Abstract
The milk oligosaccharides were studied for two species of the Carnivora: the American black bear (Ursus americanus, family Ursidae, Caniformia), and the cheetah, (Acinonyx jubatus, family Felidae, Feliformia). Lactose was the most dominant saccharide in cheetah milk, while this was a minor saccharide and milk oligosaccharides predominated over lactose in American black bear milk. The structures of 8 neutral saccharides from American black bear milk were found to be Gal(β1–4)Glc (lactose), Fuc(α1–2)Gal(β1–4)Glc (2′-fucosyllactose), Gal(α1–3)Gal(β1–4)Glc (isoglobotriose), Gal(α1–3)[Fuc(α1–2)]Gal(β1–4)Glc (B-tetrasaccharide), Gal(α1–3)[Fuc(α1–2)]Gal(β1–4)[Fuc(α1–3)]Glc (B-pentasaccharide), Fuc(α1–2)Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–3)Gal(β1–4)Glc (difucosyl lacto-N-neotetraose), Gal(α1–3)Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–3)Gal(β1–4)Glc (monogalactosyl monofucosyl lacto-N-neotetraose) and Gal(α1–3)Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc (Galili pentasaccharide). Structures of 5 acidic saccharides were also identified in black bear milk: Neu5Ac(α2–3)Gal(β1–4)Glc (3′-sialyllactose), Neu5Ac(α2–6)Gal(β1–4)GlcNAc(β1–3)[Fuc(α1–2)Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (monosialyl monofucosyl lacto-N-neohexaose), Neu5Ac(α2–6)Gal(β1–4)GlcNAc(β1–3)[Gal(α1–3)Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (monosialyl monogalactosyl lacto-N-neohexaose), Neu5Ac(α2–6)Gal(β1–4)GlcNAc(β1–3){Gal(α1–3)Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–6)}Gal(β1–4)Glc (monosialyl monogalactosyl monofucosyl lacto-N-neohexaose), and Neu5Ac(α2–6)Gal(β1–4)GlcNAc(β1–3){Gal(α1–3)[Fuc(α1–2)]Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–6)}Gal(β1–4)Glc (monosialyl monogalactosyl difucosyl lacto-N-neohexaose). A notable feature of some of these milk oligosaccharides is the presence of B-antigen (Gal(α1–3)[Fuc(α1–2)]Gal), α-Gal epitope (Gal(α1–3)Gal(β1–4)Glc(NAc)) and Lewis x (Gal(β1–4)[Fuc(α1–3)]GlcNAc) structures within oligosaccharides. By comparison to American black bear milk, cheetah milk had a much smaller array of oligosaccharides. Two cheetah milks contained Gal(α1–3)Gal(β1–4)Glc (isoglobotriose), while another cheetah milk did not, but contained Gal(β1–6)Gal(β1–4)Glc (6′-galactosyllactose) and Gal(β1–3)Gal(β1–4)Glc (3′-galactosyllactose). Two cheetah milks contained Gal(β1–4)GlcNAc(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (lacto-N-neohexaose), and one cheetah milk contained Gal(β1–4)Glc-3’-O-sulfate. Neu5Ac(α2–8)Neu5Ac(α2–3)Gal(β1–4)Glc (disialyllactose) was the only sialyl oligosaccharide identified in cheetah milk. The heterogeneity of milk oligosaccharides was found between both species with respect of the presence/absence of B-antigen and Lewis x. The variety of milk oligosaccharides was much greater in the American black bear than in the cheetah. The ratio of milk oligosaccharides-to-lactose was lower in cheetah (1:1–1:2) than American black bear (21:1) which is likely a reflection of the requirement for a dietary supply of N-acetyl neuraminic acid (sialic acid), in altricial ursids compared to more precocial felids, given the role of these oligosaccharides in the synthesis of brain gangliosides and the polysialic chains on neural cell adhesion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactose (Gal(β1–4)Glc) has long been considered the predominant saccharide in the milks of eutherian mammals [1], and its synthesis is believed to regulate milk volume via osmotic effects in the secretory vesicles of lactocytes [2]. However, in the milks of several species of the order Carnivora, oligosaccharides predominate over lactose [3]. For example, the milks or colostra of members of the Caniformia, such as bears [4,5,6,7,8], raccoon [9], seals [10,11,12,13], white-nosed coati [14], striped skunk [15] and mink [16] contain substantial amounts of oligosaccharides in addition to a lesser amount of lactose, while lactose predominates over oligosaccharides in milks or colostra of domestic dogs [17, 18], spotted hyena [19], African lion [20] and clouded leopard [20]. We, therefore, hypothesized that the milks of Caniformia other than the domestic dog contain more oligosaccharides than lactose, while the milks of Felidae contain more lactose than oligosaccharides, even though the physiological significance is unknown [3, 9]. The domestic dog is an exception to this, as its milk saccharide ratio more closely aligns with that of Feliformia than Caniformia [17, 18], but this may be associated with the domestication process and/or reliance on artificial and human-prescribed diets. To examine further this hypothesis we examined the milks of two additional carnivores, a member of the Caniformia (American black bear) and of the Felidae (cheetah) in this study.
The milk oligosaccharides have been studied in several bear species including, Ezo brown bear [4], Japanese black bear [5, 8], polar bear [6, 7] and giant panda [21], while those of American black bear have not been characterized. However, American black bear milk has been hypothesized to be rich in oligosaccharides based on its greater colorimetric response to non-specific sugar assays such as phenol-sulfuric and picric acid assays than enzymatic lactose methods [22, 23]. A significant feature of bear milk oligosaccharides is the presence of A (GalNAc(α1–3)[Fuc(α1–2)]Gal), B (Gal(α1–3)[Fuc(α1–2)]Gal) or H (Fuc(α1–2)Gal) antigens, depending on the bear species, and α-Gal epitope (Gal(α1–3)Gal(β1–4)GlcNAc) in the non reducing end, as well as Lewis x (Gal(β1–4)[Fuc(α1–3)]GlcNAc) [4,5,6,7,8]. In the milks of Feliformia, the milks of African lion and clouded leopard contain A-tetrasaccharide (GalNAc(α1–3)[Fuc(α1–2)]Gal(β1–4)Glc) [20], while spotted hyena has B-tetrasaccharide (Gal(α1–3)[Fuc(α1–2)]Gal(β1–4)Glc) [19]. The milks of spotted hyena and clouded leopard contain isoglobotriose (Gal(α1–3)Gal(β1–4)Glc) [19, 20]. The acidic milk oligosaccharides of the Caniformia, for those species which have been studied, contain only Neu5Ac sialic acid, whereas milk oligosaccharides of two felids (African lion and clouded leopard) contained only Neu5Gc [3, 20]. Most of the milk oligosaccharides of Caniformia species contain lactose, lacto-N-neotetraose (Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc) and lacto-N-neohexaose (Gal(β1–4)GlcNAc(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc as core units, while in Felidae milk oligosaccharides contain only lactose as a core unit [3, 9, 19, 20]. The diversity of milk oligosaccharides is usually greater among Caniformia than in Feliformia.
In this study, we observed similar differences between the American black bear, a caniform, and the cheetah, a feliform, as well as the differences among species of each taxonomic group. We also discuss the possibility that the observed differences in the relative proportion of oligosaccharides to lactose are related to developmental state at birth among carnivorans.
Materials and methods
Milk samples
American black bear milk
Milk (colostrum) samples were obtained from wild American black bears in the Poconos Mountains of Pennsylvania in January 1984, as part of a long-term study of black bear biology by the Pennsylvania Game Commission [24], Milk were collected 0–2 days postpartum as described by Oftedal et al. [22]. Milk was manually expressed from the lactating bears after they were chemically immobilized with about 440 mg ketamine hydrochloride and 220 mg xylazine hydrochloride per 100 kg body weight; they were also injected with oxytocin (30–40 IU) and their nipples cleaned with distilled water. Milk was expressed from one or more nipples, but samples from individual nipples were kept separate. Individual samples were frozen in air-tight vials at −20 °C and remained frozen until thawed for pooling and extraction (see below). Due to the small volume of colostrum obtained from individual nipples, we pooled five-colostrum samples representing one nipple sample per bear. The ID numbers of these bears, assigned by the Pensylvania Game Commision, were 3411, 4018, 5406, 5463 and 6495.
The carbohydrate fraction was extracted at the Smithsonian National Zoological Park, Washington DC and brought to Obihiro University of Agriculture and Veterinary Medicine.
The carbohydrate fraction was extracted at the Smithsonian zoological institution and brought to Obihiro University of Agriculture and Veterinary Medicine.
Milk samples
Cheetah milk (colostrum)
The milks were opportunistically collected from three captive cheetahs of wild origin, housed at the Breeding Centre for Endangered Arabian Wildlife Centre (United Arab Emirates). The cheetah required immobilisation for medical intervention. Anaesthesia was induced using a combination of 0.05 mg/kg of medetomidine and 2–3 mg/kg of ketamine, they were also injected with 20-25 IU of oxytocin during the procedure. The milk was manually expressed from each cleaned nipple and pooled into one vial. In cheetah 1 CM(a): The milk (10 mL) was collected at one day post partum in March 2006 for cheetah 2 CM(b): milk (21 mL) was collected at two days post partum in November 2006, and for the third cheetah CM(c): milk (10 mL) was collected at one day post partum in 2009. The samples were stored at −20 °C and remained frozen until thawing for extraction of the carbohydrate fractions.
Materials
Fuc(α1–2)Gal(β1–4)Glc (2′-fucosyllactose), Neu5Ac(α2–3)Gal(β1–4)Glc (3’-SL) and Neu5Ac(α2–8)Neu5Ac(α2–3)Gal(β1–4)Glc (disialyllactose) were purchased from Sigma (St. Louis, MO, USA). Gal(α1–3)Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc (Galili pentasaccharide) and Neu5Ac(α2–6)Gal(β1–4)GlcNAc(β1–3)[Gal(α1–3)Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (monosialyl monogalactosyl lacto-N-neohexaose) were separated from both mink and striped skunk milk [15, 16]. B-Tetrasaccharide, Gal(α1–3)Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–3)Gal(β1–4)Glc (monogalactosyl monofucosyl lacto-N-neotetraose), Neu5Ac(α2–6)Gal(β1–4)GlcNAc(β1–3){Gal(α1–3)Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–6)}Gal(β1–4)Glc (monosialyl monogalactosyl monofucosyl lacto-N-neohexaose) and Neu5Ac(α2–6)Gal(β1–4)GlcNAc(β1–3){Gal(α1–3)[Fuc(α1–2)]Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–6)}Gal(β1–4)Glc (monosialyl monogalactosyl difucosyl lacto-N-neohexaose) were purified from Japanese black bear milk [5, 8]. Fuc(α1–2)Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–3)Gal(β1–4)Glc (difucosyl lacto-N-neotetraose) was purified from Ezo brown bear milk [4]. Isoglobotriose, Gal(β1–3)Gal(β1–4)Glc (3′-galactosyllactose) and Gal(β1–6)Gal(β1–4)Glc (6′-galactosyllactose) were separated from caprine colostrum [25]. Lacto-N-neotetraose, lacto-N-neohexaose and Gal(α1–3)[Fuc(α1–2)]Gal(β1–4)[Fuc(α1–3)Glc (B-pentasaccharide) were purchased from Seikagaku Co. (Tokyo, Japan). Gal(β1–4)Glc-3’-O-SO3 was separated from dog milk [17].
Isolation of neutral milk oligosaccharides from American black bear milk
The milk samples were thawed, pooled and extracted with four volumes of chloroform/methanol (2:1, v/v). The emulsion was centrifuged at 4 °C and 4000 Xg for 30 min, and the lower chloroform layer and denatured proteins were discarded. The methanol was removed from the upper layer by rotary evaporation, and the lyophilized residue was designated as the carbohydrate fraction.
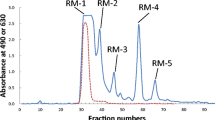
After the carbohydrate fraction was divided into three portions, one portion was dissolved in 2 mL of water and the solution passed through a BioGel P-2 column (<45 μm, 2.5 × 100 cm, Bio-Rad Laboratories, Hercules, CA) that had been calibrated with 2 mg each of galactose (monosaccharide), lactose (disaccharide), and raffinose (trisaccharide). The gel was washed with 0.1 HCl and 0.1 M NaOH before use. Elution was done with distilled water at a flow rate of 15 mL/h, and fractions of 5 mL were collected. Aliquots (0.5 mL) of each fraction were analyzed for hexose with phenol-H2SO4 [26] and for sialic acid with periodate-resorcinol [27]. Peak fractions were pooled as shown in Fig. 1 and freeze-dried. The saccharides in the peak fraction, designated as BB-1 to BB-8 (see Fig. 1), were checked by thin layer chromatography using acetone/2-propanol/0.1 M lactic acid (2:2:1, v/v/v) as a developing solvent. Detection of the spots was done by spraying with 5% H2SO4 in ethanol and heating. Gel filtration was repeated three times with the other two portions of the carbohydrate fraction.
Gel chromatogram of the carbohydrate fraction from American black bear milk on a BioGel P-2 column (2.5 × 100 cm). Elution was done with distilled water at a flow rate of 15 mL/h and fractions of 5.0 mL were collected. Each fraction was monitored by the phenol-H2SO4 method at 490 nm (solid line) and the periodate-resorcinol method at 630 nm (broken line)
The components in BB-4 to BB-8 were separated by high-performance liquid chromatography (HPLC). The Hitachi 7000 series HPLC system (Tokyo) consisted of an autosampler L-7200, a column oven L-7300, a pump L-7100, and an evaporation light scattering detector SEDEX-75 with a system controller D-7100. The HPLC stationary phase was a 7 μm Hypercarb column (100 × 4.6 mm i.d.; Thermo Fisher Scientific), and the mobile phase comprised acetonitrile in distilled water run at 40 °C. The LC gradient was delivered at 1.0 mL/min and consisted of an initial linear increase from 5% to 30% acetonitrile over 80 min. The chromatograms are shown in Fig. 2. The components in BB-4-1 to BB-4-6 (from BB-4, see Fig. 2), BB-5-1 and BB-5-4 (from BB-5), BB-6-1 to BB-6-3 (from BB-6), BB-7-1 to BB-7-3 (from BB-7), and BB-8-1 to BB-8-3 (from BB-8) were each collected, concentrated by rotary evaporation, and subjected to 1H-NMR spectroscopy and MALDI-TOF MS. When NMR produces an identical “fingerprint” pattern to a known oligosaccharide with known NMR results, then it is not essential to follow up with mass spectroscopy [5, 8].
High performance liquid chromatography of the neutral oligosaccharide fractions BB-4, BB-5. BB-6, BB-7 and BB-8 separated from the carbohydrate fraction of American black bear milk by gel chromatography (Fig. 1). The Hitachi 7000 series HPLC system (Tokyo) consisted of autosampler L-7200, a column oven L-7300, a pump L-7100, and an evaporation light scattering detector SEDEX-75 with a system controller D-7100. The stationary phase was a 7 μm Hypercarb column (100 × 4.6 mm i.d.; Thermo Fisher Scientific), while the mobile phase was acetonitrile in distilled water run at 40 °C. The LC gradient was delivered at 1.0 mL/min and consisted of an initial linear increase from 5% to 30% acetonitrile over 80 min
Isolation of acidic oligosaccharides from American black bear milk
The components in peak BB-2 of the gel chromatogram (see Fig. 1) which reacted positively with both periodate-resorcinol (630 nm) and phenol-H2SO4 (490 nm) were dissolved in 2 mL of 50 mmol/L Tris hydroxyaminomethane-HCl buffer solution (pH 8.7) and subjected to anion exchange chromatography on a DEAE-Sephadex A-50 column (2.0 × 35 cm; GE Healthcare, Uppsala, Sweden), which was equilibrated and eluted with the same solution. Elution was done at a flow rate of 15 mL/h and fractions were analyzed for hexose using the phenol-H2SO4 method [26]. Figure 3 shows that the ion exchange chromatography separated the BB-2 fraction into three peaks. The components in the second peak, designated as BB-2-2, were pooled, lyophilized, dissolved in 2 mL of water, and passed through a column (2.0 × 35 cm) of BioGel P-2 to remove salts as described above.
Anion exchange chromatography of BB-2 (Fig. 1) separated from American black bear milk carbohydrate by chromatography on BioGel P-2. A DEAE-Sephadex A-50 column (2.0 × 35 cm) equilibrated with 50 mmol/L. Tris hydroxyaminomethane-HCl buffer (pH 8.7) was used. Elution was done with 250 mL of the buffer. The flow rate was 15 mL/h and fractions of 5 mL were collected. They were monitored by the phenol-H2SO4 method
The components in BB-2-2 were then subjected to HPLC on a TSK gel Amide-80 column (4.6 × 250 mm, pore size 80 Å, particle size 5 μm; Tosoh, Japan) (chromatogram in Fig. 4). The HPLC was performed by using mobile phase 80% and 50% (vol/vol) acetonitrile (CH3CN) in 15 mmol/L potassium phosphate buffer (pH 5.2) denoted as buffer A and B, respectively. Elution was done using linear gradient from buffer A to buffer B with the following condition: buffer A = 100%, B = 0% at 0 min; A = 50%, B = 50% at 15 min; and A = 0%, B = 100% at 80 min at 60 °C and flow rate of 1 mL/min. The eluates were monitored by measuring the absorbance at 195 nm. The peaks designated as BB-2-2-1 to BB-2-2-17 (Fig. 4) were each pooled, concentrated by rotary evaporation, and subjected to 1H-NMR spectroscopy and MALDI-TOF MS to determine their structures.
High performance liquid chromatogram of fraction BB-2-2 (see Fig. 3). The HPLC was done using Shimadzu LC-10 ATVP pump (Shimadzu, Tokyo, Japan) on a TSK-gel Amide-80 column (4.6 × 250 mm, pore size 80 Å, particle size 5 μm; Tosoh, Tokyo, Japan). It was performed by using mobile phase 80% and 50% (vol/vol) acetonitrile (CH3CN) in 15 mmol/L potassium phosphate buffer (pH 5.2) denoted as buffer A and B, respectively. Elution was done using linear gradient from buffer A to buffer A with the following condition: buffer A = 100%, B = 0% at 0 min; A = 50%, B = 50% at 15 min; and A = 0%, B = 100% at 80 min at 60 °C and flow rate of 1 mL/min. The eluates were monitored by measuring the absorbance at 195 nm
Isolation of milk oligosaccharides and lactose from cheetah milk
The milk samples were mixed with four volumes of chloroform/methanol (2:1, v/v) and stirred. The solution was then centrifuged at 3500 rpm for 30 min and the upper layer collected. The methanol was evaporated off with a rotary evaporator and the residue dissolved with a small amount of water for transport to the laboratory for analysis.
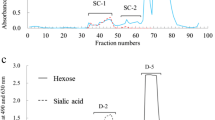
The carbohydrate fractions were shipped to Obihiro University of Agriculture and Veterinary Medicine, and then freeze-dried. Each (CM(a), CM(b) and CM(c)) of the carbohydrate fractions were dissolved in 2 mL of water and separated by gel filtration on BioGel P-2 column (2.5 × 100 cm) as similar to that of bear milk carbohydrate. The CM(b) was divided into two equal volume of the solution and subjected to the gel filtration twice. These carbohydrate fractions were separated as in Fig. 5. The saccharides in each pooled fraction were detected by thin layer chromatography as above. The saccharides in the fraction of CM(a)-4, CM(b)-4, CM(c)-2, and CM(b)-2 were characterized by 1H-NMR spectroscopy. The neutral oligosaccharides in fractions CM(a)-3, CM(b)-3, CM(c)-3 and CM(c)-4 were separated and purified by HPLC with Hypercarb column as above (chromatograms in Fig. 6). The separated components were characterized by 1H-NMR.
High performance liquid chromatography of the neutral oligosaccharides fractions (a) CM(a)-3, (b) CM(b)-3 and (c) CM(c)-3 separated from cheetah milk by gel filtration (see Fig. 5) using a 7 μm Hypercarb column (100 × 4.6 mm i.d.: Thermo Fisher Scientific)
The fraction CM(a)-1 was dissolved in 2 mL of 50 mM Tris-hydroxyaminomethane-HCl buffer (pH 8.7) and subjected to anion exchange chromatography using a DEAE Sephadex A-50 column (1.5 X 35 cm) equilibrated with the same buffer (chromatogram in Fig. 7). The unadsorbed components were eluted with 250 mL of the buffer, after which elution was continued using a linear gradient of 0–0.5 M NaCl in the buffer. Elution was done at a flow rate of 15 mL/h and fractions were analyzed for hexose. The peak fractions (CM(a)-1–1, CM(a)-1–2, CM(a)-1–3) were pooled, concentrated, and passed through a column (2.0 × 35 cm) of BioGel P-2 to remove salts. The components in the adsorbed fraction CM(a)-1–3 (see Fig. 7) were separated and purified by HPLC with Amide-80 column as above (chromatogram in Fig. 8). The separated components were characterized by 1H-NMR.
Anion exchange chromatography of CM(a)-1 (Fig. 6) separated from cheetah milk carbohydrate by chromatography on BioGel P-2. A DEAE-Sephadex A-50 column (2.0 × 35 cm) equilibrated with 50 mmol/L Tris hydroxyaminomethane-HCl buffer (pH 8.7) was used. The unadsorbed components were eluted with 250 mL of the buffer, after which elution was continued using a linear gradient of 0–0.5 M NaCl in the buffer. The flow rate was 15 mL/h and fractions of 5 mL were collected. They were monitored by the phenol-H2SO4 method
High performance liquid chromatography of CM(a)-1–3 (see Fig. 3) using a TSK-gel Amide-80 column (4.6 × 250 mm, pore size 80 Å, particle size 5 μm; Tosoh, Tokyo, Japan)
1H-NMR spectroscopy
Nuclear magnetic resonance spectra were recorded in D2O (99.96 atom D%; Sigma-Aldrich, Milwaukee, WI) at 500 or 600 MHz for 1H-NMR with a JEOL ECP-500 Fourier transform-NMR (Jeol, Tokyo, Japan) or a Varian INOVA 600 specrometer (Varian Inc., Palo Alto, CA) operated at 293.1 K. Chemical shifts are expressed as change relative to internal 3-(trimethyl)-1-propane sulfuric acid, sodium salt, but measured by reference to internal acetone (δ = 2.225).
Mass spectrometry
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was performed on the oligosaccharide fractions, using Autoflex II TOF/TOF mass spectrometer (Brucker Daltonics, Bremen, Germany). Lyophilized oligosaccharide fractions were dissolved in 5 μL of milli-Q water. The oligosaccharide solution was mixed with an equal volume of 10 mg/mL. SDHB (Brucker Daltonics), which is a mixture of 2,5-dihydrobenzoic acid and 2-hydroxy-5-methoxybenzoic acid, saturated in milli-Q water, spotted on a MTP 384 target plate ground steel TF (Bruker Daltnics), and dried. Mass spectra were obtained using a pre-installed method, RP_0–2 kDa (a reflector positive ion mode focusing on the mass range up to 2 kDa). Peptide calibration standard II (Bruker Daltonics) was used for external calibration of the mass spectrometer.
Results
Characterization of neutral saccharides of American black bear milk
The crude carbohydrate fraction from American black bear milk separated into several peaks during gel filtration on BioGel P-2 (Fig. 1). The fractions in each peak were pooled. As the second peak, designated BB-2, reacted positively with periodate – resorcinol, it was concluded that the components in this peak contained sialic acid. The components in BB-4 to BB-8 were subjected to HPLC using a Hypercarb column, and the resulting peaks were designated as shown in Fig. 2. The separated peak components obtained by HPLC were characterized by 1H-NMR spectroscopy.
Identification of galactose
Thin layer chromatography showed that fraction BB-9 contained galactose
Identification of lactose
As the 1H-NMR spectrum (chemical shifts in Table 1) of BB-8-1 was identical to that of lactose, the saccharide in this fraction was characterized to be Gal(β1–4)Glc.
Identification of 2′-fucosyllactose
As the 1H-NMR spectra (chemical shifts in Table 1) of BB-7-2 and BB-7-3 were identical to the published data [19] for authentic 2’-FL, the oligosaccharide in these fractions was characterized to be Fuc(α1–2)Gal(β1–4)Glc.
Identification of isoglobotriose
The 1H-NMR spectrum (chemical shifts in Table 1) of BB-7-1 showed that this fraction contained two oligosaccharides. As the chemical shifts of one oligosaccharide were identical to those of BB-7-2, it was characterized to be 2’-FL. As the other chemical shifts of another oligosaccharide (BB-7-1-2) were identical to the published data for isoglobotriose [19, 25], it was characterized to be Gal(α1–3)Gal(β1–4)Glc.
Identification of B-tetrasaccharide
The 1H-NMR spectrum (chemical shifts in Table 1) of BB-6-1 was identical to the published data for B-tetrasaccharide [5, 28] separated from Japanese black bear milk; it was therefore characterized to be Gal(α1–3)[Fuc(α1–2)]Gal(β1–4)Glc.
Identification of B-pentasaccharide
Since the 1H-NMR patterns of BB-5-1 and BB-5-2 were identical, it was concluded that these two peaks contained the same saccharide, which separated into α- and β-anomer isomers during the Hypercarb column chromatography. As the 1H-NMR spectra of BB-5-1 and BB-5-2 (chemical shifts in Table 1) were identical to the published data for that of authentic B-pentasaccharide [5, 28], the oligosaccharide in these peaks was characterized to be Gal(α1–3)[Fuc(α1–2)]Gal(β1–4)[Fuc(α1–3)]Glc.
Characterization of difucosyl lacto-N-neotetraose
The MALDITOF MS of BB-4-1 had the ions of 1022.355 and 1038.325 of [M + Na] and [M + K], respectively, indicating a monosaccharide composition of [Hex]3[HexNAc]1[deoxyHex]2.
The 1H-NMR spectrum (Supplementary Fig. 1, chemical shifts in Table 1) had the H-1 shifts of α(1–2) linked Fuc, α-Glc, α(1–3) linked Fuc, β(1–3) linked GlcNAc, β-Glc and two β(1–4) linked Gal at δ 5.278, 5.219, 5.116, 4.710, 4.662, 4.513 and 4.438, respectively, H-4 of β(1–4) linked Gal, which was substituted at OH-3, at δ 4.144, H-5 of α(1–2) linked Fuc at δ 4.252, and H-6 of α(1–2) and α(1–3) linked Fuc at δ 1.265 and 1.236, respectively, and NAc of β(1–3) linked GlcNAc at δ 2.025. The H-1 shift of α(1–2) linked Fuc at δ 5.278 showed the presence of Fuc(α1–2)Gal(β1–4)GlcNAc, while the H-1 shift of α(1–3) linked Fuc at δ 5.116 showed the presence of Gal(β1–4)[Fuc(α1–3)]GlcNAc [4]. From these observations, the oligosaccharide in BB-4-1 was characterized to be Fuc(α1–2)Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–3)Gal(β1–4)Glc (difucosyl lacto-N-neotetraose).
Characterization of monogalactosyl monofucosyl lacto-N-neotetraose
The MALDITOF MS of BB-4-2 had the ion of 1038.322 of [M + K], indicating a monosaccharide composition of [Hex]4[HexNAc]1[deoxyHex]1.
The 1H-NMR spectrum (chemical shifts in Table 1) had the H-1 shifts of α-Glc, α(1–3) linked Gal, α(1–3) linked Fuc, β(1–3) linked GlcNAc, β-Glc and two β(1–4) linked Gal at δ 5.219, 5.143, 5.138, 4.710, 4.662, 4.535 and 4.433, respectively, H-4 of β(1–4) linked Gal, which was substituted at OH-3 by α(1–3) linked Gal, at δ 4.204, H-5 of α(1–3) linked Gal at δ 4.193, H-4 of another β(1–4) linked Gal, which was substituted at OH-3 by β(1–3) linked GlcNAc, at δ 4.159, H-6 of α(1–3) linked Fuc at δ 1.180, and NAc of β(1–3) linked GlcNAc at δ 2.022. From these observations and the similarity for the published data [4,5,6], the oligosaccharide in BB-4-2 was characterized to be Gal(α1–3)Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–3)Gal(β1–4)Glc (monogalactosyl monofucosyl lacto-N-neotetraose).
Identification of Galili pentasaccharide
The MALDITOF MS of this fraction had the ions of 892.293 of [M + Na], indicating a monosaccharide composition of [Hex]4[HexNAc]1.
As the 1H-NMR patterns of BB-4-5 and BB-4-6 were identical, it was concluded that these two peaks contained the same saccharides. The 1H-NMR spectrum (Supplementary Fig. 2, chemical shifts in Table 1) had the H-1 shifts of α-Glc, α(1–3) linked Gal, β(1–3) linked GlcNAc, β-Glc and two β(1–4) Gal at δ 5.219, 5.145, 4.704 and 4.701, 4.663, 4.553 and 4.437, respectively, H-5 of α(1–3) linked Gal at δ 4.194, H-4 of β(1–4) linked Gal, which was substituted at OH-3 by α(1–3) linked Gal, at β 4.186, H-4 of β(1–4) linked Gal, which was substituted at OH-3 by β(1–3) linked GlcNAc, at δ 4.157, and NAc of β(1–3) linked GlcNAc at δ 2.034. As the spectrum was essentially similar to the published data for Galili pentasaccharide [6, 15, 16], the oligosaccharide in this fraction was characterized to be Gal(α1–3)Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc.
Analysis of fractions BB-4-3, BB-4-4, BB-5-3, BB-5-4, BB-6-2, BB-6-3, BB-8-2 and BB-8-3
As clearly resolved chemical shifts were not observed in the 1H-NMR of fractions BB-4-3, BB-4-4, BB-5-3, BB-5-4, BB-6-2, BB-6-3, and BB-8-2, the saccharides in these fractions were not characterized in this study. It was assumed that the unresolved 1H-NMR spectra were due to insufficient material. It was concluded from its 1H-NMR spectrum that the component in BB-8-3 was not a saccharide.
Analysis of fraction BB-3
The oligosaccharides in this fraction were not studied in this study because of insufficient material.
Analysis of fraction BB-1
It was assumed that this fraction contained glycoproteins/glycopeptides; these were not characterized in this study.
Characterization of acidic oligosaccharides of American black bear milk
Fraction BB-2 separated into three peaks during ion exchange chromatography, as shown in Fig. 3. The first peak was thought to contain a mixture of high molecular neutral oligosaccharides, which were not investigated. The components in the second peak, designated as BB-2-2, were further separated by HPLC as shown in Fig. 4. The oligosaccharides in BB-2-2-1 to BB-2-2-16 were characterized by 1H-NMR. It was assumed that the third eluted fraction, designated as BB-2-3 contained disialyl or more highly sialylated oligosaccharides; these were not characterized.
Identification of 3′-sialyllactose
As the 1H-NMR spectrum (chemical shifts in Table 2) of BB-2-2-1 was identical to that of authentic 3’-SL [19], it was characterized to be Neu5Ac(α2–3)Gal(β1–4)Glc.
Characterization of monosialyl monofucosyl lacto-N-neohexaose
The MALDITOF MS of this fraction had the ions of 1548.280 and 1586.281 of [M + K] and [M + 2 K-H], respectively, indicating a monosaccharide composition of [Neu5Ac]1[Hex]4[HexNAc]2[deoxyHex]1.
The 1H-NMR spectrum (chemical shifts in Table 2) had the H-1 shifts of α(1–2) linked Fuc, α-Glc, β(1–3) linked GlcNAc, β-Glc, β(1–6) linked GlcNAc, and three β(1–4) linked Gal, at δ 5.308, 5.219, 4.723, 4.666, 4.597, 4.542, 4.455 and 4.433, respectively, H-4 of β(1–4) linked Gal, which was substituted at OH-3, at δ 4.151, H-5 and H-6 of α(1–2) linked Fuc at δ 4.225 and 1.230, respectively, and NAc of β(1–3) and β(1–6) linked GlcNAc at δ 2.051 and 2.064, respectively. The spectrum had the H-3 axial, H-3 equatorial and NAc shifts of α(2–6) linked Neu5Ac at δ 1.724, 2.667 and 2.027, respectively. As this pattern was essentially identical to that of the published data [11] for monosialyl monofucosyl lacto-N-neohexaose, this oligosaccharide (BB-2-2-6-1) was characterized to be Neu5Ac(α2–6)Gal(β1–4)GlcNAc(β1–3)[Fuc(α1–2)Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc.
However, the 1H-NMR spectrum of this fraction had many other chemical shifts which arose from other saccharides in this fraction. It was concluded that one of these contained α(1–3) linked Fuc, as the spectrum had the H-1 and H-6 at δ 5.129 and 1.175, respectively.
Characterization of monosialyl monogalactosyl lacto-N-neohexaose
The MALDITOF MS of BB-2-2-8 had the ions of 1564.292 and 1602.272 of [M + K] and [M + 2 K-H], respectively, indicating a monosaccharide composition of [Neu5Ac]1[Hex]5[HexNAc]2.
The 1H-NMR spectrum (chemical shifts in Table 2) had the H-1 shifts of α-Glc, α(1–3) linked Gal, β(1–3) linked GlcNAc β-Glc, β(1–6) linked GlcNAc and three β(1–4) linked Gal at δ 5.219, 5.145, 4.726, 4.668, 4.641, 4.545, 4.455 and 4.432, H-5 of α(1–3) linked Gal at δ 4.196 and H-4 of β(1–4) linked Gal, which was substituted at OH-3 by α(1–3) linked Gal, at δ 4.183. The spectrum had H-4 of β(1–4) linked Gal, which was substituted at OH-3 by β(1–3) linked GlcNAc, at δ 4.149, and NAc of β(1–3) and β(1–6) linked GlcNAc at δ 2.051 and 2.062, respectively. This also had the H-3 axial, H-3 equatorial and NAc shifts of α(2–6) linked Neu5Ac at δ 1.725, 2.667 and 2.027, respectively. As these 1H-NMR chemical shifts were essentially similar for the published data for monosialyl monogalactosyl lacto-N-neohexaose [15, 16], the oligosaccharide in BB-2-2-8 was characterized to be Neu5Ac(α2–6)Gal(β1–4)GlcNAc(β1–3)[Gal(α1–3)Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc.
Characterization of monosialyl monogalactosyl monofucosyl lacto-N-neohexaose
The MALDITOF MS of BB-2-2-9 had the ions of 1710.214 and 1748.218 of [M + K] and [M + 2 K-H], respectively, indicating a monosaccharide composition of [Neu5Ac]1[Hex]5[HexNAc]2[deoxyHex]1.
The 1H-NMR spectrum (Supplementary Fig. 3, chemical shifts in Table 2) had the H-1 shifts of α-Glc, α(1–3) linked Gal, α(1–3) linked Fuc, β(1–3) linked GlcNAc, β-Glc, β(1–6) linked GlcNAc and three β(1–4) linked Gal at δ 5.219, 5.142, 5.115, 4.724, 4.668, 4.640, 4.526, 4.455 and 4.432, respectively, H-5 of α(1–3) linked Gal at δ 4.197 and H-4 of β(1–4) linked Gal, which was substituted at OH-3 by α(1–3) linked Gal, at δ 4.160. The spectrum had H-4 of β(1–4) linked Gal, which was substituted at OH-3 by β(1–3) linked GlcNAc, at δ 4.143, H-6 of α(1–3) linked Fuc at δ 1.179, and NAc of β(1–3) and β(1–6) linked GlcNAc at δ 2.051. This also had the H-3 axial, H-3 equatorial and NAc shifts of α(2–6) linked Neu5Ac at δ 1.725, 2.668 and 2.027, respectively. As these 1H-NMR chemical shifts were essentially similar to the published data for monosialyl monogalactosyl monofucosyl lacto-N-neohexaose [8, 29], the oligosaccharide in BB-2-2-9 was characterized to be Neu5Ac(α2–6)Gal(β1–4)GlcNAc(β1–3){Gal(α1–3)Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–6)}Gal(β1–4)Glc.
Characterization of monosialyl monogalactosyl difucosyl lacto-N-neohexaose
The MALDITOF MS of BB-2-2-10 had the ions of 1858.156 and 1894.122 of [M + K] and [M + 2 K-H], respectively, indicating a monosaccharide composition of [Neu5Ac]1[Hex]5[HexNAc]2[deoxyHex]2.
The 1H-NMR spectrum (chemical shifts in Table 2) had the H-1 of α(1–2) linked Fuc, α(1–3) linked Gal, α-Glc, α(1–3) linked Fuc, β(1–3) linked GlcNAc, β-Glc, β(1–6) linked GlcNAc and three β(1–4) linked Gal at δ 5.283, 5.245, 5.219, 5.107, 4.723, 4.668, 4.612, 4.583, 4.445 and 4.434, respectively, H-5 of α(1–3) linked Gal at δ 4.225 and H-4 of β(1–4) linked Gal, which was substituted at OH-3 by α(1–3) linked Gal, at δ 4.266. The spectrum had H-4 of β(1–4) linked Gal, which was substituted at OH-3 by β(1–3) linked Gal, at δ 4.147, H-5 and H-6 of α(1–2) linked Fuc at δ 4.330 and 1.292, respectively, H-6 of α(1–3) linked Fuc at δ 1.247, and NAc of β(1–6) and β(1–3) linked GlcNAc at δ 2.056 and 2.051, respectively. This also had the H-3 axial, H-3 equatorial and NAc of α(2–6) linked Neu5Ac at δ 1.725, 2.668 and 2.027, respectively. As these 1H-NMR chemical shifts were essentially similar to the published data for monosialyl monogalactosyl difucosyl lacto-N-neohexaose [8], the oligosaccharide in BB-2-2-10 was characterized to be Neu5Ac(α2–6)Gal(β1–4)GlcNAc(β1–3){Gal(α1–3)[Fuc(α1–2)]Gal(β1–4)[Fuc(α1–3)]GlcNAc(β1–6)}Gal(β1–4)Glc.
Analysis of fractions BB-2-2-3, BB-2-2-4, BB-2-2-5 and BB-2-2-7
The saccharides in these fractions were not characterized because they yielded unresolved 1H-NMR spectra. It was assumed that this was due to insufficient material.
Analysis of fractions BB-2-2-11, BB-2-2-12, BB-2-2-13, BB-2-2-14, BB-2-2-15, BB-2-2-16 and BB-2-2-17
Clearly resolved 1H-NMR spectra were not obtained for the saccharides in these fractions because of their high molecular weights.
Characterization of neutral saccharides and lactose sulfate of cheetah milk
The carbohydrate fraction from three cheetah milk separated into several peaks, designated CM(a)-1 to CM(a)-4, CM(b)-1 to CM(b)-4, and CM(c)-1 to CM(c)-4, during gel filtration on BioGel P-2 (Fig. 5). Since the components in CM(a)-2 to CM(a)-4, CM(b)-2 to CM(b)-4 and CM(c)-2 to CM(c)-4 did not react positively with periodate – resorcinol they were considered to be neutral oligosaccharides. The saccharides in CM(a)-4, CM(b)-4, CM(c)-2 and CM(b)-2 were characterized by 1H-NMR. The oligosaccharides in the fractions of CM(a)-3, CM(b)-3 and CM(c)-3 were further purified by HPLC as pooled in Fig. 6.
Identification of lactose
The 1H-NMR spectra (chemical shifts in Table 3) of the saccharides in CM(a)-4 and CM(b)-4 were identical with that of Gal(β1–4)Glc, these are lactose. The 1H-NMR spectrum of fraction CM(c)-4 showed that this contained a contaminant as well as lactose; it was therefore purified by HPLC with a Hypercarb column. After the purification, the 1H-NMR spectrum was identical with that of lactose.
Identification of isoglobotriose
The 1H-NMR spectra (Chemical shifts in Table 3) of the saccharides in CM(a)-3–1 and CM(c)-3–1 were identical with that of Gal(α1–3)Gal(β1–4)Glc [19, 25], these are isoglobotriose.
Identification of 6′-galactosyllactose and 3′-galactosyllactose
The 1H-NMR spectrum (chemical shifts in Table 3) of CM(b)-3–1 showed the presence of two saccharides in this fraction; a major and a minor. The spectrum had the anomeric shifts of α-Glc, β-Glc, β(1–6) linked Gal and β(1–4) linked Gal at δ 5.221, 4.668, 4.484 and 4.459, respectively. As this pattern was similar to that of 6′-galactosyllactose (6′-GL) [25], the major saccharide in this fraction was characterized to be Gal(β1–6)Gal(β1–4)Glc.
The spectrum, in addition, had the small H-1 shifts of β(1–3) linked Gal and β(1–4) linked Gal at δ 4.612 and 4.510, and the characteristic H-4 shift of β(1–4) linked Gal, which was substituted by β(1–3) linked Gal, at δ 4.197. From these observations and the similarity for the published data [30], the minor saccharide in this fraction was characterized to be Gal(β1–3)Gal(β1–4)Glc (3′-galactosyllactose, 3′-GL).
Identification of lacto-N-neohexaose and lactose 3’-O-sulphate
The 1H-NMR spectrum (chemical shifts in Table 3) of CM(c)-2 showed the presence of two saccharides in this fraction; a major and a minor. The spectrum had the anomeric shifts of α-Glc, two β(1–3) linked Gal, β-Glc, two β(1–6) linked Gal, and three β(1–4)linked Gal at δ 5.219, 4.704 and 4.701, 4.667, 4.644 and 4.637, and 4.480, 4.471 and 4.428, respectively. The spectrum had the characteristic H-4 of β(1–4) linked Gal, which was substituted by β(1–3) linked GlcNAc, at δ 4.146, and NAc shifts of β(1–3) and β(1–6) linked GlcNAc at 2.031 and 2.061, respectively. As this pattern was similar to that of lacto-N-neohexaose [31], the major saccharide in this fraction was characterized to be Gal(β1–4)GlcNAc(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc.
The spectrum (chemical shifts in Table 3), in addition, had the H-1 shift of α-Glc and β(1–4)Gal at δ 5.228 and 4.568, and characteristic down field shifts of H-3 and H-4 of β(1–4) linked Gal at δ 4.334 and 4.295, respectively. As this pattern was similar to the published data [17] of lactose-3’-O-sulphate, the minor saccharide in this fraction was characterized to be Gal(β1–4)Glc-3’-O-SO3.
As the 1H-NMR spectrum of CM(b)-2 had essentially similar shifts with those of the major saccharide in CM(c)-2, this fraction contained lacto-N-neohexaose, too. However, there were no shifts caused by lactose-3’-O-sulphate in this fraction.
The saccharide in CM(a)-2 was not characterized in this study, as the clear NMR spectrum was not obtained
Characterization of sialyl saccharide of cheetah milk
The fraction CM(a)-1 separated into the unadsorbed fractions, designated CM(a)-1–1 and CM(a)-1–2, and the adsorbed fractions, designated as CM(a)-1–3 and CM(a)-1–4 during the ion exchange chromatography on DEAE-Sephadex A-50 as shown in Fig. 7. The fraction CM(a)-1–3 was separated into CM(a)-1–3-1 to CM(a)-1–3-6 during HPLC on an Amide-80 column (Fig. 8), and the component in the separated peaks were characterized by 1H-NMR.
Identification of disialyllactose
The 1H-NMR spectrum (chemical shifts in Table 3) of CM(a)-1–3-4 had the anomeric shifts of α-Glc, β-Glc and β(1–4) linked Gal at δ 5.223, 4.661 and 4.522, respectively, showing that the core structure is Gal(β1–4)Glc. The spectrum had the chemical shifts of H-3 axial at δ 1.739, H-3 equatorial at δ 2.776 and 2.676, and NAc shifts of Neu5Ac at δ 2.066 and 2.029, showing the presence of two Neu5Ac residues. The signal intensity of the shift at δ 1.739 corresponded to two protons. As the 1H-NMR pattern was essentially similar to that of authentic disialyllactose, the oligosaccharides in CM(a)-1–3-4 was characterized to be Neu5Ac(α2–8)Neu5Ac(α2–3)Gal(β1–4)Glc.
As the concentrations of oligosaccharides in CM(a)-1–1 and CM(a)-1–2 were small, these were not characterized in this study. As the clear 1H-NMR spectra were not obtained for CM(a)-1–3-1, CM(a)-1–3-2, CM(a)-1–3-3, CM(a)-1–3-5 and CM(a)-1–3-6, these were not characterized in this study. The components in CM(a)-1–4 were not studied in this study.
Discussion
The ratio of oligosaccharides (OS) to lactose in Caniformia and Feliformia
From the profiles obtained by gel filtration of the carbohydrate fraction (see Figs. 1 and 5), we conclude that milk oligosaccharides predominate over lactose in American black bear milk, while lactose is the predominant saccharide in cheetah milk. Based on relative peak area of the gel chromatograms, the ratio of milk oligosaccharides to lactose was estimated to be 21:1 in American black bear milk (0–2 days postpartum), as compared to 1:1.1, 1:1.1 and 1:2.1 in the milks of the three cheetahs (CM(a), CM(b) and CM(c), respectively) at 1–2 days postpartum. These ratios are consistent with previous observations that milk oligosaccharides predominate over lactose in the milks of most species of the Caniformia, while lactose is the predominant saccharide in the milks of the Feliformia [3]. Per our current and previous estimates, the ratios of milk oligosaccharides to lactose among Caniformia are as follows: a) 52:1 in milk of Japanese black bear (37 days post partum), b) 32:1 in milk of raccoon, c) 21:1 in milk of American black bear (0–2 days postpartum), d) 13:1 in milk of polar bear at 27 months postpartum, e) 10:1 in milk of giant panda at 13 days post partum, f) 7:1 in milk of striped skunk at 20–48 days post partum, g) 5:1 in milk of mink at 15 days postpartum, h) 4:1 in milk of high arctic harbor seal, i) 2:1 in milk of white-nosed coati at 17 days postpartum, and finally, j) 1:6 in milk of domestic dog at 13 days postpartum. By contrast, among Feliformia, the ratio is a) 1:1 in colostrum of spotted hyena at 2 days post partum, b) 1:1 in colostrum of clouded leopard, c) 1:1–1:2 in milk of cheetah at 0–2 days postpartum, and d) 1:2 in milk of African lion at 127 days postpartum.
Our data indicate that oligosaccharides predominate over lactose in milks of Caniformia species, such as American black bear, but not in milks of Feliformia such as the cheetah. However, the extent to which this hypothesis applies to other Carnivore milks should be tested with data from many other Carnivora milks; the milk OS to lactose ratio may also differ at different lactation stages after birth. It would also be interesting to examine highly altricial carnivores, such as giant pandas. The variation of this ratio during the course of lactation has been observed in the case of humans. It has been reported that the OS concentration is 22 ~ 24 g/L in colostrum and 12 ~ 13 g/L in mature milk, respectively [32]; namely the ratio is around 1: 4.6 in colostrum and 1: 2.6 in mature milk. This suggests that the ratio may vary during the course of lactation and inter-specific comparisons should be conducted with milk samples from the same lactation stage.
The biological significance of oligosaccharides (OS) proportion
The biological significance of the differing proportions of lactose, acidic and neutral oligosaccharides observed among mammals is not certain. The hypothesis that oligosaccharides serve an anti-bacterial and prebiotic function in neonatal digestive tracts, and thus should be selected for in social species with larger group size and more avenues for social transmission of pathogens [33], does not appear to apply to carnivorans, as highly social lions and dogs have the lowest, not highest, OS:lactose ratios, whereas high OS:lactose ratios are found in predominantly solitary carnivorans, such as raccoons, bears, mink and striped skunk (see above).
An alternative hypothesis is that oligosaccharides, as transporters of specific saccharide constituents, may be important for species with altricial young. Certainly, altricial neonates are characterized by immaturity of physiological and biochemical functions, and when this is combined with rapid growth and the need to rapidly synthesize complex tissues, a situation might arise in which rates of tissue synthesis of a particular constituent are insufficient to meet requirements without dietary (i.e. milk) supply. For example, in altricial rat pups, the rate-limiting enzyme in sialic acid synthesis (UDP-N-acetylglucosamine N-acetylmannosamine epimerase/kinase) has low liver activity in neonates [34]. Based on gene expression profiles of various tissues of rat pups, Duncan et al. [35] concluded that sialic acid was absorbed from milk and compensated for low neonatal rates of sialic acid synthesis. Radiolabelled exogenous sialic acid has also been shown to be deposited in the tissues of growing rat pups, including the brain, which has an especially high sialic acid content [36, 37]. It has been argued that a dietary supply of N-acetyl neuraminic acid (sialic acid), a major constituent of acidic oligosaccharides in milk, may be essential for synthesis of brain gangliosides and the polysialic chains on neural cell adhesion molecules in preterm and rapidly growing infants [38, 39]. This argument is especially pertinent to mammals with altricial young, whose brains at birth may be at an even less advanced stage of development than that of early (e.g., 24 week) preterm human infants. We hypothesize that milk oligosaccharides and especially sialic acid-containing oligosaccharides should be enriched to carnivoran species with altricial young that complete a large proportion of brain and organ growth postnatally.
However, it appears that the concentration of sialyl oligosaccharides in milk is lower in the American black bear compared to two of the cheetah (see Figs. 1 and 5). To test our hypothesis regarding the importance of sialyl oligosaccharides across a broader array of mammals it will be necessary to investigate the OS ratios in the milks of many different species at several lactation stages. For example, in cows, the concentration of sialyl oligosaccharides in milk is high in colostrum within 48 h post partum, but then rapidly decreases to very small level [40]. This may suggests a specific need for sialic acid during neonatal developmental stages.
A classic method for determining physical maturity at birth, is to assess water content of lean tissues, as this parameter gradually declines with development. When compared at birth across terrestrial (altricial) carnivorans it ranges from 81.0% (dog puppies), 82.0% (domestic cat kittens), 83.0% (mink kits) to 84.0% (American black bear cubs) [22]. Comparable values for precocial marine carnivores (Caniformia: Pinnipedia) are 71–74% (n = 4 species) [41]. The higher water content of lean tissue is related to the less mature state of the neonates. However, little if any data are available for neonates of other carnivorans, whereby, such data are not sufficient for broad comparisons to oligosaccharide patterns.
An alternative approach is to examine, via mass assessment, postnatal development of the body, or specific organs, in relation to adult mass. For example, brain mass at birth, expressed relative to adult mass, is considered a measure of the degree of neonatal maturity [42]. In order to maximize useful data, we examined total mass at birth of neonates, expressed as a percentage of maternal mass, with the assumption that a smaller neonate at birth will in general be less developed than larger neonates. Using species-specific data assembled by Oftedal and Gittleman [43], the birth mass percentage of cheetahs (0.48) was considerably higher than American black bears (0.29), and this difference also holds at a familial level Felidae (cats) (mean = 1.60 ± standard deviation (SD) 0.80, n = 13) vs Ursidae (bears) (mean = 0.30 ± 0.08 SD, n = 4. Thus, these patterns are consistent with our predictions: altricial ursids have high OS:lactose ratios (10:1–51:1) compared to more precocial felids (1:1 to 1:2, see above).
Our data were sufficient to examine patterns among other caniform and feliform families. For example, three Caniform families known to have high OS:lactose ratios (Mephtitidae, Mustelidae, Procyonidae) have relatively high but varied birth mass percentages (averaging 2.67 ± 0.45, n = 2; 2.05 ± 1.26, n = 13; and 4.03 ± 3.08, n = 4; respectively). Thus, their milks are higher in oligosaccharides than would be predicted from birth mass percentages. Further research is needed on both physiological maturity and postnatal development to determine correlations to milk oligosaccharide composition.
Unlike other Caniform species studied, lactose predominates over oligosaccharides in the milk of domestic dogs [17]. The neonatal dog is rather immature and does not open its eyes until around 10 days of age, but calculating the proportional size of puppies relative to the adult body weight is complicated by the extreme variation in size represented by different breeds of dogs. For example, puppies of giant breeds such as the English Mastiff may represent <1% of adult BW, whereas puppies of toy breeds such as Papillon may be >5% of adult BW at birth [44,45,46]. Using Oftedal and Gittlemen’s data for dogs [43], a medium size dog has a neonatal weight representing 1.67% of maternal weight, aligning with our hypothesis that OS:lactose is lower in species having higher proportional mass at birth. However, the domestication of this species by humans may have influenced its lactational physiology, and may have reduced the concentration of oligosaccharides in milk relative to other Caniforms. To clarify this, future studies are needed to characterize the milk of non-domesticated canid species such as wolf, coyote, red fox, bush dog.
Specific oligosaccharide structures found in milk of the American black bear and related Caniformia
The neutral as well as acidic milk oligosaccharides in American black bear milk, characterized in this study, are shown in Fig. 9. It was concluded that the significant feature of these oligosaccharides is the presence of B (Gal(α1–3)[Fuc(α1–2)]Gal), H (Fuc(α1–2)Gal), α-Gal epitope (Gal(α1–3)Gal(β1–4)GlcNAc), B Lewis x (Gal(α1–3)[Fuc(α1–2)]Gal(β1–4)[Fuc(α1–3)]GlcNAc), Lewis y (Fuc(α1–2)Gal(β1–4)[Fuc(α1–3)]GlcNAc) or α-Gal Lewis x (Gal(α1–3)Gal(β1–4)[Fuc(α1–3)]GlcNAc) in the structure. B-pentasaccharide, monosialyl monogalactosyl difucosyl lacto-N-neotetraose, monogalactosyl monofucosyl lacto-N-neotetraose, and monosialyl monogalactosyl monofucosyl lacto-N-neohexaose, which have B Lewis x or α-Gal Lewis x, have also been found in the milk of Japanese black bear milk [5, 8]; this likely reflects the homology of milk oligosaccharides between these bear species. However, Galili pentasaccharide, difucosyl lacto-N-neotetraose, monosialyl monogalactosyl lacto-N-neohexaose, and monosialyl monofucosyl lacto-N-neohexaose were not detected in Japanese black bear milk [5, 8]. As Galili pentasaccharide and monosialyl monogalactosyl lacto-N-neohexaose, are most likely precursors in the synthesis of monogalactosel monofucosyl lacto-N-neotetraose and monosialyl monogalactosyl monofucosyl lacto-N-neohexaose, respectively. These two oligosaccharides may exist in Japanese black bear milk but in such trace amounts that we did not detect them. Difucosyl lacto-N-neotetraose has been found in Ezo brown bear milk [4], while monosialyl monofucosyl lacto-N-neohexaose and monosialyl monogalactosyl lacto-N-neohexaose have been detected in milks of harbor seal, and mink and stripped skunk, respectively [11, 15, 16]. Galili pentasaccharide has also been found in the milk of polar bear, mink and striped skunk [6, 15, 16]. Thus, it is likely that there is homology in milk oligosaccharides between American black bear and other Ursidae species, and also between this black bear and other Caniformia species. In our previous study of milk oligosaccharides of Ezo brown bear, Japanese black bear and polar bear, the oligosaccharides, whose core units were lacto-N-neotetraose or lacto-N-neohexaose, always contained Lewis x excepting for one polar bear [4,5,6,7,8]. As some oligosaccharides did not contain Lewis x in their structures in this study, this may be a difference between the American black bear and other Ursidae. It is assumed that this must have been caused by the relatively weak activity of α3fucosyltransferase in lactating mammary glands of this American black bear compared to that of other bear species. All oligosaccharides other than those containing lactose core unit had only type II (Gal(β1–4)GlcNAc) but not type I (Gal(β1–3)GlcNAc). This is a consistent feature of all other studied Caniformia including the Ursidae [3, 47].
Specific oligosaccharide structures found in milk of the cheetah and related Feliformia
The characterized cheetah milk oligosaccharides are shown in Fig. 10. Isoglobotriose was detected in milk of animals 1 and 3, but was not found in that of animal 2. 3′-GL and 6′-GL were detected in the milk of animal 2, but not in those of 1 and 3. The milks or colostra of many Caniformia species contain isoglobotriose, but not the milks of dog, raccoon, seals and African lion [3, 46]. Lactose 3’-O-sulphate was detected only in milk of animal 3. Among Carnivora milk, this has been found only in dog milk [17]. Lacto-N-neohexaose was detected in the milks of animals 2 and 3, but not in that of animal 1. Thus, the heterogeneity of these oligosaccharides was observed in milk among the individual cheetahs. In the milks of Felidae, which had been studied before, all oligosaccharides had only lactose as a core unit [19, 20]. Thus the presence of lacto-N-neotetraose is the first case that the milk oligosaccharides contain another core unit than lactose in the Felidae. The milk of Carnivora including Felidae usually have the oligosaccharides containing A, B or H antigens in the non-reducing end [3, 29], but these were not detected in these cheetah milks. This must be due to the lack of α2fucosyltransferase activity in cheetah mammary glands, at least early in lactation, because H antigen is the precursor for the biosynthesis of A or B antigens.
With regard to variation among milk oligosaccharides within a species it is well known that human milk oligosaccharides vary depending on donor’s secretor or Lewis blood group. For example non secretor donor’s milk does not include the oligosaccharides containing non reducing α(1–2) linked Fuc residue such as Fuc(α1–2)Gal(β1–4)Glc (2’-FL), Fuc(α1–2)Gal(β1–3)GlcNAc(β1–3)Gal(β1–4)Glc (LNFP-I) or Fuc(α1–2)Gal(β1–3)[Fuc(α1–4)]GlcNAc(β1–3)Gal(β1–4)Glc (LNDFH-1), etc., because of the non expression of the enzyme FUT2, even though these are dominant saccharides in secretor donor’s milk [32, 48]. In addition, Lewis negative donor’s milk does not contain oligosaccharides containing α(1–4) linked Fuc residue such as Gal(β1–3)[Fuc(α1–4)]GlcNAc(β1–3)Gal(β1–4)Glc (LNFP-II) and LNDFH-1, etc., due to the non expression of FUT3 [32, 48]. However it is thought that the individual variation of the oligosaccharides in cheetah milk, clarified in this study, should be different from that of human donors. We speculate that the individual variation of the oligosaccharides among cheetahs are caused by the presence/absence of α- or β-galactosyltransferase, sulfonyltransferase and/or β3N-acetylglucosaminyltransferase in lactating mammary glands. Whether milk oligosaccharides can be classified into some groups, as in human milk, will require study of many additional samples of cheetah milk.
Disialyllactose (Neu5Ac(α2–8)Neu5Ac(α2–3)Gal(β1–4)Glc) was found in the cheetah milk. In our previous studies of the Felidae, Neu5Gc(α2–3)Gal(β1–4)Glc was found in milk of African lion and clouded leopard [20], while Neu5Ac(α2–3)Gal(β1–4)Glc was not detected. The colostrum of spotted hyena contained Neu5Ac(α2–3)Gal(β1–4)Glc, but not Neu5Gc(α2–3)Gal(β1–4)Glc [19]. It appears that the presence/absence of Neu5Ac or Neu5Gc in milk sialyloligosaccharides varies among species of the Felidae, while only Neu5Ac has been found in milk or colostrum of species of the Caniformia.
Specific milk oligosaccharides: Are they important for artificial feeding of neonates?
Despite the recognition that the aggregate amount of oligosaccharides in milk may exceed that of lactose, and the proliferation of information about the neutral and acidic oligosaccharide structures found among carnivoran milks, there is still little certainty about the physiological, nutritional, immune-defense or developmental necessity of these constituents for neonates (but see discussion of sialic acid, above). Studies are complicated by the need to be species-specific and the uncertainty about the extent to which findings are applicable to other, even closely related species. Although milk replacers continue to be produced by various manufactures for dogs and cats, it is unlikely that these products match the oligosaccharide profiles of dog or cat milk, let alone the profiles of the milks of other zoo animals and wildlife for which they are employed. It is not known if this creates health or developmental problems. However, given that strong selective evolutionary pressures have apparently maintained the synthesis of a great diversity of oligosaccharide structures in carnivoran milks, especially in taxa with immature neonates, it seems likely that the oligosaccharides are important to neonatal development.
Among endangered species captive breeding programs, the artificial rearing of neonates occurs for a range of reasons, but primarily following maternal ill-health or poor mothering. For example, the cheetah is often reared on milk replacer formulated for domestic canine or feline requirements, but often uses bovine milk as its basis [49, 50]. But the ratio of milk oligosaccharides to lactose and also the profile of the oligosaccharide in milk differ between cow and these Carnivora species. Bovine mature milk contains only trace amount of oligosaccharides [47], suggesting that some artificial oligosaccharides should be incorporated in milk replacers used for carnivore species.
References
Jenness, R.E., Regehr, E.A., Sloan, R.E.: Comparative studies of milks. II. Dialyzable carbohydrates. Comp. Biochem. Physiol. 13, 339–352 (1964)
Shennan, D.B., Peaker, M.: Transport of milk constituents by the mammary gland. Physiol. Rev. 80, 925–951 (2000)
Urashima, T., Messer, M., Oftedal, O.T.: Comparative biochemistry and evolution of milk oligosaccharides of monotremes, marsupials, and eutherians. In: Pontarotti, P. (ed.) Evolutionary Biology: Genome Evolution, Speciation, Coevolution and Origin of Life, Pp. 3–33. Springer, Swiyzerland (2014)
Urashima, T., Kusaka, Y., Nakamura, T., Saito, T., Maeda, N., Messer, M.: Chemical characterization of milk oligosaccharides of the brown bear, Ursus arctos yesoensis. Biochim. Biophys. Acta. 1334, 247–255 (1997)
Urashima, T., Sumiyoshi, W., Nakamura, T., Arai, I., Saito, T., Komatsu, T., Tsubota, T.: Chemical characterization of milk oligosaccharides of the Japanese black bear, Ursus thibetanus japonicus. Biochim. Biophys. Acta. 1472, 290–306 (1999)
Urashima, T., Yamashita, T., Nakamura, T., Arai, I., Saito, T., Derocher, A.E.: O. Wiig, O.: chemical characterization of milk oligosaccharides of the polar bear, Ursus maritimus. Biochim. Biophys Acta. 1475, 395–408 (2000)
Urashima, T., Nagata, H., Nakamura, T., Arai, I., Saito, T., Imazu, K., Hayashi, T., Derocher, A.E., Wiig, O.: Differences in oligosaccharides pattern of a sample of polar bear colostrum and mid-lactation milk. Comp. Biochem. Physiol. B136, 887–896 (2003)
Urashima, T., Nakamura, T., Teramoto, K., Arai, I., Saito, T., Komatsu, T., Tsubota, T.: Chemical characterization of sialyl oligosaccharides in milk of the Japanese black bear, Ursus thibetanus japonicus. Comp. Biochem. Physiol. B139, 587–595 (2004)
Urashima, T., Yamaguchi, E., Ohshima, T., Fukuda, K., Saito, T.: Chemical structures of oligosaccharides in milk of the raccoon (Procyon lotor). Glycoconj. J. 35, 275–286 (2018)
Urashima, T., Arita, M., Yoshida, M., Nakamura, T., Arai, I., Saito, T., Arnould, J.P.Y., Kovacs, K.M., Lydersen, C.: Chemical characterization of the oligosaccharides in hooded seal. (Cystophora cristata) and Australian fur seal (Arctocephalus pusillus doriferus) milk. Comp. Biochem. Physiol. B128, 307–323 (2001)
Urashima, T., Nakamura, T., Yamaguchi, K., Munakata, J., Arai, I., Saito, T., Lydersen, C., Kovacs, K.M.: Chemical characterization of the oligosaccharides in milk of high Arctic harbour seal (Phoca vitulina vitulina). Comp. Biochem. Physiol. A135, 549–563 (2003)
Urashima, T., Nakamura, T., Nakagawa, D., Noda, M., Arai, I., Saito, T., Lydersen, C., Kovacs, K.M.: Characterization of oligosaccharides in a milk of bearded seal (Erignathus barbatus). Comp. Biochem. Physiol. B138, 1–18 (2004)
Kinoshita, M., Ohta, H., Higaki, K., Kojima, Y., Urashima, T., Nakajima, K., Suzuki, M., Kovacs, K.M., Lydersen, C., Hayakawa, T., Kakehi, K.: Structural characterization of multi-branched oligosaccharides from seal milk by combination of off-line HPLC-MALDI-TOF MS and sequential exoglycosidase digestion. Anal. Biochem. 388, 242–253 (2009)
Urashima, T., Yamamoto, M., Nakamura, T., Arai, I., Saito, T., Namiki, M., Yamaoka, K., Kawahara, K.: Chemical characterisation of the oligosaccharides in a sample of milk of a white-nosed coati, Nasua narica (Procyonidae: Carnivora). Comp. Biochem. Physiol. A123, 187–193 (1999)
Taufik, E., Sekii, N., Senda, A., Fukuda, K., Saito, T., Eisert, R., Oftedal, O.T., Urashima, T.: Neutral and acidic milk oligosaccharides of the striped skunk (Mephitidae: Mephitis mephitis). Anim. Sci. J. 84, 569–578 (2013)
Urashima, T., Nakamura, T., Ikeda, A., Asakuma, S., Arai, I., Saito, T., Oftedal, O.T.: Characterization of oligosaccharides in milk of a mink, Mustela vison. Comp. Biochem. Physiol. A142, 461–471 (2005)
Bubb, W.A., Urashima, T., Kohso, K., Nakamura, T., Arai, I., Saito, T.: Occurrence of an unusual lactose sulfate in dog milk. Carbohydr. Res. 318, 123–128 (1999)
Rostami, M.S., Benet, T., Spears, J., Reynolds, A., Satyaraj, E., Sprenger, N., Austin, S.: Milk oligosaccharides over time of lactation from different dog breeds. PLoS One e99824 (2014)
Uemura, Y., Takahashi, S., Senda, A., Fukuda, K., Saito, T., Oftedal, O.T., Urashima, T.: Chemical characterization of milk oligosaccharides of a spotted hyena (Crocuta crocuta). Comp. Biochem. Physiol. A152, 158–161 (2009)
Senda, A., Hatakeyama, E., Kobayashi, R., Fukuda, K., Uemura, Y., Saito, T., Packer, C., Oftedal, O.T., Urashima, T.: Chemical characterization of milk oligosaccharides of an African lion (Panthera leo) and a clouded leopard (Neofelis nebulosa). Anim. Sci. J. 81, 687–693 (2010)
Nakamura, T., Urashima, T., Mizukami, T., Fukushima, M., Arai, I., Senshu, T., Imazu, K., Nakao, T., Saito, T., Ye, Z.: H. Zuo, H., Wu, K.: composition and oligosaccharides of a milk sample of the giant panda, Ailuropoda melanoleuca. Comp. Biochem. Physiol. B135, 439–448 (2003)
Oftedal, O.T., Alt, G.L., Widdowson, E.M., Jakubasz, M.R.: Nutrition and growth of suckling black bears (Ursus americanus) during their mothers’ winter fast. Brit J Nutr. 70, 59–79 (1993)
Oftedal, O.T., Iverson, S.J.: Phylogenetic variation in the gross composition of milks. In: Jensen, R. (ed.) Handbook of Milk Composition, Pp. 749–789. Academic Press, New York (1995)
Alt, G.L. Reproductive biology of female black bears and early growth and development of cubs in northeastern Pennsylvania. PhD Thesis, West Virginia University. (1989)
Urashima, T., Bubb, W.A., Messer, M., Tsuji, Y., Taneda, Y.: Studies of the neutral trisaccharides of goat (Capra hircus) colostrum and of the one- and two-dimensional 1H and 13C NMR spectra of 6’-N-acetylglucosaminyllactose. Carbohydr. Res. 262, 173–184 (1994)
Dubois, M., Gill, K.A., Hamilton, J.K., Roberts, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956)
Jourdian, G.W., Dean, L., Roseman, S.: The sialic acid XI. A periodate – resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J. Biol. Chem. 256, 430–435 (1971)
Oftedal, O.T., Nicol, S.C., Davies, N.W., Sekii, N., Taifik, E., Fukuda, K., Saito, T., Urashima, T.: Can an ancestral condition for milk oligosaccharides be determined? Evidence from the Tasmanian echidna (Tachyglossus aculeatus setosus). Glycobiology. 24, 826–839 (2014)
Uemura, Y., Asakuma, S., Yon, L., Saito, T., Fukuda, K., Arai, I., Urashima, T.: Structural determination of the oligosaccharides in the milk of an Asian elephant (Elephas maximus). Comp. Biochem. Physiol. A145, 468–478 (2006)
Urashima, T., Messer, M., Bubb, W.A.: Biosynthesis of marsupial milk oligosaccharides II: characterizeation of a β6-N-acetylglucosaminyltransferase in lactating mammary glands of the tammar wallaby, Macropus eugenii. Biochim. Biophys. Acta. 1117, 223–231 (1992)
Urashima, T., Kawai, Y., Nakamura, T., Arai, I., Saito, T., Namiki, M., Yamaoka, K., Kawahara, K., Messer, M.: Chemical characterisation of six oligosaccharides in a sample of colostrum of the brown capuchin, Cebus apella (Cebidae: primate). Comp. Biochem. Physiol. A123, 187–193 (1999c)
Bode, L.: Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 22, 11147–11162 (2012)
Tao, N., Wu, S., Kim, J., Joo An, H., Hinde, K., Power, M., Gagneux, P., German, J.B., Lebrilla, C.B.: Evolutionary Glycomics: characterization of milk oligosaccharides in primates. J. Proteome Res. 10, 1548–1557 (2011). https://doi.org/10.1021/pr1009367
Gal, B., Ruano, M.J., Puente, R., Garcia-Pardo, L.A., Rueda, R., et al.: Developmental changes in UDPN-acetylglucosamine 2-epimerase activity of rat and Guinea-pig liver. Comp. Biochem. Physiol. B Biochem.Mol. Biol. 118, 13–15 (1997)
Duncan, P.I., Raymond, F., Fuerholz, A., Sprenger, N.: Sialic acid utilization and synthesis in the neonatal rat revisited. PLoS One. 4(12), e8241 (2009). https://doi.org/10.1371/journal.pone.0008241
Wang, B., Brand-Miller, J., McNeil, Y., McVeagh, P., et al.: 1998 Sialic acid concentration of brain gangliosides: variation among eight mammalian species. Comp. Biochem. Physiol. 119A, 435–439 (1998)
Sprenger, N., Duncan, P.I.: Sialic acid utilization. Adv Nutr. 3(3), 392S–397S (2012). https://doi.org/10.3945/an.111.001479
Wang, B., Brand-Miller, J.: The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutrit. 57, 1351–1369 (2003). https://doi.org/10.1038/sj.ejcn.1601704
Wang, B.: Sialic acid is an essential nutrient for brain development and cognition. Annu. Rev. Nutr. 29, 177–222 (2009). https://doi.org/10.1146/annurev.nutr.28.061807.155515
Nakamura, T., Kawase, H., Kimura, K., Watanabe, Y., Ohtani, M., Arai, I., Urashima, T.: Changes in bovine colostrum and milk sialyloligosaccharides during early lactation. J. Dairy Sci. 86, 1315–1320 (2003)
Oftedal, O.T., Bowen, W.D., Boness, D.J.: Lactation performance and nutrient deposition in pups of the harp seal, Phoca groenlandica, on ice floes off Southeast Labrador. Physiol. Zool. 69, 635–657 (1996)
Eisert, R., Potter, C.W., Oftedal, O.T.: Brain size in neonatal and adult Weddell seals: Costs and consequences of having a large brain. Marine Mammal Sci. 30(1), 184–205 (2014). https://doi.org/10.1111/mms.12033
Oftedal, O.T., Gittleman, J.G.: Patterns of energy output during reproduction in carnivores. In: Gittleman, J.G. (ed.) Carnivore Behavior, Ecology and Evolution, pp. 375–378. Cornell University Press, Ithaca, N.Y (1989)
Groppetti, D., Pecile, A., Palestrini, C., Marelli, S.P., Boracchi, P.: A national census of birth weight in purebred dogs in Italy. Animal. 7, 43–63 (2017)
Hawthome, A.J., Booles, D., Nugent, P.A., Gettinby, G., Wilkinson, J.: Body-weight changes during growth in puppies of different breeds. J. Nutr. 134, 2027S–2030S (2004)
Scantlebury, M., Butterwick, R., Speakman, J.R.: Energetics of lactation in domestic dog (Canis familiaris) breeds of two sizes. Comp. Biochem. Physiol. A127, 197–210 (2000)
Urashima, T., Messer, M., Oftedal, O.T.: Oligosaccharides in the milk of other mammals. In: McGuire, M.I., McGruire, M.A., Bode, L. (eds.) Prebiotics and probiotics in human milk, pp. 45–139. Academic Press, Amsterdam (2016)
Kobata, A.: Structures and application of oligosaccharides in human milk. Proc. Jpn. Acad. B. 86, 1–7 (2010)
Bell, K.M., Rutherfurd, S.M., Cottam, Y.H., Hendriks, W.H.: Evaluation of two milk replacers fed to hand-reared cheetah cubs (Acinonyx jubatus): nutrient composition, apparent total tract digestibility, and comparison to maternal cheetah milk. Zoo Biology. 29, 1–15 (2010). https://doi.org/10.1002/zoo.20344
Bell, K.M., Rutherfurd, S.M., Morton, R.H.: Growth rates and energy intake of hand-reared cheetah cubs (Acinonyx jubatus) in South Africa. J. Anim. Physiol. Anim. Nutr. 96, 182–190 (2012). https://doi.org/10.1111/j.1439-0396.2011.01133.x
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
American black bear miks were collected In 1984 by, and according to research protocols of, the Pennsylvania Game Commission (Oftedal et al. [22]). The cheetah samples were collected for purposes other than research (e.g. veterinary diagnostics) prior to this study commencing. Samples were subsequently made available to the researchers on an opportunistic basis and ethical review undertaken by the relevant groups determined the study to be low risk.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PPTX 1.29 mb)
Rights and permissions
About this article
Cite this article
Urashima, T., Umewaki, M., Taufik, E. et al. Chemical structures of oligosaccharides in milks of the American black bear (Ursus americanus americanus) and cheetah (Acinonyx jubatus). Glycoconj J 37, 57–76 (2020). https://doi.org/10.1007/s10719-019-09899-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-019-09899-7