Abstract
High temperature is a major factor affecting grain yield and plant senescence in wheat growing regions of central and east China. In this study, two different wheat cultivars, Yangmai 9 with low-grain protein concentration and Xuzhou 26 with high-grain protein concentration, were exposed to different temperature regimes in growth chambers during grain filling. Four day/night temperature regimes of 34°C/22°C, 32°C/24°C, 26°C/14°C, and 24°C/16°C were established to obtain two daily temperatures of 28 and 20°C, and two diurnal day/night temperature differences of 12 and 8°C. Concentration of a lipid peroxidation product malondialdehyde (MDA), activities of the antioxidants superoxide dismutase (SOD) and catalase (CAT), chlorophyll concentration (SPAD) in flag leaves and kernel weight were determined. Results show that activities of SOD and CAT in leaves increased markedly on 14 days after anthesis (DAA) for the high-temperature treatment (34°C/22°C) and then declined. As a result, MDA concentration in leaves increased significantly under high temperature (34°C/22°C and 32°C/24°C). Compared with optimum temperature treatment, high temperature reduced the concentration of soluble protein and SPAD values in flag leaves. Grain-filling rate increased slightly initially, but decreased significantly during late grain filling under high temperature. As a result, final grain weight was reduced markedly under high temperature. Decreases in the activities of SOD and CAT and increases in MDA concentration in leaves were more pronounced with a 12°C of day/night temperature difference when under high temperatures. Kernel weight was higher under 12°C of day/night temperature difference under optimum temperatures (24°C/16°C and 26°C/14°C). The responses to high-temperature regimes appeared to differ between the two wheat cultivars with different grain protein concentrations. It is concluded that a larger diurnal temperature difference hastened the senescence of flag leaves under high-temperature conditions, but retarded senescence under optimum temperature treatments of 26°C/14°C and 24°C/16°C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The optimum temperature to achieve maximum yields of wheat is generally considered to be between 15 and 20°C during grain growth (Dupont and Altenbach 2003). Temperatures greater than 30°C during grain filling are known to reduce individual kernel mass in wheat (Randall and Moss 1990; Wardlaw 1994). Low-kernel mass has been documented in some wheat growing regions of China (North Wheat Dry-hot Wind Research Group 1998), where high-temperature stress frequently occurs during grain filling.
Loss of leaf viability during senescence results in a close link between the duration of photosynthetically active leaf area and grain yield in wheat (Simpson 1968; Rawson et al. 1983; Ellen 1987). Leaf senescence is very sensitive to environmental conditions, particularly high temperature (Paulsen 1994). Sustained chlorophyll concentrations during maturation have been used as an efficient indicator of heat tolerance in wheat cultivars (Reynolds et al. 1994). When plants are exposed to high-temperature stress, chlorophyll biosynthesis is inhibited (Tewari and Tripathy 1998). Loss of chlorophyll is usually attributed to membrane damage and leaf senescence (Simon 1974; Liu and Huang 2000; Huang et al. 2001).
Heat injury is related to the production of active oxygen species, which cause oxidative damage to cell membranes and results in the dysfunction of the active oxygen-scavenging systems during stress (Dhindsa and Matowe 1981; Elstner 1982; Bowler et al. 1992). High temperatures are known to induce the production of active oxygen species, which are highly reactive and can bring about peroxidation of membrane lipids, leading to membrane damage (Levitt 1980; Dhindsa et al. 1981). It has been shown that leaf senescence in wheat is associated with the increase in active oxygen (Bai 1994) and the disturbed balance between producing and quenching active oxygen species (Bai 1994). Moderately elevated temperatures accelerate senescence, reducing the duration of photosynthetically active leaf area and diminished carbon gain (Al-Khatib and Paulsen 1989; Harding et al. 1990).
There are few studies on the physiological effects of heat stress on grain growth in wheat including day–night temperature difference. Even less information is available on differential responses of wheat cultivars with respected to leaf senescence and kernel weight influenced by high-temperature conditions during grain filling. Thus, the present study was undertaken to determine the effects of prolonged high-temperature stress and day/night temperature differences on membrane peroxidation concentration in flag leaves and kernel weight in two wheat cultivars.
Materials and methods
Plant materials and temperature treatments
A pot experiment was carried out at Nanjing Agricultural University, using two winter wheat (Triticum aestivum L.) cultivars Yangmai 9 and Xuzhou 26, with average grain protein concentration of about 11 and 15%, respectively. Pots with a diameter of 23 cm and height of 25 cm were each filled with 8 kg pot−1 sieved yellow–brown soil. The soil contained total available N of 29.2 μg g−1, available P2O5 of 29.5 μg g−1, and available K2O of 72.3 μg g−1. Fifteen seedlings per pot were initially planted, and then thinned to five seedlings per pot at the third-leaf stage. The nitrogen fertilization rate used was 1.5 kg N pot−1 according to local standard for high-yielding wheat cultivation, and P2O5 and K2O were 0.5 and 1.5 kg N pot−1, respectively. Water was added as needed to soil relative water content, SRWC = 75–80%.
Plants were initially grown in a greenhouse until 7 days after anthesis (DAA), and then moved to controlled growth chambers for application of the temperature treatments. The chambers were set for four day/night temperature regimes of 34°C/22°C, 32°C/24°C, 26°C/14°C, and 24°C/16°C. This resulted in mean daily temperatures of 28 and 20°C, and two day/night temperature differences of 12 and 8°C. Day and night temperatures were maintain for 12 h with a photo period lasting from 6:00 to 18:00, at a PPFD of around 800 μmol m−2 s−1. Relative humidity in the chambers was set at ±65%.
Plant sampling
Heads flowering on the same day were tagged. From 7 DAA, the tagged heads were sampled at 7-day intervals until maturity. Flag leaves were detached and immediately submerged in liquid nitrogen for and stored at −40°C until the enzyme assays. Since plants under high temperature (34°C/22°C and 32°C/24°C) were already matured at 32 DAA, the leaf samples from these treatments at 35 DAA were not used for the enzyme assays. Grain-filling rate was estimated by obtaining dry kernel weight from the periodical samples after anthesis. At each sampling, grains were detached from three pots per treatment with five main stems per pot and oven-dried at 80°C until constant weight. At maturity, grains were harvested from three pots per treatment and sun-dried for grain weight.
Measurement
Chlorophyll concentration (SPAD value)
SPAD values of flag leaves were determined using a chlorophyll meter (Minolta SPAD-502) every 7 DAA till 35 DAA.
Enzyme extraction
Enzyme extract for CAT and SOD was prepared by grinding 2 g fresh leaves with 5 ml extraction buffer (0.1 M phosphate, pH 7.5, containing 0.5 mM EDTA and 1 Mm ascorbic acid) in ice bath and centrifuged at 13,000g for 20 min. The supernatant was used to measure enzyme activities. Superoxide dismutase (SOD) and CAT are the most effective antioxidant enzymes, and their combined effect averts cellular damage.
CAT activity
Catalase activity was assayed by measuring the initial rate of H2O2 disappearance (Kato and Shimizu 1987). The reaction mixture of 3 ml contained 0.1 M sodium phosphate buffer (pH 7), and 2 mM H2O2 and 0.1 ml of crude extract. The breakdown of H2O2 was followed by measuring the absorbance change at 240 nm and the enzyme activity was calculated using the extinction coefficient (40 mM cm−1 at 240 nm) for H2O2.
SOD activity
Superoxide dismutase activity was assayed by measuring its ability to inhibit the photoreduction of nitro blue tetrazolium (NBT) following the method of Giannopolitis and Ries (1977). The reaction mixture of 3 ml contained 50 μM NBT, 13 mM methionine, 75 μM NBT chloride, 0.1 mM EDTA, 50 Mm phosphate buffer (pH 7.8), 50 Mm sodium carbonate, and 0.1 ml enzyme solution. Test tubes containing the reaction solution were irradiated under a light bank (15 fluorescent lamps) at 78 μmol m−2 s−1 for 15 min. Absorbances of the irradiated and non-irradiated solutions at 560 nm were determined with a spectrophotometer (Hitachi U-1100, Tokyo, Japan). One unit of SOD activity was defined as the amount of enzyme that inhibits 50% of NBT photoreduction.
Soluble protein concentration
Soluble protein concentration of the crude extract was measured by the Bright Blue G-250 solution (Bai and Tang 1993). Protein concentration was expressed as mg g−1 fresh weight.
MDA concentration
Following the similar procedures of enzyme extraction, MDA concentration was measured by TBA method. The extraction of 3 ml and 0.5% TBA of 5 ml were incubated for 10–15 min in boiling-water bath, and then centrifuged at 1,800g for 10 min after cooling to room temperature. Supernatant was collected to measure the absorbance at 450, 532, and 600 nm, respectively (Zhao et al. 1994). Absorbance of the clear solution was measured at 532 nm and corrected for non-specific turbidity by subtracting the absorbance at 600 nm. The amount of accumulated MDA was estimated by using an absorption coefficient of 155 mM cm−3.
Results
MDA concentration
From 7 to 28 or 35 DAA, MDA concentration in leaves increased for both cultivars under all temperature regimes after 14 DAA of treatment (Fig. 1). Compared with optimum temperature, high temperature (34°C/22°C and 32°C/24°C) enhanced leaf MDA concentration significantly from 14 to 28 DAA. Under both high (34°C/22°C and 32°C/24°C) and optimum temperature (26°C/14°C and 24°C/16°C), higher diurnal temperature difference enhanced MDA concentration in the leaves of both cultivars.
SOD activity
High temperatures enhanced leaf SOD activity significantly for both wheat cultivars on 14 DAA. Leaf SOD activity then declined at prolonged high temperatures until 28 DAA (Fig. 2). Under high temperatures, SOD activity under 32°C/24°C was higher than that under 34°C/22°C in Yangmai 9 14 DAA, but the opposite response was observed with Xuzhou 26. Under optimum temperatures, SOD activity under 26°C/14°C was higher than that under 24°C/16°C in Xuzhou 26, but the reversed was true for Yangmai 9.
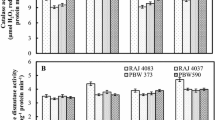
CAT activity
High temperature enhanced CAT activity on 14 DAA in leaves for both wheat cultivars. CAT activity declined with grain filling over all temperature treatments (Fig. 3). However, high temperatures in leaves of both cultivars showed enhanced CAT activity 14 DAA. Reductions in CAT activity were greater with 34°C/22°C and 32°C/24°C than either 26°C/14°C or 24°C/16°C. CAT activity for both cultivars was also influenced by diurnal temperature differences under high temperatures. The larger the diurnal temperature difference, the lower the CAT activity under high temperatures. Xuzhou 26 had higher leaf CAT activity than Yangmai 9 from 14 to 28 or 35 DAA.
Soluble protein concentration
High temperatures enhanced soluble protein concentration in flag leaves for both cultivars on 14 DAA, however it declined when compared with optimum temperature treatments (Fig. 4). Also from 14 DAA, soluble protein concentrations were similar between two diurnal temperatures under high temperature, but were markedly higher for 26°C/14°C than for 24°C/16°C under optimum temperatures.
SPAD value
High temperatures (34°C/22°C and 32°C/24°C) significantly reduced SPAD values in flag leaves during grain-filling period (Fig. 5). Under optimum temperatures, SPAD value increased by 14 DAA, and then declined until maturity. During later filling stage, high temperatures significantly reduced SPAD and shortened the duration of photosynthetic function in flag leaves. Under the different diurnal temperatures, SPAD values were similar under both high and optimum temperatures. Yangmai 9 had higher SPAD values than Xuzhou 26 under all temperature treatments.
Grain-filling rate
Grain-filling rate increasing during early grain filling until 21 DAA and then declined with all temperature treatments. Compared with optimum temperatures, high temperatures enhanced grain-filling rates slightly before 21 DAA, but they declined during later filling. Furthermore, the greater the diurnal temperature difference, the faster the grain filling rate declined under high temperatures, whereas under optimum temperatures, grain filling rates differed only with daily temperature differences. In addition, grain-filling rate in Xuzhou 26 was obviously higher than in Yangmai 9.
Grain weight
Single grain weight at maturity was reduced by high temperature in both cultivars (Fig. 6), largely due to the reduced grain-filling period (Fig. 7). Grain weight was also reduced by the large diurnal temperature difference at high temperature, but was enhanced by large diurnal temperature difference under the optimum temperature. Consistent with the pattern of grain-filling rate, Xuzhou 26 had a higher grain weight than Yangmai 9 under different temperature conditions.
On 14, 21, and 28 DAA, grain weight was positively related to SOD activity, CAT activity and soluble protein concentration in leaves of both cultivars, however on 21 and 28 DAA, the correlation between grain weight and MDA concentration was negative (Table 1). All correlation coefficients were greater 21 DAA than those 14 and 28 DAA, determined with the except for the correlation between grain weight and MDA concentration.
Discussion
Heat stress is known to adversely affect grain yield in wheat (Stone and Nicolas 1994). Plaut et al. (2004) reported that high temperature reduced the rate of transport of dry matter from vegetative organs to kernel, but sensitivity to high-temperature stress differed with varieties. Shah and Paulsen (2003) found that wheat grain weight was constant 1 week earlier at high temperature compared to an apparent optimum temperature. In this study, high temperature enhanced grain-filling rate during early grain filling, but reduced it sharply after reaching maximum on 21 DAA. This resulted in a significant decrease in final grain weight. The effect of high-temperature stress on kernel weight was similar for both cultivars, although the kernel weight was consistently higher in Xuzhou 26 than in Yangmai 9. At high temperature, kernel weight was greater for the 32°C/24°C treatment with lower day/night temperature difference. At the optimum temperature, kernel weight was greater at 26°C/14°C with higher temperature difference. But grain weight was more responsive to higher temperature difference at high temperatures than at optimum temperatures.
Superoxide dismutase and catalase are two key enzymes in the active-oxygen scavenging system that can quench super oxygen free radicals and H2O2, respectively, and thus suppress the production of active oxygen species (Elstner 1982; Bowler et al. 1992). Guo et al. (1998) reported that SOD activity in flag leaves of wheat increased during an initial period of heat stress, but decreased rapidly following prolonged heat stress. In this study, SOD activity increased transiently around 14 DAA but decreased rapidly under high-temperature treatments. This suggested that around 14 DAA, the observed decrease in SOD activity would cause O2 −accumulation and possible oxidative damage. In addition, diurnal temperature difference affected SOD activity differently between two cultivars. During late grain filling, higher temperature differences enhanced SOD activity in Xuzhou 26 with its high-grain protein concentration, but reduced SOD activity in Yangmai 9 with its low-grain protein concentration.
As CAT breaks down H2O2, a decrease in its activity would result in accumulation of H2O2, causing oxidative membrane damage. High temperature enhanced CAT activity around 14 DAA, but subsequently reduced it. In addition, the response of SOD activity to diurnal temperature difference differed between two cultivars. Response of CAT activity to diurnal temperature difference was similar for both cultivars. The present study indicated that SOD and CAT activities were positively related to grain weight, and thus reducing SOD and CAT activities generally reduced grain weight in wheat.
Malondialdehyde is a final product of peroxidation of unsaturated fatty acids in phospholipids that is often used as a measure of level of lipid peroxidation (Halliwell and Gutteridge 1989). High temperature enhanced MDA concentrations in leaves for both cultivars, which was associated with the increase in cell membrane permeability. This result was consistent with the report by Liu et al. (2005) in wheat. But MDA concentrations were elevated by larger diurnal temperature difference under both high and optimum temperatures. This implied that lipid peroxidation was built up in leaves under enlarged diurnal temperature difference, which would affect biochemical metabolism and hasten senescence progress in wheat.
Chlorophyll concentration of flag leaves was used as a measure of leaf senescence and its acceleration by heat stress (Al-Khatib and Paulsen 1984). Premature loss of chlorophyll due to heat sensitivity in wheat crop has been reported earlier (Reynolds et al. 1994). Significant reduction in chlorophyll concentration (SPAD) under high temperature was observed in both cultivars in this study. However, at high and optimum temperatures, larger diurnal temperature difference tended to give higher SPAD than lower diurnal temperature difference. Loss of chlorophyll is usually attributed to membrane damage and leaf senescence (Liu and Huang 2000). The results suggested that high temperature accelerated leaf senescence in wheat, but optimum temperature with larger temperature difference would delay leaf senescence, as seen in Xuzhou 26.
High temperatures enhanced soluble protein concentrations in flag leaves on 14 DAA, this was probably resulted from enhanced proteolysis or nitrogen assimilation in leaves. After 14 DAA, however, soluble protein concentration declined rapidly at high temperature, similar to the finding in flag leaves of rice (Peng et al. 2005). The decrease of soluble protein concentration under high temperature might cause the decline of photosynthetic rate in leaves. The results also found that larger diurnal temperature difference enhanced soluble protein concentration and delayed senescence of flag leaves at optimum temperature, but reduced soluble protein concentration and hastened leaf senescence at high temperature. Also, at high temperature, soluble protein concentration in Xuzhou 26 with its high-grain protein concentration was more responsive to diurnal temperature difference than that in Yangmai 9 with its low-grain protein concentration.
In conclusion, the results of this study suggested that high temperature during grain filling accelerated leaf senescence in wheat. As a result, the membrane lipid peroxidation in flag leaf was hastened and kernel weight was reduced significantly. The responses to high temperature appeared different between two wheat cultivars with different grain protein concentrations. Moreover, larger diurnal temperature difference was harmful for leaf functioning and grain filling in wheat at high temperature, but relatively favorable at optimum temperature. Further studies should be undertaken to explore appropriate approaches and measures for regulating the impact of high-temperature stress on leaf senescence in wheat. This would lead to minimizing damage of high temperature by enhancing leaf function and improving grain growth under heat stress during grain filling.
Abbreviations
- MDA:
-
Malondiadehyde
- SOD:
-
Superoxide dismutate
- CAT:
-
Catalase
- DAA:
-
Days after anthesis
- PPFD:
-
Photosynthetic photon flux density
References
Al-Khatib K, Paulsen GM (1984) Mode of high temperature injury to wheat during grain development. Physiol Plant 61:363–368
Al-Khatib K, Paulsen GM (1989) Enhancement of thermal injury to photosynthesis in wheat plants and thylakoids by high light intensity. Plant Physiol 90:1041–1048
Bai BZ (1994) Plant physiology. China Science Press, Beijing, pp 89–90 (in Chinese)
Bai BZ, Tang XJ (1993) Plant physiology testing technology. China Science Press, Beijing, pp. 99–100, 156–157 (in Chinese)
Bowler C, Van Montagu M, Inze D (1992) Superoxide dismutase and stress tolerance. Ann Rev Plant Physio Plant Mol Biol 43:83–116
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defense against lipid peroxidation. J Exp Bot 32:79–91
Dhindsa RA, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence correlated with increased permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 126:93–101
Dupont FM, Altenbach SB (2003) Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis. J Cereal Sci 38:133–146
Ellen J (1987) Effects of plant density and nitrogen fertilization in winter wheat (Triticum aestivum L.): I. Production pattern and grain yield. Neth J Agr Sci 35:137–153
Elstner EF (1982) Oxygen activation and oxygen toxicity. Ann Rev Plant Physio 33:73–96
Giannopolitis CN, Ries SK (1977) Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol 59:309–314
Guo TC, Wang CY, Zhu YJ, Wang HC, Li JX, Zhou JZ (1998) Effects of high temperature on the senescence of root and top-part of wheat plant in the later stage. Acta Agron Sin 24(6):957–962 (in Chinese with English abstract)
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine, 2nd edn. Oxford University Press, Oxford, UK
Harding SA, Guikema JA, Paulsen GM (1990) Photosynthetic decline from high temperature stress during maturation of wheat. I. Interaction with senescence processes. Plant Physiol 92:648–653
Huang BR, Liu XZ, Xu QZ (2001) Supraoptimal soil temperature induced oxidative stress in leaves of creeping bentgrass cultivars differing in heat tolerance. Crop Sci 41:430–435
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves: phenolic-dependent peroxidative degradation. Can J Bot 65:729–735
Levitt J (1980) Responses of plants to environmental stress, 2nd edn. Academic, New York
Liu P, Guo WS, Pu HC, Feng CN, Zhu XK, Peng YX (2005) Effects of high temperature during grain filling period on antioxidant enzymes and lipid peroxidation in flag leaves of wheat. Sci Agric Sin 38:2403–2407 (in Chinese with English abstract)
Liu X, Huang B (2000) Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci 40:503–510
North Wheat Dry-hot Wind Research Group (1998) Wheat dry-hot wind. Weather Press, Beijing
Paulsen GM (1994) High temperature responses of crop plants. In: Boote KJ et al (eds) Physiology and determination of crop yield. ASA, CASSA, and SSSA, Madison, WI
Peng CL, Ou ZY, Liu N, Lin GZ (2005) Response to high temperature in flag leaves of super high-yielding rice Pei’ai 64S/E32 and Liangyoupeijiu. Rice Sci 12(3):179–186
Plaut Z, Butow BJ, Blumenthal CS, Wrigley CW (2004) Transport of dry matter into developing wheat kernels and its contribution to grain yield under post-anthesis water deficit and elevated temperature. Field Crops Res 86:185–198
Randall PJ, Moss HJ (1990) Some effects of temperature regime during grain filling on wheat quality. J Agric Res 41:603–617
Rawson HM, Hindmarsh JH, Fisher RA, Stockman YM (1983) Changes in leaf photosynthesis with plant ontogeny and relationships with yield per ear in wheat cultivars and 120 progeny. Aust J Plant Physiol 10:503–514
Reynolds MP, Balota M, Delgado MIB, Amani I, Fischer RA (1994) Physiological and morphological traits associated with spring wheat yield under hot, irrigated conditions. Aust J Plant Physiol 21:717–730
Shah NH, Paulsen1 GM (2003) Interaction of drought and high temperature on photosynthesis and grain-filling of wheat. Plant Soil 257:219–226
Simon EW (1974) Phospholipids and plant membrane permeability. New Phytol 73:377–420
Simpson GM (1968) Association between grain yield per plant and photosynthetic area above the flag-leaf node in wheat. Can J Plant Sci 48:253–260
Stone PJ, Nicolas ME (1994) Wheat cultivars vary widely their responses of grain yield and quality to short periods of post-anthesis heat stress. Aust J Plant Physiol 21:887–900
Tewari AK, Tripathy BC (1998) Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol 117:851–858
Wardlaw IF (1994) The effect of high temperature on kernel development in wheat: variability related to pre-heading and post-anthesis conditions. Aust J Plant Physiol 21:731–739
Zhao SJ, Xu ZC, Zou Q, Meng QW (1994) Improvement of method for measurement of malondialdehvdein plant tissues. Plant Physiol Commun 30:207–210 (in Chinese with English abstract)
Acknowledgements
This research was supported by the National Natural Science Foundation of China (30200166) and the Natural Science Foundation of Jiangsu Province, China (BK2005212).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, H., Dai, T., Jing, Q. et al. Leaf senescence and grain filling affected by post-anthesis high temperatures in two different wheat cultivars. Plant Growth Regul 51, 149–158 (2007). https://doi.org/10.1007/s10725-006-9157-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-006-9157-8