Abstract
Drought is one of the major abiotic stress that limits crops yield. Here we investigated photosynthetic performance in flag leaves of a hybrid rice (Oryza sativa. L) LYPJ exposed to prolonged drought stress during leaf senescence. Our results highlighted an architecture remodeling of thylakoid membrane proteins characterizing by amplified PSI-LHCII and PSII dimers supercomplexes and LHCII assemblies played crucial role in drought resistance. Coordination of PSII and LHCII protein phosphorylation, thermal dissipation, and CEF around PSI sustained photo-equilibrium when LEF was suppressed by decreased Cytb6f complex. CO2 limitation resulted in degradation of Rubisco content and loss of Rubisco activity, but up-regulated the enzymes responsible for C4 photosynthesis pathway. It was collectively pointed to that redox poise was a pivotal and ATP/NADPH ratio was probably a linker of light reaction, CO2 assimilation and other metabolism pathways. In general, our results provided additional insights into the role of thylakoid membrane plasticity in long-term ambient acclimation and complex coordination of drought responses in rice LYPJ during the senescence stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In oxygenic photosynthetic organisms, the thylakoid membrane system harbors the photosynthetic electron transfer chain that converts solar energy absorbed by pigments into chemical energy, in the form of ATP and reducing equivalents NADPH mainly consumed in CO2 fixation (Garab 2014). For higher plants, such effective photosynthetic machinery is employed by photosystem I (PSI) and photosystem II (PSII), which act in series under sophisticated photo-equilibrium to maintain appropriated ATP/NADPH ratio. It is well established that the serially cooperating photosystems have lateral heterogeneity in their distribution within the thylakoid membranes, PSII predominantly localize in appressed regions, i.e. grana stacks, PSI and ATP synthase are confined to the non-appressed, including grana ends, grana margins and stromal lamellae, while Cytb6f complex is find unevenly in both, with small electron carriers, plastoquinone (PQ) and plastocyanin (PC) diffused back and forth (Kirchhoff 2014; Pribil et al. 2014). Both PSI and PSII are multisubunit membrane–protein complexes laterally associated with respective peripheral antenna systems, namely pigments-binding light harvesting complexes (LHCs). Actually, the photosynthetic apparatus is more complicated in nature as a result of hierarchical organizations into various supercomplexes and megacomplexes, along with membrane fluidity, offering the flexibility to allow protein reorganization and redistribution (Kirchhoff 2014; Pribil et al. 2014; Garab 2014). Such plasticity is vital for plants adaptation to ambient changes, maintaining essential photosynthetic rate when illumination or CO2 is altered and performing effective protection or repair processes to minimize the photodamage induced by excess energy (Suorsa et al. 2015).
Plentiful investigations on structure and dynamics of the thylakoid membrane under various conditions have been carried out to reveal the underlying regulative functions. Most prominently, structural and functional plasticity of LHCII plays important regulatory roles in photosynthetic acclimation through mediating state transition by phosphorylation/dephosphorylation (Garab 2014). This process is initiated when the redox poise of the PQ pool is perturbed by unequal excitation of PSII and PSI. Preferential excitation of PSII results in protonation of PQ and facilitates the interaction of PQH2 and the Q0 site of Cytb6f complex, thereupon activating the STN7 kinase responsible for LHCII phosphorylation, which consequently disassociates from PSII and exhibits higher affinity to PSI (state II) (Goldschmidt-Clermont and Bassi 2015). Conversely, when PQ is deprotonated, STN7 becomes inactive and LHCII is dephosphorylated by the constitutively active TAP38/PPH1 phosphatase, thus recombined with PSII (state I). It is noteworthy that state transition was considered as a short-term response while changes in PSII/PSI stoichiometry were cardinal in long-term acclimation (Chow 1990). However, other evidences pointed out that LHCII trimer served as an intrinsic light harvester for PSI, and state transition was actually redox-controlled dynamics in PSII-LHCII, LHCII pool and PSI-LHCII (Galka et al. 2012; Grieco et al. 2015). Furthermore, the PSII repair cycle, another crucial photoprotective process, also relies on phosphorylation-dependent disassembly of PSII-LHCII supercomplexes. The disassembly leads to a partial destacking of grana and swelling of lumen, which favors PC-mediated electron transport between Ctyb6f and PSI, mobilization of photodamaged D1 proteins to stroma lamellae and their accessibility to proteases, and of course LHCII migration in state transition (Puthiyaveetil et al. 2014; Giovanardia et al. 2018). Compared with the rapid turnover of PSII, PSI seems to be more vulnerable to photodamage as its recovery is much slower. To address the possible redox pressure at PSI such as accepter limitation caused by CO2 dearth, plants have evolved an additional redox-controlled electron transport pathway independent of state transition, cyclic electron flow (CEF) (Takahashi et al. 2013). Two CEF systems in plants have been proposed: the chloroplast NADH dehydrogenase-like (NDH) complex-dependent pathway and the PGR5-PGRL1 protein-dependent pathway. The latter has been indicated the dominating CEF that generates additional ATP and sustains the proton gradient (ΔpH) across thylakoid membrane (Shikanai 2016). However, it has been suggested that the NDH-dependent CEF pathway also contributed to proton motive force (pmf) for ATP synthesis at low light condition, and was activated by H2O2 (Strand et al. 2015; Yamori et al. 2015). Notably, lumenal acidification ascribed to ΔpH is also vital for protective mechanisms in PSII, the non-photochemical quenching (NPQ) which depends on ΔpH and is facilitated by the PsbS protein and activation of the xanthophyll cycle (Sylak-Glassman et al. 2014). It has been suggested PsbS protonation promoted disassembly and aggregation of LHCII by interaction with Lhcb1(Correa-Galvis et al. 2016). Besides, alternative electron transport activities mediated by diverse plastid terminal oxidase also act as a safety valve for excess excitation energy (Laureau et al. 2013). Nevertheless, intriguingly, PSI with damaged FeS clusters could conduct a self-rescue transformation, where it functions as a powerful non-photochemical energy quencher to alleviate the photoinhibition caused by excess electrons from PSII (Tiwari et al. 2016). Briefly, when linear electron transport is precluded, redox state of PQ initiates relevant protective mechanisms, concomitantly with the suppression of Cytb6f complex triggered by lumenal acidification and co-regulation of ATP synthase to modulate the photo-equilibrium, basing on alteration of thylakoid membrane architecture. It has been collectively pointed to a functional integrity of PSI and PSII in spite of their distinct photoprotective manners (Schottler et al. 2015; Yamori et al. 2011).
So far, dynamics of the thylakoid membrane have been mostly investigated under different light conditions and results mainly elucidated short-term acclimation, while much less information has been known in other cases, especially in long-term stress (Kaiser et al. 2015; Ruban and Johnson 2015). The dynamics of thylakoid membranes have been detected under cadmium stress and iron deficiency (Basa et al. 2014), salt stress (Shu et al. 2015) and drought stress (Chen et al. 2016). Among various abiotic and biotic stress, drought is one of the major stress factors that limit crop yield (Cominelli et al. 2013). Drought response network consists of multiple morphological, biochemical and physiological processes. The effects largely depend on duration and severity of drought as well as genotypes and developmental stages of plants (Barnabas et al. 2008). Long-term drought responses of crops have been emphasized on proton and electron transport (Zivcak et al. 2014), protein expression and chloroplast ultrastructure (Vassileva et al. 2012) and enzymatic antioxidant capacity (Huseynova 2012). However, few studies have ascertained the constitutive dynamics of thylakoid membranes to long-term drought stress in rice plants, which is prone to suffer from frequent water deficit. Here, we hypothesized that the re-architecture of thylakoid membrane played a central role in long-term photosynthetic acclimation to drought stress. In view of this, we employed a widely cultured hybrid rice ‘Liangyoupeijiu’ (LYPJ, Oryza. sativa L.), well known for its high yield and good grain quality in China and South Asia, and monitored the photosynthetic properties of its flag leaf, the pivotal contributor of grain filling under prolonged drought stress (Yu et al. 1997). Our previous work showed that flag leaves of LYPJ maintains relatively high maximum quantum yield of primary photochemistry (only 0.1–0.2% lower than well-watered group) and stable end electron accepter reduction in PSI (98–99% of that in control and not-fall, but-rise on 35 days) under drought stress during leaf senescence (Wang et al. 2017a). It was reliable to underline that drought resistance of LYPJ underwent remodeling of photosynthetic apparatus. In the present work, we extended the analyses by examining the effects of prolonged drought stress on thylakoid membrane component and function to probe elucidation of the antecedent observations. Additionally, given the inevitable CO2 limitation caused by stomatal closure when encountering water deficit, coordination of photo-carbon balance was also analyzed through assays the activity of photosynthetic enzymes relevant to carbon fixation.
Materials and methods
Plant materials and stress treatment
The seeds of rice (O. sativa L.) genotype LYP9 were obtained from the Institute of Agricultural Sciences of Jiangsu Nanjing, China. The experiments were conducted during 10 August (first flag leaves expansion) to 26 September (the harvest time) in 2014 and 2015 in Nanjing Normal University, Jiangsu Province, China. According to Wang et al. (2017b), seeds were surface sterilized in 30% H2O2 for 10 min, followed by three thorough washing with sterile distilled water. Subsequently, the seeds were soaked in water for 72 h in dark to induce germination. Seedlings were grown in a climate chamber (16/8 h light [350 μmol m−2 s−1 photosynthetic photon flux density (PPFD PAR)]/dark periods, 30/25 °C and 80% relative humidity) until the four-leaf stage. Afterwards, the seedlings were transplanted into 12 plastic pots [two blocks, six pot (four plants each pot) for well-watered condition and six for drought treatment] containing soil from the paddy field. The loam soil was optimally fertilized with available nitrogen, phosphorus and potassium. The total N fertilizer applied was 3.375 t ha−1, with an N-P-K ratio of 1: 0.6: 0.6. Drought were initiated on rice with flag leaves fully expanded by withholding water based on appearing drought symptoms such as leaf angle change. Optimal water was supplied to DS plants until the soil was white and cracking, and leaves were wilting and withered. During the experimental period where the whole senescence stage of flag leaves was concluded, samples were collected at about 7-day intervals and stored at − 80 °C until analysis.
Chlorophyll a fluorescence
Measurements of chlorophyll (chl) fluorescence was performed with a pocket fluorometer (Handy PEA, Hansatech, UK) between 9:00 AM and 10:00 AM on sunny days in uppermost, fully expanded flag leaves of rice plants as described by Zhang et al. (2015). Leaf clips were dark adapted for 30 min (to obtain open reaction centers) and then irradiated with red light at 3000 μmol m−2 s−1 for 1 s. The professional PEA Plus and Biolyzer HP3 software were employed to calculated the fluorescence parameters. The formulas of fluorescence parameters were referred to those mentioned in Zhang et al. (2015). For the well-watered group and drought stress group, ten flag leaves in different plants for each group were made respectively.
Two-dimensional blue-native/SDS–polyacrylamide gel electrophoresis
Separation of thylakoid membrane complexes was according to the method of Järvi et al. (2011) with some modifications. Thylakoids were isolated from flag leaves ground in grinding buffer optimized for rice. The chlorophyll concentration of thylakoid fractions was determined as described in Porra et al. (1989). The thylakoid membrane was resuspended into ice-cold storage buffer [25 mM Bis–Tris/HCl (pH 7.0) and 20% (w/v) Glycerol] to a chlorophyll concentration of 1.0 mg ml−1. An equal volume of detergent solution was added to a final concentration of 1.5% (w/v) dodecyl-β-d-maltoside (DM) (Sigma, USA). Separation of the thylakoid membrane protein complexes by BN-PAGE was obtained by using 5–12.5% separation gel and 4% stacking gel with electrophoresis system suggested by Järvi et al. (2011). Two-dimensional Blue-native/SDS–polyacrylamide gel electrophoresis (2D BN/SDS-PAGE) was carried out as described by Wittig et al. (2006) with minor modifications. When the BN-PAGE was completed, gel strips of 3 mm wide were cut out from BN-PAGE lanes, and incubated with gentle shaking in solubilizing buffer [66 mM Na2CO3, 2% (w/v) SDS and 0.67% β-Mercaptoethanol] at 25 °C for 30 min, following with briefly rinsed in distilled water to remove excessive β-mercaptoethanol. Subsequently, the strips were attached to the top of the denaturing gel [15% (w/v) polyacrylamide and 6 M urea] and overlayed in SDS running buffer [25 mM Tris, 190 mM glycine and 0.1% SDS] containing 0.5% (w/v) agarose (Laemmli 1970). Following the electrophoresis, Protein spots were stained with Coomassie Brilliant Blue R-250.
Image acquisition and data analysis
The stained gels were scanned using the Image scanner III (GE Healthcare). The images were analyzed with Imagemaster™ 2D Platinum software version 6.0 (GE Healthcare). Three gels for each treatment from three independent experiments were used for the analysis. The intensities of spots were quantified based on the ratio of the volume of a single spot to the whole set of spots. Only spots with quantitative changes of at least 1.5-fold in abundance that were reproducible in three replicates were used for mass spectrometry.
MALDI-TOF/TOF MS analysis and database searching
Differentially expressed protein spots were extracted, processed, and analyzed using an ABI 4800 Proteomics Analyzer MALDI-TOF/TOF MS (Applied Biosystems, Foster City, CA). Internal mass calibration was performed using trypsin autodigestion products. Database searching and peptide mass fingerprinting (PMF) were performed using the Mascot server (http://www.matrixscience.com). Sequences data of differential expressed protein data were searched from NCBInr (http://www.ncbi.nlm.nih.gov) database, with the MOWSE search parameter criteria as follows: trypsin specificity, one missed cleavage site, cysteine carbamidom ethylation, acrylamide modified cysteine, methionine oxidation, similarity of pI, relative molecular mass specified, and minimum sequence coverage of 15%. All identified proteins had a MASCOT score greater than a significance level of p < 0.05.
SDS-PAGE and immunoblotting
The isolated thylakoid membrane solution was boiled with loading buffer [125 mM Tris–HCl (pH 6.8), 2 mM EDTA, 30 mM mercaptoethanol, 5% (w/v) SDS, 5% (w/v) sucrose and 0.02% bromophenol blue] for 5 min. For protein immunoblot analysis, isolated thylakoid protein samples with same amount of Chl were first separated by 12.5% SDS-PAGE, then transferred to PVDF membranes and immunoblotted with various antibodies (Agrisera, Sweden).
CO2 assimilation enzyme activity assays
The activities of phosphoenolpyruvate carboxylase (PEPC) and NADP-dependent malic enzyme (NADP-ME) were determined according to Ryšlavá et al. (2003). Total Rubisco activity was cited as described in Wang et al. (2017b).
Statistical analysis
All data were statistically analyzed with SPSS (SPSS Inc., version 18.0, Chicago, USA). The vertical bars represent standard deviations. Differences between treatment means were compared using the LSD at the 0.05 probability level. Single and double asterisks refer to significant difference between the drought and the control at 0.05 and 0.01, respectively.
Results
Effects of drought on PSII reaction centers
The amount of PSII reaction centers per excited cross section (RC/CSm) and the total number of active reaction center per absorption (10RC/ABS) were measured to dissect the status of PSII reaction centers of rice flag leaves with and without drought treatment. We found that there were significant decreases in both RC/CSm (Fig. 1A) and 10RC/ABS (Fig. 1B) with the onset of drought. Both total number of reaction centers and active reaction centers showed extremely significant differences in DS plants and WW plants after 21 days drought treatment. Decrease in active reaction centers was more pronounced in DS plants compared with that of total reaction centers. This phenomenon indicated that less energy was used to drive electron transport in this cultivar LYPJ in this drought condition. Hence, this energy would be dissipated by non-photochemical mechanisms.
Changes in the amount of PSII reaction centers per excited cross section (RC/CSm) (A) and the total number of active reaction center per absorption (10RC/ABS) (B) of flag leaves during senescence stage in high-yield hybrid rice LYPJ exposed to drought stress. Vertical bars represent standard deviations (n = 10). Single and double asterisks refer to significant difference between the drought and the control at 0.05 and 0.01, respectively
Effects of drought on PSII photochemical performance
The PSII photochemical performances of rice flag leaves of DS plants and WW plants were characterized by the phenomenological fluxes parameters, including absorption flux of photons per cross section (ABS/CSm), fluxes for trapping per cross section (TR0/CSm), potential electron transport per cross section (ET0/CSm) and dissipation per cross section (DI0/CSm). As evident by Fig. 2, we could notice that, when exposed to drought stress for 28 days, the only significant increase in ABS/CSm was concomitant with inhibition of ET0/CSm and increment in DI0/CSm. In general, ABS/CSm was relatively little affected by drought but ET0/CSm and DI0/CSm seemed to be down- and up-regulated in DS plants, respectively. The up-regulated dissipation indicators (DI0/CSm) in DS rice plants in this study suggested that energy dissipation was enhanced in order to protect LYPJ leaves in senescence stage from photo damage.
Changes in the phenomenological fluxes parameters approximated by FM. A Absorption flux of photons per cross section (ABS/CSm); B fluxes for trapping per cross section (TR0/CSm); C potential electron transport per cross section (ET0/CSm); D dissipation per cross section (DI0/CSm) of flag leaves during senescence stage in high-yield hybrid rice LYPJ exposed to drought stress. Vertical bars represent standard deviations (n = 10). Single and double asterisks refer to significant difference between the drought and the control at 0.05 and 0.01, respectively
Drought enhanced the thermal dissipation of PSII
Three thermal dissipation related parameters, thermal dissipation quantum yield (φD0), dissipation per cross section (DI0/CS0) and dissipation energy flux per PSII reaction center (DI0/RC), were presented in Fig. 3. When the treatment time extended, DI0/RC of DS plants increased gradually. Likewise, φD0 changed to a higher grade in DS plants and more pronounced when stress accumulated. These changes indicated a lower energy utilization efficiency in DS plants, and consistence with the former parameters such as DI0/CSm.
Changes in related thermal dissipation parameters. A Thermal dissipation quantum yield (φD0); B dissipation per cross section, approximated by Fo (DI0/CS0); C dissipation energy flux per PSII reaction center (DI0/RC) of flag leaves during senescence stage in high-yield hybrid rice LYPJ exposed to drought stress. Vertical bars represent standard deviations (n = 10). Single and double asterisks refer to significant difference between the drought and the control at 0.05 and 0.01, respectively
Effects of drought on thylakoid membrane proteins analyzed by BN/SDS-PAGE
To get an insight into the relationship between thylakoid regulation systems and thylakoid pigment-protein complex dynamics, the patterns of protein complexes from WW and DS rice plants were analyzed. After the first dimension separation by BN-PAGE, five major protein complex bands were observed (Fig. 4), defined as PSI core, ATPase/Cytb6f, CP43 less PSII core, LHCII trimer and LHCII monomer. The CP43 less PSII core complex was significantly down-regulated in DS plants. Another primarily change was that drought appeared to decrease the level of LHCII monomer, but with LHCII trimer barely affected. In light of our results, it appeared that PSII core was a preferential target of drought stress and the state of LHCII played an essential role in drought response. Intriguingly, drought induced distinct increment in several bands with high molecular mass. These supercomplexes were further analyzed by mass spectrometry, and subunits of both PSI and PSII were identified, as well as subunits of LHCII.
Information of identified protein spots by BN/SDS-PAGE (21 days-drought-treated rice plants) was shown in Table 1. Of these, six proteins in drought-intensified supercomplexes were found dominated, including CP43 (spots 48 and 50), D1 (spot 46), oxygen-evolving complex protein (OEC) 1 (spots 44 and 45), PsaA (spots 53 and 54), PsaB (spot 55) and chlorophyll a-b binding protein (spot 35). Formation of PSI-LHCII supercomplex and PSII core dimer could be speculated. In PSI core complex, the level of PsaD (spot 0) decreased in DS plants. For PSII core complex, core proteins—D1 (spot 20) and D2 (spots 14 and 15), reduced by drought stress, as well as photosystem II 44 kDa protein (spot 23) and OEE1 (spot 17). Additionally, antenna proteins Lhcb4 (CP29) (spot 41) and Lhcb5 (CP26) (spot 43) were amplified in stress condition while Lhcb1 (CP28) (spot 11) was diminished. The dominant subunit of ATPase—alpha (spot 50) and beta (spot 51), were found to be up-regulated in DS plants. Meanwhile, Cytb6 (spot 32), which was a critical subunit of Cytb6f complex, showed remarkable degradation. Last but not least, several non-photosynthetic proteins (spots 31, 36, 37, 38, 40, 42 and 49) located in chloroplast were found differentially expressed under drought stress, which might have regulatory roles.
Effects of drought on thylakoid membrane proteins analyzed by western blot
To identify drought stress responsive changes in thylakoid membrane proteins and testify the results obtained by BN/SDS-PAGE, we performed western blot analysis of subunits of PSI and PSII complexes as well as those of Cytb6f complexes and ATP synthase complexes in DS and WW rice plants (Fig. 5). Both PSI core (PsaA) and antenna subunits (Lhca1-2) pronounced little changes. Antenna subunits of PSII (Lhcb2, and Lhcb5) degraded at different levels. The compositions of OEC complex, PsbO, but not PsbP, PsbQ and PsbR, showed stability to drought stress (Fig. 5). Moreover, Cytbf declined with stress aggravated. Likewise, beta subunit of ATPase decreased when stress deteriorated but increment was observed in early (Fig. 5).
Effects of drought on photosynthetic enzyme activities
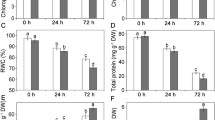
The activities of key enzymes in C3 pathway represented by Rubisco and C4 pathway characterized by PEPC and NADP-ME were assessed in WW and DS plants as evident by Fig. 6. Drought stress caused a decrease in the activity of Rubisco, with significant difference arose at the outset in our previous work (Wang et al. 2017b). At translation level, the expression of RcbL was inhibited under drought condition and more obvious after 14 days (Fig. 6C). Conversely, both PEPC and NADP-ME activities increased in DS plants, although significant difference was not found until 21 days (Fig. 6A, B). Moreover, the expression pattern of PEPC was consistent with enzyme activity detection (Fig. 6C).
Activities PEPC (A), NADP-ME (B) and immunoblot with antibodies against large subunit of Rubisco complexes and PEPC subunits (C) in flag leaves during senescence stage in high-yield hybrid rice LYPJ exposed to drought stress. Vertical bars represent standard deviations (n = 3). Single and double asterisks refer to significant difference between the drought and the control at 0.05 and 0.01, respectively
Discussion
To improve crop drought tolerance for global food security, we need more profound understandings of strategies adopted by plants to mitigate detrimental effects caused by drought stress (Barnabas et al. 2008; Cominelli et al. 2013). In this study, we attempted to comprehending the mechanism of drought tolerance in the hybrid rice LYPJ, which exhibited impervious photosynthetic efficiency under prolonged drought stress during reproductive stage as previously mentioned, emphasizing on photosynthetic processes. It is interesting to underline that high hierarchical organization of photosynthetic apparatus, PSII core dimer and PSI-LHCII supercomplex were more pronounced in DS plants (Fig. 4). Therefore, it was convincible to propose that the remodeling of thylakoid membrane played a central role in long-term photosynthetic acclimation to drought stress.
Photo-equilibrium depended on architecture switch of thylakoid membrane complexes
The efficiency of photosynthesis depends on the coordinated interaction of PSII and PSI in the electron-transport. Plants subjected to the water deficit showed lower values of ET0/CSm characterizing limitation on linear electron flow (LEF) (Fig. 2). This observation could be partially ascribed to deficit of intersystem electron transporter, Cytb6f complex (Fig. 5, Table 1). Consequently, it would lead to increase of redox poise at PSII acceptor side and PSI donor side, which further gave rise to reactive oxygen species (ROS). To avoid possible photodamage caused by ROS, DS plants underwent serial architecture switch of thylakoid membrane complexes (illustrated by responsive pattern of 21 days drought-treated plants) (Fig. 5, Table 1). First of all, the CP43 less PSII core protein complex was significantly down-regulated, consisting of D1 protein (spot 20) and D2 protein (spots 14 and 15). Conversely, D1 protein in PSII core dimer (spot 46) was higher expressed (Table 1). However, reverse observations were acquired recently in Arabidopsis thaliana exposed to long-term drought stress, where PSII dimer but not PSII monomer decreased with the onset of stress (Chen et al. 2016). This discrepancy might be attributed to the level of D1 phosphorylation which suggested to stabilize PSII dimer, as they reported remarkable reduction of P-D1 in drought-treated plants (Kruse et al. 1997). Therefore, we hypothesized that P-D1 level was sustained or even enhanced in DS plants. Additionally, D1 phosphorylation is also a prerequisite for controlled turnover of the PSII, and thus rapid PSII repaired cycle could be facilitated (Tikkanen et al. 2008). On the other hand, LHCII assemblies altered in DS plants, characterizing by significant decrease in LHCII monomers i.e. decrease in LHCII monomer to trimer ratio. Similar results in drought-treated Arabidopsis thaliana were reported by Chen et al. (2016), while preferential attack of LHCII trimer was observed in Beta vulgaris under cadmium stress and iron deficiency, and decrease in LHCII monomer concomitant with LHCII trimer increase was claimed in salt-treated Cucumis sativus (Basa et al. 2014; Shu et al. 2015). This indicated that changes of LHCII assemblies may be stress-specific and/or species-specific.
The possible elucidation of the different performance of monomer and trimer in this work was disassembling of PSII-LHCII supercomplexes suggested by shrinkage and destacking of grana thylakoids (Fig. 7), which partially confirmed by increase of PSII dimers. It was suggested that energy absorbed by monomeric Lhcs can be more easily quenched than trimers (Garab et al. 2002). Intriguingly, although monomeric Lhcb1 showed reduction in DS plants, Lhcb4 and Lhcb5 were significantly amplified (Table 1). Phosphorylation of Lhcb4 by STN7 protein kinase has been indicated an extra dissipative process except PsbS-mediated NPQ in rice (Betterle et al. 2015). Hence, more pronounced monomeric Lhcb4 (probably P-Lhcb4) and Lhcb5 favored the energy quenching process, in agreement with enhanced thermal dissipation indicated by φD0 (Fig. 3). Meanwhile, decrease in monomeric Lhcb1, we supposed, signified LHCII trimer accumulation although LHCII trimer band showed no difference in DS plants. The elevated PSI-LHCII supercomplex supported the inference (Fig. 5). Both thermal dissipation and formation of PSI-LHCII supercomplex were dependent on phosphorylation of LHCII. P-LHCII, primarily Lhcb1 and Lhcb2 (Crepin and Caffarri 2015; Longoni et al. 2015), concomitantly with PSII core protein phosphorylation, facilitated the dissociation of PSII-LHCII which was an imperative step for PSII repair cycle. More important is the benefit that P-LHCIIs tend to associate with PSI, then readjusting the spectral and spatial absorption cross-sections of the two photosystems and ensuring homeostatic regulation of the redox poise of electron transfer chain (Galka et al. 2012).
Scheme of drought resistance on photosynthesis in flag leaf of high-yield hybrid rice LYPJ showing structural and functional readjustments of thylakoid membrane complexes and enzymes. Under drought stress, stomatal closure imposes restriction on available CO2. The co-regulation of Cytb6f complex and ATP synthase cut down the normal supply of ATP and NADPH. Inactivation of PSII reaction center and disassembly of PSII-LHCII favor the thermal dissipation, resulting in destacking of grana. Phosphorylated LHCII trimers migrate from PSII to PSI, maintaining the cross section homeostasis of dynamic photo-equilibrium. Enhanced cyclic electron flow (CEF) in margins generated additional ATP and sustained the proton gradient (ΔpH) characterizing by lumen expansion, facilitating the ΔpH-dependent thermal dissipation and diffusion-dependent electron transport by PQ and PC. STN8 protein kinase phosphorylates PSII core, stabilizing its dimeric formation, and involves in regulation of CEF and Ca2+ signal transduction. The high expression of PEPC and NADP-ME may compensate the decreased Rubisco for CO2 assimilation and benefit other metabolism pathways
It should be pointed out that only a fraction of P-LHCII migrated to PSI and most disconnected LHCIIs probably formed aggregations at energy-dissipative state, which were more efficient in dissipation than monomeric and dimeric complexes (Goldschmidt-Clermont and Bassi 2015; van Oort et al. 2007). Whereas in DS plants Lhcbs decreased to varying degrees (Fig. 5) and PSII reaction centers were impaired (Fig. 1), constraints on energy absorption and trapping could yet be regarded as a protection from photodamage. These results suggested that drought posed limitation on electron transport and the excess excitation energy absorption in PSII could be dissipated as thermal dependent on changes in LHCII assemblies.
Limitation of LEF and CO2 and increment in antenna size though LHCII binding brought pressure for PSI (Tsabari et al. 2015). As already mentioned, the reduction of end electron accepter of PSI in DS plants was relatively impervious even though CO2 assimilation was limited. Hence, it was convincible that CEF operated for protecting PSI from photoinhibition. It has been well-documented that CEF plays a crucial role in regulating photo-equilibrium, as it generates additional ATP and sustains ΔpH (Kohzuma et al. 2009). Although Takahashi et al. (2013) demonstrated that CEF was independent of state transition, yet we found they had cooperation in homeostatic regulations. The observed typical thylakoid structure of state II (Fig. 7) with swollen lumen supported the CEF in grana margins through accelerated the diffusion of PQ (Kirchhoff 2014). Likewise, PSII repair taking place in stroma lamellae was facilitated. Lumen acidification was not only a positive feedback of CEF, but also an imperative of PsbS-mediated NPQ. Nonetheless, we still noticed some minor but interesting alterations in PSI. On the one hand, PsaD protein in PSI core complex was down-regulated while PsaA was steady. As PsaD has been indicated of impact on electron transfer between FeS-clusters in PsaC, the changes might play a regulatory role in CEF. Recently, Tiwari et al. (2016) has reported that damage of FeS-clusters transformed into energy quencher in the absence of ΔpH-dependent regulatory mechanisms in pgr5 mutant. However, whether proteins of PSI participate in controlling CEF has not been determined. On the other hand, Lhca3 decreased in early stress but increased latter, whereas other Lhcas remained unaffected (Fig. 5A). Here we speculated that Lhca3 was a potential regulator of dynamics between PSI-LHCII and LHCII pool, as it was required for complete state transition (Benson et al. 2015). Additionally, TPR repeated containing protein (spot 42), which protects PSI from oxidative disruption during assembly (Heinnickel et al. 2016), showed specific expression in DS plants, along with peptide chain release factor 2 (spot 38) and BAG family molecular chaperone regulator 8 (spot 37). These auxiliary proteins were probably important for the stabilization of PSI-LHCII supercomplex. Briefly, increased PSI-LHCII complex and CEF around PSI were vital for balance of excitation energy and ΔpH-dependent protective mechanisms in PSII. Furthermore, we found that PsbO was not so sensitive to drought stress as PsbP, PsbQ and PsbR (Fig. 5B).
Afterwards, contradictory results arose in ATP synthase, where protein immunoblotting pointed to reduction in expression level, but BN/SDS-PAGE supported an increase pattern (Fig. 5A, Table 1). Kohzuma et al. (2009) considered that ATP synthase was the key control point of photosynthetic proton circuit, while Yamori et al. (2011) demonstrated that LEF was more limited by Cytb6f complex. Most documents supported the view that contents of ATP synthase and Cytb6f complex were strictly co-regulated (Schottler et al. 2015). Decline of ATP synthase benefited the build-up of ΔpH utilized by NPQ. From another aspect, sufficient ATP was essential for other protective processes such as photorespiration (Silva et al. 2015). Clearly further studies on expression level and direct assay on activity of ATP synthase are in demand.
In conclusion, these results highlight the importance of dynamics of thylakoid membrane complex in rice plant adaptation to water deficit. The redox-controlled and phosphorylation-dependent remodeling of thylakoid membrane lay the foundation for photo-equilibrium, and sustaining ΔpH seemed to a critical necessity for photoprotective process. NPQ and state transition were concomitant in drought response, incompatible with the argument that state transition functioned under low light condition while NPQ was dominant in high light stress (Tikkanen et al. 2010). In favor of Vallon et al. (1991), our results suggested that state transition was not only a light-acclimation mechanism but also contributed to rerouting of photosynthetic electron flow, accompanied by CEF under drought stress.
Photo-carbon balance demanded enzymes responsible for C4 photosynthesis
As already discussed, balanced activity of the two photosystems was warranted by the remodeling of thylakoid membrane complex. In particular, co-regulation of Cytb6f complex and ATP synthase could be considered as an automatic adaptation for CO2 limitation caused by stomatal closure. Here we noticed remarkable decrease of Rubisco activity in DS plants in our previous work (Wang et al. 2017b), in agreement with results observed in drought-stressed wheat emerged from Vassileva et al. (2012). According to Galmes et al. (2013), Rubisco-related parameters were not sensitive to mild-to-moderate drought stress and inhibition of Rubisco activity was ascribed to both decreased Rubisco content and increased tight-binding inhibitors (Parry et al. 1997). Likewise, loss of Rubisco activity and content were more pronounced after 21 days when stress was severe. Yamane et al. (2012) demonstrated that Rubisco could be excluded from chloroplast and degraded though this pathway in salt-treated rice. We proposed that it was partially responsible for decrease of RbcL (Fig. 6C). By virtue of Rubisco degradation, drought-induced nutrient starvation in DS plants might be alleviated as Rubisco was a N pool due to its high abundance (Warren et al. 2004). Notably, potentiated PEPC and NADP-ME activities in DS plants indicated an alternative pathway to address CO2 limitation. PEPC and NADP-ME are typical enzymes responsible for C4 photosynthesis, yet C3 plants possess all of these genes although expression levels are much lower than those in C4 species (Hibberd and Quick 2002). Doubnerová and Ryšlavá (2011) have reviewed the functions of these C4 enzymes in C3 plants and concluded that these enzymes are more important for plants under stress conditions. Besides compensation for CO2 assimilation, non-photosynthetic counterparts of these enzymes play important roles in replenishment of Krebs cycle intermediates, facilitating the biosynthesis of amino acids and other compounds and NADPH for the antioxidant system (Doubnerová and Ryšlavá 2011). However, the increase of C4 enzymes seemed to be latter than loss of Rubisco activity, mechanistic regulation of C4 pathway in C3 plants has not been clearly determined. By the way, BAG family molecular chaperone regulator 8 (spot 37), a specific protein induced by drought, is a chaperone regulator for heat shock protein (HSP) 70. Vassileva et al. (2012) suggested an important role of HSP70 in drought resistance. Apart from the hypothesis that HSP70 helped stabilize PSII dimer and PSI-LHCII supercomplexes, it was of interest to mention that HSP70 could associate with NADP-ME and modulated its catalytic properties (Lara et al. 2005), and thereby HSP70 was probably a candidate regulator of C4 photosynthesis in C3 plants under drought stress. Nevertheless, we failed to know the status of photorespiration which is important in stress acclimation as it also provides intermediates for secondary metabolism, such as phenolic compounds observed in cell wall of DS plants which was beneficial for drought resistance. It could be speculated that photorespiration rate changed in DS plants because of alteration in [CO2]/[O2] and it participated in the regulation network (Silva et al. 2015). Given the importance of ATP/NADPH ratio in controlling these processes but uncertainty of changes in ATP synthase, further investigations should be designed to detect how the ATP/NADPH budget counted.
Conclusion
Efficient electron transfer reactions require a balanced excitation energy distribution between PSII and PSI. Our results provide evidence that the interactions between PSII and LHCII protein phosphorylation, thermal dissipation, and CEF around PSI function in balancing the excitation energy distribution between PSII and PSI though architecture remodeling of thylakoid membrane proteins. Additionally, enhanced C4 photosynthesis may have dual role that it provides compensation for carbon fixation when both CO2 and Rubisco activity are limited and favors the antioxidant system though supplying important counterparts. In general, more efforts should be donated to determine how ATP/NADPH influenced the integrated mobilization of photosynthesis, CO2 assimilation, antioxidant system and other energy metabolism pathways in drought responses.
References
Barnabas B, Jager K, Feher A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31:11–38
Basa B, Lattanzio G, Solti Á, Tóth B, Abadía J, Fodor F, Sárvári É (2014) Changes induced by cadmium stress and iron deficiency in the composition and organization of thylakoid complexes in sugar beet (Beta vulgaris L.). Environ Exp Bot 101:1–11
Benson SL, Maheswaran P, Ware MA, Hunter CN, Horton P, Jansson S, Ruban AV, Johnson MP (2015) An intact light harvesting complex I antenna system is required for complete state transitions in Arabidopsis. Nat Plant 1:15176
Betterle N, Ballottari M, Baginsky S, Bassi R (2015) High light-dependent phosphorylation of photosystem II inner antenna CP29 in monocots is STN7 independent and enhances nonphotochemical quenching. Plant Physiol 167:457–471
Chen YE, Liu WJ, Su YQ, Cui JM, Zhang ZW, Yuan M, Zhang HY, Yuan S (2016) Different response of photosystem II to short and long term drought stress in Arabidopsis thaliana. Physiol Plant 158:225–235
Chow WS (1990) Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc Natl Acad Sci USA 87:7502–7506
Cominelli E, Conti L, Tonelli C, Galbiati M (2013) Challenges and perspectives to improve crop drought and salinity tolerance. New Biotechnol 30:355–361
Correa-Galvis V, Poschmann G, Melzer M, Stühler K, Jahns P (2016) PsbS interactions involved in the activation of energy dissipation in Arabidopsis. Nat Plant 2:15225
Crepin A, Caffarri S (2015) The specific localizations of phosphorylated Lhcb1 and Lhcb2 isoforms reveal the role of Lhcb2 in the formation of the PSI-LHCII supercomplex in Arabidopsis during state transitions. BBA-Bioenergetics 1847:1539–1548
Doubnerová V, Ryšlavá H (2011) What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci 180:575–583
Galka P, Santabarbara S, Khuong TT, Degand H, Morsomme P, Jennings RC, Boekema EJ, Caffarri S (2012) Functional analyses of the plant photosystem I-light-harvesting complex II supercomplex reveal that light-harvesting complex II loosely bound to photosystem II is a very efficient antenna for photosystem I in state II. Plant Cell 24:2963–2978
Galmes J, Aranjuelo I, Medrano H, Flexas J (2013) Variation in Rubisco content and activity under variable climatic factors. Photosynth Res 117:73–90
Garab G (2014) Hierarchical organization and structural flexibility of thylakoid membranes. BBA-Bioenergetics 1837:481–494
Garab G, Cseh Z, Kovacs L, Rajagopal S, Varkonyi Z, Wentworth M, Mustárdy L, Dér A, Ruban AV, Papp E, Holzenburg A, Horton P (2002) Light-induced trimer to monomer transition in the main light-harvesting antenna complex of plants: thermo-optic mechanism. Biochemistry-US 41:15121–15129
Giovanardia M, Pantaleonia L, Ferronia L, Paglianob C, Albaneseb P, Baldisserottoa C, Pancaldia S (2018) In pea stipules a functional photosynthetic electron flow occurs despite a reduced dynamicity of LHCII association with photosystems. BBA-Bioenergetics 1859:1025–1038
Goldschmidt-Clermont M, Bassi R (2015) Sharing light between two photosystems: mechanism of state transitions. Curr Opin Plant Biol 25:71–78
Grieco M, Suorsa M, Jajoo A, Tikkanen M, Aro EM (2015) Light-harvesting II antenna trimers connect energetically the entire photosynthetic machinery—including both photosystems II and I. BBA-Bioenergetics 1847:607–619
Heinnickel M, Kim RG, Wittkopp TM, Yang W, Walters KA, Herbert SK, Grossman AR (2016) Tetratricopeptide repeat protein protects photosystem I from oxidative disruption during assembly. Proc Natl Acad Sci USA 113:2774–2779
Hibberd JM, Quick WP (2002) Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415:451–454
Huseynova IM (2012) Photosynthetic characteristics and enzymatic antioxidant capacity of leaves from wheat cultivars exposed to drought. BBA-Bioenergetics 1817:1516–1523
Järvi S, Suorsa M, Paakkarinen V, Aro EM (2011) Optimized native gel systems for separation of thylakoid protein complexes: novel super- and mega-complexes. Biochem J 439:207–214
Kaiser E, Morales A, Harbinson J, Kromdijk J, Heuvelink E, Marcelis LF (2015) Dynamic photosynthesis in different environmental conditions. J Exp Bot 66:2415–2426
Kirchhoff H (2014) Diffusion of molecules and macromolecules in thylakoid membranes. BBA-Bioenergetics 1837:495–502
Kohzuma K, Cruz JA, Akashi K, Hoshiyasu S, Munekage YN, Yokota A, Kramer DM (2009) The long-term responses of the photosynthetic proton circuit to drought. Plant Cell Environ 32:209–219
Kruse O, Zheleva D, Barber J (1997) Stabilization of photosystem two dimers by phosphorylation: implication for the regulation of the turnover of D1 protein. FEBS Lett 408:276–280
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lara MV, Drincovich MF, Muller GL, Maurino VG, Andreo CS (2005) NADP-malic enzyme and Hsp70: co-purification of both proteins and modification of NADP-malic enzyme properties by association with Hsp70. Plant Cell Physiol 46:997–1006
Laureau C, De Paepe R, Latouche G, Moreno-Chacon M, Finazzi G, Kuntz M, Corni G, Streb P (2013) Plastid terminal oxidase (PTOX) has the potential to act as a safety valve for excess excitation energy in the alpine plant species Ranunculus glacialis L. Plant Cell Environ 36:1296–1310
Longoni P, Douchi D, Cariti F, Fucile G, Goldschmidt-Clermont M (2015) Phosphorylation of the light-harvesting complex II isoform Lhcb2 is central to state transitions. Plant Physiol 169:2874–2883
Parry MAJ, Andralojc PJ, Parmar S, Keys AJ, Habash D, Paul MJ, Alred R, Quick WP, Servaites JC (1997) Regulation of Rubisco by inhibitors in the light. Plant Cell Environ 20:528–534
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with atomic absorption spectroscopy. BBA-Bioenergetics 975:384–394
Pribil M, Labs M, Leister D (2014) Structure and dynamics of thylakoids in land plants. J Exp Bot 65:1955–1972
Puthiyaveetil S, Tsabari O, Lowry T, Lenhert S, Lewis RR, Reich Z, Kirchhoff H (2014) Compartmentalization of the protein repair machinery in photosynthetic membranes. Proc Natl Acad Sci USA 111:15839–15844
Ruban AV, Johnson MP (2015) Visualizing the dynamic structure of the plant photosynthetic membrane. Nat Plant 1:15161
Ryšlavá H, Müller K, Semorádová Š, Synková H, Čeřovská N (2003) Photosynthesis and activity of phosphoenolpyruvate carboxylase in Nicotiana tabacum L. leaves infected by Potato virus A and Potato virus Y. Photosynthetica 41:357–363
Schottler MA, Toth SZ, Boulouis A, Kahlau S (2015) Photosynthetic complex stoichiometry dynamics in higher plants: biogenesis, function, and turnover of ATP synthase and the cytochrome b 6 f complex. J Exp Bot 66:2373–2400
Shikanai T (2016) Regulatory network of proton motive force: contribution of cyclic electron transport around photosystem I. Photosynth Res 129:253–260
Shu S, Yuan Y, Chen J, Sun J, Zhang W, Tang Y, Zhong M, Guo S (2015) The role of putrescine in the regulation of proteins and fatty acids of thylakoid membranes under salt stress. Sci Rep 5:14390
Silva EN, Silveira JAG, Ribeiro RV, Vieira SA (2015) Photoprotective function of energy dissipation by thermal processes and photorespiratory mechanisms in Jatropha curcas plants during different intensities of drought and after recovery. Environ Exp Bot 110:36–45
Strand DD, Livingston AK, Satoh-Cruz M, Froehlich JE, Maurino VG, Kramer DM (2015) Activation of cyclic electron flow by hydrogen peroxide in vivo. Proc Natl Acad Sci USA 112:5539–5544
Suorsa M, Rantala M, Mamedov F, Lespinasse M, Trotta A, Grieco M, Vuorio E, Tikkanen M, Järvi S, Aro EM (2015) Light acclimation involves dynamic re-organization of the pigment-protein megacomplexes in non-appressed thylakoid domains. Plant J 84:360–373
Sylak-Glassman EJ, Malnoë A, De Re E, Brooks MD, Fischer AL, Niyogi KK, Fleming GR (2014) Distinct roles of the photosystem II protein PsbS and zeaxanthin in the regulation of light harvesting in plants revealed by fluorescence lifetime snapshots. Proc Natl Acad Sci USA 111:17498–17503
Takahashi H, Clowez S, Wollman FA, Vallon O, Rappaport F (2013) Cyclic electron flow is redox-controlled but independent of state transition. Nat Commun 4:1954
Tikkanen M, Nurmi M, Kangasjarvi S, Aro EM (2008) Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. BBA-Bioenergetics 1777:1432–1437
Tikkanen M, Grieco M, Kangasjarvi S, Aro EM (2010) Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol 152:723–735
Tiwari A, Mamedov F, Grieco M, Suorsa M, Jajoo A, Styring S, Tikkanen M, Aro EM (2016) Photodamage of iron–sulphur clusters in photosystem I induces non-photochemical energy dissipation. Nat Plant 1:6035
Tsabari O, Nevo R, Meir S, Carrillo LR, Kramer DM, Reich Z (2015) Differential effects of ambient or diminished CO2 and O2 levels on thylakoid membrane structure in light-stressed plants. Plant J 81:884–894
Vallon O, Bulte L, Dainese P, Olive J, Bassi R, Wollman FA (1991) Lateral redistribution of cytochrome b 6 /f complexes along thylakoid membranes upon state transition. Proc Natl Acad Sci USA 88:8262–8266
van Oort B, van Hoek A, Ruban AV, van Amerongen H (2007) Aggregation of light-harvesting complex II leads to formation of efficient excitation energy traps in monomeric and trimeric complexes. FEBS Lett 581:3528–3532
Vassileva V, Demirevska K, Simova-Stoilova L, Petrova T, Tsenov N, Feller U (2012) Long-term field drought affects leaf protein pattern and chloroplast ultrastructure of winter wheat in a cultivar-specific manner. J Agr Crop Sci 198:104–117
Wang Y, Xu C, Wu M, Chen GX (2017a) Characterization of photosynthetic performance during reproductive stage in high-yield hybrid rice LYPJ exposed to drought stress probed by chlorophyllafluorescence transient. Plant Growth Regul 81:489–499
Wang Y, Xu C, Zhang BB, Wu M, Chen GX (2017b) Physiological and proteomic analysis of rice (Oryza sativa L.) in flag leaf during flowering stage and milk stage under drought stress. Plant Growth Regul 82:201–218
Warren CR, Livingston NJ, Turpin DH (2004) Photosynthetic responses and N allocation in Douglas-fir needles following a brief pulse of nutrients. Tree Physiol 24:601–608
Wittig I, Braun HP, Schagger H (2006) Blue native PAGE. Nat Protoc 1:418–428
Yamane K, Mitsuya S, Taniguchi M, Miyake H (2012) Salt-induced chloroplast protrusion is the process of exclusion of ribulose-1,5-bisphosphate carboxylase/oxygenase from chloroplasts into cytoplasm in leaves of rice. Plant Cell Environ 35:1663–1671
Yamori W, Takahashi S, Makino A, Price GD, Badger MR, von Caemmerer S (2011) The roles of ATP synthase and the cytochrome b 6 /f complexes in limiting chloroplast electron transport and determining photosynthetic capacity. Plant Physiol 155:956–962
Yamori W, Shikanai T, Makino A (2015) Photosystem I cyclic electron flow via chloroplast NADH dehydrogenase-like complex performs a physiological role for photosynthesis at low light. Sci Rep 5:e13908
Yu SB, Li JX, Xu CG, Tan YF, Gao YJ, Li XH, Zhang Q, Saghai Maroof MA (1997) Importance of epistasis as the genetic basis of heterosis in an elite rice hybrid. Proc Natl Acad Sci USA 94:9226–9231
Zhang M, Shan Y, Kochian L, Strasser RJ, Chen G (2015) Photochemical properties in flag leaves of a super-high-yielding hybrid rice and a traditional hybrid rice (Oryza sativa L.) probed by chlorophyll a fluorescence transient. Photosynth Res 126:275–284
Zivcak M, Kalaji HM, Shao HB, Olsovska K, Brestic M (2014) Photosynthetic proton and electron transport in wheat leaves under prolonged moderate drought stress. J Photoch Photobiol B 137:107–115
Acknowledgements
The authors thank Prof. Lv ChuanGen for providing material. This work was supported by Natural Science Foundation of Jiangsu province of China (Grant No. BK20171034), the Agricultural Independent Innovation Foundation of Jiangsu Province (Grant No. CX173022) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
CGX conceived and designed the experiments. XC performed the experiments. WYW analyzed the data. LH contributed analysis tools. WYW and XC contributed to the writing of the manuscript. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Lei, H., Xu, C. et al. Long-term drought resistance in rice (Oryza sativa L.) during leaf senescence: a photosynthetic view. Plant Growth Regul 88, 253–266 (2019). https://doi.org/10.1007/s10725-019-00505-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-019-00505-2