Abstract
Nitric oxide (NO) is a key signal molecule that is involved in plant response to various abiotic stresses. The present study was conducted to investigate the effects of NO on Typha angustifolia which has been subjected to cadmium stress. Our results showed that sodium nitroprusside (SNP, a NO donor) treatment could significantly mitigate both the Cd (CdCl2·2.5H2O)-induced increase of malondialdehyde content in the root and relative electrolyte leakage in the root and leaf. NO demonstrated a reduced or counteractive effect on the Cd-induced increase in the activities of some typical antioxidant enzymes. The Cd-induced changes on the contents of non-protein thiol and phytochelatins in the root could also be reversed by NO. The protected effect of NO is likely achieved through trapping Cd in the root and decreasing Cd accumulation in the leaf and sheath. Furthermore, NO enhanced the allocation of Cd in the cell wall and reduced the distribution of Cd in the soluble fraction of the root and leaf. Differential biosynthesis of ascorbic acid content in some tissues might also be responsible for the protected effect of NO. This study concludes that NO counteracts Cd toxicity strongly in T. angustifolia via regulating antioxidant metabolism and enhancing Cd accumulation in the cell wall of the root.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a highly toxic and persistent environmental poison for plants and animals. It can be found in soil and water, and is readily uptaken by plants. Thus, with regards to agricultural plants growing in these affected areas, the sustentative consumption of high-Cd plant tissue constitutes a significant threat to human health (Wang et al. 2013; Wu et al. 2014). As a non-essential nutrient, Cd has been shown to influence the absorption, transportation, and usage of both water and essential nutrients while also causing various visible symptoms including chlorosis, wilting and root browning (Ding et al. 2013; Gallego et al. 2012; Garcia et al. 2006; Gratao et al. 2012). To cope with Cd stress, plants adopt many ways to avoid Cd stress or enhance tolerance to Cd toxicity (Baker 1987). The cell wall, which is the first barrier against Cd stress, could provide important ligands to immobilize Cd when it enters plants (Huguet et al. 2012). Moreover, cell wall deposition and vacuolar compartmentalization also play an important role in the detoxifying of metals (Verbruggen et al. 2009). When the cell wall is saturated with heavy metals, the excess of heavy metal ions could be transferred into the vacuole storage (Krämer 2000; Przymusinski and Wozny 1985; Wierzbicka 1987). Cd stress could cause the overproduction of reactive oxygen species (ROS) which subsequently leads to lipid peroxidation (Smeets et al. 2008). Excess ROS could be cleared by antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione peroxidase (GPX) and non-enzymatic antioxidant such as ascorbic acid (AsA), which reduce glutathione and other molecules capable of quenching ROS. In addition, the excess of heavy metal ions can be chelated by some peptide substances, such as the non-protein thiol (NPT), phytochelatins (PCs) which can be induced by Cd stress (Cobbett 2000; Xiang and Oliver 1998).
Nitric oxide (NO) is a bioactive molecule that functions in numerous physiological processes in plants including plant growth and development (Durner et al. 1998). Besides its role, as an important signaling molecule, NO has been shown to be involved in the physiological responses of various plants to biotic and abiotic stresses such as drought, salt, heavy metal and heat (Arasimowicz and Floryszak-Wieczorek 2007). Two main mechanisms were proposed to be responsible for the protected effect of NO. First of all, NO could directly scavenge the overproduced ROS in cells. Secondly, NO might function as a signaling molecule to modulate antioxidative gene expression (Verma et al. 2013). However, accumulating evidence shows that NO is a double edged sword in plants. Low and high content of NO could accelerate and inhibit growth, respectively (Wink and Mitchell 1998). Apparently conflicting results have also been published on NO physiology. For example, the Cd-mediated induction (Bartha et al. 2005) or inhibition (Rodríguez-Serrano et al. 2009) of NO production has been found to be involved in the regulation of Cd cytotoxicity (Luo et al. 2012).
The Typha genus, important aquatic plants widely distributed in wetlands, show great capacity to absorb heavy metal so that it can be used in phytoremediation. Mojiri et al. (2013) suggests that Typha domingensis is an effective accumulator plant for phytoremediation of some heavy metals (Pb, Ni and Cd). Typha angustifolia has been proven to be strongly tolerant to Cd stress, and adding Cd could even increase its height and biomass (Bah et al. 2011). Therefore, Typha plants can be used in phytoremediation for Cd-polluted water. Our previous experiments also demonstrated that T. angustifolia was a metal-hyperaccumulator which showed high heavy metal tolerance (Wu et al. 2007, 2008). Therefore, T. angustifolia could be an excellent model system to explore the mechanisms involved in its high Cd tolerance. However, related studies were lacking. The effect of NO on plant tolerance to Cd stress is conflicted in different species and there is little information about it in T. angustifolia. Hence, we studied the role of NO in plant response to Cd stress by evaluating changes in various metabolic processes via application of a NO donor in T. angustifolia. This present study will be of significance as an elucidator of the functionality of NO in T. angustifolia, which has not yet been explored. As a result, this research may deepen our understanding of the mechanisms of NO-mediated amelioration of Cd toxicity in plants and can provide a theoretical basis for ameliorating phytoremediation.
Materials and methods
Plant materials and treatment
Typha angustifolia seedlings were grown in the growth chamber at 30/25 °C (day/night), with a light intensity of 200 μmol m−2 s−1 and a 12 h photoperiod. The Hoagland nutrient solution (pH 6.0) was refreshed every 2 d. After growing for 20 d, seedlings were incubated in six groups as follows: (1) CK; (2) 444.8 μM Cd (CdCl2·2.5H2O); (3) 444.8 μM Cd + 100 μM sodium nitroprusside (SNP, a NO donor); (4) 444.8 μM Cd + OLD SNP; (5) 444.8 μM Cd + 100 μM SNP + 0.1 % Hemoglobin bovine (Hb, a NO scavenger, w:v); (6) 444.8 μM Cd + 0.1 % Hb (w:v). Cd concentration had been screened in preliminary experiments. An old SNP solution (presumably containing equimolar amounts of ferrocyanide, nitrate and nitrite, two normal products of NO decomposition) was obtained as a negative control by maintaining a 100 μM SNP solution for 10 days in the light to eliminate NO before its application (Tossi et al. 2009). After 4 days, root, sheath and leaf were harvested separately according to various physiological indexes. After 7 days, we took a photo for four groups which including CK, Cd, Cd + SNP and Cd + Hb. The concentrations of above chemicals used in this study were determined in pilot experiments from where the effective responses were obtained.
Assay of antioxidant enzyme
0.2 g fresh plant tissue (root, leaf or sheath) was homogenized in 1.6 mL 50 mM sodium phosphate buffer (PBS, pH 7.8) using a pre-chilled (4 °C) mortar and pestle. Extractions were centrifuged at 12,000×g for 20 min at 4 °C. The supernatant was used for enzyme activity assay. SOD activity was determined as previously described by Zhou et al. (1997). CAT activity was determined by measuring the changes in absorbance at 470, 240 and 290 nm, respectively (Aebi 1984).
Determination of malondialdehyde (MDA) content and electrolyte leakage
MDA content was measured on the basis of Devi and Prasad (1998) with minor modifications. Plant tissues (0.2 g) were homogenized in 3 mL of 0.5 % (w/v) Trichloroacetic acid (TCA) and then centrifuged at 3,000×g for 10 min. 2 mL of supernatant was mixed with 2 mL 0.67 % (w/v) 2-Thiobarbituric acid (TBA). The mixture was heated at 100 °C for 30 min, quickly cooled on ice, and then centrifuged at 3,000×g for 10 min. The absorbance of the supernatant was read at 450, 532 and 600 nm.
To measure electrolyte leakage, 0.2 g fresh tissues were floated on 50 mL of deionized water with continuous shaking for 24 h at room temperature. The electrolyte content in the solution was measured immediately (C0). Then samples were boiling for 20 min and electrolyte content was determined (C1). Results were expressed as percentage of electrolyte leakage: relative electrolyte leakage = (C0/C1) × 100 % (Blum and Ebercon 1981).
Determination of Cd content
After 4 days of Cd treatment, seedlings were rinsed thrice with deionized water. Seedlings of each treatment were divided into two groups, one of which was dried at 105 °C for 30 min and then at 80 °C for 24 h. The plant material was digested with HNO3:HClO4 (87:13, v:v). The other group was used for the determination of subcellular Cd distribution, with some modification (Wang et al. 2008). 1 g sample was homogenized in 30 mL of extraction medium containing 250 mM sucrose, 50 mM Tris(hydroxymethyl)aminomethane(Tris–HCl) (pH 7.5) and 1 mM DL-Dithiothreitol (DTT). The homogenate was centrifuged at 300×g for 5 min to isolate the cell wall. The supernatant was centrifuged at 20,000×g for 45 min. The precipitate was cell organelles and the supernatant was the soluble fraction. All aforementioned steps were carried out at 4 °C. Cd content was determined by ICP (ICP-AES, Thermo Elemental, USA).
Determination of plant metabolites
Plant tissues (0.2 g) were homogenized in 2 mL of 5 % 5-Sulfosalicylic acid dehydrate and then centrifuged at 8000×g for 10 min. The NPT content was measured in the supernatant. The reaction mixture contained 200 μL of the supernatant, 2 mL of 0.2 M Tris–HCl (pH 8.2) and 0.15 mL of 10 mM 5,5′-Dithiobis-(2-nitrobenzoic acid) (DTNB) and was incubated for 20 min. After incubation, the absorbance was measured at 412 nm. An aliquot without DTNB was used to adjust the spectrophotometer to zero absorbance and GSH was used as standard (Devi and Prasad 1998).
Statistical analysis
The resulting data were analyzed through analysis of variance (ANOVA) while the difference (Duncan) test was employed to determine significant differences among the treatments at the P < 0.05 level.
Results
NO protects T. angustifolia against Cd toxicity
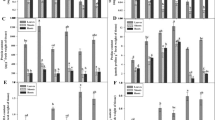
Under Cd stress, T. angustifolia seedlings showed symptoms of Cd toxicity: growth inhibition and severe oxidative damage (Fig. 1). Lipid peroxidation caused by Cd stress was monitored via measuring MDA, a product of peroxidation. After 4 days’ treatment, Cd exposure caused significant increases in the MDA content of the leaf and sheath but it seemed to have little effect on the root (Fig. 2a). On the contrary, exogenous NO treatment significantly reduced Cd-induced MDA accumulation in root but caused slightly decreased MDA accumulation in the leaf and sheath. When combining with Cd, SNP and Hb demonstrated opposing tendencies in the sheath and root. The MDA content of the Cd + OLD SNP (old SNP containing no NO but ferrocyanide, nitrate and nitrite) and Cd + Hb treatment were close to the Cd treatment alone. This observation implies that NO can alleviate the damage of Cd by reducing the content of MDA in the root.
Effect of Nitric oxide (NO) on seedling growth in the leaf, sheath and root of T. angustifolia. Plants were treated with Hoagland nutrient solution (control, CK), Cadmium (Cd, 444.8 μM), Cd (444.8 μM) + sodium nitroprusside (SNP, a NO donor, 100 μM) and Cd (444.8 μM) + 0.1 % Hemoglobin bovine (Hb, a NO scavenger, w:v) for 4 days. Photo was taken 7 days after treatment. Vertical bars represent standard error of the mean (n = 3). Vertical bars represent standard error of the mean (n = 3). Bars with different letters are significantly different within each group at P < 0.05 according to Duncan’s multiple test
Effect of sodium nitroprusside (SNP, a NO donor) on malondialdehyde (MDA) a content and relative electrolyte leakage b in the leaf, sheath and root of T. angustifolia. Plants were treated with Hoagland nutrient solution (control, CK), Cd (444.8 μM), Cd (444.8 μM) + SNP (100 μM), Cd (444.8 μM) + OLD SNP, Cd (444.8 μM) + SNP (100 μM) + 0.1 % Hb (w:v), Cd (444.8 μM) + 0.1 % Hb (w:v). Vertical bars represent standard error of the mean (n = 3). Vertical bars represent standard error of the mean (n = 3). Bars with different letters are significantly different within each group at P < 0.05 according to Duncan’s multiple test
Relative electrolyte leakage was measured to evaluate the degree of Cd injury in T. angustifolia. Under Cd stress, relative electrolyte leakage was significantly higher than the control in the root and leaf of T. angustifolia while there seemed to be little effect in the sheath (Fig. 2b). SNP treatment significantly blocked the increase of electrical conductivity triggered by Cd stress in the leaf while adding Hb significantly relieved the effect of NO. Our data shows that the relative electrolyte leakage levels of plants treated with OLD SNP or Hb was not significantly different than Cd-exposed plants. In short, the aforementioned results proved that the application of exogenous NO exhibited protective effects against Cd-induced oxidative damage in T. angustifolia.
Effects of NO on activities of antioxidant enzymes and AsA content
The combined results from our lipid peroxidation and electrical conductivity assays suggested that SNP treatment enhanced plant tolerance to Cd stress probably through reducing ROS accumulation and subsequent oxidative damage. Thus, it was necessary to examine the antioxidant responsible for ROS scavenging. Subsequent analyses were carried out to investigate SOD and CAT in T. angustifolia plants which were subjected to Cd stress, with or without the SNP or Hb treatments. Our results showed that Cd treatment significantly increased SOD and CAT activities in T. angustifolia compared with CK (Fig. 3). However, we observed that SNP counteracted the Cd-induced increases to the activities of SOD and CAT. Likewise, adding Hb counteracted the effect of SNP on the activities of SOD and CAT. Among the two antioxidant enzymes studied, SNP treatment had insignificant or diametric effects on enzyme activities in T. angustifolia under Cd stress.
Effect of sodium nitroprusside (SNP, a NO donor) on SOD and CAT activities in the leaf, sheath and root of T. angustifolia exposed to Cd in the presence of SNP. Plants were treated with Hoagland nutrient solution (control, CK), Cd (444.8 μM), Cd (444.8 μM) + SNP (100 μM) and Cd (444.8 μM) + 0.1 % Hb (w:v). Vertical bars represent standard error of the mean (n = 3). Bars with different letters are significantly different within each group at P < 0.05 according to Duncan’s multiple test
Ascorbic acid (AsA) is one of the most important water-soluble non-enzymatic antioxidants in plants, which are related to the detoxification of reactive oxygen species (Chen and Gallie 2012). In T. angustifolia, the content of AsA was induced by SNP treatment compared with Cd treatment, while Hb treatment reversed counteracted this increase (Fig. 4).
Effect of sodium nitroprusside (SNP, a NO donor) on ascorbic acid (AsA) content in the leaf, sheath and root of T. angustifolia. Plants were treated with Hoagland nutrient solution (control, CK), Cd (444.8 μM), Cd (444.8 μM) + SNP (100 μM) and Cd (444.8 μM) + 0.1 % Hb (w:v). Vertical bars represent standard error of the mean (n = 3). Bars with different letters are significantly different within each group at P < 0.05 according to Duncan’s multiple test
Effect of NO on the accumulation of metabolites
Both PCs and NPT are peptides which can relieve Cd stress by chelating Cd in cells. As shown in Fig. 5, Cd treatment significantly increased the contents of PCs and NPT in the root, while it had only slight effects on the leaf and sheath. SNP treatment was found to significantly counteract Cd-induced increases to PCs and NPT in the root. After application of Hb to plants under Cd stress, PCs and NPT content had only slight changes in the leaf and sheath, but there was an obvious reduction in the root compared with Cd stress. These results suggest that the protected effect of SNP might not work through chelating Cd ions in cells by PCs or NPT.
Effect of sodium nitroprusside (SNP, a NO donor) on non-protein thiol (NPT) and phytochelatins (PCs) in the leaf, sheath and root of T. angustifolia. Plants were treated with Hoagland nutrient solution (control, CK), Cd (444.8 μM), Cd (444.8 μM) + SNP (100 μM) and Cd (444.8 μM) + 0.1 % Hb (w:v). Vertical bars represent standard error of the mean (n = 3). Bars with different letters are significantly different within each group at P < 0.05 according to Duncan’s multiple test
NO increase Cd accumulation in cell walls
Analysis of Cd content revealed that additional Cd in nutrient solution significantly increased Cd accumulation in different tissues of T. angustifolia. The uptake of Cd in the SNP-treated seedlings exhibited a significant decrease in the leaf and sheath (Table 1). However the accumulation of Cd in the root was significantly increased. When applied together with Cd and Hb, the accumulation of Cd had a crosscurrent with SNP treatment, but close to Cd stress. We further determined Cd content in the subcellular fractions of different tissues (Table 2). As expected, in Cd + SNP treated leaves, Cd content in different subcellular fractions decreased when compared with samples subjected to Cd stress alone. Adding SNP under Cd treatment, the accumulation of Cd had slightly increased in cell wall and organelles, but it was obviously reduced in the soluble fraction of the sheath when compared with plants that wrer only subjected to Cd stress. In the root, supplying SNP resulted in increased Cd accumulation in the cell wall and decreased Cd content in the soluble fraction of cells. The Cd content in different subcellular fractions of the leaf increased under Cd + Hb treatment when compared with the Cd + SNP treated, and the Cd content in the organelles of Cd + Hb treated was significantly higher than it was in the leaf under Cd stress alone. Hb promoted the accumulation of Cd in the soluble fraction of root samples compared with SNP treatment.
Discussion
Cd is one of the toxic heavy metals and has become a widespread pollutant in the environment. Cd is easily accumulated in higher plants and causes severe inhibition of plant growth (Wagner 1993). It has been well documented that Cd stress can indirectly cause the increased generation of ROS, which subsequently results in oxidative damage to plant tissues (De Michele et al. 2009). MDA, a product of lipid peroxidation, is often measured as an indicator of oxidative stress. It has been reported in wheat root that MDA content increases under Cd stress and supplementary SNP could decrease MDA content and thus alleviate Cd stress (Singh et al. 2008). When applied together with Cd, SNP and Hb had an opposite tendency compared to Cd + SNP treatment. Consistent with this, our results showed that under Cd stress, adding SNP relieved Cd stress via decreasing MDA content in the root (Fig. 2a). An electrolyte leakage assay is frequently used to measure the degree of cell damage. Our data show that Cd exposure caused significant changes in REL in different tissues. We estimated the electrolyte leakage of the different tissues and found that SNP treatment could significantly block the increase of electrical conductivity triggered by Cd stress (Fig. 2b). However, application of Cd + SNP + Hb treatment increased levels of REL when compared with Cd + SNP treatment. Additionally, plants subjected to the Cd + OLD SNP treatment yielded opposing results compared with the Cd + SNP treatment. The increased levels of REL indicated that Cd exposure induced the generation of ROS, causing cellular damage in plants (Apel and Hirt 2004). This study found that SNP-treatment relieved the increase of MDA content and levels of REL, and it showed that exogenous NO could protect or repair effect of the plant cell membrane.
In normal conditions, production and scavenging of ROS are in equilibrium. Adversity stress causes ROS metabolic imbalance and leads to excessive accumulation of reactive oxygen species which thereby cause oxidative damage. Under Cd stress, plants scavenge excessive accumulation of ROS through the products of antioxidant enzymes and non-enzymatic antioxidants, thereby mitigating cell injury (Schützendübel et al. 2001; Wu et al. 2003). In this work, the activities of antioxidant enzymes including SOD and CAT increased in different tissues of T. angustifolia following Cd exposure, which illustrates that Cd stress leads to oxidative stress and which implies that the T. angustifolia is able to better tolerate Cd treatment by enhancing the activities of antioxidant enzymes (Fig. 3). However, the activities of SOD and CAT were decreased by Cd + SNP treatment, compared with plants which were subjected to Cd exposure alone. Similarly, it has been reported in wheat root that SNP supply resulted in a reduction in Cd-induced increased activities of SOD and CAT (Singh et al. 2008). Laspina et al. (2005) found that NO could prevent Cd-induced by increasing the activity of SOD in sunflower leaf. Under Cd exposure, Hb treatment could reverse the effect of SNP on the activities of SOD and CAT, and even higher than Cd exposure alone in the root. This effect might be ascribed to the capability of NO to scavenge Cd toxicity in other ways but antioxidant enzymes, which then hindered the increase in the activities of the antioxidant enzymes SOD and CAT.
We subsequently analyzed the changes of non-enzymatic antioxidants in T. angustifolia. The content of AsA, one of the most abundant and important non-enzyme antioxidants, was obviously induced by NO application in Cd exposed plants. However, the content of AsA had little increased by Hb treatment upon Cd exposure while comparing with Cd stress only (Fig. 4). These results indicated that the reduction of Cd toxicity might be aroused by antioxidant properties of NO, and SNP-induced AsA content might be involved in maintaining the balance of ROS.
Apart from scavenging of Cd-induced overproduced ROS, chelation of metals in the cytosol by high-affinity ligands is potentially a rather important mechanism of heavy-metal detoxification and tolerance. Some NPT compounds, comprised of several acid-soluble sulfhydryl components, such as cysteine and PCs, are known to be important for the detoxification or homeostasis of heavy metals (Clemens 2001). The PCs are the most widely studied high-affinity compounds in plants, especially in relation to Cd tolerance (Zenk 1996). The detoxification role of PCs has been supported by a range of biochemical and genetic evidence (Maier et al. 2003; Cobbett and Goldsbrough 2002). Once PCs are synthesized, they can be transported into the vacuole, where they then form high molecular weight complexes. These complexes are the ultimate and more stable storage form of cadmium (Mendoza-Cozatl and Moreno-Sanchez 2006). In this study, NPT and PCs contents in the root were significantly increased in plants exposed to Cd. However, the addition of SNP with Cd resulted in diametrically opposed changes in the NPT and PCs contents when compared to Cd exposure alone (Fig. 5). Application of Hb upon Cd stress significantly decreased PCs content in the root compared with simply Cd exposed plants.
We further analyzed Cd content in seedlings upon Cd stress. Our results showed that the highest content of Cd was accumulated in the root (Table 1). This is consistent with previous studies, which conclude that Cd mainly gathered in the root and accumulated lower Cd in ground (Küpper et al. 2000). Exogenous NO application increased Cd accumulation in the root and decreased its content in the leaf and sheath (Table 1). Namdjoyan and Kermanian (2013) found that treatment with 100 μM SNP considerably reduced root-to-shoot translocation of arsenic in watercress plants. Cd + Hb treatment resulted in increased accumulation of Cd in the leaf and sheath while decreasing Cd content in the root. Our results indicated that exogenous NO relieved Cd toxicity by reducing the transportation of Cd to the ground. The accumulation of Cd in different subcellular tissues was also analyzed. Table 2 showed that exogenous NO treatment enhanced Cd accumulation in the cell walls of the root in T. angustifolia seedlings, while it decreased Cd accumulation in the cell walls, organelles and the soluble fraction of the leaf. This decreased Cd content within cells resulted in less NPT and PCs being induced, which is responsible for the reduction of endogenous NPT and PCs contents upon Cd + SNP treatment. As might be expected, Cd + Hb treatment increased the Cd content in the soluble fraction of the root and leaf. Recalling basic botany, the cell wall is the first barrier to prevent Cd from entering into plant cells. Therefore, the accumulation of Cd in cell walls is one of the important mechanisms which plants use to mitigate Cd toxicity. Bringezu et al. (1999) indicated that a protein with oxalate oxidase activity in the cell walls could bind heavy metals. Thus, the uptake into the cells is restricted as only very small amounts were detected in the cytoplasm and in the organelles. It has also been previously reported that adding exogenous NO could relieve Cd stress by increasing pectin and hemicellulose contents in the root cell wall, increasing Cd accumulation in the rice root, and decreasing Cd accumulation in the rice leaf (Xiong et al. 2009). These results showed that exogenous NO could enhance cell wall holding functions to lessen Cd content in the soluble fraction which could ensure the normal order of various physiological and biochemical metabolism in the cytoplasm under Cd stress. We speculate that there might be an NO-dependent mechanism to exclude heavy metals from the cell and to prevent toxic concentrations from accumulating in the cytoplasm in T. angustifolia, but this model would need further investigation before it can be validated.
In summary, our study suggests that NO alleviates Cadmium toxicity in T. angustifolia mainly by enhancing the inherent ability of cell walls to chelate Cd. This study shows that the induced effect of NO on antioxidant enzymes may also play a role in maintaining ROS level balance. As a signaling molecule, NO could also change enzyme activity of cell wall and thus affect the chemical properties of the cell wall (An et al. 2005). We speculate that NO might clearly regulate excess ROS by changing antioxidant enzyme activities of the cell wall; however, this theory requires further investigation. Collectively, our data imply that NO functions as a protector in T. angustifolia under Cd stress primarily via regulating antioxidant metabolism and enhancing Cd accumulation in the root cell wall.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
An LZ, Liu YH, Zhang MX, Chen T, Wang XL (2005) Effects of nitric oxide on growth of maize seedling leaves in the presence or absence of ultraviolet-B radiation. J Plant Physiol 162:317–326
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Arasimowicz M, Floryszak-Wieczorek J (2007) Nitric oxide as a bioactive signalling molecule in plant stress responses. Plant Sci 172:876–887
Bah AM, Dai HX, Zhao J, Sun HY, Cao FB, Zhang GP, Wu FB (2011) Effects of Cadmium, Chromium and lead on growth, metal uptake and antioxidative capacity in Typha angustifolia. Biol Trace Elem Res 142:77–92
Baker AJM (1987) Metal tolerance. New Phytol 106:93–111
Bartha B, Kolbert Z, Erdei L (2005) Nitric oxide production induced by heavy metals in Brassica juncea L. Czern. and Pisum sativum L. Acta Biol Szeged 49:9–12
Blum A, Ebercon A (1981) Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci 21:43–47
Bringezu K, Lichtenberger O, Leopold I, Neumann D (1999) Heavy metal tolerance of Silene vulgaris. J Plant Physiol 154:536–546
Chen Z, Gallie DR (2012) Induction of monozygotic twinning by ascorbic acid in tobacco. PLoS ONE 7:e39147
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832
De Michele R, Vurro E, Rigo C, Costa A, Elviri L, Di Valentin M, Careri M, Zottini M, di Toppi LS, Lo Schiavo FL (2009) Nitric oxide is involved in Cadmium-induced programmed cell death in Arabidopsis suspension cultures. Plant Physiol 150:217–228
Devi SR, Prasad MNV (1998) Copper toxicity in Ceratophyllum demersum L. (coontail), a free floating macrophyte: response of antioxidant enzymes and antioxidants. Plant Sci 138:157–165
Ding YF, Qu AL, Gong SM, Huang SX, Lv B, Zhu C (2013) Molecular identification and analysis of Cd-responsive microRNAs in rice. J Agric Food Chem 61:11668–11675
Durner J, Wendehenne D, Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA 95:10328–10333
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Lannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Garcia JS, Gratão PL, Azevedo RA, Arruda MAZ (2006) Metal contamination effects on sunflower (Helianthus annuus L.) growth and protein expression in leaves during development. J Agric Food Chem 54:8623–8630
Gratao PL, Monteiro CC, Carvalho RF, Tezotto T, Piotto FA, Peres LE, Azevedo RA (2012) Biochemical dissection of diageotropica and Never ripe tomato mutants to Cd-stressful conditions. Plant Physiol Biochem 56:79–96
Huguet S, Bert V, Laboudigue A, Barthès V, Isaure MP, Llorens I, Schat H, Sarret G (2012) Cd speciation and localization in the hyperaccumulator Arabidopsis halleri. Environ Exp Bot 82:54–65
Krämer U (2000) Cadmium for all meals-plants with an unusual appetite. New Phytol 145:1–3
Küpper H, Lombi E, Zhao FJ, McGrath SP (2000) Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperacculator Arabidopsis halleri. Planta 212:75–84
Laspina NV, Groppa MD, Tomaro ML, Benavides MP (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169:323–330
Luo BF, Du ST, Lu KX, Liu WJ, Lin XY, Jin CW (2012) Iron uptake system mediates nitrate-facilitated cadmium accumulation in tomato (Solanum lycopersicum) plants. J Exp Bot 63:3127–3136
Maier EA, Matthews RD, McDowell JA, Walden RR, Ahner BA (2003) Environmental cadmium levels increase phytochelatin and glutathione in lettuce grown in chelator-buffered nutrient solution. J Environ Qual 32:1356–1364
Mendoza-Cozatl DG, Moreno-Sanchez R (2006) Control of glutathione and phytochelatin synthesis under cadmium stress. Pathway modeling for plants. J Theor Biol 238:919–936
Mojiri A, Aziz HA, Zahed MA, Aziz SQ, Selamat MRB (2013) Phytoremediation of heavy metals from urban waste leachate by southern cattail (Typha domingensis). Int J Sci Res Environ Sci 1:63–70
Namdjoyan S, Kermanian H (2013) Exogenous nitric oxide (as sodium nitroprusside) ameliorates arsenic-induced oxidative stress in watercress (Nasturtium officinale R. Br.) plants. Sci Hortic-Amst 161:350–356
Przymusinski R, Wozny A (1985) The reactions of lupin roots on the presence of lead in the medium. Biochem Physiol Pflanzen 180:309–318
Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, del Rio LA, Sandalio LM (2009) Cellular responses of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide and calcium. Plant Physiol 150:229–243
Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine root. Plant Physiol 127:887–898
Singh HP, Batish DR, Kaur G, Arora K, Kohli RK (2008) Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat root. Environ Exp Bot 63:158–167
Smeets K, Ruytinx J, Semane B, Van Belleghem F, Remans T, Van Sanden S, Vangronsveld J, Cuypers A (2008) Cadmium-induced transcriptional and enzymatic alterations related to oxidative stress. Environ Exp Bot 63:1–8
Tossi V, Lamattina L, Cassia R (2009) An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol 181:871–879
Verbruggen N, Hermans C, Schat H (2009) Molecular mechanisms of metal hyperaccumulation in plants. New Phytol 181:759–776
Verma K, Mehta SK, Shekhawat GS (2013) Nitric oxide (NO) counteracts cadmium induced cytotoxic processes mediated by reactive oxygen species (ROS) in Brassica juncea: cross-talk between ROS, NO and antioxidant responses. Biometals 26:255–269
Wagner GJ (1993) Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron 51:173–212
Wang X, Liu YG, Zeng GM, Chai LY, Song XC, Min ZY, Xiao X (2008) Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ Exp Bot 62:389–395
Wang QH, Liang X, Dong YJ, Xu LL, Zhang XW, Hou J, Fan ZY (2013) Effects of exogenous nitric oxide on cadmium toxicity, element contents and antioxidative system in perennial ryegrass. Plant Growth Regul 69:11–20
Wierzbicka M (1987) Lead translocation and localization in Allium-cepa roots. Can J Bot 65:1851–1860
Wink DA, Mitchell JB (1998) Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radical Biol Med 25:434–456
Wu FB, Zhang GP, Dominy P (2003) Four barley genotypes respond differently to cadmium: lipid peroxidation and activities of antioxidant capacity. Environ Exp Bot 50:67–78
Wu XL, Luo YM, Xu YC, Yan GL, Zhao Y (2007) Effects of cadmium stress on some physiological indexes of Typha angustifolia (in chinese). J Plant Res Environ 16:74–76
Wu XL, Luo YM, Xu YC, Yan GL (2008) The kinetics characteristic of Cd2+ absorption of Typha angustifolia (in chinese). Guihaia 28:511–514
Wu M, Wang PY, Sun LG, Zhang JJ, Yu J, Wang YW, Chen GX (2014) Alleviation of cadmium toxicity by cerium in rice seedlings is related to improved photosynthesis, elevated antioxidant enzymes and decreased oxidative stress. Plant Growth Regul 74:251–260
Xiang CB, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10:1539–1550
Xiong J, An LY, Lu H, Zhu C (2009) Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230:755–765
Zenk MH (1996) Heavy metal detoxification in higher plants-a review. Gene 179:21–30
Zhou W, Zhao D, Lin X (1997) Effects of waterlogging on nitrogen accumulation and alleviation of waterlogging damage by application of nitrogen fertilizer and mixtalol in winter rape (Brassica napus L.). J Plant Growth Regul 16:47–53
Acknowledgments
This work was supported by the Funding of Natural Science of Jiangsu Province, China (BK2011640) and Funding of agricultural science and technology innovation of Jiangsu Province, China (CX(12)5075). We specially thank Cade Guthrie in Plant Biology of U C Davis and Lydia Wooldridge in University of Missouri (Columbia) for reading and revising this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, H., Jin, Q., Wang, Y. et al. Effects of nitric oxide on alleviating cadmium stress in Typha angustifolia . Plant Growth Regul 78, 243–251 (2016). https://doi.org/10.1007/s10725-015-0089-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-015-0089-z