Abstract
Mammalian milk/colostrum usually contains oligosaccharides along with the predominant disaccharide lactose. It has been found that the number and identity of these milk oligosaccharides varies among mammalian species. Oligosaccharides predominate over lactose in the milk/colostrum of Arctoidea species (Carnivora), whereas lactose predominates over milk oligosaccharides in Artiodactyla including cow, sheep, goat, camel, reindeer and pig. To clarify whether heterogeneity of a variety of milk oligosaccharides is found within other species of Artiodactyla, they were studied in the milk of giraffe, sitatunga, deer and water buffalo. The following oligosaccharides were found: Neu5Ac(α2–3)[GalNAc(β1–4)]Gal(β1–4)Glc (GM2 tetrasaccharide), and Gal(α1–3)Gal(β1–4)Glc (isoglobotriose) in giraffe milk; Neu5Ac(α2–3)Gal(β1–4)Glc (3’-SL), Neu5Ac(α2–6)Gal(β1–4)Glc (6’-SL), Gal(α1–4)Gal(β1–4)Glc (globotriose) and isoglobotriose in sitatunga colostrum; Gal(β1–3)Gal(β1–4)Glc (3′-GL), Gal(β1–6)Gal(β1–4)Glc (6′-GL), isoglobotriose, Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc (lacto-N-neotetraose, LNnT), Gal(β1–4)Glc-3’-O-SO3 (3’-O-lactose sulphate) in deer milk; 3′-GL, isoglobotriose and Gal(β1–3)Gal(β1–3)Gal(β1–4)Glc (3′,3″-digalactosyllactose, DGL) in water buffalo colostrum. Thus it was shown that the milk oligosaccharides are heterogeneous among these Artiodactyla species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammalian milk or colostrum usually contains lactose (Gal(β1–4)Glc) as the predominant carbohydrate as well as lower amounts of a variety of oligosaccharides, most of which contain a lactose unit at their reducing ends [1,2,3]. The monosaccharide units contained in these milk oligosaccharides include N-acetylglucosamine (GlcNAc), galactose (Gal), N-acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), N-acetylgalactosamine (GalNAc) and fucose (Fuc). However, the amounts and type of these oligosaccharides vary between mammalian species.
For example, human milk contains 12 ~ 13 g/L of milk oligosaccharides, which constitutes 20% of its carbohydrate fraction along with 80% of lactose. More than 240 of these oligosaccharides have been separated from human milk or colostrum; 162 of their chemical structures have been characterized to date [4]. These oligosaccharides have been grouped into 19 series based on their core structures. Neutral oligosaccharides, especially fucosyl oligosaccharides, predominate over acidic oligosaccharides in human milk or colostrum.
It is generally believed that human milk oligosaccharides function as prebiotics that stimulate the growth of beneficial bacteria in the infant colon, and as decoy receptors that inhibit the attachment of pathogenic microorganisms to the colonic mucosa. In addition, small amounts are absorbed into the circulation and may act as immune modulators [5].
It has been found that several Arctoidea species of Carnivora, other than house dog, are exceptional among eutherians in that milk oligosaccharides predominate over lactose in their milk or colostrum. [1, 6]. These oligosaccharides have been characterized in the milk or colostrum of dog, striped skunk, mink, raccoon, whitenosed coati, bears, seals, spotted hyena, African lion and spotted hyena [1, 6].
Within Artiodactyla species, the oligosaccharides have been characterized in the milk/colostrum of cows [1, 7,8,9,10,11], sheep [1, 11,12,13,14], goats [1, 11, 15, 16], camels [1, 11, 17], pigs [1, 11], reindeer [1, 18] and addax [19]. In these species the milk/colostrum contains lactose as the predominant carbohydrate: this situation differs from that in the Arctoidea, in which oligosaccharides predominate. A major ratio of milk oligosaccharides have a lactose core unit, whereas a minor ratio of them have lacto-N-neotetraose (Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc), lacto-N-novopentaose I (Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc) or lacto-N-neohexaose (Gal(β1–4)GlcNAc(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc) core units [1].
In this study, the chemical structures of the oligosaccharides were characterized in the milk or colostrum of the following Artiodactyla species: giraffe (Giraffa camelopardalis), sitatunga (Tragelaphus spekii), deer (Cervus nippon yesoensis) and water buffalo (Bubalus bubalis) We have compared the features of the chemical structures of Artiodactyla milk oligosaccharides with those of Arctoidea and discuss their heterogeneity as well as their homology.
Materials and methods
Materials
The giraffe milk samples (total 77 mL) were collected from a lactating female bred at Kyoto city zoo, Kyoto, Japan, during the whole lactating period from August 2016 – July 2017. The sitatunga colostrum sample (10 mL) was collected from a lactating female at 1 day postpartum in April, 2017. This animal had been bred at a local zoo named Himeji Central Park, in Himeji, Japan. The deer milk samples (32 mL) were collected from several lactating slaughtered females in May – August, 2017, at a local deer farm named Shiretoko Ezoshika Farm, Shari, east Hokkaido, Japan. The water buffalo colostrum sample (100 mL) was collected from a 1st parity lactating female in Deli Serdang District, North Sumatra Province, Sumatra Island, Indonesia, on February 13th, 2017 at 09.00 AM about 2 h after parturition.
Isolation of milk oligosaccharides and lactose
The above volumes of the milk/colostrum samples were each thawed and extracted with four volumes of chloroform/methanol (2:1, v/v). The emulsion was centrifuged at 4 °C and 5000 rpm for 30 min, and the lower chloroform layer and the denatured proteins were discarded. Methanol was removed from the upper layer by rotary evaporation, and the residue was dissolved in 5 ml water, followed by the addition of three volumes of ethanol. The solution was kept overnight at 4 °C and the resulting precipitant was removed by centrifugation at 4 °C and 5000 rpm for 30 min. The ethanol was removed by rotary evaporation and the residue was dissolved in 5 ml water, followed by freeze-drying. The resulting white powder represented the carbohydrate fraction. The carbohydrate fractions from milk /colostrum of giraffe, sitatunga, deer and water buffalo were named as the fraction SCG, SC, D and B, respectively.
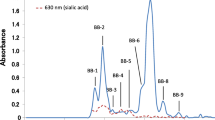
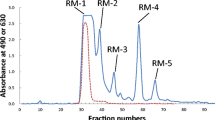
The carbohydrate fraction of each sample was dissolved in 2 mL of water and the solution passed through a BioGel P-2 (<45 μm, Bio Rad Laboratories, Hercules, CA, USA) column (2.5 × 100 cm) at a flow rate of 0.25 mL/min. The elution solvent was distilled water. Fractions of 5 mL were collected and aliquots of each fraction were analyzed for hexose by the phenol-sulfuric acid method [20] at 490 nm and for sialic acid by the periodate-resorcinol method [21] at 630 nm. Peak fractions were pooled and freeze-dried. Figure 1a–d show the elution profiles of the carbohydrate fractions SCG, SC, D and B extracted from these milk or colostrum samples. The saccharides in the peak fractions SCG-4, SC-4, D-5 and B-4 (see Fig. 1) were checked by thin layer chromatography using acetone/2-propanol/0.1 mol lactic acid (2:2:1, v/v/v) as a developing solvent (chromatogram in Supplementary Fig. 1). Detection of the spots was done by spraying with 5% H2SO4 in ethanol and heating. The saccharides in the peak fractions SC-3 and D-4 (see Fig. 1b, c) were subjected to proton nuclear magnetic resonance (1H-NMR) spectroscopy to determine their chemical structures.
Gel chromatograms of the carbohydrate fractions from (a) giraffe milk, (b) sitatunga colostrum, (c) deer milk and (d) water buffalo colostrum on a BioGel P-2 column (2.5 × 100 cm). Elution was done with distilled water at a flow rate of 0.25 mL/min and fractions of 5.0 mL were collected. Each fraction was monitored by the phenol-H2SO4 method at 490 nm (solid line) and the periodate-resorcinol method at 630 nm (dotted line)
The components in SC-1, D-2, B-2 and B-3 (see Fig. 1b–d) were further separated by high-performance liquid chromatography (HPLC) on a TSK gel Amide-80 column (4.6 × 250 mm, pore size 80 Å, particle size 5 μm; Tosoh, Tokyo, Japan) using LC-10ATVP pump (Shimadzu, Tokyo, Japan). As for the components in SCG-1, SCG-2 and SCG-3 (See Fig. 1a) were separated by HPLC on a TSK gel Amide-80HR column (4.6 × 250 mm, pore size 80 Å, particle size 5 μm; Tosoh, Tokyo, Japan) using a L-2130 pump (Hitachi, Tokyo, Japan). Both HPLC were done by using mobile phase 80% and 50% (v/v) acetonitrile (CH3CN) in 15 mmol/L potassium phosphate buffer (pH 5.2) denoted as buffer A and buffer B, respectively. Elution was done using linear gradient of acetonitrile from buffer A to buffer B with the following condition: buffer A = 100%, B = 0% at 0 min.; A = 50%, B = 50% at 15 min.; and A = 0%, B = 100% at 80 min. at 60 °C and flow rate of 1 mL/min.
The eluates were monitored by measuring the absorbance at 195 nm. The peak fractions of oligosaccharides were pooled, concentrated by rotary evaporation, and subjected to 1H-NMR to determine the structures.
1H-NMR spectroscopy
Nuclear magnetic resonance spectra were recorded in D2O (99.96 atom D%; Sigma-Aldrich, Milwaukee, WI) at 500 or 600 MHz for 1H-NMR with a JEOL ECP-500 Fourier transform-NMR (Jeol, Tokyo, Japan) or a Varian INOVA 600 spectrometer (Varian Inc., Palo Alto, CA) operated at 293.1 K. Chemical shifts are expressed as change relative to internal 3-(trimethyl)-1-propane sulfuric acid, sodium salt, but measured by reference to internal acetone (δ = 2.225).
Mass spectrometry
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was performed using an Autoflex II TOF/TOF mass spectrometer (Brucker Daltonics, Bremen, Germany). Lyophilized oligosaccharide fractions were dissolved in 1 μL of milli-Q water. The oligosaccharide solution was mixed with an equal volume of 10 mg/mL. SDHB (Brucker Daltonics), which is a mixture of 2,5-dihydrobenzoic acid and 2-hydroxy-5-methoxybenzoic acid, saturated in milli-Q water, spotted on a MTP 384 target plate ground steel TF (Bruker Daltnics), and dried. Mass spectra were obtained using a pre-installed method, RP_0–2 kDa (a reflector positive ion mode focusing on the mass range up to 2 kDa). Peptide calibration standard II (Bruker Daltonics) was used for external calibration of the mass spectrometer.
Results
Separation of oligosaccharides from milk or colostrum of giraffe, sitatunga, deer and water buffalo
Gel filtrations
The carbohydrate fractions extracted from the milk or colostrum were separated by gel filtration on BioGel P-2 as shown in Fig. 1. The fraction from giraffe milk separated into four peaks, designated as SCG-1 to SCG-4 (Fig. 1a), while that from sitatunga colostrum separated into four peaks, designated as SC-1 to SC-4 (Fig. 1b). The peak fractions from deer milk were pooled as D-1 to D-5 as in Fig. 1c, while those from water buffalo colostrum were pooled as B-1 to B-4 as in Fig. 1d. It was assumed that only the fractions SCG-1 (giraffe), SC-1 (sitatunga), D-1 and D-2 (deer), and B-1 and B-2 (water buffalo) contained sialyl saccharides, because these reacted positively with periodate – resorcinol. It was shown by thin layer chromatography that each of the large peaks SCG-4 (giraffe), SC-4 (sitatunga), D-5 (deer) and B-4 (water buffalo) contained a disaccharide, lactose (see Supplementary Fig. 1).
Normal phase high performance liquid chromatography
The components in SCG-1, SCG-2 and SCG-3 from giraffe milk separated into several peaks by normal phase HPLC as shown in Fig. 2. The components in SC-1 from sitatunga colostrum separated into several peaks by HPLC as shown in Fig. 3a, while those in D-2 from deer milk separated as in Fig. 3b. The components in D-1 and D-3 (deer) were not separated by the HPLC, as the amounts in these fractions were too small. The components in B-2 and B-3 from water buffalo colostrum separated into the peaks as in Fig. 4a, b, respectively. The components in B-1 were not separated by HPLC, because of the small amount in this fraction.
High performance liquid chromatograms of the fractions (a) SCG-1, (b) SCG-2, and (3) SCG-3 separated from the carbohydrate fraction of giraffe milk by gel chromatography (see Fig. 1a). The high performance liquid chromatography was done using a L-2130 pump (Hitachi, Tokyo, Japan) on a TSK gel Amide-80HR column (4.6 × 250 mm, pore size 80 Å, particle size 5 μm; Tosoh, Tokyo, Japan). The HPLC were done by using mobile phase 80% and 50% (v/v) acetonitrile (CH3CN) in 15 mmol/L potassium phosphate buffer (pH 5.2) denoted as buffer A and buffer B, respectively. Elution was done using linear gradient of acetonitrile from buffer A to buffer B with the following condition: buffer A = 100%, B = 0% at 0 min.; A = 50%, B = 50% at 15 min.; and A = 0%, B = 100% at 80 min. at a 60 °C and flow rate of 1 mL/min
High performance liquid chromatograms of the fractions (a) SC-1 and (b) D-2 separated from the carbohydrate fractions of sitatunga colostrum and deer milk, respectively, by gel chromatography (see Fig. 1b, c). The high performance liquid chromatography was done using Shimadzu LC-10 ATVP pump (Shimadzu, Tokyo, Japan) on a TSK-gel amide-80 column (4.6 × 250 mm, pore size 80 Å, particle size 5 μm; Tosoh, Tokyo, Japan). The elution was done by similar condition as Fig. 2
Characterization of the oligosaccharides
The oligosaccharides in the separated fractions were characterized by 1H-NMR and MALDI-TOFMS, as follows.
Giraffe
SCG-3_6
The oligosaccharide in fraction SCG-3_6 was characterized by comparison of its 1H-NMR spectrum with that of authentic isoglobotriose. The spectrum (chemical shifts in Table 1) had the H-1 shifts of Glc α, α(1–3) linked Gal, Glc β and β(1–4) linked Gal at δ 5.225, 5.146, 4.668 and 4.525, respectively. It had the H-4 shift of β(1–4) linked Gal, which was substituted by α(1–3) linked Gal, at δ 4.185 and H-5 of α(1–3) linked Gal at δ 4.197. As these were similar to those of authentic isoglobotriose, the saccharide in this fraction was identified to be Gal(α1–3)Gal(β1–4)Glc.
SCG-1_10
The oligosaccharide in fraction SCG-1_10 was characterized by comparison of the 1H-NMR spectrum with that of the published data [22] for GM2 tetrasaccharide and its MALDI-TOFMS spectrum. The 1H-NMR spectrum (chemical shifts in Table 1, spectrum in Supplementary Fig. 2) had the characteristic H-3 axial triplet and H-3 equatorial doublet doublet shifts at δ 1.926 and 2.657 of sialic acid, respectively; these chemical shifts were different from the H-3 axial and equatorial shifts of both Neu5Ac(α2–3)Gal(β1–4)Glc and Neu5Ac(α2–6)Gal(β1–4)Glc. The spectrum had the H-3 doublet doublet shift of β(1–4) linked Gal, which was substituted by α(2–3) linked sialic acid at δ 4.150, showing the presence of a Sia(α2–3)Gal(β1–4) unit. The spectrum had the characteristic H-4 shift of β(1–4) linked Gal at δ 4.117, and NAc shifts of Neu5Ac and GalNAc at δ 2.031 and 2.013, respectively. As all these shifts were also found in the published data for the 1H-NMR of GM2 tetrasaccharide, which had been separated from bottlenose dolphin milk, it was considered that the oligosaccharide in this fraction could be GM2 tetrasaccharide. However, because of the overlapping with HDO shift, the anomeric shifts of the components were not clearly resolved. Therefore a MALDI-TOFMS spectrum of the fraction was obtained. The [M + Na]+ and [M + K]+ ions at 859.298 and 875.254 (mass spectrum in Supplementary Fig. 3) indicated a monosaccharide composition corresponding to Hex2HexNAc2Neu5Ac1. From these observations, the saccharide in this fraction was identified to be Neu5Ac(α2–3)[GalNAc(β1–4)]Gal(β1–4)Glc.
The 1H-NMR spectrum indicated that the other components in the separated HPLC peaks (Fig. 2) were not saccharides.
Sitatunga
SC-3
The spectrum (chemical shifts in Table 2, spectrum in Supplementary Fig. 4) of SC-3 showed that this fraction contained two oligosaccharides, major and minor. The spectrum had the H-1 shifts, arising from a major saccharide, of Glc α, α(1–3) lined Gal, Glc β and β(1–4) linked Gal at δ 5.224, 5.146, 4.669 and 4.524, respectively, and H-4 shift of β(1–4) linked Gal at δ 4.184 and H-5 shift of α(1–3) linked Gal at δ 4.197. As these are similar to those of authentic isoglobotriose, one saccharide (SC-3_1) in this fraction was identified to be Gal(α1–3)Gal(β1–4)Glc. The spectrum had the H-1 shifts, that arose from a minor saccharide, of α(1–4) linked Gal, and β(1–4) linked Gal at δ 4.947 and 4.510, respectively and H-5 of α(1–4) linked Gal at δ 4.371. As these are essentially similar to those of authentic globotriose and the published data [22], the minor saccharide (SC-3_2) was characterized to be Gal(α1–4)Gal(β1–4)Glc. Based on comparison of the intensity of the chemical shifts at δ 5.146 and δ 4.947, it was estimated that the ratio of isoglobotriose to globotriose in this fraction was 6:1. In addition, it was shown that this fraction contained a small quantity of nucleotides, because the spectrum had shifts at δ 7.9, 5.99 and 5.98.
SC-1_1
The spectrum (chemical shifts in Table 2, spectrum in Supplementary Fig. 5) had the H-1 shifts of Glc α, Glc β, and β(1–4) linked Gal at δ 5.222, 4.664 and 4.531, respectively, and H-3 axial, H-3 equatorial and NAc shifts of α(2–3) linked Neu5Ac at δ 1.800, 2.756 and 2.029, respectively, and H-3 shift of β(1–4) linked Gal, which was substituted by α(2–3) linked Neu5Ac, at δ 4.116. As these are similar to those of authentic 3’-SL, it was thought that this fraction contained 3’-SL. However, the spectrum had an additional shift at δ 4.452. As we suspected that this shift might have been caused by an additional H-1 shift of this component, a MALDI-TOFMS spectrum was obtained (mass spectrum in Supplementary Fig. 6). As the [M + Na]+ and [M + K]+ ions at 656.187 and 672.153, respectively, showed the monosaccharide composition to be Hex2Neu5Ac1, the saccharide in this fraction was characterized to be Neu5Ac(α2–3)Gal(β1–4)Glc. It was concluded that the shift at δ 4.452 was due to a contaminant.
SC-1_2
The 1H-NMR spectrum (chemical shifts in Table 2) had the H-1 shifts at δ 5.224, 4.669 and 4.427 of Glc α, Glc β and β(1–4) linked Gal, respectively, and H-3 axial, H-3 equatorial and NAc shifts of α(2–6) linked Neu5Ac at, δ 1.745, 2.712 and 2.028, respectively. As these were similar to authentic 6’-SL, the oligosaccharide in this fraction was characterized to be Neu5Ac(α2–6)Gal(β1–4)Glc.
As the 1H-NMR spectra of other fractions separated by HPLC were not clearly resolved, these were not characterized in this study.
Deer
D-4
Some oligosaccharides in this fraction were characterized based on the H-1 and other chemical shifts in its 1H-NMR spectrum. The spectrum (chemical shifts in Table 3, spectrum in Supplementary Fig. 7) had the H-1 shifts of Glc α and Glc β of all oligosaccharides at δ 5.224 and 4.667 in this fraction. The spectrum had the characteristic down field shifts of H-3 doublet doublet and H-4 doublet of β(1–4) linked Gal at δ 4.335 and 4.293, respectively, and H-1 shift of this residue at δ 4.568. As this pattern was similar to those of the published data [23] for 3’-O-lactose sulphate, separated from dog milk, the first saccharide (D-4_1) in this fraction was characterized to be Gal(β1–4)Glc-3’-O-sulphate. The spectrum had the characteristic H-1 and H-5 of α(1–3) linked Gal at δ 5.145 and 4.193, respectively. As these are similar to that of authentic isoglobotriose, the second saccharide (D-4_2) was characterized to be Gal(α1–3)Gal(β1–4)Glc. In addition, the spectrum had the H-1 of β(1–3) and β(1–4) linked Gals at δ 4.612 and 4.511, respectively, which would have arisen from 3′-GL [15], and H-1 of β(1–4) and β(1–6) linked Gals at δ 4.459 and 4.486, respectively, which should from 6′-GL [15]; these indicated that the structure of the third saccharide (D-4_3) was Gal(β1–3)Gal(β1–4)Glc and that of the fourth saccharide (D-4_4) Gal(β1–6)Gal(β1–4)Glc.
D-2_10, D-2_11
The 1H-NMR spectra of D-2_10 and D-2_11 were essentially similar to each other. The 1H-NMR spectrum of D-2_10 (chemical shifts in Table 3) had the H-1 shifts of Glc α, β(1–3) linked GlcNAc, Glc β, and two β(1–4) linked Gal at δ 5.221, 4.705 and 4.701, 4.664, and 4.480 and 4.438, respectively. The spectrum also had H-4 shift of β(1–4) linked Gal, which was substituted by β(1–3) linked GlcNAc, at δ 4.157 and NAc shift of β(1–3) linked GlcNAc at δ 2.033. As these are similar to those of the authentic lacto-N-neotetraose, the oligosaccharides in these fractions were identified to be Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc.
D-2_3
The 1H-NMR spectrum had the shifts at δ 7.959, 5.984, 5.876, 5.970 and 5.967, which arose from uracil, the shifts at δ 4.373, 4.365 and 4.359 from ribose, and the H-1 shifts of hexose or N-acetylhexosamine at δ δ 5.510, 5.445 and 5.421. These observations showed the presence of sugar nucleotides which were not identified.
The components in the other peaks could not been characterized, because of low resolution of the chemical shifts in their 1H-NMR spectra.
Water buffalo
B-3_8
The 1H-NMR spectrum (chemical shifts in Table 4) had the H-1 shifts of Glc α, Glc β, β(1–3) linked Gal, and β(1–4) linked Gal at δ 5.226, 4.668, 4.615 and 4.613, and 4.511, respectively, and H-4 of β(1–4) linked Gal, which was substituted by β(1–3) linked Gal, at δ 4.198. As these are essentially similar to those of authentic 3′-GL and its published data [15], the saccharide in the fraction B-3_8 was characterized to be Gal(β1–3)Gal(β1–4)Glc.
B-3_7
The 1H-NMR spectrum (chemical shifts in Table 4) showed that this fraction contained two oligosaccharides. The spectrum had the H-1 shifts of Glc α, Glc β, β(1–3) linked Gal, and β(1–4) linked Gal at δ 5.225, 4.668, 4.615 and 4.614, and 4.512, respectively, and H-4 of β(1–4) linked Gal, which was substituted by β(1–3) linked Gal, at δ 4.198. As these are essentially similar to those of authentic 3′-GL and its published data [15], one saccharide (B-3_7–1) in this fraction was characterized to be Gal(β1–3)Gal(β1–4)Glc.
The spectrum also had the H-1 shifts of α(1–3) linked Gal, Glc β, and β(1–4) linked Gal at δ 5.145, 4.664, and 5.524, respectively. The spectrum had the H-5 shift of α(1–3) linked Gal at δ 4.198, which was overlapped by that of H-4 of β(1–4) linked Gal of B-3_7–1, and H-4 of β(1–4) linked Gal, which was substituted by α(1–3) linked Gal, at δ 4.187. As these data are essentially similar to those for authentic isoglobotriose, the second saccharide (B-3_7–2) in the fraction was characterized to be Gal(α1–3)Gal(β1–4)Glc.
B-3_1 and B-3_2
From the absence of α anomeric shifts of reducing ends, it was concluded that these fractions did not contain free saccharides.
B-3_3
The 1H-NMR spectrum had the characteristic doublet doublet shift of H-1 shift at δ 5.604. This suggested that this fraction contained a phosphorylated saccharide, but this component was not characterized because of absence of a suitable reference compound for the 1H-NMR spectrum.
B-3_5
The 1H-NMR had the anomeric shifts at δ 5.225, 5.207, 4.751, 4.667, 4.651 and 4.452, and NAc shift of N-acetylhexosamine at δ 2.063. However, the saccharide in this fraction could not been characterized because of the absence of a suitable reference compound.
B-2_3
As the 1H-NMR spectrum was similar to that of authentic Neu5Ac, the saccharide in this fraction was identified to be free N-acetylneuraminic acid.
B-2_8, B-2_10, B-2_11 and B-2_12
The 1H-NMR spectra of B-2_8, B-2_10, B-2_11 and B-2_12 were essentially similar to each other. The 1H-NMR spectrum of B-2_10 (chemical shifts in Table 4, spectrum in Supplementary Fig. 8) had the H-1 shifts of Glc α, β(1–3) linked internal Gal, Glc β, β(1–3) linked external Gal and β(1–4) linked Gal at δ 5.226, 4.679, 4.668, 4.620 and 4.512, respectively, and H-4 of β(1–3) and β(1–4) linked Gal, which were substituted by β(1–3) linked Gal, at δ 4.202, and 4.196, respectively. As these were essentially similar to those of the published data [24] for 3′, 3″-digalactosyllactose, separated from brushtail possum milk, the saccharides in these fractions were characterized to be Gal(β1–3)Gal(β1–3)Gal(β1–4)Glc.
The components of the other peak fractions were not characterized because the 1H-NMR did not show clearly resolved chemical shifts.
Discussion
In this study the disaccharide lactose was found to be the predominant saccharide in the milk or colostrum of giraffe, sitatunga, deer and water buffalo, as shown by the thin layer chromatography of the contents of the large peaks obtained during gel filtration of their carbohydrate fractions (Fig. 1). The ratio of the yields of milk oligosaccharides to lactose in these Artiodactyla species milk or colostrum was determined by estimation of peak areas in Fig. 1, as follows; 1:12 in giraffe milk, 1:4.2 in sitatunga colostrum, 1:7 in deer milk, 1:5 in water buffalo colostrum. Oligosaccharides were present only at low levels, especially in giraffe milk. Previous studies had similarly shown that lactose is the predominant saccharide in the milk/colostrum of several species of Artiodactyla including cow [11], sheep [11], goat [11], camel [11, 17], reindeer [18] and pig [11]. This is in contrast to Carnivora, in which oligosaccharides predominate over lactose in the milk/colostrum of raccoon [25], bears [26,27,28], seals [29,30,31], mink [32], striped skunk [33] and whitenosed coati [34].
The neutral as well as acidic oligosaccharides in the milk or colostrum of giraffe, sitatunga, deer and water buffalo characterized in this study are summarized in Table 5. Isoglobotriose was found in all 4 of these Artiodactyla milks or colostrum. As this trisaccharde has been identified in milk/colostrum of many Artiodactyla species including cow [9,10,11], goat [11, 15], sheep [11, 14], dromedary camel [11], and pig [11], it can be concluded that most or all Artiodactyla milk/colostrum contain isoglobotriose. This trisaccharide has also been shown to be present in the milk or colostrum of many Carnivora species including mink [32], striped skunk [33], whitenose coati [34], three species of bears [26,27,28], giant panda [35], spotted hyena [36] and clouded leopard [37], but has not identified in some other Carnivora such as dog [23], raccoon [25], hooded and bearded seals [29, 31], and lion [37].
Globotriose was identified in sitatunga colostrum. Bottlenose dolphin is the only other species whose milk contains this trisaccharide, among the mammalian species characterized to date [22]. Evidently this trisaccharide is present only in the milk/colostrum of specific species, in contrast to isoglobotriose.
β3’-Galactosyllactose (3’-GL) was identified in the milk or colostrum of deer and water buffalo, while 3′,3″-digalactosyllactose (DGL) was found only in water buffalo milk. The trisaccharide has previously been identified in the milk or colostrum of cow [8, 9, 11], sheep [11, 14], goat [11, 15], camel [11, 17], reindeer [18] and pig [11]. These galactosyllactoses are characteristically prominent in the milk of marsupials, [24, 38,39,40,41,42,43], and it is noteworthy that the detection of DGL in water buffalo colostrum is the first finding in the milk/colostrum of species other than marsupials. β6′-galactosyllactose (6’-GL) was identified in deer milk in this study. This trisaccharide had also been found in the milk/colostrum of cow [8, 9, 11], sheep [11, 14], goat [11, 15], camel [11, 17], reindeer [18] and pig [11].
Other than lacto-N-neotetraose (LNnT) in deer milk, all milk oligosaccharides described in this study contain lactose as a core unit. However, LNnT or its derivatives have been identified in milk or colostrum of cow [10, 11], sheep [11], goat [11], camel [11], reindeer [18] and pig [11]. Some milk oligosaccharides of cow [10, 11], sheep [11], goat [11], camel [11, 17] and pig [11] contain lacto-N-neohexaose (Gal(β1–4)GlcNAc(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc) and lacto-N-novopentaose 1 (Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc) and their derivatives. Although these were not identified in this study, it is possible that they were present at very low levels.
Lactose-3’-O-sulphate was identified only in deer milk. This saccharide had not been previously found in milk or colostrum of cow [10, 11], sheep [11,12,13], goat [11, 16], camel [11, 17] and pig [11], but its presence was recently described in bovine colostrum [44]. However, it has been identified in milk or colostrum of dog [23], Corquel’s sifaka [45], and Hamadryas baboon [46].
3’-SL and 6’-SL, which were identified in sitatunga colostrum, have been found in milk or colostrum of Artiodactyla species including cow [10, 11], goat [11, 16], sheep [11], camel [11, 17] and pig [11] and of bottlenose dolphin [22] and some cetacean species [47], which are also closely related to Artiodactyla. Based on the peak area in the HPLC, it is likely that the concentration of 3’-SL in sitatunga colostrum is similar to that of 6’-SL. 3’-SL predominates over 6’-SL in milk or colostrum among most of the Artiodactyla and cetaceans [1, 2], while 6’-SL predominates over 3’-SL in caprine colostrum [1, 2, 16].
GM2 tetrasaccharide, which was identified in giraffe milk, had previously been found in the milk of bottlenose dolphin [22] and rhesus macaque [46]. It is likely that this oligosaccharide is found in milk and colostrum of only specific species since, phylogenetically, these three species are not at all closely related.
Neu5Gc containing oligosaccharides were not identified in our Artiodactyla samples. It has been shown that among Artiodactyla the sheep is the only species in which Neu5Gc containing milk oligosaccharides predominate over those containing Neu5Ac [11,12,13].
Summarising, it would appear that the presence of 3’-SL, 6’-SL, 3′-GL, 6′-GL and isoglobotriose is common in the milk/colostrum of not only Artiodactyla species but also of other eutherians, while GM2 tetrasaccharide, globotriose, DGL and 3’-O-lactose sulphate are more species specific. Evidently there is a diversity of milk oligosaccharides even within the Artiodactyla.
Our structural characterizations indicate that the average molecular size of the milk oligosaccharides in these Artiodactyla is small, with trisaccharides predominating over larger saccharides. This might be due to the lack or low activity of β3N-acetylglucosaminyltransferase, an enzyme which catalyses elongation of oligosaccharides, within the lactating mammary glands.
It has been shown that among many Carnivora species their milk oligosaccharides contain A (GalNAc(α1–3)[Fuc(α1–2)]Gal), B (Gal(α1–3)[Fuc(α1–2)]Gal) or H (Fuc(α1–2)Gal) antigens at their non reducing ends [25,26,27,28,29,30,31,32,33,34,35,36,37], whereas these were not found in our Artiodactyla milks. The milk/colostrum of other Artiodactyla including cow [10], sheep [11], goat [11, 15], camel [11] and pig [11] contain only trace amounts of 2’-FL (Fuc(α1–2)Gal(β1–4)Glc), which is a precursor of A or B tetrasaccharides, while the tetrasaccharide, (GalNAc(α1–3)[Fuc(α1–2)]Gal(β1–4)Glc), has been identified in bovine colostrum in only two cases [10, 11]. This appears to be a significant difference between the milk oligosaccharides of Artiodactyla and Carnivora.
It is well known that the glycolsyltransferases which are involved in the biosynthesis of A or B antigens transfer GalNAc or Gal from UDP-GalNAc or UDP-Gal, respectively, to the potential acceptors of H antigen containing glycolconjugates such as 2’-FL or LNFP-1 (Fuc(α1–2)Gal(β1–3)GlcNAc(β1–3)Gal(β1–4)Glc). It has been shown that milk from human non secretor donors, which comprise 15% of lactating women, do not contain 2’-FL, LNFP-1 or LNDFH-I (Fuc(α1–2)Gal(β1–3)[Fuc(α1–4)]GlcNAc(β1–3)Gal(β1–4)Glc); this is due to the absence of the activity of α2 fucosyltransferase (FUT 2), which transfers Fuc from GDP-Fuc to Gal(β1–4)GlcNAc (N-acetyllactosamine) or lactose, in the lactating mammary glands of these women. When Kobata et al. performed the glycosyltransferase reaction using non secretor women’s milk as the enzyme source and with UDP-GalNAc or UDP-Gal as a donor and 2’-FL or LNFP-1 as an acceptor, the formation of A antigen containing oligosaccharide was observed as the reaction product using A blood group milk, while B antigen containing oligosaccharide was obtained with B blood group milk [48]. It can be hypothesized that A or B antigen containing oligosaccharides, which are present in the milks of many varieties of carnivorous mammals, are biosynthesized by A or B transferase with H antigen containing oligosaccharides as precursors, in their lactating mammary glands. However, in this study, no A tetrasaccharide or B tetrasaccharide or 2’-FL was found in the milk or colostrum of four Artiodactyla species. It can be assumed that these absences are due to lack of FUT2 activity on their lactating mammary glands as in the case of human non secretor women.
It is generally accepted, as mentioned in the Introduction, that milk oligosaccharides have biological significance for human neonates insofar as they act as prebiotics and as decoy receptors and immuno modulators [5]. These functions are probably important in other species as well, but the extent may depend on the maturity of their neonates. Most Artiodactyla are relatively precocious at birth in comparison with newborn humans and Carnivora, and therefore their milk oligosaccharides are likely to be less significant in terms of function. The finding that the milk and colostrum oligosaccharides of Artiodactyla are less abundant and less varied than those of other species is consistent with this hypothesis.
One should note that a limitation of this study is that it was done with only a small number of milk or colostrum samples. To further clarify the features of milk oligosaccharides of the Artiodactyla, including the above-mentioned four species, additional studies should be performed with a greater number of samples.
References
Urashima, T., Messer, M., Oftedal, O.T.: Oligosaccharides in milk of other mammals. In: McGuire, M., McGuire, M., Bode, L. (eds.) Prebiotics and Probiotics in Human Milk, pp. 45–139. Academic Press, Amsterdam (2016)
Jenness, R.E., Regehr, E.A., Sloan, R.E.: Comparative studies of milks. II. Dialyzable carbohydrates. Comp. Biochem. Physiol. 13, 339–352 (1964)
Urashima, T., Saito, T., Nakamura, T., Messer, M.: Oligosaccharides of milk and colostrum in non-human mammals. Glyconj. J. 18, 357–371 (2001)
Urashima, T., Hirabayashi, J., Sato, S., Kobata, A.: Human milk oligosaccharides as essential tools for basic and application studies on galectins. Trends Glycosci. Glycotechnol. 30, SE51–SE65 (2018)
Bode, L.: Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 22, 1147–1162 (2012)
Urashima, T., Messer, M., Oftedal, O.T.: Comparative biochemistry and evolution of milk oligosaccharides of monotremes, marsupials, and eutherians. In: Pontarotti, P. (ed.) Evolutionary Biology: Genome Evolution, Speciation, Coevolution and Origin of Life, pp. 3–33. Springer, Cham (2014)
Saito, T., Itoh, T., Adachi, S.: Presence of two neutral disaccharides containing N-acetylhexosamine in bovine colostrum as free forms. Biochim. Biophys. Acta. 801, 147–150 (1984)
Saito, T., Itoh, T., Adachi, S.: Chemical structure of three neutral trisaccharides isolated in free forms from bovine colostrum. Carbohydr. Res. 165, 43–51 (1987)
Urashima, T., Saito, T., Ohmisya, K., Shimazaki, K.: Structural determination of three neutral oligosaccharides in bovine (Holstein-Friesian) colostrum, including the novel trisaccharide, GalNAcα1-3Galβ1-4Glc. Biochim. Biophys. Acta. 1073, 225–229 (1991)
Marino, K., Lane, J.A., Abrahams, J.L., Struwe, W.B., Harvey, D.J., Morotta, M., Hickey, R.M., Rudd, P.M.: Method for milk oligosaccharide profiling by 2-aminobenzamide labeling and hydrophilic interaction chromatography. Glycobiology. 21, 1317–1330 (2011)
Albrecht, S., Lane, J.A., Marino, K., Al Busadah, K.A., Carrington, S.D., Hickey, R.M., Rudd, P.M.: A comparative study of free oligosaccharides in the milk of domestic animals. Br. J. Nutr. 111, 1313–1328 (2014)
Nakamura, T., Urashima, T., Nakagawa, M., Saito, T.: Sialyllactose occurs as free lactones in ovine colostrum. Biochim. Biophys. Acta. 1381, 286–292 (1998)
Sasaki, M., Nakamura, T., Hirayama, K., Fukuda, K., Saito, T., Urashima, T., Asakuma, S.: Characterization of two novel sialyl N-acetyllactosaminyl nucleotides separated from ovine colostrum. Glycoconj. J. 33, 789–796 (2016)
Urashima, T., Saito, T., Nishimura, J., Ariga, H.: New galactosyllactose containing α-glycosidic linkage isolated from ovine (Booroola dorset) colostrum. Biochim. Biophys. Acta. 992, 375–378 (1989)
Urashima, T., Bubb, W.A., Messer, M., Tsuji, Y., Taneda, Y.: Studies of the neutral trisaccharides of goats (Capra hircus) colostrum and of the one- and two-dimentional 1H and 13C NMR spectra of 6’-N-acetyllactosaminyllactose. Carbohydr. Res. 262, 173–184 (1994)
Urashima, T., Murata, S., Nakamura, T.: Structural determination of monosialyl trisaccharides obtained from caprine colostrum. Comp. Biochem. Physiol. B116, 431–435 (1997)
Fukuda, K., Yamamoto, A., Ganzorig, K., Khuukhenbaatar, J., Senda, A., Saito, T., Urashima, T.: Chemical characterization of the oligosaccharides in Bacterian camel (Camelus Bactrianus) milk and colostrum. J. Dairy Sci. 93, 5572–5587 (2010)
Taufik, E., Ganzorig, K., Nansalmaa, M., Fukuda, R., Fukuda, K., Saito, T., Urashima, T.: Chemical characterization of saccharides in the milk of a reindeer (Rangifer tarandus tarandus). Int. Dairy J. 34, 104–108 (2014)
Ganzorig, K., Asakawa, T., Sasaki, M., Saito, T., Suzuki, I., Fukuda, K., Urashima, T.: Identification of sialyl oligosaccharides including an oligosaccharide nucleotide in colostrum of an addax (Addax nasomaculatus) (subfamily Antelopinae). Anim. Sci. J. 89, 167–175 (2018)
Dubois, M., Gills, K.A., Hamilton, J.K., Roberts, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956)
Jourdian, G.W., Dean, L., Roseman, S.: The sialic acid XI. A periodate – resorcinol method for the quantitative estimateon of free sialic acids and their glycolsides. J. Biol. Chem. 256, 430–435 (1971)
Uemura, Y., Asakuma, S., Nakamura, T., Arai, I., Taki, M., Urashima, T.: Occurrence of a unique sialyl tetrasaccharide in colostrum of a bottlenose dolphin (Tursiops trunkatus). Biochim. Biophys. Acta. 1725, 290–297 (2005)
Bubb, W.A., Urashima, T., Kohso, K., Nakamuta, T., Arai, I., Saito, T.: Occurrence of an unusuall lactose sulfate in dog milk. Carbohydr. Res. 318, 123–128 (1999)
Urashima, T., Fujita, S., Fukuda, K., Nakamura, T., Saito, T., Cowan, P., Messer, M.: Chemical characterization of milk oligosaccharides of the common brushtail possum (Trichosurus vulpecula). Glycoconj. J. 31, 387–399 (2014)
Urashima, T., Yamaguchi, E., Ohshima, T., Fukuda, K., Saito, T.: Chemical structures of oligosaccharides in milk of the raccoon (Procyon lotor). Glycoconj. J. 35, 275–286 (2018)
Urashima, T., Kusaka, Y., Nakamura, T., Saito, T., Maeda, N., Messer, M.: Chemical characterization of milk oligosaccharides of the brown bear, Ursus arctos yesoensis. Biochim. Biophys. Acta. 1334, 247–255 (1997)
Urashima, T., Sumiyoshi, W., Nakamura, T., Arai, I., Saito, T., Komatsu, T., Tsubota, T.: Chemical characterization of milk oligosaccharides of the Japanese black bear, Ursus thibetanus japonicus. Biochim. Biophys. Acta. 1472, 290–306 (1999)
Urashima, T., Yamashita, T., Nakamura, T., Arai, I., Saito, T., Derocher, A.E., Wiig, O.: Chemical characterization of milk oligosaccharides of the polar bear, Ursus maritimus. Biochim. Biophys. Acta. 1475, 395–408 (2000)
Urashima, T., Arita, M., Yoshida, M., Nakamura, T., Arai, I., Saito, T., Arnould, J.P.Y., Kovacs, K.M., Lydersen, C.: Chemical characterization of the oligosaccharides in hooded seal (Cystophora cristata) and Australian fur seal (Arctocephalus pusillus doriferus) milk. Comp. Biochem. Physiol. B128, 307–323 (2001)
Urashima, T., Nakamura, T., Yamaguchi, K., Munakata, J., Arai, I., Saito, T., Lydersen, C., Kovacs, K.M.: Chemical characterization of the oligosaccharides in milk of high Arctic harbor seal (Phoca vitulina vitulina). Comp. Biochem. Physiol. A135, 549–563 (2003)
Urashima, T., Nakamura, T., Nakagawa, D., Noda, M., Arai, I., Saito, T., Lydersen, C., Kovacs, K.M.: Characterization of oligosaccharides in milk of bearded seal (Erignathus barbatus). Comp. Biochem. Physiol. B138, 1–18 (2004)
Urashima, T., Nakamura, T., Ikeda, A., Asakuma, S., Arai, I., Saito, T., Oftedal, O.T.: Characterization of oligosaccharides in milk of a mink, Mustela vison. Comp. Biochem. Physiol. A142, 461–471 (2005)
Taufik, E., Sekii, N., Senda, A., Fukuda, K., Saito, T., Eisert, R., Oftedal, O.T., Urashima, T.: Neutral and acidic milk oligosaccharides of the striped skunk (Mephitidae: Mephilis mephilis). Anim. Sci. J. 84, 569–578 (2013)
Urashima, T., Yamamoto, M., Nakamura, T., Arai, I., Saito, T., Namiki, M., Yamaoka, K., Kawahara, K.: Chemical characterisation of the oligosaccharides in a sample of milk of a white-nosed coati, Nasua narica (Procyonidae: Carnivora). Comp. Biochem. Physiol. A123, 187–193 (1999)
Nakamura, T., Urashima, T., Mizukami, T., Fukushima, M., Arai, I., Senshu, T., Imazu, K., Nakao, T., Saito, T., Ye, Z., Zuo, H., Wu, K.: Composition and oligosaccharides of a milk sample of the giant panda, Ailuropoda melanoleuca. Comp. Biochem. Physiol. B135, 439–448 (2003)
Uemura, Y., Takahashi, S., Senda, A., Fukuda, K., Saito, T., Oftedal, O.T., Urashima, T.: Chemical characterization of milk oligosaccharides of a spotted hyena (crocuta crocuta). Comp. Biochem. Physiol. A152, 158–161 (2009)
Senda, A., Hatakeyama, E., Kobayashi, R., Fukuda, K., Uemura, Y., Saito, T., Packer, C., Oftedal, O.T., Urashima, T.: Chemical characterization of milk oligosaccharides of an African lion (Panthera leo) and a clouded leopard (Neofelis nebulosa). Anim. Sci. J. 81, 687–693 (2010)
Urashima, T., Messer, M.: Evolution of milk oligosaccharides and their function in monotremes and marsupials. In: Pontarotti, P. (ed.) Evolutionary Biology: Self/Nonself Evolution, Species and Complex Traits Evolition, Methods and Concepts, pp. 237–256. Springer, Swizzerland (2017)
Collins, J.G., Bradbury, J.H., Trifonoff, E., Messer, M.: Structures of four new oligosaccharides from marsupial milk, determined mainly by 13C-NMR spectroscopy. Carbohydr. Res. 92, 136–140 (1981)
Urashima, T., Taufik, E., Fukuda, R., Nakamura, T., Fukuda, K., Saito, T., Messer, M.: Chemical characterization of milk oligosaccharides of the koala (Phascolarctos cineus). Glycoconj. J. 30, 801–811 (2013)
Hirayama, K., Taufik, E., Kikuchi, M., Nakamura, T., Gukuda, F., Saito, K., Newgrain, K., Green, B., Messer, M., Urashima, T.: Chemical characterization of milk oligosaccharides of the common wombat (Vombatus ursinus). Anim. Sci. J. 87, 1167–1177 (2016)
Urashima, T., Sun, Y., Fukuda, K., Hirayama, K., Taufik, E., Nakamura, T., Saito, T., Merchant, J., Green, B., Messer, M.: Chemical characterization of milk oligosaccharides of the eastern quoll (Dasyusus viverrinus). Glycoconj. J. 32, 361–370 (2015)
Urashima, T., Yamamoto, T., Hirayama, K., Fukuda, K., Nakamura, T., Saito, T., Newgrain, K., Merchant, J., Green, B., Messer, M.: Chemical characterization of milk oligosaccharides of the tiger quoll (Dasyurus maculatus), a marsupial. Glycoconj. J. 33, 797–807 (2016)
Vicaretti, S., Mohtarudin, N., Garner, A., Zandberg, W.F.: Cappilary electrophoresis analysis of bovine milk oligosaccharides permits an assessment of the influence of diet and the discovery of nine abundant sulfated analogues. J. Agr. Food Chem. 66, 8574–8583 (2018). https://doi.org/10.1021/acs.jafc.8b01041
Taufik, E., Fukuda, K., Senda, A., Saito, T., Williams, C., Tilden, C., Eisert, R., Oftedal, O.T., Urashima, T.: Structural characterization of neutrall and acidic oligosaccharides in the milks of strepsirrhine primates: greater galago, aye-aye, Coquerel’s sifaka and mongoose lemur. Glycoconj. J. 29, 119–134 (2012)
Goto, K., Fukuda, K., Senda, A., Saito, T., Kimura, K., Glander, K.E., Hinde, K., Dittus, W., Milligan, L.A., Power, M.L., Oftedal, O.T., Urashima, T.: Chemical characterization of oligosaccharides in the milk of six species of new and old world monkeys. Glycoconj. J. 27, 703–715 (2010)
Urashima, T., Kobayashi, M., Asakuma, S., Uemura, Y., Arai, I., Fukuda, K., Saito, T., Mogoe, T., Ishikawa, H., Fukui, Y.: Chemical characterization of the oligosaccharides in Bryde’s whale (Balaenoptera edeni) and sei whale (Balaenoptera borealis lesson) milk. Comp. Biochem. Physiol. B146, 153–159 (2007)
Kobata, A.: Structures and application of oligosaccharides in human milk. Proc. Jpn. Acad. Ser. B86, 731–747 (2010)
Acknowledgements
We thank to Mr. Katsumasa Tomita of Shiretoko Ezoshika Farm for supplying deer milk. This work was supported by a Grant-in-Aid for Scientific Research ©, grant No. 16 K08003, from the Japan Society for the Promotion of Science, Sports and Culture (Japan), and a grant from Yotsuba Milk Products Co. (Japan). We thank Directorate of Higher Education of Republic Indonesia through foreign collaboration research scheme 2018 for supporting the collection and analysis of water buffalo colostrum.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
Supplementary Fig. 1
Thin layer chromatogram of the fractions of SCG-4, SC-4, D-5 and B-4 separated from milk or colostrum of giraffe, sitatunga, deer and water buffalo, respectively, by gel filtration on BioGel P-2 (see Fig. 1). Acetone/2-propanol/0.1 mol lactic acid (2:2:1, v/v/v) was used as a developing solvent. Detection of the spots was done by spraying with 5% H2SO4 in ethanol and heating. The lanes are as follows: a, authentic lactose; b, SCG-4; c, SC-4; d, D-5; e, B-4. (PDF 97 kb)
Supplementary Fig. 2
1H-NMR spectrum of the oligosaccharide in fraction of SCG-1_10 separated from giraffe milk (see Fig. 2a). The spectrum was obtained in D2O at 600 MHz with a Varian INOVA 600 spectrometer operated at 293 K. Chemical shifts are expressed relative to internal 3-(trimethylsilyl)-1-propane sulfuric acid, sodium salt, but were actually measured by reference to internal acetone (δ = 2.225). (PDF 176 kb)
Supplementary Fig. 3
Matrix-assisted, laser-desorption time-of-flight mass spectrum of the oligosaccharide in fraction of SCG-1_10. The mass spectrometry was performed using an Autoflex II TOF/TOF mass spectrometer (Brucker Daltonics, Bremen, Germany). Lyophilized oligosaccharide fractions were dissolved in 1 μL of milli-Q water. The oligosaccharide solution was mixed with an equal volume of 10 mg/mL. SDHB (Brucker Daltonics), which is a mixture of 2,5-dihydrobenzoic acid and 2-hydroxy-5-methoxybenzoic acid, saturated in milli-Q water, spotted on a MTP 384 target plate ground steel TF (Bruker Daltnics), and dried. Mass spectra were obtained using a pre-installed method, RP_0–2 kDa (a reflector positive ion mode focusing on the mass range up to 2 kDa). (PDF 98 kb)
Supplementary Fig. 4
1H-NMR spectrum of the oligosaccharide in fraction of SC-3 separated from sitatunga colostrum (see Fig. 1b). The spectrum was obtained in D2O at 500 MHz with a JEOL ECP-500 Fourier transform-NMR spectrometer operated at 293 K. (PDF 163 kb)
Supplementary Fig. 5
1H-NMR spectrum of the oligosaccharide in fraction of SC-1_1 separated from sitatunga colostrum (see Fig. 3a). The spectrum was obtained in D2O at at 600 MHz with a Varian INOVA 600 spectrometer operated at 293 K. (PDF 183 kb)
Supplementary Fig. 6
Matrix-assisted, laser-desorption time-of-flight mass spectrum of the oligosaccharide in fraction of SC-1_1. (PDF 87 kb)
Supplementary Fig. 7
1H-NMR spectrum of the oligosaccharide in fraction of D-4 separated from deer milk (see Fig. 1c). The spectrum was obtained in D2O at 500 MHz with a JEOL ECP-500 Fourier transform-NMR spectrometer operated at 293 K. (PDF 272 kb)
Supplementary Fig. 8
1H-NMR spectrum of the oligosaccharide in fraction of B-2_10 separated from water buffalo colostrum (see Fig. 4a). The spectrum was obtained in D2O at 500 MHz with a JEOL ECP-500 Fourier transform-NMR spectrometer operated at 293 K. (PDF 222 kb)
Rights and permissions
About this article
Cite this article
Mineguchi, Y., Miyoshi, M., Taufik, E. et al. Chemical characterization of the milk oligosaccharides of some Artiodactyla species including giraffe (Giraffa camelopardalis), sitatunga (Tragelaphus spekii), deer (Cervus nippon yesoensis) and water buffalo (Bubalus bubalis). Glycoconj J 35, 561–574 (2018). https://doi.org/10.1007/s10719-018-9849-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-018-9849-0