Abstract
The main objective of the present review is to provide a compilation of published data of the effects of several climatic conditions on Rubisco, particularly its activity, state of activation, and concentration, and its influence on leaf gas exchange and photosynthesis. The environmental conditions analyzed include drought, salinity, heavy metals, growth temperature, and elevated [O3], [CO2], and ultraviolet-B irradiance. The results show conclusive evidence for a major negative effect on activity of Rubisco with increasing intensity of a range of abiotic stress factors. This decrease in the activity of Rubisco is associated with down-regulation of the activation state of the enzyme (e.g., by de-carbamylation and/or binding of inhibitory sugar phosphates) in response to drought or high temperature. On the contrary, the negative effects of low temperature, heavy metal stress (cadmium), ozone, and UV-B stress on Rubisco activity are associated with changes in the concentration of Rubisco. Notably, in response to all environmental factors, the regulation of in vivo CO2 assimilation rate was related to Rubisco in vitro parameters, either concentration and/or carboxylation, depending on the particular stress. The importance of the loss of Rubisco activity and its repercussion on plant photosynthesis are discussed in the context of climate change. It is suggested that decreased Rubisco activity will be a major effect induced by climate change, which will need to be considered in any prediction model on plant productivity in the near future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic stress is the principal cause of crop failure, decreasing the average yields of most major crops by more than 50 % and threatening the sustainability of agriculture worldwide. Crop productivity is primarily dictated by the plant carbon balance, which is determined from the difference between the rate of photosynthetic CO2 assimilation and respiration. Within the photosynthetic process, ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) is the enzyme responsible of CO2 fixation, the importance of which on the primary productivity has been estimated to be above 1011 tons of atmospheric CO2 being annually fixed (Field et al. 1998).
Engineering Rubisco to improve its catalytic capacity is envisaged as one of the most suitable means for improving global plant productivity and agricultural yields (Parry et al. 2013). In particular, increasing the carboxylase catalytic turnover rate and/or the ratio of carboxylation to the apparently futile oxygenation reaction would improve photosynthesis and yield (Whitney et al. 2011). Owing to its importance, significant knowledge has been gained over the past decades on the biochemistry and the molecular biology of Rubisco. It is now well established that in vivo Rubisco activity is rapidly regulated to control the flux through the photosynthetic carbon reduction cycle in response to fluctuations in the environment. This regulation consists in the spontaneous carbamylation of a lysine residue and the subsequent stabilization of the carbamate by Mg2+ ions (Cleland et al. 1998). Premature binding of RuBP to uncarbamylated Rubisco or binding of day- and night-time inhibitors results in inactive complex (Parry et al. 2008). Release of sugar phosphates from active and inactive Rubisco sites is catalyzed by the nuclear encoded enzyme Rubisco activase, the activity of which is modulated by stromal ATP/ADP and redox changes, thus facilitating carbamylation and regulating Rubisco activity according to the metabolic demands of photosynthesis (Portis 2003).
Although the mode of regulation is well established at the molecular level, much less is known on the precise modulation of Rubisco activity under varying environmental conditions, with apparently controversial results being published. For instance, there is discrepancy on the effects of drought on the Rubisco activity, with some studies showing no effect (Sharkey and Seemann 1989; Tezara et al. 1999), while other studies found it to be affected, hypothesizing that Rubisco activity plays a central role in the drought-induced depression of photosynthesis (Parry et al. 2002; Zhou et al. 2007). Most likely, the trigger of the decrease of the Rubisco activity depends on the severity and/or duration of the stress imposed (Flexas et al. 2006a; Galmés et al. 2011a). In addition, when activity is impaired, the precise underlying mechanisms (i.e., decreased Rubisco content, activation state, or presence of inhibitors) seem to depend on the species analyzed (Bota et al. 2004) and the rate of drought imposition (Flexas et al. 2006b). Other authors have suggested that decreased Rubisco activity under drought stress is a direct consequence of secondary oxidative stress, which in turn depends on the prevailing levels of irradiance (Zhou et al. 2007). With respect to other environmental stresses, there is much less information, although a similar lack of generalized patterns of response of Rubisco has been observed under conditions of salinity (e.g., Delfine et al. 1998; Feng et al. 2007; Singh et al. 2007), high temperature (e.g., Gesch et al. 2003; Kim and Portis 2005, Prasad et al. 2009), low temperature (Savitch et al. 2000; Aranjuelo et al. 2005; Zhou et al. 2006), increased O3 (e.g., Fontaine et al. 1999; Di Cagno et al. 2001; Leitao et al. 2007), increased CO2 (e.g., Sicher and Bunce 1997; Centritto and Jarvis 1999; Aranjuelo et al. 2011) increased UV (Correia et al. 2005) or heavy metal toxicity in soils (e.g., Chaffei et al. 2004; Dhir et al. 2009; Ying et al. 2010).
Improving our understanding on how Rubisco activity is regulated under varying environmental conditions is crucial to design the best Rubisco to be engineered to improve photosynthesis and yield under a climate change scenario. For instance, climate change perspectives predict increased temperature and CO2 and decreased water availability in many areas of the world (Gornall et al. 2010). As mentioned, the response of Rubisco to each of these three environmental conditions separately is not fully understood, for which there is still much to be learned before the effects of the three factors interacting together can be envisaged.
The aim of the present study was to compile a large dataset from the literature on the effects of environmental variables on Rubisco activity and its main components. Data analysis intends to answer the following questions: (i) is there a general pattern of response of Rubisco activity to each independent environmental factor? (ii) is there a common general response to all environmental factors? (iii) is it possible, with the data available, to have a hint of the regulatory mechanisms for environmentally induced down-regulation of Rubisco activity?, i.e., is it mostly regulated through decreased concentration, activation, or other factors? and (iv) what is the actual relationship between stress-induced decreases of Rubisco activity as determined in vitro and the in vivo down-regulation of gas exchange and photosynthetic activity?
Materials and methods
Data on the effects of eight environmental factors (drought stress, salinity, heavy metals, high and low growth temperatures, and elevated [O3], [CO2], and ultraviolet-B (UV-B) irradiance) on in vitro Rubisco parameters were compiled by surveying the peer-reviewed literature on the web of science (Thompson-ISI, Philadelphia, USA). Eight databases were created, one for each environmental factor, with the following data: (i) descriptive information (article, species, and environmental conditions regarding the treatment), in vitro Rubisco parameters (initial and total activities, carbamylation state, and concentration), and in vivo light-saturated net CO2 assimilation rate (A N).
Within each article × species interaction, a control treatment was considered when plants were grown under optimal conditions. Although plant response to stress can be influenced by other factors related to plant developmental stage, duration of the stress and growth, and sampling conditions, those data were not considered during data analysis. Nevertheless, care was taken, during data extraction from original sources, to ensure minimal differences in these additional factors between control and non-control treatments.
Units for the initial and total Rubisco activities, as well as for Rubisco concentration, varied among studies, with unit interconversion being sometimes problematic or even impossible. Therefore, for comparative purposes, these parameters were expressed as a percentage of the control values. Consequently, for scaling purposes, A N data were also transformed into percentage of the control values.
Data were arranged in averages extracted from tables, text, and/or figures of each article, and compiled into spreadsheets specific for each environmental factor. Then, data were classified according to arbitrarily established intensity degrees of stress, as explained below, and averages and standard errors calculated. All publications and species whose data have been used in the present review are listed in Table 1, classified according to the different environmental factors.
Drought is the environmental factor under which Rubisco has been characterized in more detail (Table 1). Comparing results from different studies and species is difficult because of interspecific differences in the response of photosynthesis to leaf water potential and/or leaf or soil relative water content, the parameters most commonly used to assess the degree of drought. In an attempt to solve this problem, the relationship between stomatal conductance (g s) and photosynthesis has been used, since g s has been described as a valid indicator of the intensity of drought stress (Flexas and Medrano 2002; Chaves et al. 2009). Therefore, only articles providing gs values were considered in this database. Plants were grouped under mild, moderate, severe, and extreme drought stress when the g s was above 70 %, between 70 and 40 %, between 40 and 10 %, and less than 10 % of control values, respectively.
For salinity studies, plants were grouped depending on the concentration of NaCl in the irrigation solution: mild stress when [NaCl] < 100 mM, moderate stress when [NaCl] was between 100 and 400 mM, and severe stress at [NaCl] > 400 mM.
The temperature databases consisted of data from plants grown (i.e., acclimated) either under low or high temperatures. Hence, the aim was to study the effects of the growth temperature, but not of the measuring temperature. Therefore, in vitro activities and in vivo gas-exchange data from both control and stressed treatments were measured at the same temperature (typically between 20 and 30 °C). This rule for comparable temperature of measurement was generalized for all databases.
For high-temperature stress, data were grouped under mild, moderate, and severe heat stress. To define the different intensities of stress, the absolute increase in Celsius degrees between the treatment and the control was multiplied by a factor considering the temperature achieved during the light period. This factor had a value of 1 when the temperature during the light period was <30 °C, 2 when this temperature was between 30 and 38 °C, and 3 when >38 °C. For mild, moderate, and severe heat stress intensities, the product between the maximum temperature factor and the absolute increase in Celsius degrees was <10, 10–20, and >20, respectively.
With respect to low-temperature stress, because the growth temperature in all control treatments was similar (between 20 and 30 °C), the absolute decrease in Celsius degrees was directly used to define the two intensities of stress: mild-to-moderate stress when the temperature decrease <10 °C, and severe stress when the decrease >10 °C.
The search for literature reporting data on the effect of heavy metals on the in vitro Rubisco parameters showed that most experiments were performed on cadmium toxicity. Therefore, our review on heavy metal effects is mainly focused in studies dealing with Cd. Two different intensities of Cd toxicity were considered: mild/moderate, and severe stress intensities, which corresponded to [Cd] in the irrigation solution lower and higher than 50 μM, respectively.
Similar to heavy metals, data on O3 were separated in two groups of intensity. Mild/moderate stress included [O3] < 120 nL L−1, whereas severe stress included studies where the plants were exposed to [O3] > 120 nL L−1. The data from articles dealing with elevated [CO2] were separated into studies where elevated [CO2] was <200 and >200 % than the corresponding ambient [CO2] treatment. Finally, the low amount of data available on the effects of higher UV-B irradiance on the in vitro Rubisco parameters precluded its grouping under different intensities.
Statistical analyses
Univariate analysis of variance (ANOVA) was used to study the effect of the different stress intensities on the in vitro parameters. Significant differences between means were revealed by Duncan test (P < 0.05). These analyses were performed using the SPSS 12.0 software package (SPSS Inc., Chicago, IL, USA). Regression analyses were performed with the 11.0 Sigma Plot software package (Sigma, St Louis, MO, USA).
Results and discussion
Drought and salt stress
There is a general consensus that drought and salinity limit photosynthesis predominantly through increases in the leaf resistances to CO2 transport, while metabolic impairment may occur when the intensity of stress becomes more severe (Flexas et al. 2004). In spite of this, variability of data on Rubisco parameters indicates that there is some controversy in literature, which has been attributed to differences in the velocity of stress imposition and to species-specific responses (Parry et al. 2002; Flexas et al. 2006a; Galmés et al. 2011a).
Data collected in this review essentially support the general consensus, with no significant or only minor changes in any of the Rubisco-related parameters under mild-to-moderate drought, followed by decreases in Rubisco activity at higher intensities of stress, when g s was below 40 % of control treatment values (Fig. 1a). Initial and total Rubisco activities decreased by about 70 % under severe stress, although the carbamylation of Rubisco was not significantly modified. In principle, decreased activity with unequal change in carbamylation can be explained by decreased Rubisco content. However, this was not the case as the concentration of Rubisco under severe stress was not significantly different from that reported under nonstressed conditions (Fig. 1a). This suggests that, under severe drought, a percentage of the catalytic sites of Rubisco are blocked by tight-binding of inhibitors which decrease the concentration of sites catalytically available for carboxylation. An increase in the concentration of tight-binding inhibitors has been reported in several species under moderate-to-severe drought intensities (Parry et al. 2002; Carmo-Silva et al. 2010). The decrease in the CO2 concentration at the sites of carboxylation in the chloroplastic stroma observed under moderate-to-severe drought intensities (Flexas et al. 2012), and the concurrent increase in the CO2/O2 ratio may lead to an increase in the production of misfire products, like d-glycero-2,3-diulose-1,5-bisphosphate (Parry et al. 2008). This finding supports the view that carbamylation is not the only parameter modulating Rubisco activity and that the presence of unknown inhibitors can control the number of Rubisco sites that can be activated by carbamylation (Eichelmann et al. 2009).
Effect of a drought and b salt stress on Rubisco parameters. As for drought, four different intensities of stress were established according to the stomatal conductance (g s) values expressed as a percentage of the control treatment. Mild stress g s > 70 %; moderate stress 70 % < g s < 40 %; severe stress 40 % < g s < 10 %; extreme stress g s < 10 %. As for salt stress, three different intensities were considered according to the concentration of NaCl in the solution used to irrigate the treated plants. Number of replicates is indicated at the bottom of each column. See Table 1 for articles and species considered in the analyses. Different letters denote statistically significant differences among different treatments through Duncan test (P < 0.05), being control treatment a
There is evidence showing that decreased ATP limits RuBP production under drought stress (Tezara et al. 1999), and inhibitors binding to Rubisco sites may occur especially at subsaturating RuBP concentration. The interaction of Rubisco with tight-binding inhibitors has been hypothesized to prevent the degradation by proteases of the Rubisco not being used for in vivo catalysis, especially in stressed leaves (Parry et al. 2008). Nevertheless, this protection to proteolysis would not be sufficient under extreme drought intensities, when the concentration of Rubisco drops to about half of that measured under control conditions (Fig. 1a). However, under these extreme drought intensities, the initial activity (at about 50 % with respect to control values) decreases below total activity (Fig. 1a). This fact suggests that ca. 25 % of Rubisco becomes inactivated because of decarbamylation of catalytic sites, probably mediated by the lower CO2 availability induced by further decreases in the stomatal and mesophyll conductances to CO2 (Galmés et al. 2011a). The degree of deactivation of Rubisco sites in drought-stressed plants has been related to its functional type, which could explain, at least partially, the apparent discrepancies on the effects of drought on Rubisco in vitro parameters between different species (Galmés et al. 2011a).
The effects of soil salinity on plants have been divided into osmotic and ionic phases (Munns and Tester 2008). The osmotic phase is caused by a decrease in the soil and the intracellular water potential, and its consequences on the physiology of plants are similar to those triggered by drought stress (Munns and Tester 2008). With respect to photosynthesis, osmotic effects of salinity have been described to primarily affect leaf CO2 diffusion through decreases of g s and g m (Flexas et al. 2004), thereby potentially decreasing Rubisco activation because of decarbamylation of catalytic sites, as reported under moderate concentration of NaCl in the soil (100–400 mM) (Fig. 1b). Moreover, similar to the effects of mild drought stress, Rubisco carbamylation state was not affected at lower salt stress intensities ([NaCl] < 100 mM) (Fig. 1b), although decreases in the concentration of Rubisco have been reported (Delfine et al. 1998). This is clearly illustrated in Fig. 2a, where in rice subjected to salt stress, differences between initial and total activities become evident at moderate salt concentrations, but not at concentrations below 100 mM. However, data availability for some Rubisco parameters under salt stress is yet scarce, and more studies are required to confirm the trends described here.
a The relationship between the concentration of NaCl in the irrigation solution and the Rubisco total and initial activities of Oryza sativa (Feng et al. 2007). b The relationship between the concentration of NaCl in the irrigation solution and the Rubisco total activity, for three species differing in the tolerance to salt stress: Cakile maritima (high tolerance, from Debez et al. 2006), O. sativa (medium tolerance, from Feng et al. 2007), and Cicer arietinum (low tolerance, from Soussi et al. 1998)
At higher salt concentrations or under prolonged stress, the osmotic phase is followed by an ionic phase, when metabolic impairment of the photosynthetic machinery is more likely to occur (Munns and Tester 2008). However, distinctions have to be made among species differing in their tolerance to salt stress. Hence, the effect of increasing soil salinity on the Rubisco total activity of the halophyte Cakile maritima is lower in comparison to rice and especially the glycophyte chickpea (Fig. 2b). Differences in the effects of salinity on the Rubisco activity among these species seem to be exclusively ascribed to their ability to control salt uptake by the roots and transport within the plant, and particularly to avoid salt accumulation to toxic levels in the cytosol and certain subcellular organelles like chloroplast (Flowers and Colmer 2008).
In vitro assays showed that Na+ begins to inhibit most enzymes at concentrations around 100 mM (Greenway and Osmond 1972; Parida and Das 2005). Rubisco seems to be among the most sensitive enzymes, with sharp declines of its carboxylase activity starting even at lower salt concentrations in the assay media (Sivakumar et al. 2000). The precise biochemical causes of NaCl toxicity of Rubisco activity have yet to be explained, although it does not seem to be related to the disruption of the interaction between subunits. In fact, it has been reported that NaCl stimulated the oxygenase activity measured in vitro (Sivakumar et al. 2000). No difference in the sensitivity of Rubisco extracted from halophytes and glycophytes to NaCl has been documented yet (Sivakumar et al. 1998), unlike what has been observed for other enzymes (Munns et al. 2006).
A distinctive response between water and salt stresses at high stress intensities can be described for Rubisco concentration. Thus, while under drought it decreases, under salt stress it increases (Fig. 1). This increase in the content of Rubisco in response to extremely high concentration of NaCl, which is particular to halophyte plants and other photosynthetic organisms (Takabe et al. 1988), may be a compensatory mechanism for the reduced activation of Rubisco.
Growth temperature
Rubisco parameters were not significantly affected by mild heat stress, except for a slight (~20 %) decrease in total activity (Fig. 3a). This small decrease is likely to be due to increased inhibitors blocking Rubisco sites, given the minor decrease in the Rubisco concentration which would not explain the decrease in the total activity (Fig. 3a).
Effects of a high- and b low-temperature stresses on Rubisco parameters. It is important to remark that high and low temperatures refer to the growth temperature and not to the measuring temperature. Data on Rubisco activity were measured at the thermal optimum (i.e., between 20 and 30 °C), and always at the same temperature as the respective control. As for high-temperature stress, to define the different intensities of stress, the absolute increase in Celsius degrees was multiplied by a factor considering the maximum temperature achieved during the treatment. This factor had a value of 1 when the maximum temperature was <30 °C, 2 when the maximum temperature was between 30 and 38 °C, and 3 when the maximum temperature >38 °C. For mild, moderate, and severe stress intensities, the product between the maximum temperature factor and the absolute increase in Celsius degrees was <10, 10–20, and >20, respectively. As for low-temperature stress, the absolute decrease in Celsius degrees was used to define the two intensities of stress: mild-to-moderate stress when the decrease <10 °C, and severe stress when the decrease >10 °C. Number of replicates is indicated at the bottom of each column. See Table 1 for articles and species considered in the analyses. Different letters denote statistically significant differences among different treatments through Duncan test (P < 0.05), being control treatment a
At moderate stress intensities, decreases in Rubisco activity became stronger, and correlated with decreases of the Rubisco concentration (Fig. 3a). Lower expression of Rubisco per area under heat stress has been reported in various species, concomitantly to decreases of its protein content (Vu et al. 2002; Pérez et al. 2011), and may be partly explained by changes in leaf anatomy, such as decreases in leaf mass area and the surface of chloroplasts facing the internal air spaces (Kogami et al. 2001; Yamori et al. 2005).
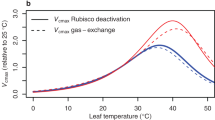
For the temperature-stress analyses, it should be highlighted that growth temperature was considered to be the treatment while measurements of Rubisco activity were made at the same temperature for control and stress treatment plants. Inhibition of enzymatic activity tends to be reversible at mild-to-moderate supra-optimal temperatures, whereas at severely high temperatures inhibition tends to be irreversible (Haldimann and Feller 2004; Sharkey and Zhang 2010). Therefore, it is noteworthy that some parts of the effect of mild-to-moderate heat stress on Rubisco activity and carbamylation state could have been lost when performing the assays at an optimal temperature (Fig. 3a). Under severe heat stress, however, strong irreversible decreases in Rubisco initial (<40 %) and total activity (~50 %) are typically measured (Fig. 3a).
The decrease in total activity under severe heat stress is probably due to both decreased concentration of Rubisco and increased production of inhibitors (Fig. 3a). It has been shown that the production of side-products from RuBP by Rubisco is stimulated in heat stressed leaves (Sharkey et al. 2001; Kim and Portis 2006). However, it is not clear whether increased inhibitors production would result in a stronger inhibition fallover, because a faster release of inhibitors from Rubisco sites may occur at high temperatures (Schrader et al. 2006). Furthermore, at severe heat stress, Rubisco initial activity decreased to <40 % because of decreased carbamylation state (Fig. 3a). This result is in agreement with previous reports identifying Rubisco activase as one of the most heat-sensitive components of the photosynthetic apparatus (Feller et al. 1998; Salvucci et al. 2001), thus leading to Rubisco deactivation at moderate to extreme heat stress intensities. Whether the decline in Rubisco activation state at high temperature is due to limitation in the electron transport capacity rather than a consequence of a direct effect of heat on the integrity of Rubisco activase is still an unresolved question (Hikosaka et al. 2006; Sage and Kubien 2007; Yamori and von Caemmerer 2009). Deactivation due to decreasing CO2 concentrations can be, in principle, discarded because leaf conductances tend to increase up to moderate heat-stress intensities, and because starvation CO2 levels have not been documented even in plants exposed to extreme heat stress (Flexas et al. 2012).
According to the compiled data, the activity of Rubisco measured in vitro at non-stressful temperatures is not significantly affected by low growth temperatures (Fig. 3b). However, when growing at low temperature, the in vivo carboxylase capacity is diminished because of the direct effect of low temperature on the Rubisco kinetic constants, particularly the decreased carboxylase turnover rate (k ccat ) (Sage 2002). In order to compensate for these effects, plants grown under severe cold stress have an increased concentration of Rubisco (Fig. 3b) (Hikosaka et al. 2006). The higher concentration of Rubisco occurs simultaneously with increments of its leaf mass area and leaf N content per area in plants grown at low temperature (Yamori et al. 2010). This effect has also been described for other photosynthetic enzymes (Holaday et al. 1992). Other reported response mechanisms to low growth temperature include acclimation of Rubisco kinetic constants, which may compensate for some of the negative effects of decreased activity under low temperatures (Cavanagh and Kubien 2013).
Overall, the data compiled from the literature demonstrate that the regulation of Rubisco activity differs in plants grown at different temperatures. Heat stress causes a decline in the activity of Rubisco through alteration of the activity of Rubisco activase, concentration of catalytic sites and/or presence of inhibitors, depending on the intensity of the stress. The effects of cold stress on Rubisco were limited to a trend for an increased concentration when growth temperature was severely diminished. Irrespective of the observed trends, the species included in the analysis may differ in their optimum temperature for photosynthesis (Hikosaka et al. 2006), and therefore in their specific response to a given change in the growth temperature.
Heavy metals
Among the studies analyzing the effects of heavy metals on photosynthesis, and particularly on Rubisco, those using toxic concentrations of cadmium are by far the most abundant (Chaffei et al. 2004; Krantev et al. 2008; Ying et al. 2010).
Data from the literature show that Rubisco performance under Cd exposure is conditioned by the concentration of this toxic element in the media (Fig. 4). Rubisco initial activity is almost unchanged at Cd values below 50 μM. On the other hand, Rubisco initial activity is negatively affected after exposure to Cd concentration above 50 μM (Fig. 4). These changes in activity were explained, in some cases, by alterations in the concentration of Rubisco (Pietrini et al. 2003; Chaffei et al. 2004; Ying et al. 2010), although our compilation did not report significant differences in the concentration of the enzyme, when compared to control plants (Fig. 4).
Effects of the concentration of Cd2+ on the initial and total activity, and concentration of Rubisco. Number of replicates is indicated at the bottom of each column. See Table 1 for articles and species considered in the analysis. Different letters denote statistically significant differences among different treatments through Duncan test (P < 0.05), being control treatment a
Different mechanisms have been suggested to explain the influence of Cd on the Rubisco activity. An irreversible dissociation of the Rubisco large and small subunits has been observed under high Cd concentration, thus leading to total inhibition of the enzyme (Malik et al. 1992). Alternatively, it is likely that Cd2+ lowers carboxylase activity by replacing Mg2+ in its catalytic sites (Dias et al. 2013). Indeed, alteration of Rubisco kinetics, including a shift of activity toward RuBP oxygenation after binding to bivalent cations others than Mg2+ was described earlier (Christeller and Laing 1979; Robison et al. 1979). Another mechanism leading to decreased Rubisco carboxylase activity has been related to limited chlorophyll synthesis under Cd stress (Pietrini et al. 2003). Finally, it is not excluded that thiol residues of Rubisco activase could bind Cd, thus inducing Rubisco inactivation (Portis 2003).
The causes of the decline in the total activity that occurred at [Cd] < 50 μM with no concurrent decreases in the concentration of Rubisco (Fig. 4) remains to be elucidated. Increased concentration of inhibitors in the low Cd treatment cannot be discarded, but it is unlikely that plants under low Cd increased the content of Rubisco sites and blocked them by enhancing the production of inhibitors. It is worth noting that the data shown in Fig. 4 are with a relatively low number of species, and data for initial and total activities came from species with different sensitivities to Cd toxicity, thus precluding conclusive trends on the effects of Cd on Rubisco in vitro. Indeed, similar to what occurred with salinity stress, the effects of heavy metals on Rubisco are species specific, and depend on the divergent strategies to cope with the toxicity of heavy metals. For instance, at [Cd2+] around 50 μM, the hyper-accumulator Picris divaricata maintained Rubisco content at control levels (Ying et al. 2010), while a 50 % decrease in the Rubisco content was observed in Solanum lycopersicum (Chaffei et al. 2004).
There is very little information regarding the effect of other heavy metals on Rubisco performance (e.g., Al, Cr, Fe, Mn). From limited studies, it is clear that deleterious effects on Rubisco activity are not only dependent on the concentration, but also on nature of the toxic element (Jiang et al. 2009; Li et al. 2010; Dhir et al. 2011).
Atmospheric (O3 and CO2) and UV changes
The analysis of the compiled data on the effects of increased O3 suggests a trend relatively independent of the intensity of the stress (i.e., of the [O3]). Thus, this trend can be summarized as a similar decrease in the initial and total activities up to about 70 % of the values measured under the control conditions, which can be explained by a similar decline in the concentration of Rubisco (Fig. 5a). This result is in accordance with the observed decline in the levels of rbcS and rbcL mRNA transcripts, and large subunit polypeptides in the O3-treated plants (see Dizengremel 2001 and references therein). In addition, exposure to O3 leads to increased oxidizing stress in the chloroplast (Pell et al. 1997), probably inducing the modification of Rubisco to an unstable form that may be rapidly degraded by proteases (Pell et al. 1994).
Effect of the increase in the atmospheric concentration of a ozone and b CO2 on Rubisco parameters. As for ozone stress, the different intensities were established according to the concentration of ozone in air: mild-to-moderate stress <120 nL L−1, and severe stress >120 nL L−1. As for CO2, two different intensities were established according to the concentration of CO2 in the atmosphere of treated plants with respect to that measured under control conditions. Number of replicates is indicated at the bottom of each column. See Table 1 for articles and species considered in the analyses. Different letters denote statistically significant differences among different treatments through Duncan test (P < 0.05), being control treatment a
Although the concentration of Rubisco activase has been shown to decrease after O3 exposure (Pelloux et al. 2001), there is not a significant decrease in the carbamylation status of Rubisco in plants subjected to [O3] (Fig. 5a), possibly due to stable or increased ratio [Rubisco]/[Rubisco activase]. In spite of this general trend, the phytotoxic effects of O3 on Rubisco activity and concentration have been described to vary depending on various factors, among them, being the species or even genotypes within a given species (Enyedi et al. 1992; Wittig et al. 2007), the developmental stage of the leaf, with the older leaves being more sensitive to the pollutant (Pell et al. 1997; Lütz et al. 2000), or the duration and timing of exposure (Pell et al. 1992; Landry and Pell 1993).
Compiled data analyzing the effects of [CO2] on Rubisco showed that plants exposed to high [CO2] decreased Rubisco activity, especially when [CO2] > 200 % (with respect to the control treatment; Fig. 5b). The lack of effect on the activation state and Rubisco content when plants were fumigated with [CO2] < 200 % suggests that Rubisco inhibitors may play a key role in the depleted carboxylation activity. According to Kane et al. (1998) and Pérez et al. (2011), under elevated [CO2], the production of Rubisco binding inhibitors is enhanced to protect Rubisco from proteolytic activity.
On the contrary, the analyses carried out on studies where plants were exposed to [CO2] > 200 % (with respect to the control treatment) showed that reduced Rubisco content is the primary driver in the regulation of Rubisco activity to high [CO2] (Fig. 5b). A key process conditioning the photosynthetic performance under elevated [CO2] is the capability of plants to adjust their C sink/source balance (Ainsworth et al. 2004). According to this hypothesis, when plants exposed to elevated [CO2] have limitations in increasing C sink strength, they decrease their Rubisco content and consequently photosynthetic rates to balance C source activity and sink capacity (Aranjuelo et al. 2013).
The limited number of articles in the literature reporting Rubisco in vitro data under different UV-B irradiances precluded executing a similar analysis to those performed with the other environmental factors. In spite of this, the compiled data demonstrate a general trend of a decreased activity due to a decline in the concentration of Rubisco (He et al. 1993; Allen et al. 1998), while the carbamylation state remained relatively stable (Jordan et al. 1992; Allen et al. 1998). Prolonged exposure to UV radiation has been shown to decrease the abundance of Rubisco subunit transcripts (Jordan et al. 1992) and to induce the formation of high molecular mass aggregates of Rubisco (Ferreira et al. 1996) through photomodification (Wilson et al. 1995) and photodegradation processes (Caldwell 1993). The decline in the concentration of Rubisco correlated positively with the decrease in A N (r 2 = 0.715, P < 0.01, not shown), confirming previous suggestions that UV-induced inhibition of photosynthetic CO2 uptake is primarily because of changes in the carboxylase capacity (Lingakumar and Kulandaivelu 1993).
Drought, high temperature, and CO2 effects on Rubisco activity may determine climate change impacts on the leaf CO2 assimilation capacity
The nature of climate change has been typically summarized by higher atmospheric [CO2] and growing temperatures, and varying precipitation patterns potentially leading to more frequent and severe drought episodes (Gornall et al. 2010). The comprehension of how Rubisco responds to these main drivers of climate change and whether induced changes in Rubisco activity impact the photosynthetic capacity of plants is of pivotal importance to predict consequences of future climate on agriculture and natural ecosystems. According to data in Fig. 6, changes in Rubisco initial activity and carbamylation state induced by drought or increased growth temperature correlated positively with changes in A N (expressed as a % of the control treatment values). These results suggest that the decrease in the photosynthetic capacity of plants induced by drought and heat stress is partially explained by changes in the activity and activation state of Rubisco in vitro.
Relationship between the net CO2 assimilation rate (A N) and Rubisco initial activity (a, d, g), carbamylation (b, e, h), and concentration (c, f, i), under the following environmental conditions: drought (a, b, c), heat stress (d, e, f), and elevated CO2 (g, h, i). All parameters are expressed in percentage with respect to the control values. Regression line, altogether with regression coefficient and significance level are indicated when the relationship was significant (P < 0.05)
Correlation analyses in Fig. 6g–i indicate that changes in A N in response to increased [CO2] were significantly related to Rubisco initial activity, but not to its carbamylation state or concentration. A deeper analysis of these plots shows that regardless of the Rubisco parameters, most studies reported an increase in A N under elevated [CO2]. For instance, Rubisco initial activity decreased under elevated [CO2] in all the compiled studies, and the extent of this decrease partly determined the response of A N (Fig. 6g). Although the increase in A N under elevated [CO2] is caused, in a large proportion, by the higher intercellular CO2 availability, the importance of Calvin cycle enzymes other than Rubisco (aldolase, sedoheptulose 1,7-bisphosphatase and transketolase) may have higher control coefficient on photosynthesis (Lefebvre et al. 2005; Uematsu, et al. 2012; Aranjuelo et al. 2013). It is remarkable that even with a 50 % reduction in the Rubisco content, plants exposed to elevated [CO2] were capable to maintain and/or increase A N (Fig. 6i). Because under non-CO2 limiting conditions plants have an excess of Rubisco, redistribution of the excess of N invested in Rubisco and partitioning to other organs and limiting processes results in increased capacity for CO2 fixation (Ainsworth and Rogers 2007).
With regard to other environmental factors analyzed in this review, changes in A N due to salt stress correlated well with changes in Rubisco carbamylation and concentration (Table 2). As for heavy metals and O3, changes in A N only correlated with modifications of the Rubisco concentration. Finally, in cold acclimated plants, the alteration of A N was explained by increases in the initial activity (Table 2). A general trend is that the modification of the photosynthetic capacity under those environmental factors being categorized as phytotoxins (salt, heavy metals, and O3) are mainly associated with alterations in the concentration of Rubisco, while changes in the activity/activation of the enzyme are responsible of the affected photosynthesis under water and temperature stresses and elevated CO2.
Despite being beyond the scope of this article, the relevance of the interaction between the different environmental factors, associated with synergistic and antagonistic phenomena, needs to be mentioned. For instance, in wheat, it was observed that although elevated [CO2] and temperature affected negatively the Rubisco content, the combination of both factors induced the stimulation of k ccat (Pérez et al. 2011). Similar interactive effects have been described among almost all environmental factors included in the present review (e.g., Kytöviita et al. 1999; Pelloux et al. 2001; Tezara et al. 2002; Aranjuelo et al. 2005), along with nutrient availability (e.g., Correia et al. 2005; Guo et al. 2007; Yamori et al. 2011), and they must be considered when extrapolating results to field conditions, where usually two or more stresses take place simultaneously.
In summary, the present analysis of the effects of individual stresses associated with climate change together with the scarce but valuable knowledge on the interactions of some of these stresses, strongly suggest that decreased Rubisco activity will be a major plant response to climate change conditions, which is currently often neglected but should be considered in prediction models on plant productivity in the near future.
Concluding remarks
Acclimation of the key enzyme Rubisco to cope with fluctuations in the environment may be of great importance for plant survival and crop yield. There are data reporting differential capacities of plants from different climates, different functional types, or branches of evolution, to acclimate. However, by compiling most of the existing data on Rubisco activity under variable abiotic factors, we have demonstrated the existence of some trends describing how Rubisco activity is affected by a range of abiotic stresses. The results reveal some common patterns of response regardless of the particular stress and demonstrate that significant reductions in the activity of Rubisco take place when the intensity of the stress becomes severe (Table 3). Furthermore, the decreased state of activation of Rubisco (i.e., indicative of de-carbamylation and/or binding of inhibitory sugar phosphates) contributes to the loss of Rubisco activity particularly under drought and heat stress (Table 3). Finally, a significant relationship between Rubisco in vitro parameters and in vivo CO2 assimilation was found for all environmental factors. For those factors related to soil toxicity (salt, heavy metals, etc.), to the atmosphere (O3), or to solar irradiance (UV-B), the decrease in CO2 assimilation with stress is associated with decreased Rubisco content (Table 2). For those factors which directly affect the availability of CO2 for carboxylation (drought, [CO2]), or high temperature extremes which directly affect chloroplast function, the results indicate that the reduction in CO2 assimilation is strongly associated with reduced state of activation of Rubisco (e.g., which may occur by reduced level or function of Rubisco activase). In general, this down-regulation in Rubisco activity may occur through signal transduction processes as a consequence of the stress inhibiting some part of photosynthesis or subsequent acclimation to stress under the prevailing environmental conditions.
References
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Ainsworth EA, Rogers A, Nelson R, Long SP (2004) Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric For Meteorol 122:85–94
Allen DJ, Nogués S, Baker NR (1998) Ozone depletion and increased UV-B radiation: is there a real threat to photosynthesis? J Exp Bot 49:1775–1788
Alonso A, Pérez P, Morcuende R, Martinez-Carrasco R (2008) Future CO2 concentrations, though not warmer temperatures, enhance wheat photosynthesis temperature responses. Physiol Plantarum 132:102–112
Aranjuelo I, Pérez P, Hernández L, Irigoyen JJ, Zita G, Martínez-Carrasco R, Sánchez-Díaz M (2005) The response of nodulated alfalfa to water supply, temperature and elevated CO2: photosynthetic down-regulation. Physiol Plantarum 123:348–358
Aranjuelo I, Irigoyen JJ, Sánchez-Díaz M (2007) Effect of elevated temperature and water availability on CO2 exchange and nitrogen fixation of nodulated alfalfa plants. Environ Exp Bot 59:99–108
Aranjuelo I, Irigoyen JJ, Nogués S, Sánchez-Díaz M (2009) Elevated CO2 and water-availability effect on gas exchange and nodule development in N2-fixing alfalfa plants. Environ Exp Bot 65:18–26
Aranjuelo I, Cabrera-Bosquet L, Morcuende R, Avice JC, Nogués S, Araus JL, Martínez-Carrasco R, Pérez P (2011) Does ear C sink strength contribute to overcoming photosynthetic acclimation of wheat plants exposed to elevated CO2? J Exp Bot 62:3957–3969
Aranjuelo I, Sanz-Sáez A, Jauregui I, Irigoyen JJ, Araus JL, Sánchez-Díaz M, Erice G (2013) Harvest index, a parameter conditioning responsiveness of wheat plants to elevated CO2. J Exp Bot. doi:10.1093/jxb/ert081
Bischof K, Hanelt D, Wiencke C (2000) Effects of ultraviolet radiation on photosynthesis and related enzyme reactions of marine macroalgae. Planta 211:555–562
Bota J, Medrano H, Flexas J (2004) Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol 162:671–681
Brendley B, Pell EJ (1998) Ozone-induced changes in biosynthesis of Rubisco and associated compensation to stress in foliage of hybrid poplar. Tree Physiol 18:81–90
Bunce JA, Sicher RC (2003) Daily irradiance and feedback inhibition of photosynthesis at elevated carbon dioxide concentration in Brassica oleracea. Photosynthetica 41:481–488
Caldwell CR (1993) Ultraviolet-induced photodegradation of cucumber (Cucumis sativus L.) microsomal and soluble protein tryptophanyl residues in vitro. Plant Physiol 101:947–953
Carmo-Silva AE, Keys AJ, Andralojc PJ, Powers SJ, Arrabaça MC, Parry MAJ (2010) Rubisco activities, properties, and regulation in three different C4 grasses under drought. J Exp Bot 61:2355–2366
Carmo-Silva AE, Gore MA, Andrade-Sanchez P, French AN, Hunsaker DJ, Salvucci ME (2012) Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ Exp Bot 83:1–11
Cavaco AM, Da Silva-Bernardes A, Arrabaça MC (2003) Effects of long-term chilling on growth and photosynthesis of the C4 gramineae Paspalum dilatatum. Physiol Plantarum 119:87–96
Cavanagh AP, Kubien DS (2013) Can phenotypic plasticity in Rubisco performance contribute to photosynthetic acclimation? Photosynth Res. doi:10.1007/s11120-013-9816-3
Centritto M, Jarvis P (1999) Long-term effects of elevated carbon dioxide concentration and provenance on four clones of Sitka spruce (Picea sitchensis). II. Photosynthetic capacity and nitrogen use efficiency. Tree Physiol 19:807–814
Centritto M, Magnan F, Lee H, Jarvis P (1999) Interactive effects of elevated [CO2] and drought on cherry (Prumus avium) seedlings. II. Photosynthetic capacity and water relations. New Phytol 141:141–153
Chaffei C, Pageau K, Suzuki A, Gouia H, Ghorbel MH, Masclaux-Daubresse C (2004) Cadmium toxicity induced changes in nitrogen management in Lycopersicon esculentum leading to a metabolic safeguard through an amino acid storage strategy. Plant Cell Physiol 45:1681–1693
Chauan S, Srivalli S, Nautiyal AR, Khanna-Chopra R (2009) Wheat cultivars differing in heat tolerance show a differential response to monocarpic senescence under high-temperature stress and the involvement of serine proteases. Photosynthetica 47:536–547
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Christeller JT, Laing WA (1979) Effects of manganese ions and magnesium ions on the activity of soya-bean ribulose bisphosphate carboxylase/oxygenase. Biochem J 183:747–750
Cleland WW, Andrews TJ, Gutteridge S, Hartman FC, Lorimer GH (1998) Mechanism of Rubisco: the carbamate as general base. Chem Rev 98:549–561
Correia CM, Pereira JMM, Coutinho JF, Björn LO, Torres-Pereira JMG (2005) Ultraviolet-B radiation and nitrogen affect the photosynthesis of maize: a mediterranean field study. Eur J Agron 22:337–347
Dann MS, Pell EJ (1989) Decline of activity and quantity of ribulose bisphosphate carboxylase/oxygenase and net photosynthesis in ozone-treated potato foliage. Plant Physiol 91:427–432
Debez A, Saadaoui D, Ramani B, Ouerghi Z, Koyro HW, Huchzermeyer B, Abdelly C (2006) Leaf H+-ATPase activity and photosynthetic capacity of Cakile maritima under increasing salinity. Environ Exp Bot 57:285–295
Degl’Innocenti E, Vaccà C, Guidi L, Soldatini GF (2003) CO2 photoassimilation and chlorophyll fluorescence in two clover species showing different response to O3. Plant Physiol Biochem 41:485–493
Delfine S, Alvino A, Zacchini M, Loreto F (1998) Consequences of salt stress on conductance to CO2 diffusion, Rubisco characteristics and anatomy of spinach leaves. Aust J Plant Physiol 25:395–402
Dhir B, Sharmila P, Pardha Saradhi P, Nasim SA (2009) Physiological and antioxidant responses of Salvinia natans exposed to chromium-rich wastewater. Ecotox Environ Safe 72:1790–1797
Dhir B, Sharmila P, Pardha Saradhi P, Sharma P, Kumar R, Mehta D (2011) Heavy metal induced physiological alterations in Salvinia natans. Ecotox Environ Safe 74:1678–1684
Di Cagno R, Guidi L, De Gara L, Soldatini GF (2001) Combined cadmium and ozone treatments affect photosynthesis and ascorbate-dependent defenses in sunflower. New Phytol 151:627–636
Dias MC, Brüggemann W (2010) Limitations of photosynthesis in Phaseolus vulgaris under drought stress: gas exchange, chlorophyll fluorescence and Calvin cycle enzymes. Photosynthetica 48:96–102
Dias MC, Monteiro C, Mouthino-Pereira J, Correia C, Gonçalves B, Santos C (2013) Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiol Plant. doi:10.1007/s11738-012-1167-8
Dizengremel P (2001) Effects of ozone on the carbon metabolism of forest trees. Plant Physiol Biochem 39:729–742
Dwyer SA, Ghannoum O, Nicotra A, von Caemmerer S (2007) High temperature acclimation of C4 photosynthesis is linked to changes in photosynthetic biochemistry. Plant Cell Environ 30:53–66
Eichelmann H, Talts E, Oja V, Padu E, Laisk A (2009) Rubisco in planta kcat is regulated with photosynthetic electron transport. J Exp Bot 60:4077–4088
Enyedi AJ, Eckardt NA, Pell AJ (1992) Activity of ribulose bisphosphate carboxylase/oxygenase from potato cultivars with differential response to ozone stress. New Phytol 122:493–500
Erice G, Aranjuelo I, Irigoyen JJ, Sánchez-Díaz M (2007) Effect of elevated CO2, temperature and limited water supply on antioxidant status during regrowth of nodulated alfalfa. Physiol Plantarum 130:33–45
Feller U, Crafts-Brandner SJ, Salvucci ME (1998) Moderately high temperatures inhibit ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase-mediated activation of Rubisco. Plant Physiol 116:539–546
Feng L, Han Y, Liu G, An B, Yang J, Yang G, Li Y (2007) Overexpression of sedoheptulose-1,7-bisphosphatase enhances photosynthesis and growth under salt stress in transgenic rice plants. Funct Plant Biol 34:822–834
Ferreira RMB, Franco E, Teixeira ARN (1996) Covalent dimerization of ribulose bisphosphate carboxylase subunits by UV radiation. Biochem J 318:227–234
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240
Flexas J, Medrano H (2002) Drought-inhibition of photosynthesis in C3 plants; stomatal and non-stomatal limitations revisited. Ann Bot 89:183–189
Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD (2004) Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol 6:269–279
Flexas J, Bota J, Galmés J, Medrano H, Ribas-Carbó M (2006a) Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol Plantarum 127:343–352
Flexas J, Ribas-Carbó M, Bota J, Galmés J, Henkle M, Martínez-Cañellas S, Medrano H (2006b) Decreased Rubisco activity during water stress is induced by stomatal closure, not by decreased relative water content. New Phytol 172:73–82
Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyer E, Ferrio JP, Gago J, Gallé A, Galmés J, Kodama N, Medrano H, Niinemets U, Peguero-Pina JJ, Pou A, Ribas-Carbó M, Tomás M, Tosens T, Warren CR (2012) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci 193–194:73–84
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Fontaine V, Pelloux J, Podor M, Afif D, Gérant D, Grieu P, Dizengremel P (1999) Carbon fixation in Pinus halepensis submitted to ozone. Opposite response of ribulose-1,5-bisphosphate carboxylase/oxygenase and phosphoenolpyruvate carboxylase. Physiol Plantarum 105:187–192
Galmés J, Ribas-Carbó M, Medrano H, Flexas J (2011a) Rubisco activity in Mediterranean species is regulated by the chloroplastic CO2 concentration under water stress. J Exp Bot 62:653–665
Galmés J, Conesa MA, Ochogavía JM, Perdomo JA, Francis D, Ribas-Carbó M, Savé R, Flexas J, Medrano H, Cifre J (2011b) Physiological and morphological adaptations in relation to water use efficiency in mediterranean accessions of Solanum lycopersicum. Plant Cell Environ 34:245–260
Gérant D, Podor M, Grieu P, Afif D, Cornu S, Morabito D, Banvoy J, Robin C, Dizengremel P (1996) Carbon metabolism enzyme activities and carbon partitioning in Pinus halepensis Mill. exposed to mild drought and ozone. J Plant Physiol 14:142–147
Gesch RW, Kang I-H, Mallo-Meagher M, Vu JCV, Boote KJ, Allen LH Jr, Bowes G (2003) Rubisco expression in rice leaves is related to genotypic variation of photosynthesis under elevated growth CO2 and temperature. Plant Cell Environ 26:1941–1950
Gornall J, Betts R, Burke E, Clark R, Camp J, Willett K, Wiltshire A (2010) Implications of climate change for agricultural productivity in the early twenty-first century. Phil Trans R Soc B 365:2973–2989
Greenway H, Osmond CB (1972) Salt responses of enzymes from species differing in salt tolerance. Plant Physiol 49:256–259
Guidi L, Deg’Innocenti E, Soldatini GF (2003) Assimilation of CO2, enzyme activation and photosynthetic electron transport in bean leaves, as affected by high light and ozone. New Phytol 156:377–388
Guo S, Chen G, Zhou Y, Shen Q (2007) Ammonium nutrition increases photosynthesis rate under water stress at early development stage of rice (Oryza sativa L.). Plant Soil 296:115–124
Haldimann P, Feller U (2004) Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ 27:1169–1183
He J, Huang LK, Chow WS, Whitecross MI, Anderson JM (1993) Effect of supplementary ultraviolet-B radiation on rice and pea plants. Aust J Plant Physiol 20:129–142
He J, Huang L-K, Chow WS, Whitecrosss MI, Anderson JM (1994) Responses of rice and pea plants to hardening with low doses of ultraviolet-B radiation. Aust J Plant Physiol 21:563–574
Hikosaka K, Ishakawa K, Borjigidai A, Muller O, Onoda Y (2006) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57:291–302
Holaday AS, Martindale W, Alred R, Brooks AL, Leegood RC (1992) Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature. Plant Physiol 98:1105–1114
Hu L, Wang Z, Huang B (2009) Photosynthetic responses of bermudagrass to drought stress associated with stomatal and metabolic limitations. Crop Sci 49:1902–1909
Hu L, Wang Z, Huang B (2010) Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis from drought stress in a C3 perennial grass species. Physiol Plantarum 139:93–106
Inclán R, Gimeno BS, Dizengremel P, Sanchez M (2005) Compensation processes of Aleppo pine (Pinus halepensis Mill.) to ozone exposure and drought stress. Environ Pollut 137:517–524
Jacob J, Greitner Drake BG (1995) Acclimation of photosynthesis in relation to Rubisco and non-structural carbohydrate contents and in situ carboxylase activity in Scirpus olneyi grown at elevated CO2 in the field. Plant, Cell Environ 18:875–884
Jiang HX, Tang N, Zheng JG, Li Y, Chen LS (2009) Phosphorus alleviates aluminum-induced inhibition of growth and photosynthesis in Citrus grandis seedlings. Physiol Plantarum 137:298–311
Jordan BR, He J, Chow S, Anderson JM (1992) Changes in mRNA levels and polypeptide subunits of ribulose 1,5-bisphosphate carboxylase in response to supplementary ultraviolet-B radiation. Plant Cell Environ 15:91–98
Kane HJ, Wilkin JM, Portis AR Jr, Andrews TJ (1998) Potent inhibition of ribulose-bisphosphate carboxylase by an oxidized impurity in ribulose-1,5-bisphosphate. Plant Physiol 117:1059–1069
Kasai K, Fukayama H, Uchida N, Mori N, Yasuda T, Oji Y, Nakamura C (1998) Salinity tolerance in Triticum aestivum-Lophopyrum elongatum amphiploid and 5E disomic addition line evaluated by NaCl effects on photosynthesis and respiration. Cereal Res Commun 26:281–287
Kim K, Portis AR Jr (2005) Temperature dependence of photosynthesis in Arabidopsis plants with modifications in Rubisco activase and membrane fluidity. Plant Cell Physiol 46:522–530
Kim K, Portis AR Jr (2006) Kinetic analysis of the slow inactivation of Rubisco during catalysis: effects of temperature, O2 and Mg++. Photosynth Res 87:195–204
Kogami H, Hanba YT, Kibe T, Terashima I, Masuzawa T (2001) CO2 transfer conductance, leaf structure and carbon isotope composition of Polygonum cuspidatum leaves from low and high altitudes. Plant Cell Environ 24:529–538
Krantev A, Yordanova R, Janda T, Szalai G, Popova L (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165:920–931
Kulandaivelu G, Nedunchezhian N (1993) Synergistic effects of ultraviolet-B enhanced radiation and growth temperature on ribulose 1,5-bisphosphate carboxylase and (CO2)-C-14 fixation in Vigna sinensis L. Photosynthetica 29:377–383
Kytöviita M, Pelloux J, Fontaine V, Botton B, Dizengremel P (1999) Elevated CO2 does not ameliorate effects of ozone on carbon allocation in Pinus halepensis and Betula pendula in symbiosis with Paxillus involutus. Physiol Plantarum 106:370–377
Lal A, Ku M, Edwards G (1996) Analysis of inhibition of photosynthesis due to water stress in the C3 species Hordeum vulgare and Vicia faba: electron transport, CO2 fixation and carboxylation. Photosynth Res 49:57–69
Landry LG, Pell EJ (1993) Modification of Rubisco and altered proteolytic activity in O3-stressed hybrid poplar (Populus maximowizii x trichocarpa). Plant Physiol 101:1355–1362
Lefebvre S, Lawson T, Zakhleniuk OV, Lloyd JC, Raines CA (2005) Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol 138:451–460
Lehnherr B, Grandjean A, Machler F, Fuhrer J (1987) The effect of ozone in ambient air on ribulose bisphosphate carboxylase/oxygenase activity decreases photosynthesis and grain yield in wheat. J Plant Physiol 130:189–200
Leitao L, Bethenod O, Biolley JP (2007) The impact of ozone on juvenile maize (Zea mays L.) plant photosynthesis: effects on vegetative biomass, pigmentation, and carboxylases (PEPc and Rubisco). Plant Biol 9:478–488
Leitao L, Dizengremel P, Biolley JP (2008) Foliar CO2 fixation in bean (Phaseolus vulgaris L.) submitted to elevated ozone: distinct changes in Rubisco and PEPc activities in relation to pigment content. Ecotox Environ Safe 69:531–540
Li J, Gale J, Novoplansky A, Barak S, Volokita M (1999) Response of tomato plants to saline water as affected by carbon dioxide supplementation. II. Physiological responses. J Hort Sci Biotechnol 74:238–242
Li Q, Chen L, Jiang H, Tang N, Yang L, Lin Z, Li Y (2010) Effects of manganese-excess on CO2 assimilation, carbohydrates and photosynthetic electron transport of leaves, and antioxidant systems of leaves and roots in Citrus grandis seedlings. BMC Plant Biol 10:1–16
Lingakumar K, Kulandaivelu G (1993) Changes induced by ultraviolet-B radiation in vegetative growth, foliar characteristics and photosynthetic activities in Vigna unguiculata. Aust J Plant Physiol 20:299–308
Liu X, Huang B (2008) Photosynthetic acclimation to high temperatures associated with heat tolerance in creeping bentgrass. J Plant Physiol 165:1947–1953
Lütz C, Anegg S, Gerant D, Alaoui-Sossé B, Gérard J, Dizengremel P (2000) Beech trees exposed to high CO2 and to simulated ozone levels: effects on photosynthesis, chloroplast components and leaf enzyme activity. Physiol Plantarum 109:252–259
Malik D, Sheoran IS, Singh R (1992) Carbon metabolism in leaves of cadmium-treated wheat seedlings. Plant Physiol Biochem 30:223–229
Maroco J, Rodrigues M, Lopes C, Chaves M (2002) Limitations to leaf photosynthesis in field-grown grapevine under drought—metabolic and modelling approaches. Funct Plant Biol 29:451–459
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164:601–610
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Munns R, James RA, Läuchli A (2006) Approaches to increasing the salt tolerance of wheat and other cereals. J Exp Bot 57:1025–1043
Olesen B, Madsen T (2000) Growth and physiological acclimation to temperature and inorganic carbon availability by two submerged aquatic macrophyte species, Callitriche cophocarpa and Elodea canadensis. Funct Ecol 14:252–260
Pagter M, Bragato C, Brix H (2005) Tolerance and physiological responses of Phragmites australis to water deficit. Aquat Bot 81:285–299
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotox Environ Safe 60:324–349
Parry MAJ, Andralojc PJ, Khan S, Lea PJ, Keys AJ (2002) Rubisco activity: effects of drought stress. Ann Bot 89:833–839
Parry MAJ, Keys AJ, Madgwick PJ, Carmo-Silva AE, Andralojc PJ (2008) Rubisco regulation: a role for inhibitors. J Exp Bot 59:1569–1580
Parry MAJ, Andralojc PJ, Scales JC, Salvucci ME, Carmo-Silva AE, Alonso H, Whitney SM (2013) Rubisco activity and regulation as targets for crop improvement. J Exp Bot 64:717–730
Pell EJ, Eckardt N, Enyedi AJ (1992) Timing of ozone stress and resulting status of ribulose bisphosphate carboxylase/oxygenase and associated net photosynthesis. New Phytol 120:397–405
Pell EJ, Eckardt NA, Glick RE (1994) Biochemical and molecular basis for impairment of photosynthetic potential. Photosynth Res 39:453–462
Pell EJ, Schlagnhaufer CD, Arteca RN (1997) Ozone-induced oxidative stress: mechanisms of action and reaction. Physiol Plantarum 100:264–273
Pell EJ, Sinn JP, Brendley BW, Samuelson L, Vinten-Johansen C, Tien M, Skillman J (1999) Differential response of four tree species to ozone-induced acceleration of foliar senescence. Plant Cell Environ 22:779–790
Pelloux J, Jolivet Y, Fontaine V, Banvoy J, Dizengremel P (2001) Changes in Rubisco and Rubisco activase gene expression and polypeptide content in Pinus halepensis M. subjected to ozone and drought. Plant Cell Environ 24:123–131
Pérez P, Morcuende R, Martín del Molino I, Martínez-Carrasco R (2005) Diurnal changes of Rubisco in response to elevated CO2, temperature and nitrogen in wheat grown under temperature gradient tunnels. Environ Exp Bot 53:13–27
Pérez P, Alonso A, Zita G, Morcuende R, Martínez-Carrasco R (2011) Down-regulation of Rubisco activity under combined increases of CO2 and temperature minimized by changes in Rubisco kcat in wheat. Plant Growth Regul 65:439–447
Pietrini F, Iannelli M, Pasqualini S, Massacci A (2003) Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmites australis (Cav.) Trin. ex Steudel. Plant Physiol 133:829–837
Pons TJ (2012) Interaction of temperature and irradiance effects on photosynthetic acclimation in two accessions of Arabidopsis thaliana. Photosynth Res 113:207–219
Portis AR Jr (2003) Rubisco activase—Rubisco’s catalytic chaperone. Photosynth Res 75:11–27
Prasad PVV, Vu J, Boote K, Allen LH (2009) Enhancement in leaf photosynthesis and up regulation of Rubisco in the C4 sorghum plant at elevated growth carbon dioxide and temperature occur at early stages of leaf ontogeny. Funct Plant Biol 36:761–769
Pritchard S, Ju Z, Santen EV, Qiu J, Weaver D, Prior S, Roger H (2000) The influence of elevated CO2 on the activities of antioxidative enzymes in two soybean genotypes. Funct Plant Biol 27:1061–1068
Pushpalatha P, Sharma-Natu P, Ghildiyal MC (2009) Photosynthetic response of wheat cultivar to long-term exposure to elevated temperature. Photosynthetica 46:552–556
Robison PD, Martin MN, Tabita FR (1979) Differential effects of metal ions on Rhodospirillum rubrum ribulose bisphosphate carboxylase/oxygenase and stoichiometric incorporation of HCO3 − into a cobalt(III)-enzyme complex. Biochemistry 18:4453–4458
Rogers A, Ellsworth DS, Humphries SW (2001) Possible explanation of the disparity between the in vitro and in vivo measurements of Rubisco activity: a study in loblolly pine grown in elevated pCO2. J Exp Bot 52:1555–1561
Sage RF (2002) Variation in the k(cat) of Rubisco in C(3) and C(4) plants and some implications for photosynthetic performance at high and low temperature. J Exp Bot 53:609–620
Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant Cell Environ 30:1086–1106
Salvucci ME, Osteryoung KW, Crafts-Brandner SJ, Vierling E (2001) Exceptional sensitivity of Rubisco activase to thermal denaturation in vitro and in vivo. Plant Physiol 127:1053–1064
Savitch L, Massacci A, Gray G, Huner N (2000) Acclimation to low temperature or high light mitigates sensitivity to photoinhibition: roles of the Calvin cycle and the Mehler reaction. Funct Plant Biol 27:253–264
Schrader SM, Kane HJ, Sharkey TD, von Caemmerer S (2006) High temperature enhances inhibitor production but reduces fallover in tobacco Rubisco. Funct Plant Biol 33:921–929
Sharkey TD, Seemann JR (1989) Mild water stress effects on carbon-reduction-cycle intermediates, ribulose bisphosphate carboxylase activity, and spatial homogeneity of photosynthesis in intact leaves. Plant Physiol 89:1060–1065
Sharkey TD, Zhang R (2010) High temperature effects on electron and proton circuits of photosynthesis. J Integr Plant Biol 52:712–722
Sharkey TD, Badger MR, von Caemmerer S, Andrews TJ (2001) Increased heat sensitivity of photosynthesis in tobacco plants with reduced Rubisco activase. Photosynth Res 67:147–156
Sicher R, Bunce J (1997) Relationship of photosynthetic acclimation to changes of Rubisco activity in field-grown winter wheat and barley during growth in elevated carbon dioxide. Photosynth Res 52:27–38
Sicher RC, Bunce JA (2001) Adjustments of net photosynthesis in Solanum tuberosum in response to reciprocal changes in ambient and elevated growth CO2 partial pressures. Physiol Plantarum 112:55–61
Singh B, Usha K (2003) Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Growth Reg 39:137–141
Singh MP, Singh DK, Rai M (2007) Assessment of growth, physiological and biochemical parameters and activities of antioxidative enzymes in salinity tolerant and sensitive basmati rice varieties. J Agron Crop Sci 193:398–412
Sivakumar P, Sharmila P, Saradhi PP (1998) Proline suppresses Rubisco activity in higher plants. Biochem Biophys Res Commun 252:428–432
Sivakumar P, Sharmila P, Saradhi PP (2000) Proline alleviates salt-stress-induced enhancement in ribulose-1,5-bisphosphate oxygenase activity. Biochem Biophys Res Commun 279:512–515
Soussi M, Ocana A, Lluch C (1998) Effects of salt stress on growth, photosynthesis and nitrogen fixation in chick-pea (Cicer arietinum L.). J Exp Bot 49:1329–1337
Takabe T, Incharoensakdi A, Arakawa K, Yokota S (1988) CO2 fixation rate and Rubisco content increase in the halotolerant cyanobacterium, Aphanothece halophytica, grown in high salinities. Plant Physiol 88:1120–1124
Tezara W, Mitchell V, Driscoll SP, Lawlor DW (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401:914–917
Tezara W, Mitchell V, Driscoll SP, Lawlor DW (2002) Effects of water deficit and its interaction with CO2 supply on the biochemistry and physiology of photosynthesis in sunflower. J Exp Bot 53:1781–1791
Uematsu K, Suzuki N, Iwamae T, Inui M, Yukawa H (2012) Increased fructose 1,6-bisphosphate aldolase in plastids enhances growth and photosynthesis of tobacco plants. J Exp Bot 63:3001–3009
Vu JCV (2005) Acclimation of peanut (Arachis hypogaea L.) leaf photosynthesis to elevated growth CO2 and temperature. Environ Exp Bot 53:85–95
Vu JCV, Allen LH Jr (2009) Growth at elevated CO2 delays the adverse effects of drought stress on leaf photosynthesis of the C4 sugarcane. J Plant Physiol 166:107–116
Vu JCV, Allen LH, Bowes G (1983) Effects of light and elevated atmospheric CO2 on the Ribulose bisphosphate carboxylase activity and ribulose bisphosphate level of soybean leaves. Plant Physiol 73:729–734
Vu JCV, Allen LH, Bowes G (1987) Drought stress and elevated CO2 effects on soybean Ribulose bisphosphate carboxylase activity and canopy photosynthetic rates. Plant Physiol 83:573–578
Vu JCV, Baker J, Pennanen A, Allen L, Bowes J, Boote K (1998) Elevated CO2 and water deficit effects on photosynthesis, ribulose bisphosphate carboxylase-oxygenase, and carbohydrate metabolism in rice. Physiol Plantarum 103:327–339
Vu JCV, Gesch RW, Pennanen AH, Allen JLH, Boote KJ, Bowes G (2001) Soybean photosynthesis, Rubisco, and carbohydrate enzymes function at supraoptimal temperatures in elevated CO2. J Plant Physiol 158:295–307
Vu JCV, Newman YC, Allen LH Jr, Gallo-Meagher M, Zhang M-Q (2002) Photosynthetic acclimation of young sweet orange trees to elevated growth CO2 and temperature. J Plant Physiol 159:147–157
Wang Y, Nii N (2000) Chlorophyll, ribulose bisphosphate carboxylase-oxygenase, glycine betaine content, photosynthesis and transpiration in Amaranthus tricolor leaves during salt stress. J Hortic Sci Biotechnol 75:623–627
Wang D, Li XF, Zhou ZJ, Feng XP, Yang WJ, Jiang DA (2010) Two Rubisco activase isoforms may play different roles in photosynthetic heat acclimation in the rice plant. Physiol Plantarum 139:55–67
Whitney SM, Houtz RL, Alonso H (2011) Advancing our understanding and capacity to engineer nature’s CO2-sequestring enzyme, Rubisco. Plant Physiol 155:27–35
Wilson ML, Ghosh S, Gerhardt KE, Holland N, Babu TS, Edelman M, Dumbroff EB, Greenburg BM (1995) In-vivo photomodification of ribulose1,5-bisphosphate carboxylase/oxygenase holoenzyme by ultraviolet-B radiation—formation of a 66-kiloDalton variant of the large subunit. Plant Physiol 109:221–229
Wittig VE, Ainsworth EA, Long SP (2007) To what extent do current and projected increases in surface ozone affect photosynthesis and stomatal conductance of trees? A meta-analytic review of the last 3 decades of experiments. Plant Cell Environ 30:1150–1162
Xu Q, Huang B (2001) Morphological and physiological characteristics associated with heat tolerance in creeping bentgrass. Crop Sci 41:127–133
Yamasaki T, Yamakawa T, Yamane Y, Koike H, Satoh K, Katoh S (2002) Temperature acclimation of photosynthesis and related changes in photosystem II electron transport in winter wheat. Plant Physiol 128:1087–1097
Yamori W, von Caemmerer S (2009) Effect of Rubisco activase deficiency on the temperature response of CO2 assimilation rate and Rubisco activation state: insights from transgenic tobacco with reduced amounts of Rubisco activase. Plant Physiol 151:2073–2082
Yamori W, Noguchi K, Terashima I (2005) Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant, Cell Environ 28:536–547
Yamori W, Suzuki K, Koguchi K, Nakai M, Terashima I (2006) Effects of Rubisco kinetics and Rubisco activation state on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Environ 29:1659–1670
Yamori W, Noguchi K, Hikosaka K, Terashima I (2010) Phenotypic plasticity in photosynthetic temperature acclimation among crop species with different cold tolerances. Plant Physiol 152:388–399
Yamori W, Nagai T, Makino A (2011) The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant Cell Environ 34:764–777
Ying RR, Qiu RL, Tang YT, Hu PJ, Qiu H, Chen HR, Shi TH (2010) Cadmium tolerance of carbon assimilation enzymes and chloroplast in Zn/Cd hyperaccumulator Picris divaricata. J Plant Physiol 167:81–87
Zhou YH, Yu JQ, Mao WH, Huang LF, Song XS, Nogués S (2006) Genotypic variation of Rubisco expression, photosynthetic electron flow and antioxidant metabolism in the chloroplasts of chill-exposed cucumber plants. Plant Cell Physiol 47:192–199
Zhou Y, Lam HM, Zhang J (2007) Inhibition of photosynthesis and energy dissipation induced by water and high light stresses in rice. J Exp Bot 58:1207–1217
Zhu J, Meinzer F (1999) Efficiency of C4 photosynthesis in Atriplex lentiformis under salinity stress. Funct Plant Biol 26:79–86
Acknowledgments
This research was supported by Plan Nacional (Spain) projects AGL2009-07999 (awarded to JG) and AGL2011-30386-CO2-O2 (awarded to IA). Iker Aranjuelo was the recipient of a Ramón y Cajal research contract funded by the Economy and Competitivity Ministry (Spain). The authors would like to thank Prof. Edwards, two anonymous reviewers, and Dr. Ribas-Carbó, whose comments contributed toward significantly improving the present article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galmés, J., Aranjuelo, I., Medrano, H. et al. Variation in Rubisco content and activity under variable climatic factors. Photosynth Res 117, 73–90 (2013). https://doi.org/10.1007/s11120-013-9861-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-013-9861-y