Skoczylas Ł, Ledwozyw-SmolenI, Rakoczy R, Kopec A, Piątkowska E, Biezanowska-Kope R, Koronowicz A, Kapusta-Duch J (2016) Biofortification of carrot (Daucus carota L.) with iodine and selenium in a field experiment. Front Plant Sci 7:730.
Smoleń S, Wierzbińska J, Sady W, Kołton A, Wiszniewska A, Liszka-Skoczylas M (2015c) Iodine biofortification with additional application of salicylic acid affects yield and selected parameters of chemical composition of tomato fruits (Solanum lycopersicum L.). Sci Hortic 188:89–96. doi: 10.1016/j.scienta.2015.03.023
Song Z, Shao H, Huang H, Shen Y, Wang L, Wu F et al (2017) Overexpression of the phosphate transporter gene OsPT8 improves the Pi and selenium contents in Nicotiana tabacum. Environ Exp Bot 137:158–165.
Sosnowski J, Truba M, Redzik P, Toczyska E (2020) The effect of growth regulator Tytanit dose on Medicago x varia T. Martin and Trifolium pratense L. yield and nutritional value. Saudi J Biol Sci 27(11):2890–2901.
Soukup M, Martinka M, Bosnić D, Čaplovičová M, Elbaum R, Lux A (2017) Formation of silica aggregates in sorghum root endodermis is predetermined by cell wall architecture and development. Ann Bot 120(5):739–753.
Soundararajan P, Manivannan A, Ko CH, Muneer S, Jeong BR (2017) Leaf physiological and proteomic analysis to elucidate silicon induced adaptive response under salt stress in Rosa hybrida ‘Rock Fire’. Int J Mol Sci 18(8):1768.
Souri Z, Khanna K, Karimi N, Ahmad P (2020) Silicon and plants: current knowledge and future prospects. J Plant Growth Regul 40(3) 906–925.
Subbarao GV, Wheeler RM, Stutte GW (2000) Feasibility of substituting sodium for potassium in crop plants for advanced life support systems. Life support biosph sci 7(3):225–32.
Sun L, Zhang M, Liu X, Mao Q, Shi C, Kochian LV, Liao H (2020) Aluminium is essential for root growth and development of tea plants (Camellia sinensis). J Integr Plant Biol 62: 984–997.
Sutini W, Augustien N, Purwanto DA, Muslihatin W (2019) The production of cinnamic acid secondary metabolites through in vitro culture of callus Camellia sinensis L with the elicitor of cobalt metal ions. In AIP Conference Proceedings, AIP Publishing LLC, 1: 030028.
Thakral V, Bhat JA, Kumar N, Myaka B, Sudhakaran S, Patil G, Sonah H, Shivaraj SM, Deshmukh R (2021) Role of silicon under contrasting biotic and abiotic stress conditions provides benefits for climate smart cropping. Environ Exp Bot 189:104545. https://doi.org/10.1016/j.envexpbot.2021.104545
Tomioka R, Oda A, Takenaka C (2005) Root growth enhancement by rhizospheric aluminum treatment in Quercus serrata Thunb. seedlings. J For Res 10(4):319–324.
Tripathi D, Mani V, Pal RP (2018) Vanadium in biosphere and its role in biological processes. Biol Trace Elem Res 186(1):52–67.
Tripathi DK, Rai P, Guerriero G, Sharma S, Corpas FJ, Singh VP (2021b) Silicon induces adventitious root formation in rice under arsenate stress with involvement of nitric oxide and indole-3-acetic acid. J Exp Bot 72(12):4457–4471.
Tripathi DK, Vishwakarma K, Singh VP, Prakash V, Sharma S, Muneer S, Nikolic M, Deshmukh R, Vaculík M, Corpas FJ (2021a) Silicon crosstalk with reactive oxygen species, phytohormones and other signaling molecules. J Hazard Mater 408:124820.
Vachirapatama N, Jirakiattiku Y, Dicinoski GW, Townsend AT, Haddad PR (2011) Effect of vanadium on plant growth and its accumulation in plant tissues. Songklanakarin J Sci Technol 33(3):255–261.
Vaculík M, Lukačová Z, Bokor B, Martinka M, Tripathi DK, Lux A (2020) Alleviation mechanisms of metal (loid) stress in plants by silicon: a review. J Exp Bot 71(21): 6744–6757.
Valentinuzzi F, Cologna K, Pii Y, Mimmo T, Cesco S (2017) Assessment of silicon biofortification and its effect on the content of bioactive compounds in strawberry (Fragaria × ananassa’Elsanta’) fruits. In VIII International Symposium on Mineral Nutrition of Fruit Crops 1217 (307–312).
Val-Torregrosa B, Bundó M, San-Segundo B (2021) Crosstalk between Nutrient Signalling Pathways and Immune Responses in Rice. Agriculture 11(8):747.
Vatansever R, Ozyigit II, Filiz E (2017) Essential and beneficial trace elements in plants, and their transport in roots: a review. Appl Biochem Biotechnol 181(1):464–482.
Wadas W, Kalinowski K (2018) Effect of Tytanit® on the dry matter and microelement contents in potato tuber. J Cent Eur Agric 19(3):557–570.
Wang L, Fan XW, Pan JL, Huang ZB, Li YZ (2015) Physiological characterization of maize tolerance to low dose of aluminum, highlighted by promoted leaf growth. Planta 242:1391–1403.
Wang L, Li J, Zhou Q, Yang G, Ding XL, Li X et al (2014) Rare earthelements activate endocytosis in plant cells. Proc Natl Acad Sci USA 111:12936–12941. doi: 10.1073/pnas.1413376111
Wang M, Gao L, Dong S, Sun Y, Shen Q, Guo S (2017) Role of Silicon on Plant–Pathogen Interactions. Front Plant Sci 8:701. doi: 10.3389/fpls.2017.00701
Wang M, Wang R, Mur LAJ, Ruan J, Shen Q, Guo S (2021) Functions of silicon in plant drought stress responses. Hortic Res 8. https://doi.org/10.1038/s41438-021-00681-1
Wang Y, Li R, Li D, Jia X, Zhou D, Li Jet al. (2017) NIP1; 2 is a plasma membrane-localized transporter mediating aluminum uptake, translocation, and tolerance in Arabidopsis. Proc Nat Acad Sci 114(19):5047–5052.
Wang Y, Stass A, Horst WJ (2004) Apoplastic binding of aluminum is involved in silicon-induced amelioration of aluminum toxicity in maize. Plant Physiol 136:3762–3770.
Wang Y, Zhang B, Jiang D, Chen G (2019) Silicon improves photosynthetic performance by optimizing thylakoid membrane protein components in rice under drought stress. Environ Exp Bot 158:117–124.
Wang YD, Wang X, Won YS (2012) Proteomics analysis reveals multiple regulatory mechanisms in response to selenium in rice. J Proteome 75:1849–1866
Wei J, Zou Y, Li P, Yuan X (2020) Titanium dioxide nanoparticles promote root growth by interfering with auxin pathways in Arabidopsis thaliana. Phyton (Buenos Aires) 89(4): 883–891.
White PJ (2016) Selenium accumulation by plants. Ann Bot 117(2):217–235.
Wu Z, Yin X, Bañuelos GS, Lin Z-Q,Zhu Z, Liu Y, Yuan L, Li M (2016)Effect of Selenium on Controlof Postharvest Gray Mold of TomatoFruit and the Possible Mechanisms Involved. Front Microbiol 6:1441.doi: 10.3389/fmicb.2015.01441
Wu ZZ, Yang JY, Zhang YX, Wang CQ, Guo SS, Yu YQ (2021) Growth responses, accumulation, translocation and distribution of vanadium in tobacco and its potential in phytoremediation. Ecotoxicol Environ Saf 207:111297.
Xi JJ, Chen HY, Bai WP, Yang RC, Yang PZ, Chen RJ, Hu TM, Wang SM (2018) Sodium-Related Adaptations to Drought: New Insights From the Xerophyte Plant Zygophyllum xanthoxylum. Front Plant Sci 9:1678.
Xia J, Yamaji N, Kasai T, Ma JF (2010) Plasma membrane-localized transporter for aluminum in rice. Proc Nat Acad Sci 107(43):18381–18385.
Xia Y, Gao QM, Yu K, Lapchyk L, Navarre D, Hildebrand D et al (2009) An intact cuticle in distal tissues is essential for the induction of systemic acquired resistance in plants. Cell Host Microbe 5:151–165. doi: 10.1016/j.chom.2009.01.001
Xie, ZB, Zhu JG, Chu HY, Zhang YL, Zeng Q, Ma HL, Cao ZH (2002) Effect of lanthanum on rice production, nutrient uptake, and distribution. J Plant Nutr 25(10):2315–2331.
Xu CM, Zhao B, Wang XD et al (2007) Lanthanum relieves salinity-induced oxidative stress in Saussurea involucrata. Biol Plant 51:567–570. https://doi.org/ 10.1007 /s10535-007-0124-7
Xu N, Zhang H H, Zhong H X, Wu Y, Li J, Xin L, Yin Z, Zhu W, Qu Y, Sun G (2018) The response of photosynthetic functions of F1 cutting seedlings from Physocarpus amurensis Maxim (♀) × Physocarpus opulifolius “diabolo” (♂) and the parental leaves to salt stress. Front Plant Sci 9:714.
Yamaji N, Mitatni N, Ma JF (2008) A transporter regulating silicon distribution in rice shoots. Plant Cell 20:1381–1389.
Yamaji N, Sakurai G, Mitani-Ueno N, Ma JF (2015) Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proc Nat Acad Sci 112(36):11401–11406.
Yang H, Xiong Z, Xu Z, Liu R (2021) Interactive Effects of Lanthanum and Calcium on Cadmium Accumulation in Wheat with Special Reference to TaNramp5 Expression Regulated by Calmodulin. J Agric Food Chem 69(24):6870–6878.
Yang H, Xu Z, Liu R, Xiong Z (2019) Lanthanum reduces the cadmium accumulation by suppressing expression of transporter genes involved in cadmium uptake and translocation in wheat. Plant Soil 441(1):235–252.
Yao KS, Wang DY, Ho WY, Yan JJ, Tzeng KC (2007) Photocatalytic bactericidal effect of TiO2 thin film on plant pathogens. Surf Coat Technol 201:6886–6888. doi: 10.1016/j.surfcoat.2006.09.068
Yin J, Jia J, Lian Z, Hu Y, Guo J, Huo H et al (2019) Silicon enhances the salt tolerance of cucumber through increasing polyamine accumulation and decreasing oxidative damage. Ecotoxicol Environ Saf 169:8–17.
Yongsheng G (2005) Effects of La3+ on Antioxidant System in Wheat Seedling Leaves under Salt Stress. Journal-chinese rare earth society-chinese edition 23(4), 490.
Yoon Y, Seo DH, Shin H, Kim HJ, Kim CM, Jang G (2020) The role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants. Agronomy 10(6): 788.
Younis AA, Khattab H, Emam MM (2020) Impacts of silicon and silicon nanoparticles on leaf ultrastructure and TaPIP1 and TaNIP2 gene expressions in heat stressed wheat seedlings. Biol Plant 64:343–352.
Zahedi H, Rad AHS, Moghadam HRT (2011) Effects of zeolite and selenium applications on some agronomic traits of three Canola cultivars under drought stress. Pesqui Agropecuária Trop 41(2):179–185.
Zahoor A, Waraich EA, Barutcular C, Hossain A, Erman M, Çig F, Gharib H, El Sabagh A (2020) Enhancing drought tolerance in wheat through improving morphophysiological and antioxidants activities of plants by the supplementation of foliar silicon. Phyton 89:529.
Zargar SJ, Mahajan R, Bhat JA, Nazir M, Deshmukh R (2019) Role of silicon in plant stress tolerance: opportunities to achieve a sustainable cropping system. 3 Biotech 9:73. https://doi.org/10.1007/s13205-019-1613-z
Zhang L, Hu B, Li W, Che R, Deng K, Li H (2014) OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol 201(4):1183–1191.
Zhao H, Zhou Q, Zhou M, Li C, Gong X, Liu C, et al (2012) Magnesium deficiency results in damage of nitrogen and carbon cross-talk of maize and improvement by cerium addition. Biol Trace Elem Res 148(1):102–109.
Zhao XQ, Mitani N, Yamaji N, Shen RF, Ma JF (2010) Involvement of silicon influx transporter OsNIP2; 1 in selenite uptake in rice. Plant Physiol 153(4):1871–1877.
Zhong Y, Chen J (2020) Ameliorative effects of Lanthanum (III) on Copper (III) stressed rice (Oryza sativa) and its molecular mechanism revealed by transcriptome profiling. Plant Physiol Biochem 152:184–193.
Zhou H, Wu H, Zhang F, Su Y, Guan W, Xie Y, Giraldo JP, Shen W (2021) Molecular basis of cerium oxide nanoparticle enhancement of rice salt tolerance and yield. Environ Sci Nano 8(11):3294–3311.
Zhou J, Li Z, Zhou T, Xin Z, Wu L, Luo Y, Christie P (2020) Aluminum toxicity decreases the phytoextraction capability by cadmium/zinc hyper accumulator Sedum plumbizincicola in acid soils. Sci Total Environ 711:134591.
Zhu CQ, Cao XC, Zhu LF, Hu WJ, Hu AY, Abliz B (2019) Boron reduces cell wall aluminum content in rice (Oryza sativa) roots by decreasing H2O2 accumulation. Plant Physiol Biochem 138:80–90.
Zhu H, Shipp E, Sanchez RJ, Liba A, Stine JE, Hart PJ, Gralla EB, Nersissian AM, Valentine JS (2000) Cobalt(2+) binding to human and tomato copper chaperone for superoxide dismutase: implications for the metal ion transfer mechanism. Biochemistry 39(18):5413–21. doi: 10.1021/bi992727+
Zhu H,Shipp E, Sanchez RJ, Liba A, Stine JE, Hart PJ, Gralla EB, Nersissian AM, Valentine JS (2000) Cobalt (2) binding to tomato copper chaperone for superoxide dismutase:implications for the metal ion transfer mechanism. Biochemistry 39(18):5413–21. doi: 10.1021/bi992727+.
Zhu Y, Jiang X, Zhang J, He Y, Zhu X, Zhou X, Gong H, Yin J, Liu Y (2020) Silicon confers cucumber resistance to salinity stress through regulation of proline and cytokinins. Plant Physiol Biochem 156:209–220.
Author information
Authors and Affiliations
Contributions
Rajesh Kumar Singhal and Shah Fahad designed the study; Pawan Kumar., Prince Choyal., Talha Javed., Dinesh Jinger., Prabha Singh., Debanjana Saha. and Prathibha MD., wrote the manuscript; Bandana Bose., Akash H. and N.K. Gupta., help in diagrams. Rekha Sodani., Devanshu Dev., Dalpat Lal Suthar., Ke Liu., Matthew Tom Harrison., Adnan Noor Shah., Taufiq Nawaz and Shah Saud revised the manuscript; Supervision Rajesh Kumar Singhal. Ethics Approval: All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Consent for publication
Not applicable.
Additional information
Communicated by Tariq Aftab .
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singhal, R.K., Fahad, S., Kumar, P. et al. Beneficial elements: New Players in improving nutrient use efficiency and abiotic stress tolerance. Plant Growth Regul 100, 237–265 (2023). https://doi.org/10.1007/s10725-022-00843-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-022-00843-8