Abstract
The phytotoxin coronatine (COR) is a jasmonic acid mimic produced by several pathovars of plant pathogen. In this study, we evaluated the protective effect of COR and nitric oxide (NO) against the toxicity of sodium arsenate in sweet basil (Ocimum basilicum L.). According to the statistical analysis, arsenic had a significant adverse effect on length and biomass of plants. Seedlings that pretreated with COR and sodium nitroprusside (SNP), significantly reversed fresh and dry lose and relative water content decay induced by the metalloid. The protective effects of COR and SNP were indicated by extent of lipid peroxidation, increase glutathione (GSH), ascorbate and thiol (–SH) content, promote antioxidant enzymes and reduce H2O2 content in basil seedlings. The present observation suggested that reduction of excess arsenic As-induced toxicity in O. basilicum by COR and NO is through the activation of enzymes involved in ROS detoxification (CAT, SOD, POD, APX, GR) and maintenance contents of molecular antioxidant (GSH, ascorbate, non-protein thiol and protein-thiol). Moreover, the results revealed a mutually amplifying reaction between COR and NO in reducing As-induced damages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are important environmental pollutants and many of them are toxic even at very low concentrations. Arsenic (As) is the most toxic metalloid widely distributed in the environment and is non-essential for plants (Zhao et al. 2009). It occurs predominantly in inorganic form as As(V) and As(III) (Tripathi et al. 2007). In soils, the most abundant As species is arsenate (As(V), Garg and Singla 2011). The presence of As in irrigation water or in soil could hamper normal growth of plants with toxicity symptoms such as biomass reduction. Arsenate is easily incorporated into plant cells through the high-affinity phosphate transport system (Finnegan and Chen 2012). Exposure of plants to arsenate results in the generation of reactive oxygen species (ROS). As-induced increase in ROS production may act as a cellular signal triggering the stress response (Garg and Singla 2011). In recent years, As-contaminated groundwater is widely used for irrigating green vegetables such as basil in dry season that results in elevated arsenic accumulation in soil and plants. This is very dangerous for human health because this heavy metal is known to be a carcinogen (Gupta et al. 2009). Hence, a better understanding of the mechanisms responsible for As resistance and toxicity in plants is needed. In this context, it becomes relevant to study the impact of As on antioxidant defense system and other biochemical changes in sweet basil, and investigate the protective roles of some compounds in alleviating its toxicity. Sweet basil (Ocimum basilicum L.) is an important commercial plant, widely cultivated in many countries. It is also a medicinal plant used for several purposes, especially for therapeutical ones. Basil essential oil has been used extensively in the food industry as a flavouring agent, and in perfumery and medical industries (Tarchoune et al. 2010).
Plants have evolved mechanisms to protect subcellular systems from the effects of ROS by using enzymatic antioxidant such as SOD, CAT and APX and non-enzymatic antioxidants such as ascorbate, phenolic compounds, carotenoid and glutathione (Gupta et al. 2009). The application of growth substances could efficiently improve plant resistance or tolerance to variable stresses (Wang et al. 2009). Coronatine is a bacterial blight toxin produced by several pathovars of Pseudomonas syringae, and has received much attention in recent years as a plant growth regulator (Uppalapati et al. 2005; Xie et al. 2008). The structure of coronatine is similar to that of a class of plant hormones called jasmonates (JAs) which are involved in the biological processes of plant growth and development (Mino et al. 1980; Weiler et al. 1994; Wang et al. 2008). Jasmonate is a plant growth hormone that plays a key role in plant defense to biotic and abiotic stress (Uppalapati et al. 2005). The overall structure of coronatine, consisting of polyketide coronafacic acid (CFA) and cyclized isoleucine derivative coronamic acid (CMA), shows structural similarity to that of jasmonates, most notably jasmonyl isoleucine (JA-Ile; Koda et al. 1996; Melotto et al. 2008). CMA is a structural analog of 1-aminocyclopropane-1-carboxylic acid (ACC), an intermediate in the pathway to ethylene in higher plants (Melotto et al. 2008). The most prominent effect of coronatine is an intense spreading of chlorosis in leaves of plants (Wang et al. 2008). In addition to chlorosis, COR induces a wide array of effects in plants including anthocyanin production, alkaloid accumulation, ethylene emission, tendril coiling, inhibition of root elongation, and hypertrophy (Feys et al. 1994; Weiler et al. 1994). COR also induces volatile production and the accumulation of proteinase inhibitors, which are stress responses (Feys et al. 1994; wang et al. 2008). Several prior investigations have confirmed the activation of JA-mediated responses by COR (Fey et al. 1994; Uppalapati et al. 2005). Some research using COR-defective bacterial mutants and purified COR demonstrated that COR regulates the expression of jasmonate induced protein genes (JIPs) and stimulates the JA pathway in Arabidopsis and Tomato. Based on similarities between COR and JA, it seems likely that COR and JA interact with at least one common host receptor (Feys et al. 1994; Uppalapati et al. 2005). Furthermore, there is some evidence that COR is more active than JAs for inducing production of secondary metabolites and antioxidants which have an important role in plant resistance to stresses (Feys et al. 1994; Tamogami and Kodama 2000). An important step in JA signaling is the SCFCOI1 E3 ubiquitin ligase-dependent degradation of JAZ repressor proteins. Jasmonoyl isoleucine promotes physical interaction between JAZ1 and COI1 (the F-box component of SCFCOI1) proteins. Melotto et al. 2008 indicated that coronatine also promotes interaction between Arabidopsis COI1 and multiple JAZ proteins. On the whole, these data highlight the importance of the isoleucine moiety in JA signaling and coronatine action, and suggest that coronatine is a potent microbial mimic of JA-Ile in the formation of COI–JAZ complexes as a part of its virulence mechanism. In addition to JA and COR, nitric oxide generation is one of the common reactions of plant cells to biotic and abiotic stress (Delledonne et al. 1998; Durner et al. 1998). Wendehenne et al. (2004) demonstrated that wounding or jasmonate treatment induces NO production in sweet potato and Arabidopsis thaliana epidermal cells and exogenous NO induces all of the genes that are required for JA biosynthesis. Nitric oxide is a free radical involved in numerous and diverse physiological processes in plants (Lamattina et al. 2003). Evidence has been obtained for the involvement of NO in plant growth and development, as well as in defense responses (Beligni et al. 2002; Lamattina et al. 2003; Yu et al. 2005). Recently, an increasing number of articles have reported the effects of exogenous NO on alleviating heavy metal toxicity in plants (Yu et al. 2005; Panda et al. 2011; Jin et al. 2010; Xiong et al. 2010). Besides being involved in mediating the fungal elicitor-induced secondary metabolite biosynthesis, JA and NO have also been widely tested as chemical inducers for improving secondary metabolite biosynthesis of plant cells. For example, JA and MeJA have been used to stimulate the production of Taxol, catharanthine, and baicalin of plant cells (Yukimune et al. 1996; Xu et al. 2006). On the other hand, NO was reported to induce the expression of genes related to phytoalexin biosynthesis in soybean (Glycine max) cell in culture (Durner et al. 1998). Furthermore, Xu and Dong (2008) reported that JA and NO act synergistically to induce matrine production of Sophora flavescens cells. However, we are not aware of any studies that have identified the effects of COR, with similar biological activities compared with JAs, and NO on stress induced changes in the antioxidative defense system in plants under heavy metal stresses. Thus, the objective of this study was to investigate the potential of COR for enhancing stress tolerance in sweet basil and evaluation of amplifying reactions between COR and NO in reducing As-induced damages in this plant.
Materials and methods
Plant material, growth condition and treatment procedures
The seeds of O. basilicum washed with sterile distilled water and transferred to plastic pots containing perlite. Fresh nutrient solution (pH 5.7 ± 0.1) for irrigation was prepared every time from the stock solutions (Hoagland and Arnon 1950). The plants were kept in greenhouse with photoperiod 16/8 h light/dark, 28/18 °C day/night and 50–60 % relative humidity. Coronatine (C8115) was purchased from Sigma. At the three-leaved stage, seedlings were treated with COR at concentrations of 0 (control) and 100 nM and SNP, as a nitric oxide donor, at 0 and 150 μM added to the nutrient solution, for 72 h. Our preliminary experiment showed that these concentrations created the most measurable effects on As-stressed basil seedlings. 35 day-old seedlings were subjected to sodium arsenate (Na2HASO4) in a 1/10 modified Hoagland solution at 0 (control) and 300 μM. 14 days after As treatment, the plants were collected and all parameters were measured. Each treatment contained three replicates.
Determination growth parameters and relative water content (RWC)
At the end of the experiments, plants were uprooted and shoot/root length and FW was recorded. The samples were dried in oven at 70 °C and DW was obtained. The leaf RWC was measured on the youngest fully expanded leaves following the method of Turner (1981).
Lipid peroxidation and H2O2 assay
Lipid peroxidation was estimated from the levels of malondialdehyde (MDA) and other aldehydes production using the thiobarbituric acid (TBA) method as described by Heath and Packer (1968) and (Meirs et al. 1992). The H2O2 content of both control and treated plants was determined after reaction with potassium iodide (KI) according to Velikova et al. (2000).
Measurement of total and non-protein thiol content
Thiol content was measured according to Sedlak and Lindsay (1968). Samples were homogenized in 0.02 M EDTA in an ice bath. Aliquots of 0.5 ml of the homogenates were mixed with 1.5 ml of 0.2 M Tris buffer (pH 8.2) and 0.1 ml of 0.01 M DTNB. The mixture was brought to 10 ml by the addition of 7.9 ml of absolute methanol. Color was allowed to develop for 15 min. Absorbance of the clear supernatant was read at 412 nm. Total sulfhydryl groups were calculated from the extinction coefficient of 13.1 mM cm−1. For measuring non-protein thiols, 5 ml of the homogenates were mixed with 4 ml distilled water and 1 ml of 50 % TCA. The contents were mixed and after 15 min the tubes were centrifuged for 15 min. 2 ml of the supernatant was mixed with 4 ml of 0.4 M Tris buffer (pH 8.9), 0.1 ml of DTNB and absorbance was read within 5 min at 412 nm against a reagent blank. The protein bound thiols (PT) were calculated by subtracting the non-protein thiols (NPT) from total thiols (TT).
Determination of phenolic compounds and anthocyanin
The total amount of phenolic compounds in the sweet basil leaves was determined using Folin–Ciocalteu’s reagent according to the method of Singleton and Rossi (1965). Fifty microliters of the methanolic extract was mixed with 450 μl of distilled water and 250 μl of 2 N Folin–Ciocalteu’s reagent. The mixture added to 1.25 ml of 20 % Na2CO3 was incubated at 25 °C for 20 min and then centrifuged at 2,000g for 10 min. The absorbance of the supernatant was measured at 735 nm and the standard curve was prepared using the gallic acid. Anthocyanin was extracted by grinding 0.1 g leaf samples with 10 ml of methanol containing 1 % (v/v) HCl and determined according to Wagner (1979).
Measurement of ascorbate (ASA), dehydroascorbate (DHA) and GSH content
The plants were homogenized with 5 % metaphosphoric acid at 4 °C. The homogenate was centrifuged at 20,000g for 15 min at 4 °C and the supernatant was collected for analysis of ascorbate and glutathione. ASA and DHA were determined according to the method of Kampfenkel et al. (1995). Briefly, total ascorbate was determined after reduction of DHA to ASC with DTT, and the concentration of DHA was estimated from the difference between total ascorbate pool (ASC plus DHA) and ASC. A standard curve was developed based on ascorbate in the range of 0–50 μg/ml. GSH content was determined at 412 nm using DTNB, according to the spectrophotometric method of (Ellman 1959).
Antioxidant enzyme activity
For determination of antioxidant enzyme activity, samples were homogenized in extraction buffer containing 50 mM phosphate buffer (pH 7.4), 1 mM PMSF, 1 mM EDTA and 1 % PVP in a pestle and mortar under frozen condition using liquid nitrogen. The homogenate was centrifuged at 22,000g for 30 min at 4 °C and the resulted supernatant was used for the assays of protein content and the activity of antioxidative enzymes. The total protein content was determined according to the method of Bradford (1976). All spectrophotometric analyses of enzyme were conducted in a Carry UV/visible light spectrophotometer. SOD activity was determined by measuring its ability to inhibit the photochemical reduction of nitro blue tetrazolium chloride (NBT) (Giannopolitis and Ries 1977). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.4), 0.1 mM Na-EDTA, 75 μM riboflavin, 13 mM methionine and 0.05 ml the enzyme extract. One unit of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition of NBT reduction at 560 nm. CAT activity was determined by measuring the rate of disappearance of hydrogen peroxide using the method of Dhindsa et al. (1981). The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7.4), 15 mM H2O2 and 100 μl enzyme extract. The decrease in hydrogen peroxide was inferred from the decline in absorbance at 240 nm. POD (GPX) activity was determined by measuring the change of absorption at 470 nm due to guaiacol oxidation following Putter (1974). The reaction mixture containing 50 mM potassium phosphate (pH 7.4), 0.3 % H2O2, 1 % guaiacol and 20 μl enzyme extract. The activity of APX was measured by monitoring the rate of ascorbate oxidation at 290 nm according to the modified method of Nakano and Asada (1981). The reaction mixture contained 50 mM potassium phosphate (pH 7.4), 0.1 mM EDTA, 0.15 mM H2O2 and 150 μl enzyme extract. Because APX is labile in the absence of ascorbate, 0.5 mM ascorbate was added to the reaction mixture which used for APX determination. One unit of APX was defined as the quantity of enzyme required to oxidize 1 μmol of ascorbate per min (extinction coefficient, 2.8 mM−1 cm−1). GR activity was assayed by monitoring the glutathione-dependent oxidation of NADPH at 340 nm by the method of Foyer and Halliwell (1976).
PAL extraction and assay
phenylalanine ammonia lyase g enzyme (PAL) was extracted from 0.3 g sample with 6.5 ml of 50 mM Tris–HCl buffer (pH 8.8) containing 15 mM β-mercaptoethanol in an ice-cooled mortar. The homogenate was centrifuged at 10,000g for 15 min, and the supernatant was collected for enzyme assay. PAL activity was measured by a modified method of Tanaka et al. (1974). Briefly, 1 ml of the extraction buffer, 0.5 ml of 10 mM l-phenylalanine, 0.4 ml of double-distilled water and 0.1 ml of enzyme extract were incubated at 37 °C for 1 h. The reaction was terminated by the addition of 0.2 ml of 25 % TCA and the cinnamic acid (CA) concentration was quantified with the absorbance measured at 290 nm. One unit of PAL activity is equal to 1 μmol of CA produced per min.
Statistical analysis
The data were analyzed by one-way ANOVA, using SPSS software, Version 18 for Windows. Duncan’s multiple range test (DMR) was used to separate means for significant treatment (p < 0.05). The reported values are means of three replicates ± SE.
Results
Effect of treatments on growth parameters and RWC
As shown in Table 1, it is clear that arsenic exposure had a large reducing effect on the shoot and root fresh/dry weight, length and leaf relative water content in As-treated plants. COR and SNP alone did not affect the growth of plants, but alleviated height reduction and increased shoots/roots fresh and dry weight under arsenic treatment. The leaves of the plants pretreated with COR and SNP maintained a much higher RWC under As stress. The beneficial effects of COR and NO were seen by all growth parameters and were shown to be statistically significant especially when these compounds supplied together.
Lipid peroxidation and hydrogen peroxide content
Table 2 compares malondialdehyde and H2O2 contents in basil plants subjected to arsenic with or without COR and SNP pretreatment. MDA and other aldehyde contents indicate lipid peroxidation and increased upon As exposure in leaves of the COR-, SNP-free controls, but by less than 31.04, 15.2 and 65.5 % in seedlings previously exposed to COR, SNP and COR+SNP, respectively. Arsenic stress caused significant accumulations (p < 0.05) of H2O2 in basil leaves, Compared to the control. Application of COR, SNP and COR+SNP considerably reduced the accumulation of H2O2 in leaves by about 30.2, 28 and 57.03 %, respectively (Table 2).
Total soluble phenols, anthocyanin content and PAL activity
In this study, arsenic exposure decreased total soluble phenols and anthocyanin content in the leaves of treated plants. However, it was observed that exogenously applied COR and SNP in O. basilicum not only compensated the reduction of these compounds but also enhanced significantly phenols pool in As+COR+SNP treated plants (Table 3).
Ascorbate, glutathione, thiols and protein content
The ASC, GSH and thiol content of O. basilicum leaves exposed to As with or without pretreatments are presented in Table 3. Arsenic caused a significant decrease in ASC, GSH, protein- and NP-thiol content in absence of any pretreatment. Similar trend was observed for leaf protein content (Table 3). However, these negative effects were not observed in plants pretreated with COR and SNP, separately. This effect was significantly greater in the plants pretreated with both COR and SNP in compared with the samples exposed to these compounds, separately. These results show that coronatine and nitric oxide may act synergistically to increase sweet basil resistance against heavy metal toxicity by enhancing non-enzymatic antioxidant pool.
Activities of PAL and antioxidant enzymes
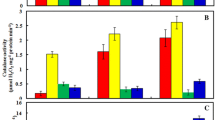
In order to investigate the mechanism of difference of phenolic content by treatments in this study, we determined PAL activity in the leaves of sweet basil under experimental conditions. As shown in Fig. 1a, PAL activity of plants treated with arsenic is decreased compare with the control. Pretreatment with COR, SNP and COR+SNP before As supply, significantly increased PAL activity in the leaves by 90.79, 76.64 and 198.3 %, respectively.
The change in the activities of representative enzymes responsible for O2 − dismutation (SOD), H2O2 detoxification (CAT, APX and POD) and antioxidant redox couples GSH regeneration (GR) after treatments are shown in Figs. 1, 2 and 3. In the arsenic exposed plants, there was a significant increase in the activities of CAT, POD and SOD compared to the control (Figs. 1b, 2a, b). The APX and GR activity decreased upon metal treatment (Fig. 3a, b). However, it was observed that in plants were pretreated with 100 nM COR and 150 μM NO before As exposure, the activities of all antioxidant enzymes were higher than those that did not have any pretreatment. Under normal condition, COR and SNP had negligible effects on the activity of antioxidant enzymes in O. basilicum but under As supply, significantly increased these activities, especially when COR and SNP were applied together (Figs. 1, 2, 3).
Discussion
According to the statistical analysis, arsenic had a significant adverse effect on length and biomass (root, shoot) of basil seedlings. This result indicates that the basil seedling is sensitive to As and the growth of root and shoot inhibit by arsenic exposure (at 300 μM in this study). One of the most effects of heavy metal toxic influence on plants is largely a strong and fast inhibition of growth process of the above and underground parts (Garg and Singla 2011). Some workers believed that the excess heavy metal induced disturbance in mineral nutrition (Mascher et al. 2002; Finnegan and Chen 2012). Arsenic has no known beneficial function, and its presence in the plant interferes with the metabolism and alters the uptake of other essential elements (Shri et al. 2009). Gomes et al. (2012) indicated that arsenic supply caused disturbances in the concentrations and distribution of nutrients in the roots and shoots of Anadenanthera peregrine. Whenever uptake of nutrition was inhibited in roots, the growth of the whole plant was constrained and the plant biomass decreased finally (Chun-xi et al. 2007). Arsenate is known to interfere on phosphate (P) uptake by plant cells since P transporters had high affinity to As, therefore competing with P anions (Finnegan and Chen 2012). Competition between As and P physiologically results in blocking the electron transport chain at the level of cell membranes and therefore, ATP synthesis is significantly inhibited (Pigna et al. 2009). This leads to the disruption of energy flows in cells and finally represses the growth and development of plants.
The formation of TBARs in plant exposed to heavy metals is an indicator of free-radical formation in the tissues (Singh et al. 2006). The present results show an increase in the level of MDA and H2O2 in the leaves with As exposure, indicating that arsenic induces oxidative stress in sweet basil. Increasing H2O2 and lipid peroxidation has been obtained in other plants under arsenic stress (Singh et al. 2006; Jin et al. 2010; Gupta et al. 2013; Hasanuzzaman and Fujita 2013). Recently, Gupta et al. (2013) demonstrated that As causes oxidative stress in Arabidopsis by inducing glycolate oxidase (H2O2 producers). It has been suggested that the inhibition of key enzyme systems, together with electron leakage during the conversion of As (V) to As (III), lead to formation of ROS, which in turn causes lipid peroxidation (Zhao et al. 2009). One likely reason for increasing H2O2 content in As-treated basil is that, H2O2 is a major product of photorespiration, a pathway whose activity greatly increases to abiotic stress (Panda et al. 2011). Plant cell membranes are generally considered to be primary sites of metal injury (Jin et al. 2010). It is possible that increasing H2O2 formation with metal toxicity can decrease cell wall extensibility and such results into lipid peroxidation (Foreman et al. 2003). Stimulating effects of 300 μM As on MDA and ROS level in O. basilicum indicated plants encountered oxidative stress causing cellular damage, inhibition of the growth as well as the loss in phenolic compounds and thiol content. Some workers believed that Arsenic induces oxidative stress by over-accumulation of ROS and reduction of nitric oxide content in plants (Gupta et al. 2013). In opposite, Jin et al. (2010) believed that arsenic stress elevated endogenous NO level in tall fescue leaves and NO might act as a signaling molecule to protect against injuries caused by arsenic toxicity. Several studies have evidenced the capacity of NO to counteract oxidative damages (Beligni et al. 2002; Wang and Wu (2005). However, there is still limited information regarding the functional role of endogenously produced NO in plants challenged with heavy metals. Singh et al. (2009) demonstrated that exogenously supplied NO significantly provides resistance to rice against As-induced toxicity and has an ameliorating effect against As-induced oxidative stress. As an antioxidant, NO could be directly quenches the ROS and modulates various cellular physiological processes to limit oxidative injury (Wendehenne et al. 2004; Singh et al. 2009). ROS can react with NO to form peroxynitrite, witch in turn may react with H2O2 to yield nitrite ion and oxygen (Laspina et al. 2005). NO supplied exogenously provides resistance against stress induced by heavy metals like Cd in sunflower and rice (Laspina et al. 2005; Panda et al. 2011), Cu in rice (Yu et al. 2005) and As in rice and fescue (Singh et al. 2009; Jin et al. 2010). It was in agreement with the results obtained in present study that an increased NO level upon SNP addition paralleled a decrease in H2O2 content and lesser MDA accumulation. In addition to a direct ROS scavenging activity, NO may also modulate lipid peroxidation by inhibiting lipoxygenase activity (Innocenti et al. 2007). COR diminished lipid peroxidation probably through the stimulation of non-enzymatic (ascorbate and glutathione) antioxidant machinery responsible for a tight regulation of ROS balance during heavy metal stress.
Cellular antioxidants such as phenolic compounds play an important role in inducing resistance of plants to metals by protecting macromolecules and membrane against attacks induced via free radicals (Sanchez-viveros 2010). Antioxidant action of phenolic compounds is due to their high tendency to chelate metals. There is new evidence that indicates the metal-binding activity of many flavonoids (Winkel-Shirley 2002). The roots of many plants exposed to heavy metals exude high levels of phenolics which inactivate free radicals by suppressing the superoxide-driven Fenton reaction (Michalak 2006). The negative effects of As on the growth of basil might be related to impaired synthesis of soluble phenols and other antioxidant compounds such as GSH and ASC by which, the sweet basil was not able to alleviate the oxidative stress induced by the accumulation of this metalloid in tissues. There have been many reports of induced accumulation of phenolic compounds, PAL and peroxidase activity in plants treated with high concentration of metals (Michalak 2006). The induction of phenolic compound biosynthesis was observed in pepper in response to Cu (Diaz et al. 2001) and in maize in response to Al (Winkel-Shirley 2002). In this study, exogenously applied COR and SNP increased the content of phenolic compounds in basil leaves. Induction of phenolic content after coronatine application has been reported in Tomato (Mino et al. 1980). Tamogami and Kodama (2000) also reported that coronatine elicits phytoalexin production in rice leaves (Oryza sativa L.) in the same manner as jasmonic acid. According to Kovacik et al. (2009) exogenously apply of SNP cause accumulation of soluble phenol by stimulating PAL activity in N-deficient Matrica chamomilla roots. In a study with tobacco bright-yellow 2 cells, only the combined application of NO and ROS generators caused a remarkable increase in PAL activity. Both ROS and NO may stimulate expression of the pal gene (De Pinto et al. 2002). In addition, Durner et al. (1998) reported that NO induced activation of PAL gene (pal) expression and PAL enzyme activity in tobacco. In Taxus chinensis cells, the combined application of MeJA and NO scavenger decreased NO content, PAL activity and taxol production but increased H2O2 accumulation (Kovacik et al. 2009). However, we found no data focused on the changes in PAL activity or phenolic metabolism-related enzymes in COR-treated plants.
The glutathione level has been shown to correlate with the plant adaptation to extreme heavy metal stress and reduced GSH pool shows marked alterations in response to metal stress (Singh et al. 2006). Flora (2011) reported that arsenic increased oxidative stress in plants by disturbing the pro/antioxidant balance. During the present study, reduced levels of protein-thiol, NP-thiol, ascorbate and glutathione were observed in As-treated plants. This result is similar with those of Hasanuzzaman and Fujita (2013), who reported a decrease in ASC and GSH content and the GSH/GSSG ratio in As-treated wheat (Triticum aestivum). This might be attributed to the toxicity of the arsenic (Sanchez-viveros 2010). Therefore, measured decline in the content of ASC and GSH in O. basilicum could be partially due to its consumption while acting as antioxidant to limit lipid peroxidation. Moreover, Glutathione as an sulfur-containing tripeptide thiol is involved in the plant protections against heavy metals as a precursor in the synthesis of phytochelatins (PCs) and in the scavenging of ROS by the ascorbate–glutathione cycle (Sharma and Dietz 2006; Xiong et al. 2010). Glutathione levels in plant tissues are known to increase under metal stress (Koricheva et al. 1997). In this study, decreasing GSH content in sweet basil may be caused by increase conversion of GSH to PC in the response of As toxicity. Recently, Gupta et al. (2009) demonstrated that PC synthesis is stimulated by As supply in Brassica juncea due to the overexpression of PCs gene. The PC–metal complex is often sequestered in the vacuoles (Sharma and Dietz 2006). The chemical structure of PCs contains repetitions of the dipeptide Glu–Cys. NO is able to react with Cys residues (S-nitrosylation), especially when these are surrounded by acidic amino acids like Glutamate, and NO reacts rapidly with glutathione, the precursor of PCs, to form S-nitrosoglutathione (Stamler et al. 1997). However, Michele et al. (2009) indicated that PCs might similarly be nitrosylated. They believed that a mechanism through which NO might modulate the PC capability to chelate heavy metals is by direct nitrosylation. Modulation of cellular thiols for protection against ROS is a therapeutic strategy of plants against arsenic. In plants pretreated with COR/SNP in this research, thiol contents increased significantly. Our hypothesis is supported by observation that JA stimulated significantly accumulation of phytochelatins in Wolffia arrhiza in response to Pb leading to enhance plant tolerance to stresses (Piotrowska et al. 2009). In addition, a considerable decrease in GR which act in GSH regeneration in ASC–GSH cycle may be affected the decrease in glutathione content under As stress. GSH in reaction with ROS, generated in the presence of As, is oxidized to GSSG and a decrease in re-reduction of GSH by GR contributes to the decrease in GSH pool in plants (Xiong et al. 2010). Although multiple factors can affect the GSH pool in plants, some conditions are known to modify the GSH biosynthesis pathway at the transcriptional level. However, such regulation was observed in response to heavy metals, JA and SNP treatments (Sasaki-Sekimoto et al. 2005; Innocenti et al. 2007). Our data provided that GSH content is increased in plants by NO and COR application. This result is similar with those of Innocenti et al. (2007), who reported an increase of GSH content in SNP-treated Medicago. They believed that NO triggered an increase of the endogenous GSH amount in M. truncatula roots through the stimulation of GSH synthesis gen transcript accumulation. In addition to a direct ROS scavenging activity and to the modulation of lipid peroxidation, NO may also protect cells against oxidative processes by stimulating GSH synthesis (Innocenti et al. 2007). Xiang and Oliver (1998) showed that JA does potentiate and enhance the capacity for GSH synthesis due to stimulation the expression of the GSH synthesis genes and JA-pretreated plants produced more GSH when challenged with cadmium or copper. They mentioned that the up-regulation of GSH metabolic genes by JA and heavy metal largely controlled at the transcriptional level. Shan and Liang (2010) also reported that JA treatment increased the transcript level of γ-ECS (γ-glutamyl cysteine synthetase) and GSH2 genes, as well as GR in Agropyron cristatum under water stress. Moreover, these researchers indicated that JA increased the transcript levels and activities of APX and GR as well as the content of ASA and GSH in the leaves. Our results indicated the existence of cross talks between NO and COR in the GSH accumulation in similar with JA and NO. Ascorbate is also known to operate as an antioxidant either in direct chemical interaction with ROS or during the reaction catalyzed by APX (Singh et al. 2006). Since, ASC and GSH are able to detoxify ROS by a direct scavenging or by acting as substrate in the enzymatic reactions (APX and GR), an elevation or protection in their contents with COR and SNP pretreatments enhanced the tolerance against As-induced oxidative stress in O. basilicum.
Plants possess several antioxidative defense systems to scavenge toxic free radicals to protect themselves from the oxidant stress. In this experiment, arsenic supply lead to a significant increase in the activity of some antioxidant enzymes such as CAT, POD and SOD and decrease in the activity of APX, PAL and GR in the leaves of O. basilicum. The function of SOD in antioxidative defense is to eliminate active oxygen species (ROS), which generates H2O2, while the resulting H2O2 can be removed by CAT, APX, and GPX enzymes. Increased activities of these enzymes commonly have been reported in plants exposed to metals (Koricheva et al. 1997). Some of the enzymes are sensitive to inhibition by heavy metal like Cu and As which react with thiol groups, due to the presence of thiol groups at the active site of the enzymes (Garg and Singla 2011). Thus, the reduced activity of GR and PAL may be due to inactivation of the enzyme by As ions. Some researchers believed that this reduction could be due to different effects of heavy metals like As and Cd at the transcriptional and post-transcriptional levels (Romero-puertas et al. 2007; Gupta et al. 2013). The increase in POD, SOD and CAT activity is related with the increase production of reactive oxygen species or expression of genes encoding these enzymes. Gupta et al. (2013) reported that all SOD isoforms (mainly CuZn–SODs) are induced by arsenic in wild type A. thaliana. Other study also indicated As-induced POD, SOD and CAT activity in a number of plants (Cao et al. 2004; Chun-Xi et al. 2007; Gupta et al. 2009; Shri et al. 2009). Optimum activities of CAT and POD and severely depressed activities of APX and GR under As toxicity suggest that excessive H2O2 in leaves was eliminated by CAT and POD mainly and the enzymes of ascorbate–glutathione cycle are more prone to inhibition by excess As supply in O. basilicum. APX along with CAT and SOD are considered to be key enzymes within the antioxidative defense mechanism, which directly determines the cellular concentration of oxygen radicals (Tripathi et al. 2007). There is some evidence that the exogenous application of NO rendered the plants more tolerant to As-induced oxidative damage by enhancing their antioxidant defense and glyoxalase system. Hasanuzzaman and Fujita 2013 reported that the treatment with SNP increased the RWC, chl and proline contents, AsA and GSH contents and the GSH/GSSG ratio as well as the activities of MDHAR, DHAR, GR, GPX, CAT, glyoxalase I and glyoxalase II in the wheat seedlings subjected to As stress. Pronounced increase in antioxidant enzymes and reduce the level of H2O2 and MDA in samples that pretreated with COR and SNP indicated that these compounds alleviated oxidative injuries through raising antioxidant enzymes activity to scavenge newly-produced ROS. In addition, increase APX and GR activity supported by significant increase of GSH and ASC content in COR+SNP-pretreated plants suggest that, ascorbate–glutathione cycle has a pivotal role in arsenic detoxification, apart from the role of PCs in the chelation of metals.
Conclusion
The reduction of growth and some antioxidant materials and induction of enzymatic antioxidative system indicated that excess As induces oxidative stress in sweet basil. Application of COR and SNP before As exposure supported a considerably higher non-enzymatic antioxidant contents and promoted antioxidant enzyme activities in the basil leaves, especially when seedlings were subjected to both compounds (COR+SNP). In summary, our results suggest that there was a mutually amplifying reaction between COR and NO in reducing As-induced damages. However, whether COR and NO participate synergically in regulation the transcript level and the activity of key enzymes remains unknown and further research is required to understand the signaling relationships between COR and NO in plant defenses against heavy metal stress.
Abbreviations
- As:
-
Arsenic
- APX:
-
Ascorbate peroxidase
- COR:
-
Coronatine
- CAT:
-
Catalase
- DW:
-
Dry weight
- DTNB:
-
5,5-dithiobis-2-nitrobenzoic acid
- FW:
-
Fresh weight
- EDTA:
-
Ethylendiamine tetraacetic acid
- GR:
-
Glutathione reductase
- GSH:
-
Glutathione
- JA:
-
Jasmonic acid
- JA-Ile:
-
Jasmonyl isoleucine
- MDA:
-
Malondialdehyde
- NO:
-
Nitric oxide
- H2O2 :
-
Hydrogen peroxide
- RWC:
-
Relative water content
- PMSF:
-
Phenyl methane sulfonyl fluoride
- PVP:
-
Poly vinyl pyrrolidone
- SOD:
-
Superoxide dismutase
- SNP:
-
Sodium nitroprusside
- TW:
-
Turgid weight
References
Beligni MV, Fath A, Bethake PC, Lamattina L, Jones RL (2002) Nitric oxide acts as an antioxidant and delays programmed cell death in barley aleurone layers. Plant Physiol 129:1642–1650
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Chem 72:248–254
Cao X, Ma L, Tu C (2004) Antioxidative responses to arsenic in the arsenic-hyperaccumulator Chinese brake fern (Pteris vittata L.). Environ Pollut 128:317–325
Chun-xi L, Shu-li F, Yan SH, Li-na J, Xu-yang L, Xiao-li H (2007) Effects of arsenic on seed germination and physiological activities of wheat seedlings. J Environ Sci 19:725–732
De Pinto MC, Tommasi F, De Gara L (2002) Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco bright-yellow 2 cells. Plant Physiol 130:698–708
Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a secondary signal in plant disease resistance. Nature 394:585–588
Dhindsa RS, Plumb-Dhindsa P, Thrope TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid per oxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:43–101
Diaz J, Bernal A, Pomar F, Merino F (2001) Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci 161:179
Durner J, Wendehenne D, Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic CMP and cyclic ADP-ribose. Proc Natl Acad Sci USA 95:10328–10333
Ellman Gl (1959) Tissue sulfydryl groups. Arch Biochem Biophys 82:70–77
Feys BJF, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6:751–759
Finnegan PM, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol 3(182):1–18
Flora SJS (2011) Arsenic-induced oxidative stress and its reversibility. Free Rad Biol Med 51(2):257–281
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownelee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplast: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Garg N, Singla P (2011) Arsenic toxicity in crop plants: physiological effects and tolerance mechanisms. Environ Chem Lett 9:303–321
Giannopolitis CN, Ries SK (1977) Superoxide dismutase I: occurrence in higher plants. Plant Physiol 59:309–314
Gomes MP, Duarte DM, Miranda PLS, Barreto LC, Matheus MT, Garcia QS (2012) The effects of arsenic on the growth and nutritional status of Anadenanthera peregrina, a Brazilian savanna tree. J Plant Nutr Soil Sci 175(3):466–473
Gupta M, Sharma P, Sarin NB, Sinha AK (2009) Differential response of arsenic stress in two varieties of Brassica juncea L. Chemosensory 74:1201–1208
Gupta DK, Inouheb M, Rodríguez-Serrano M, Romero-Puertas MC, Sandalio LM (2013) Oxidative stress and arsenic toxicity: role of NADPH oxidases. Chemosphere 90(6):1987–1996
Hasanuzzaman M, Fujita M (2013) Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22(3):584–596
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast: I. Kinetic and stochiometry of fatty acid peroxidation. Biochem Biophys 125:189–190
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:1–3
Innocenti G, Pucciariello Ch, Gleuher ML, Hopkins J, Stefano MD, Delledonne M, Puppo A, Baudouin E, Frendo P (2007) Glutathione synthesis is regulated by nitric oxide in Medicago truncatula roots. Planta 225:1597–1602. doi:10.1007/s00425-006-0461-3
Jin JW, Xu YF, Huang YF (2010) Protective effect of nitric oxide against arsenic-induced oxidative damage in tall fescue leaves. Afr J Biotechnol 9(11):1619–1627
Kampfenkel K, Van Montagu M, Inzb D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167
Koda Y, Takahashi K, Kikuta Y, Greulich F, Toshima H, Ichihara A (1996) Similarities of the biological activities of coronatine and coronafacic acid to those of jasmonic acid. Phytochemistry 41:93–96
Koricheva J, Roy S, Vranjic JA, Haukioja E, Hughes PR, Han-ninen O (1997) Antioxidant responses to simulated acid rain and heavy metal deposition in birch seedlings. Environ Pollut 95:249–258
Kovacik J, Klejdus B, Backor M (2009) Nitric oxide signals ROS scavenger-mediated enhancement of PAL activity in nitrogen-deficient Matricaria chamomilla roots: side effects of scavengers. Free Radic Biol Med 46:1686–1693
Lamattina L, Garca-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Ann Rev Plant Biol 54:109–136
Laspina NV, Groppa MD, Tomaro ML, Benavides MP (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169:323–330
Mascher R, Lippmann B, Holzinger S, Bergmann H (2002) Arsenate toxicity: effects on oxidative stress response molecules and enzymes in red clover plants. Plant Sci 163:961–969
Meirs S, Philosophhadas S, Aharoni N (1992) Ethylene increased accumulation of fluorescent lipid peroxidation products detected during senescence of parsley by a newly developed method. J Am Soc Hortic Sci 117:128–132
Melotto M, Mecey Ch, Niu Y, Chung HS, Katsir L, Yao J, Zeng W, Thines B, Staswick P, Browse J, Howe GA, He ShY (2008) A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J 55:979–988
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J Environ Stu 15(4):523–530
Michele RD, Vurro E, Rigo Ch, Costa A, Elviri L, Valentin MD, Careri M, Zottini M, Toppi LS, Schiavo FL (2009) Nitric oxide is involved in cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol 150:217–228
Mino Y, Sakai R, Uchino K, Sasabuchi T (1980) Effects of coronatine on the metabolism of phenolics in the discs of potato tuber. Ann Phytopathol Soc 46:510–516
Nakano V, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate–specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Panda P, Nath Sh, Chanu ThTh, Sharma GD, Panda SK (2011) Cadmium stress-induced oxidative stress and role of nitric oxide in rice (Oryza sativa L.). Acta Physiol. doi:10.1007/s11738-011-0710-3
Pigna M, Cozzolino V, Violante A, Meharg AA (2009) Influence of phosphate on the arsenic uptake by wheat (Triticum durum L.) irrigated with arsenic solutions at three different concentrations. Water Air Soil Pollut 197:371–380
Piotrowska A, Bajguza A, Godlewska-Zylkiewicz B, Czerpak R (2009) Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lemnaceae). Environ Exp Bot 66:507–513
Putter J (1974) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2. Academic Press, New York, pp 685–690
Romero-Puertas MC, Corpas FJ, Rodríguez-Serrano M, Gomez M, Rio LAD, Sandalio LM (2007) Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. Plant Physiol 164:1346–1357
Sanchez-Viveros G (2010) Short term effects of As-induced toxicity on growth, chlorophyll and carotenoid contents and total content of phenolic compounds of Azolla filiculoides. Water Air Soil Pollut. doi:10.1007/s11270-010-0600-0
Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Hirai MY, Noji M, Saito K, Masuda T, Takamiya K, Shibata D, Ohta H (2005) Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J 44:653–668
Sedlak J, Lindsay RH (1968) Estimation of total, protein- bound, and non-protein sulfhydryl groups in tissue by Ellman’s reagent. Anal Biochem 25:192–208
Shan Ch, Liang Z (2010) Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Plant Sci 178:130–139
Sharma SS, Dietz KJ (2006) The significance of amino acid and amino acid-derived molecules in plant responses and adaption to heavy metal stress. J Exp Bot 57(4):711–726
Shri M, Kumar S, Chakrabarty D, Kumar-Trivedi P, Mallick S, Misra P, Shukla D, Mishra S, Srivastava S, Tripathi RD, Tuli R (2009) Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72:1102–1110
Singh N, Ma LQ, Srivastava M, Rathinasabapathi B (2006) Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci 170:274–282
Singh HP, Kaur Sh, Batish DR, Sharma VP, Sharma N, Kohli RK (2009) Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nitric Oxide 20:289–297
Singleton VL, Rossi IA (1965) Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Stamler JS, Toone EJ, Stuart AL, Sucher NJ (1997) NO signals: translocation, regulation, and a consensus motif. Neuron 18:691–696
Tamogami S, Kodama O (2000) Coronatine elicits phytoalexin production in rice leaves (Oryza sativa L.) in the same manner as jasmonic acid. Phytochemistry 54:689–694
Tanaka Y, Kojima M, Uritani I (1974) Properties, development and cellular-localization of cinnamic acid 4-hydroxylase in cut injured sweet potato. Plant Cell Physiol 15:843–854
Tarchoune I, Sgherri C, Izzo R, Lachaal M, Ouerghi Z, Navari-Izzo F (2010) Antioxidative responses of Ocimum basilicum to sodium chloride or sodium sulphate salinization. Plant Physiol Biochem 48:772–777
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJM (2007) Arsenic hazards; strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165
Turner NC (1981) Techniques and experimental approaches for the measurement of plant water status. Plant Soil 58:339–366
Uppalapati SR, Ayoubi P, Weng H, Palmer DA, Mitchell RE, Jones W, Bender CL (2005) The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J 42:201–217
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci 151:59–66
Wagner GJ (1979) Content and vacuole/extravacuole distribution of neutral sugars, free amino acids, and anthocyanin in protoplasts. Plant Physiol 64:88–93
Wang BQ, Li AH, Eneji AE, Tian XL, Zhai ZX, Li JM, Duan LS (2008) Effects of coronatine on growth, gas exchange traits, chlorophyll content, antioxidant enzymes and lipid peroxidation in maize (Zea mays L.) seedlings under simulated drought stress. Plant Prod Sci 11:283–290
Wang JW, Wu JY (2005) Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of taxus cells. Plant Cell Physiol 46(6):923–930
Wang L, Chen WJ, Wang Q, Eneji AE, Li ZH, Duan LS (2009) Coronatine enhances chilling tolerance in cucumber (Cucumis sativus L.) seedlings by improving the antioxidative defence system. Agron Crop Sci 195:377–383
Weiler EW, Kutchan TM, Gorba T, Brodschelm W, Niesel U, Bublitz F (1994) The Pseudomonas phytotoxin coronatine mimics octadecanoid signalling molecules of higher plants. FEBS Lett 345:9–13
Wendehenne D, Durner J, Klessig DF (2004) Nitric oxide: a new player in plant signaling and defence responses. Plant Biol 7:449–455
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Plant Biol 5:218
Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10:1539–1550
Xie ZX, Duan LS, Tian XL, Wang BQ, Eneji AE, Li ZH (2008) Coronatine alleviates salinity stress in cotton by improving the antioxidative defense system and radical-scavenging activity. J Plant Physiol 165:375–384
Xiong J, Fu G, Tao L, Zhu Ch (2010) Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch Biochem Biophys 497:13–20
Xu MJ, Dong JF (2008) Synergistic action between jasmonic acid and nitric oxide in inducing matrine accumulation of Sophora flavescens suspension cells. J Integr Plant Biol 50(1):92–101
Xu MJ, Dong JF, Zhu MY (2006) Nitric oxide mediates the fungal elicitor-induced puerarin production in Pueraria thomsonii suspension cells through a salicylic acid (SA)-dependent or a jasmonic acid (JA)-dependent signal pathway. Sci China Ser 49:379–389
Yu C, Hung KT, Kao C (2005) Nitric oxide reduces Cu toxicity and Cu-induced NH4 + accumulation in rice leaves. Plant Physiol 162:1319–1330
Yukimune Y, Tabata H, Higashi Y (1996) Methyl jasmonate-induced of overproduction of paclitaxel and baccatio in Taxus cell suspension cultures. Nat Biotechnol 14:1129–1132
Zhao FJ, Ma JF, Meharg AA, McGrath MP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saeid, Z.D., Zahra, A. & Abdolhamid, N.S. Investigation of synergistic action between coronatine and nitric oxide in alleviating arsenic-induced toxicity in sweet basil seedlings. Plant Growth Regul 74, 119–130 (2014). https://doi.org/10.1007/s10725-014-9903-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-014-9903-2