Abstract
Extremely high temperature decreases grain yield and grain quality in rice (Oryza sativa L.). To investigate the effects of short-term post-anthesis high-temperature stress on accumulation of grain protein and protein compositions in rice, 2-year experiments were carried out in phytotron with two different cultivars, Nanjing 41 (NJ41, heat-sensitive) and Wuxiangjing 14 (WJ14, heat-tolerant), under different conditions of high-temperature levels and durations during anthesis and grain-filling stages. The results showed that grain protein content and its components (albumin, globulin, and glutelin) increased with rising temperature levels and durations, while prolamin content decreased. The contents of total protein, storage protein, albumin, globulin, and prolamin in NJ41 were more sensitive to different temperature levels than those in WJ14. Moreover, the contents of albumin and prolamin in NJ41 changed greater under temperature durations than those in WJ14. The effects of high-temperature stress on accumulation of grain protein and composition were more sensitive to high-temperature levels and durations during anthesis stage than those during grain-filling stage. The results could help us further understand the physiochemical processes governing rice yield and quality under high-temperature conditions during rice anthesis and grain-filling stages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The accelerated global warming has become a growing problem owing to the greenhouse effect. It is estimated that the global air temperature has increased by 0.5 °C in the last century and will continue increasing by 1.4–5.8 °C by the end of the twenty-first century (Teixeira et al. 2013; Liao et al. 2014). High temperature has become a major limitation to crop productivity and quality (Parent et al. 2010; Liu et al. 2013a). Rice (Oryza sativa L.) is one of the most important cereal crops and provides staple food for more than half of the world population, particularly in Asia. High temperature is the principal factor determining rice growth, development, and ultimately grain yield and quality, particularly during post-anthesis reproductive stage (Teixeira et al. 2013; Wang et al. 2014).
During rice anther development, extremely high temperature results in declination in pollen viability, retention of pollen in anthers, and pollen germination (Harsant et al. 2013; Das et al. 2014). During grain-filling period, high temperature shortens grain-filling duration, accelerates plant aging, decreases grain weight, and changes the chemical ingredients of rice caryopses such as starch, storage protein, and fatty acid, affecting the quality of rice (Lin et al. 2010). Most research has focused on the effects of high temperature on starch. High temperature can reduce starch content and change starch structure. Furthermore, high temperature decreases the expression of several starch synthesis-related genes, but increases the expression of starch-consuming a-amylases gene (Nelson et al. 2011). However, the effects of high temperature on protein have been less than adequately studied in rice. Protein accounts for 6–10 % of dry matter of debraned rice grains and is important for grain quality for nutrition, cooking, and brewing. Proteins in rice are in general classified into glutelin, prolamin, globulin, and albumin, constituting about 70, 3, 7, and 5 % of rice grain nitrogen, respectively (Lin et al. 2010). High temperature can change rice grain protein content and protein composition, but the extent has been controversial. It is suggested that high temperature during rice grain-filling period is beneficial for the accumulation of Asp-family amino acids and protein components (Liang et al. 2013). Lin et al. (2010) showed that high temperature increased the accumulation of storage protein at early filling stage but decreased the accumulation of prolamin at maturation. The effects of high temperature on different classes of storage protein have not been documented in detail. Further dynamic changes of grain protein and its composition under different conditions of high-temperature levels have not been reported so far, not to mention the difference between the heat-sensitive and heat-tolerant genotypes.
Therefore, we designed a field experiment using two cultivars with different high-temperature-tolerance levels to conduct further investigation through setting different high-temperature levels and durations. The objectives of the experiment presented in this study were (1) to investigate the effects of different high-temperature levels and durations on accumulation and composition of grain protein and (2) to quantify the effects of post-anthesis high temperature on grain protein and its composition. The results could contribute to the basic theoretical research for a better understanding of the different responsive mechanisms of rice protein under high-temperature stress during anthesis and grain-filling stages and providing useful information for assessing rice quality.
Materials and methods
Experiments
The field experiments were carried out during the period of 2012–2013 in the automatic artificial climate chamber at the experimental station (32°03′N, 118°46′E) of Nanjing Agricultural University during the rice-growing season. Two japonica cultivars Wuxiangjing 14 (WJ14, heat-tolerant) and Nanjing 41 (NJ41, heat-sensitive) were evaluated in the experiments. Seedlings were raised for about 22 days in a nearby paddy field, and then transplanted to plastic pots (with an inside diameter of 28.8 cm and a height of 35.6 cm) filled with 20 kg air-dried paddy soil. Each pot was filled with 3.0 g N, 2.0 g P2O5, and 1.5 g K2O.
High-temperature treatment
The pots with plants of 50 % open flowers with dehiscent anthers in a panicle on the same day were selected to implement the treatments of different high-temperature levels and durations. The spikelets were also labeled at anthesis when at least half the spikelets of a panicle were flowering. At anthesis and 12 days after anthesis (in the early stage of grain filling), pots were transferred to four phytotron rooms severally with the maximum/minimum temperature gradients of 32/22 °C (T1), 35/25 °C (T2), 38/28 °C (T3) and 41/31 °C (T4) under natural daylight conditions (the phytotron rooms were covered with high transparent glasses having 75 % optical transparency). T1 treatment was used as a control for the experiment. Durations of high temperature under different high-temperature levels were defined as 2 d (D1), 4 d (D2), and 6 d (D3). The information of different high-temperature treatments is summarized in supplementary Table S1.
The phytotron rooms measured 4.4 m × 4.2 m × 2.8 m (L × W × H). Each chamber was equipped with an air conditioner capable of maintaining constant temperatures. Daily temperature variation in phytotron was controlled to simulate the diurnal change pattern of temperature in ambient environment, and temperature fluctuations at each given time were within 1 °C (Supplementary Fig. S1). Temperature and relative humidity (RH) in phytotron were measured once every 5 min using HOBO data loggers (Onset Computer Corp., Bourne, MA, USA) with the stand-alone sensors above plant canopy.
Sampling
The tagged grains were harvested every 6–10 days after plants were moved outdoor after high-temperature treatments in phytotron until the physiological maturity, with each sample consisting of five barrels. Grains were dried at 80 °C to constant weight and dehulled, and then ground into a fine powder with pestle for protein content and its composition determination.
Protein measurement
Total grain protein was extracted as described by Lin et al. (2010) with minor modifications. An amount of 50 mg powder was placed into a 1.5 ml Eppendorf tube with 1 ml extraction buffer (125 mM Tris–HCl buffer, pH 6.8, containing 4 M urea, 4 % SDS and 5 % 2-mercaptoethanol). The homogenates were vortexed vigorously, incubated at 60 °C for 2 h in a shaker, and then centrifuged at 20 °C and 13,000 × g for 10 min until the supernatant was clear. The extracted total protein samples were collected as the final supernatant and stored at −80 °C.
Four classes of storage proteins (albumin, globulin, prolamin, and glutelin) were extracted sequentially as described by Kim et al. (2013). 0.2 g powder of each sample was transferred into 1.5 ml Eppendorf tube, and suspended in 1 ml 10 mM Tris–HCl (pH 6.8). The suspension was incubated at room temperature for 6 h in a shaker, vortexed vigorously every 30 min, and then centrifuged at 4 °C at 13,000 × g for 15 min. The supernate was collected to measure albumin content. The precipitate was suspended with 1 ml 10 mM Tris–HCl (pH 6.8, containing 0.5 M NaCl). Then the above steps were repeated. The supernate was collected to measure the globulin content. The precipitate was suspended with 1 ml 60 % n-propanol containing 5 % 2-mercaptoethanol for extraction of prolamin, and the extraction procedure was done as above. Finally, glutelin were extracted from the previous precipitate suspended in 1 ml solution 1 % lactic acid containing 1 mM EDTA-2Na. Each extraction was repeated two times in order to remove all the proteins of each fraction. The extracted proteins were freeze-dried and stored at −80 °C.
The grain nitrogen concentration was determined by micro-Kjeldahl method and grain protein content was calculated by nitrogen concentration using a conversion coefficient of 5.95. Grain storage protein content is equal to the sum of the contents four proteins.
Statistical analysis
The data calculations were conducted according to Liu et al. (2013a, b). For cases where there were no significant differences in data across the two-year study and the interactions between year and cultivar, and year and temperature treatment, data from both years were averaged.
Results
Effects of post-anthesis high temperature on total grain protein
The total protein content of all treatments initially declined progressively, and then increased gradually. The protein content increased with the increasing high-temperature levels (Fig. 1a, b). The protein contents under T2D2, T3D2, and T4D2 treatments of both cultivars were higher than those under T1D2 treatment, except under T2D2 treatment of WJ14 at grain-filling stage. As shown in Fig. 1c–f, the total protein content of both cultivars increased with the increasing of high-temperature durations (T3D3 > T3D2 > T3D1; T4D3 > T4D2 > T4D1). The increase in WJ14 was higher than in NJ41. The extent of high-temperature-induced changes in total protein content was comparatively greater at anthesis stage than those at grain-filling stage in both rice cultivars.
Effects of high temperature on total protein content in the rice cultivar NJ41 (a, c, e) and WJ41 (b, d, f). The lines starting at 7 days after anthesis represent anthesis treatments under high temperatures. The lines starting at 18 days after anthesis represent grain-filling treatments under high temperature. Data are the mean of three replicates ± standard deviation shown by vertical error bars. The different high-temperature levels were defined as 32/22 °C (T1), 35/25 °C (T2), 38/28 °C (T3), and 41/31 °C (T4). The different durations were defined as 2 days (D1), 4 days (D2), and 6 days (D3)
Effects of post-anthesis high temperature on storage protein
As shown in Fig. 2a, b, all stress treatments had an influence on storage protein content. The storage protein content increased with the increasing -temperature levels in both stage treatments. At anthesis stage, the storage protein content of T1D2, T2D2, T3D2, and T4D2 treatments in WJ14 slightly increased, then decreased, and finally increased. The changes of storage protein content of T4D2 treatment in NJ41 showed similar trends. The storage protein content in NJ41 reached the lowest value earlier than in WJ14. At grain-filling stage, the storage protein content in NJ41 and WJ14 showed a typical “V” curve.
Effects of high temperature on storage protein content in the rice cultivar NJ41 (a, c, e) and WJ41 (b, d, f). The lines starting at 7 days after anthesis represent anthesis treatments under high temperatures. The lines starting at 18 days after anthesis represent grain-filling treatments under high temperature. Data are the mean of three replicates ± standard deviation shown by vertical error bars. The different high-temperature levels were defined as 32/22 °C (T1), 35/25 °C (T2), 38/28 °C (T3), and 41/31 °C (T4). The different durations were defined as 2 days (D1), 4 days (D2), and 6 days (D3)
From Fig. 2c–f, the same trend could be observed in two rice cultivars under high-temperature durations. The storage protein content increased with the increasing high-temperature durations (T3D3 > T3D2 > T3D1 and T4D3 > T4D2 > T4D1). The protein content in NJ41 reached the lowest value earlier than in WJ14 at anthesis and grain-filling stages. The effects of high-temperature stress at anthesis stage were higher than those at grain-filling stage in both rice cultivars.
Effects of post-anthesis high temperature on albumin
The albumin contents in all treatments first declined rapidly, and then increased gradually in both rice cultivars (Fig. 3a, b). With the increase in the high-temperature levels, the albumin content increased, except that there was no difference between T1D2 and T2D2 treatments of WJ14. The albumin content increased under high-temperature durations, but there were no obvious changes between T3D1, T3D2, and T3D3 treatments in WJ14 under grain-filling stage (Fig. 3c–f). The extents of increases were much higher in NJ41 than in WJ14. The effects of high temperature on albumin content during anthesis stage were greater than those caused during grain-filling stage in both rice cultivars.
Effects of high temperature on albumin content in the rice cultivar NJ41 (a, c, e) and WJ41 (b, d, f). The lines starting at 7 days after anthesis represent anthesis treatments under high temperatures. The lines starting at 18 days after anthesis represent grain-filling treatments under high temperature. Data are the mean of three replicates ± standard deviation shown by vertical error bars. The different high-temperature levels were defined as 32/22 °C (T1), 35/25 °C (T2), 38/28 °C (T3), and 41/31 °C (T4). The different durations were defined as 2 days (D1), 4 days (D2), and 6 days (D3)
Effects of post-anthesis high temperature on globulin
Figure 4a, b showed that the globulin content decreased first rapidly, and then increased gradually in different treatments during anthesis and grain-filling stages. The lowest values of NJ41 occurred earlier than those of WJ14. The globulin content increased with increasing temperature levels in both rice cultivars (T4D2 > T3D2 > T2D2 > T1D2). However, no difference was found between T2D2 and T1D2 treatments of WJ14 under grain-filling stage. With the increase of high-temperature durations, the globulin content increased in both rice cultivars at both stages (Fig. 4c,d,e, and f). The effects of high temperature on globulin content during anthesis were higher than those during grain-filling stage in both rice cultivars.
Effects of high temperature on globulin content in the rice cultivar NJ41 (a, c, e) and WJ41 (b, d, f). The lines starting at 7 days after anthesis represent anthesis treatments under high temperatures. The lines starting at 18 days after anthesis represent grain-filling treatments under high temperature. Data are the means of three replicates ± standard deviation shown by vertical error bars. The different high-temperature levels were defined as 32/22 °C (T1), 35/25 °C (T2), 38/28 °C (T3), and 41/31 °C (T4). The different durations were defined as 2 days (D1), 4 days (D2), and 6 days (D3)
Effects of post-anthesis high temperature on prolamin
As shown in Fig. 5a, b, a sharp decrease in prolamin content under higher high temperatures was observed at both stages compared to T1D2 treatment. The higher the temperature level, the lower the prolamin content (T4D2 < T3D2 < T2D2 < T1D2). The prolamin content increased firstly, then decreased, and finally increased, except for the treatments of NJ41 at the grain-filling stage. Prolamin content characteristically decreased with the increasing temperature durations at both stages (Fig. 5c,d,e, and f). There were no obvious changes between T3D1 and T3D2 treatments in WJ14 at grain-filling stage. The extent of inhibition in NJ41 was higher than that in WJ14 under high-temperature levels and durations. The effects of high-temperature during anthesis on prolamin content were higher than those during grain-filling stage in both rice cultivars.
Effects of high temperature on prolamin content in the rice cultivar NJ41 (a, c, e) and WJ41 (b, d, f). The lines starting at 7 days after anthesis represent anthesis treatments under high temperatures. The lines starting at 18 days after anthesis represent grain-filling treatments under high temperature. Data are the means of three replicates ± standard deviation shown by vertical error bars. The different high-temperature levels were defined as 32/22 °C (T1), 35/25 °C (T2), 38/28 °C (T3), and 41/31 °C (T4). The different durations were defined as 2 days (D1), 4 days (D2), and 6 days (D3)
Effects of post-anthesis high temperature on glutelin
Figure 6a,b,c,d,e, and f showed that the glutelin content increased first, then decreased, and finally increased, except for the treatments of NJ41 at grain-filling stage, which was similar to the changes of prolamin content. However, the glutelin content increased with the increasing high-temperature levels (T4D2 > T3D2 > T2D2 > T1D2). The high-temperature durations significantly caused the induction of glutelin content (Fig. 6c–f). The extent of induction in WJ14 was much higher than that in NJ41. The effects of high temperature during anthesis on globulin content were higher than those caused during filling stage in both rice cultivars.
Effects of high temperature on glutelin content in the rice cultivar NJ41 (a, c, e) and WJ41 (b, d, f). The lines starting at 7 days after anthesis represent anthesis treatments under high temperatures. The lines starting at 18 days after anthesis represent grain-filling treatments under high temperature. Data are the means of three replicates ± standard deviation shown by vertical error bars. The different high-temperature levels were defined as 32/22 °C (T1), 35/25 °C (T2), 38/28 °C (T3), and 41/31 °C (T4). The different durations were defined as 2 days (D1), 4 days (D2), and 6 days (D3)
Effects of post-anthesis high temperature on the ratio of prolamin to glutelin
High temperature resulted in a significant decrease in the ratio of the content of prolamin to glutelin (P/G, Fig. 7). The P/G ratio decreased with the increase of high-temperature levels and durations under both stage treatments, especially T4D3 treatment. The extent of decrease was greater under high-temperature levels and durations in NJ41 than in WJ14. The effects of high temperature during anthesis on P/G ratio were higher than those during grain-filling stage in both rice cultivars.
Effects of high temperature on the ratio of prolamin/glutelin in the rice cultivar NJ41 (a, c, e) and WJ41 (b, d, f). The lines starting at 7 days after anthesis represent anthesis treatments under high temperatures. The lines starting at 18 days after anthesis represent grain-filling treatments under high temperature. Data are the means of three replicates ± standard deviation shown by vertical error bars. The different high-temperature levels were defined as 32/22 °C (T1), 35/25 °C (T2), 38/28 °C (T3), and 41/31 °C (T4). The different durations were defined as 2 days (D1), 4 days (D2), and 6 days (D3)
Discussion
Protein content is crucial to rice grain quality and nutritional value (Kawakatsu et al. 2010). Previous studies have shown that high temperature can increase rice grain protein content (Liang et al. 2013; Liu et al. 2013b). Cooper et al. (2008) showed that although high temperature could increase the protein content of rice grain, this might not be significant. In the present study, the results showed that high temperature increased total protein content in both rice cultivars, the extent of the increase in WJ14 under high-temperature levels was lower than that in NJ41, while the changes under high-temperature durations were reversed. The results suggested that protein expression patterns vary with temperature levels and durations and genotypes. This profiling method might be able to display polymorphisms among different genotypes, and even to reveal the quality characteristics of different genotypes (Jagadish et al. 2010; Kim et al. 2013).
In recent years, enhancing rice grain storage proteins to improve rice nutritive value has gradually become one of the important targets for rice quality breeding (Liang et al. 2013). High temperature increases the accumulation of all classes of storage proteins at early filling stage, but decreases the accumulation of prolamin at maturity (Lin et al. 2010). In this study, storage protein accumulation was accelerated under high-temperature levels and durations. Rice storage proteins are in general classified into glutelin, prolamin, globulin, and albumin. Albumin and globulin have little effects on dough quality, however, they are nutritionally important because most of them contain essential amino acids for human nutrition (Gao et al. 2009; Kim et al. 2013). Liang et al. (2013) showed that albumin and globulin contents in grains and amino acid contents were significantly increased during grain filling under high-temperature stress. This study showed that the contents of albumin and globulin increased with the increasing high-temperature levels and durations. The extent of increase of albumin was higher in NJ41 than in WJ14, suggesting that the albumin content in heat-sensitive cultivars was more sensitive to high temperature than those in heat-tolerant cultivars. However, Lin et al. (2010) showed that albumin content was not significantly affected by high temperature, but globulin content was reduced. The different effects of high temperature on albumin and globulin contents may depend upon the rice genotypes, high-temperature levels, samples for determination, or the experimental time scales. A significantly low concentration of globulin has been found in the central chalky region of grains of these sake cultivars (Shizukawa et al. 2002). Low level of globulin was also found in immature and dead grains induced under high temperature (Lin et al. 2010).
Most storage proteins in rice grains are glutelins which contain high amounts of essential amino acid of lysine. Prolamin is generally rich in leucine, but poor in lysine and sulfur-containing amino acids (Shewry 2007). The present study showed that high temperature increased the content of glutelin protein, and decreased the prolamin protein, which is supported by the results of Ashida et al. (2013) who showed that rice grown under high temperature after heating tended to show lower prolamin content and higher glutelin content. In addition, our results also showed that the extent of induction of glutelin in WJ14 was higher than that in NJ41 under high-temperature levels and durations. The extent of inhibition of prolamin in NJ41 was much higher than that in WJ14 under high-temperature levels and durations. Liao et al. (2014) reported that four glutelin-related proteins (OsUP8, OsUP12, OsUP13, and OsUP14) were upregulated in both heat-sensitive and heat-tolerant rice lines under high-temperature stress during the first half of the ripening period. Lin et al. (2010) reported that the expression of six glutelin-related proteins in rice caryopsis increased under high-temperature stress during grain-filling stage. When rice is grown under high temperatures, the synthesis of 13 kDa prolamin and the expression of prolamin genes were attenuated (Yamakawa et al. 2007). The amount of prolamin is negatively related to the digestibility and palatability of rice. High-temperature-induced decrease in prolamin might change the physicochemical properties (Lin et al. 2010). The relatively consistent increase in glutelin and decrease in prolamin can lead to a lower ratio of prolamin to glutelin (P/G), which is as an indicator of grain quality for rice wine brewing in rice grains. The results suggested that high temperature may degrade grain quality for rice wine brewing (Shizukawa et al. 2002; Lin et al. 2010).
In conclusion, to the best of our knowledge, for the first time, we analyzed the dynamic changes in the accumulation profiles of protein content and composition during rice anthesis and grain-filling stages under different high-temperature levels. The results showed that high temperature during rice anthesis and grain-filling stages increased grain protein content and protein components (albumin, globulin, and glutelin), while it decreased prolamin content. The contents of total protein, storage protein, albumin, and prolamin in NJ41 were sensitive to temperature levels than WJ14. The effects of high-temperature stress at anthesis stage were greater than those at grain-filling stage. The results could help us understand the basis of physiochemical processes governing rice quality during rice post-anthesis under high-temperature conditions.
References
Ashida K, Araki E, Maruyama-Funatsuki W, Fujimoto H, Ikegami M (2013) Temperature during grain ripening affects the ratio of type-ii/type-i protein body and starch pasting properties of rice (Oryza sativa L.). J Cereal Sci 57:153–159
Cooper NTW, Siebenmorgen TJ, Counce PA (2008) Effects of night time temperature during kernel development on rice physicochemical properties. Cereal Chem 85:276–282
Das S, Krishnan P, Nayak M, Ramakrishnan B (2014) High temperature stress effects on pollens of rice (Oryza sativa L.) genotypes. Environ Exp Bot 101:36–46
Gao LY, Wang AL, Li XX, Dong K, Wang K, Appels R, Ma WJ, Yan YM (2009) Wheat quality related differential expressions of albumins and globulins revealed by two-dimensional difference gel electrophoresis (2-D DIGE). J Proteomics 73:279–296
Harsant J, Pavlovic L, Chiu G, Sultmanis S, Sage TL (2013) High temperature stress and its effect on pollen development and morphological components of harvest index in the C3 model grass Brachypodium distachyon. J Exp Bot 64:2971–2983
Jagadish SV, Muthurajan R, Oane R, Wheeler TR, Heuer S, Bennett J, Craufurd PQ (2010) Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J Exp Bot 61:143–156
Kawakatsu T, Hirose S, Yasuda H, Takaiwa F (2010) Reducing rice seed storage protein accumulation leads to changes in nutrient quality and storage organelle formation. Plant Physiol 154:1842–1854
Kim JW, Kim BC, Lee JH, Lee DR, Rehman S, Yun SJ (2013) Protein content and composition of waxy rice grains. Pak J Bot 45:151–156
Liang CG, Zhang Q, Xu GL, Wang Y, Ohsugi R, Tian L (2013) High temperature during rice grain filling enhances aspartate metabolism in grains and results in accumulation of aspartate-family amino acids and protein components. Rice Sci 20:343–348
Liao JL, Zhou HW, Zhang HY, Zhong PA, Huang YJ (2014) Comparative proteomic analysis of differentially expressed proteins in the early milky stage of rice grains during high temperature stress. J Exp Bot 65:655–671
Lin CJ, Li CY, Lin SK, Yang FH, Huang JJ, Liu YH, Lur HS (2010) Influence of high temperature during grain filling on the accumulation of storage proteins and grain quality in rice (Oryza sativa L.). J Agric Food Chem 58:10545–10552
Liu QH, Wu X, Ma JQ, Li T, Zhou XB, Guo T (2013a) Effects of high air temperature on rice grain quality and yield under field condition. Agron J 205:446–454
Liu QH, Wu X, Li T, Ma JQ, Zhou XB (2013b) Effects of elevated air temperature on physiological characteristics of flag leaves and grain yield in rice. Chil J Agric Res 73:85–90
Nelson LD, Lawson NL, Counce PA, Moldenhauer KAK, Siebenmorgen TJ, Korth KL (2011) Grain filling and gene expression in response to increased nighttime air temperatures in developing rice grains. In: Norman RJ, Moldenhauer KAK (eds) B.R. Wells Rice Research Studies 2011. Arkansas Agricultural Experiment Station, Fayetteville, Arkansas, pp 316–323
Parent B, Turc O, Gibon Y, Stitt M, Tardieu F (2010) Modelling temperature-compensated physiological rates, based on the coordination of responses to temperature of developmental processes. J Exp Bot 61:2057–2069
Shewry PR (2007) Improving the protein content and composition of cereal grain. J Cereal Sci 46:239–250
Shizukawa Y, Oohasho Y, Masumura T, Tanaka K (2002) The contents and distribution of prolamin, glutelin and globulin that constitute storage protein (protein body) in brown rice on some rice cultivars for brewery or meal. Jpn J Crop Sci 71:224–225
Teixeira EI, Fischer G, van Velthuizen H, Walter C, Ewert F (2013) Global hot-spots of heat stress on agricultural crops due to climate change. Agric For Meteorol 170:206–215
Wang P, Zhang Z, Song X, Chen Y, Wei X, Shi PJ, Tao FL (2014) Temperature variations and rice yields in China: historical contributions and future trends. Clim Change 124:777–789
Yamakawa H, Hirose T, Kuroda M, Yamaguchi T (2007) Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray. Plant Physiol 144:258–277
Acknowledgments
This study was supported by the National High-Tech Research and Development Program of China (2013AA100404), the National Natural Science Foundation of China (31571566), the Natural Science Foundation of Jiangsu province (BK20151435), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jianlin Chen and Liang Tang co-first authors with equal contribution to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
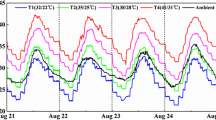
Supplementary Fig. S1
Diurnal temperature during rice growth season in artificial climate chamber (2013.8.21-2013.8.24) (TIFF 1282 kb)
Rights and permissions
About this article
Cite this article
Chen, J., Tang, L., Shi, P. et al. Effects of short-term post-anthesis high-temperature stress on dynamic process of accumulation of grain protein and its composition in rice (Oryza sativa L.). Braz. J. Bot 40, 49–58 (2017). https://doi.org/10.1007/s40415-016-0325-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-016-0325-4