Abstract
In this study on milk saccharides of the raccoon (Procyonidae: Carnivora), free lactose was found to be a minor constituent among a variety of neutral and acidic oligosaccharides, which predominated over lactose. The milk oligosaccharides were isolated from the carbohydrate fractions of each of four samples of raccoon milk and their chemical structures determined by 1H-NMR and MALDI-TOF mass spectroscopies. The structures of the four neutral milk oligosaccharides were Fuc(α1–2)Gal(β1–4)Glc (2′-fucosyllactose), Fuc(α1–2)Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc (lacto-N-fucopentaose IV), Fuc(α1–2)Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc (fucosyl para lacto-N-neohexaose) and Fuc(α1–2)Gal(β1–4)GlcNAc(β1–3)[Fuc(α1–2)Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (difucosyl lacto-N-neohexaose). No type I oligosaccharides, which contain Gal(β1–3)GlcNAc units, were detected, but type 2 saccharides, which contain Gal(β1–4)GlcNAc units were present. The monosaccharide compositions of two of the acidic oligosaccharides were [Neu5Ac]1[Hex]6[HexNAc]4[deoxy Hex]2, while those of another two were [Neu5Ac]1[Hex]8[HexNAc]6[deoxy Hex]3. These acidic oligosaccharides contained α(2–3) or α(2–6) linked Neu5Ac, non reducing α(1–2) linked Fuc, poly N-acetyllactosamine (Gal(β1–4)GlcNAc) and reducing lactose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eutherian milk or colostrum usually contains lactose (Gal(β1–4)Glc) as the predominant carbohydrate as well as lower amounts of a variety of oligosaccharides, most of which contain a lactose unit at their reducing ends [1,2,3]. In the milk of monotremes and marsupials, however, milk oligosaccharides predominate over lactose [4,5,6,7,8]. It is believed that in suckling human infants, the milk lactose is utilized as a significant energy source, whereas most of the milk oligosaccharides are resistant to digestion and absorption within the small intestine and reach the colon. There is evidence suggesting that human milk oligosaccharides can act as receptor analog that inhibit the attachment of pathogenic microorganisms to the colonic mucosa, as prebiotics, and as immune modulators as well as stimulators of nerve development [4,5,6].
Among eutherians, the milks of many species of the order Carnivora are exceptional in that they contain substantial amounts of oligosaccharides in addition to lactose; in some taxa, such as bears, the concentration of oligosaccharides greatly exceeds that of lactose [7]. The significant feature of milk oligosaccharides in Carnivora is the presence of A (GalNAc(α1–3)[Fuc(α1–2)]Gal), B (Gal(α1–3)[Fuc(α1–2)]Gal) or H (Fuc(α1–2)Gal) antigens and/or α-Gal epitope (Gal(α1–3)Gal(β1–4)Glc(NAc)) at the non reducing end, depending on the species [7]. Oligosaccharides containing Lewis x (Gal(β1–4)[Fuc(α1–3)]GlcNAc) have been found only in the milks of bears [7]. Among species of Carnivora, oligosaccharides predominate over lactose in the milks of bears [9,10,11,12,13,14], seals [15,16,17,18], whitenosed coati [19], striped skunk [20] and mink [21], while lactose predominates over oligosaccharides in the milks of dogs [22, 23], spotted hyena [24], lion [25] and clouded leopard [25]. Based on these observations, we hypothesized that the milks of Arctoidea, excepting domestic dog, contain more oligosaccharides than lactose [7], while the milks of Felidae contain more lactose than oligosaccharides [7]. However, the physiological basis of this status has not been clarified.
In this study, the chemical structures of neutral and acidic oligosaccharides were characterized in milk of the raccoon (Procyon lotor), in which milk oligosaccharides were found to predominate over lactose, as in other species of Arctoidea.
Materials and Methods
Materials
Lactating female raccoons were captured in the area near Obihiro city, as part of a study on local feral animals. The study was done in accordance with the rules of the animal ethics committee of Obihiro University of Agriculture and Veterinary Medicine. The raccoons were killed during an eradication program implemented by the municipality and samples of their milk were collected within the day after death by massage of their mammary glands. The information of the milk samples was as follows. Sample (1) 1 ml, was collected on 9/6/2014 from an animal with six placental scars whose body weight and length were 6.03 kg and 58 cm, respectively. The estimated age of the animal was greater than two years. Sample (2), 1 ml, was collected on 6/6/2014 from an animal with six placental scars whose body weight and length were 5.65 kg and 60 cm, respectively. The age of this animal was estimated to be greater than two years. Sample (3) was a combined sample collected from three animals. The first of these samples (0.5 mL) was collected on 6/5/2015 from an animal with five placental scars whose body weight and length were 5.76 kg and 54.5 cm, respectively, and whose age was estimated at one year. The second (1.1 mL) was collected on 15/5/2014 from an animal with three placental scars whose body weight and length were 4.8 kg and 56 cm, respectively, and whose age was estimated at one year. The third milk (1.7 mL) was collected on 31/5/2014 from an animal with five placental scars whose body weight and length were 5.24 kg and 57 cm, respectively, and whose age was estimated to be greater than two years. Sample (4) (1.5 ml) was collected on 29/5/2015 from an animal with three placental scars whose body weight and length were 5.45 kg and 52.5 cm, respectively, and whose estimated age was one year.
Fuc(α1–2)Gal(β1–4)Glc was purchased from Sigma (St. Louis, MO, USA). Lacto-N-neotetraose and lacto-N-neohexaose were purchased from Seikagaku Co. (Tokyo, Japan). Fuc(1–2)Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc (lacto-N-fucopentaose IV) was separated from mink milk [21].
Isolation of milk oligosaccharides and lactose from raccoon milk
Each of the raccoon milk samples was extracted with four volumes of chloroform/methanol (2:1, v/v). The emulsion was centrifuged at 4 °C and 4000 xg for 30 min, and the lower chloroform layer and the denatured proteins were discarded. Methanol was removed from the upper layer by rotary evaporation, and the residue was dissolved in 5 ml water and freeze-dried. The resulting white powder represented the carbohydrate fraction.
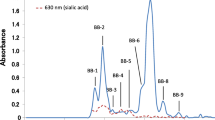
The carbohydrate fraction of each raccoon milk sample was dissolved in 2 mL of water and the solution passed through a BioGel P-2 (<45 μm, Bio Rad Laboratories, Hercules, CA, USA) column (2.5 × 100 cm) that had been calibrated with 2 mg each of galactose (monosaccharide), lactose (disaccharide) and raffinose (trisaccharide). The elution solvent was distilled water at a flow rate of 15 mL/h, and fractions of 5 mL were collected. Aliquots (0.5 mL) of each fraction were analysed for hexose by the phenol-sulfuric acid method at 490 nm [26] and for sialic acid by the periodate-resorcinol method at 630 nm [27]. Figure 1 showed the elution profile during the gel filtration of the carbohydrate fraction extracted from milk sample (4); the gel filtration profiles of the carbohydrates from milk samples (1) to (3) were very similar to each other and to the profile observed with sample (4). The saccharides in each fraction separated from the milk samples (1) to (4) by gel filtration were all examined by thin-layer chromatography (TLC) using acetone: 2-propanol: 0.1 mol/L lactic acid (2:2:1, v/v) as a developing solvent. The components were detected by spraying the thin-layer plate with a solution of 5% H2SO4 in ethanol which was then heated on a burner. As the TLC patterns of the fractions separated from milk samples (1) to (4) were very similar to each other, the neutral saccharides in the peaks designated RM-3, RM-4 and RM-5 (Fig. 1) were obtained from milk sample (4) only. They were characterized by proton nuclear magnetic resonance (1H-NMR) spectroscopy.
Gel chromatogram of the carbohydrate fraction from raccoon milk on a BioGel P-2 (2.5 × 100 cm). Elution was done with distilled water at a flow rate of 15 mL/h and fractions of 5.0 mL were collected. Each fraction was monitored for hexose by the phenol-H2SO4 method (solid line) and for sialic acid by the periodate-resorcinol (dotted line)
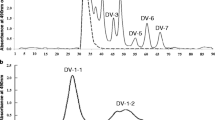
The components in the second eluted peaks (see Fig. 1) from the carbohydrates from all milk samples (1) ~ (4) were pooled (designated as RM-2) and separated by high performance liquid chromatography (HPLC) (chromatogram in Fig. 2b). The Hitachi 7000 series HPLC system (Tokyo) consisted of an autosampler L-7200, a column oven L-7300, a pump L-7100, and an evaporation light scattering detector SEDEX-75 with a system controller D-7100. The HPLC stationary phase was a 7 μm Hypercarb column (100 × 4.6 mm i.d.; Thermo Fisher Scientific, Waltham), and the mobile phase was acetonitrile in distilled water run at 40 °C. The LC gradient was delivered at 1.0 mL/min and consisted of an initial linear increase from 5 to 30% (vol/vol) acetonitrile over 80 min. The oligosaccharides in the separated fractions (RM-2-1 to RM-2-4, see Figure 2b) were pooled, lyophilized and characterized by 1H-NMR spectroscopy and MALDI-TOF mass spectrometry.
High performance liquid chromatography of the neutral oligosaccharides fractions (a) RM-1-1 separated from raccoon milk by gel filtration (see Fig. 1) and ion exchange chromatography (see Fig.3) and (b) RM-2 separated by gel filtration (see Fig. 1). The Hitachi 7000 series HPLC system (Tokyo) consisted of autosampler L-7200, a column oven L-7300, a pump L-7100, and an evaporation light scattering detector SEDEX-75 with a system controller D-7100. The stationary phase was a 7 μm Hypercarb column (100 × 4.6 mm i.d.: Thermo Fisher Scientific), while the mobile phase was acetonitrile in distilled water run at 40 °C. The LC gradient was delivered at 1.0 mL/min and consisted of an initial linear increase from 5 to 30% acetonitrile over 80 min
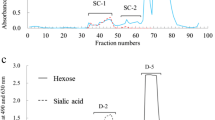
The components in the first eluted peaks (see Fig. 1) from the carbohydrates from all samples were pooled (designated as RM-1). The components in RM-1 of the gel chromatogram that reacted positively with both periodate-resorcinol (630 nm) and phenol-H2SO4 (490 nm) were dissolved in 2 mL of 50 mmol/L Tris hydroxyaminomethane-HCl buffer solution (pH 8.7) and subjected to anion exchange chromatography on a DEAE-Sephadex A-50 column (1.5 × 35 cm; GE Healthcare, Uppsala, Sweden) that was equilibrated and eluted with the same solution. Elution was done at a flow rate of 15 mL/h and fractions were analyzed for hexose using the phenol-H2SO4 method [26]. Fig. 3 shows that the ion exchange chromatography had separated the RM-1 fraction into two peaks, designated as RM-1-1 and RM-1-2. The components in RM-1-1 and RM-1-2 were separately pooled, concentrated to 2 mL, and passed though a column (2.0 × 35 cm) of BioGel P-2 to remove salts, as described above. The neutral oligosaccharides in RM-1-1 (see Fig. 3) were subjected to HPLC under conditions similar to those described above, which separated into two peaks designated as RM-1-1-1 and RM-1-1-2 (see Fig. 2a); their components were each pooled, lyophilized and characterized by 1H-NMR and MALDI-TOF mass spectrometry.
Anion exchange chromatography of RM-1 (see Fig. 1) separated from raccoon milk carbohydrate by chromatography on BioGel P-2. A DEAE-Sephadex A-50 column (2.0 × 35 cm) equilibrated with 50 mmol/L Tris hydroxyaminomethane-HCl buffer (pH 8.7) was used. Elution was done with 250 mL of the buffer. The flow rate was 15 mL/h and fractions of 5 mL were collected. They were monitored by the phenol-H2SO4 method
The components in RM-1-2 (Fig. 3) were pooled and subjected to HPLC using a Shimadzu LC-10 ATVP pump on a TSK gel Amide-80 column (4.6 × 250 mm, pore size 80 Å, particle size 5 μm; Tosoh, Tokyo, Japan (chromatogram in Figure 3). The mobile phase was 50% and 80% (vol/vol) acetonitrile in 15 mmol/L potassium phosphate buffer (pH 5.2). Elution was done using a linear gradient of acetonitrile from 80 to 50% at 60 °C at a flow rate of 1 mL/min for 80 min. The eluates were monitored by measuring the absorbance at 195 nm. The components in the peaks designated as RM-1-2-1 to RM-1-2-12 (see Fig. 4) were each pooled, concentrated and lyophilized, and characterized by 1H-NMR spectrometry and MALDI-TOF mass spectrometry.
High performance liquid chromatography of fraction RM-1-2 (see Fig. 3). The HPLC was done using Shimadzu LC-10 ATVP pump (Shimadzu, Tokyo, Japan) on a TSK-gel Amide-80 column (4.6 × 250 mm, pore size 80 Å, particle size 5 μm; Tosoh, Tokyo, Japan). The mobile phase was 50 and 80% (v/v) acetonitrile (CH3CN) in 15 mmol/L potassium phosphate buffer (pH 5.2). Elution was done using a linear gradient of CH3CN from 80 to 50% at 60 °C at a flow rate of 1 mL/min. The detection was done by UV absorption at 195 nm
1H-NMR spectroscopy
Nuclear magnetic resonance spectra were recorded in D2O (99.96 atom D%; Sigma-Aldrich, Milwaukee, WI) at 500 or 600 MHz for 1H-NMR with a JEOL ECP-500 Fourier transform-NMR (Jeol, Tokyo, Japan) or a Varian INOVA 600 specrometer (Varian Inc., Palo Alto, CA) operated at 293.1 K. Chemical shifts are expressed as change relative to internal 3-(trimethyl)-1-propane sulfuric acid, sodium salt, but measured by reference to internal acetone (δ = 2.225).
Mass spectrometry
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was performed using Autoflex II TOF/TOF mass spectrometer (Brucker Daltonics, Bremen, Germany). Lyophilized oligosaccharide fractions were dissolved in 5 μL of milli-Q water. The oligosaccharide solution was mixed with an equal volume of 10 mg/mL SDHB (Brucker Daltonics), which is a mixture of 2,5-dihydrobenzoic acid and 2-hydroxy-5-methoxybenzoic acid, saturated in milli-Q water, spotted on a MTP 384 target plate ground steel TF (Bruker Daltnics), and dried. Mass spectra were obtained using a pre-installed method, RP_0–2 kDa (a reflector positive ion mode focusing on the mass range up to 2 kDa). Peptide calibration standard II (Bruker Daltonics) was used for external calibration of the mass spectrometer.
Results
Characterization of neutral saccharides of raccoon milk
The carbohydrate fraction from raccoon milk separated into several peaks, designated RM-1 to RM-5, during gel filtration on BioGel P-2 (Fig. 1). Since the components in RM-2 to RM-5 reacted negatively with periodate – resorcinol they were considered to be neutral oligosaccharides. The saccharides in RM-3 to RM-5 were characterized by 1H-NMR. The oligosaccharides in the combined fractions of RM-2, obtained from all milk samples, were further purified by HPLC with a graphite carbon column (see Fig. 2b). The oligosaccharides in RM-2-1 to RM-2-4 were characterized by 1H-NMR and MALDI TOFMS spectroscopies.
The oligosaccharides in the combined fractions of RM-1, obtained from all milk samples, were separated into two unadsorbed peaks (RM-1-1 and RM-1-2) during ion exchange chromatography, as shown in Fig. 3. The components in the first peak designated as RM-1-1 were further purified by HPLC with a graphite carbon column (see Fig. 2a).
RM-5
The 1H-NMR spectrum (chemical shifts in Table 1) of the saccharide in RM-5 was identical with that of Gal(β1–4)Glc, i.e. lactose.
RM-4
The 1H-NMR spectrum (chemical shifts in Table 1) of the saccharide in RM-4 was identical with that of Fuc(α1–2)Gal(β1–4)Glc (2′-fucosyllactose, 2’-FL).
RM-3
The 1H-NMR spectrum (chemical shifts in Table 1) of fraction RM-3 had the anomeric shifts of α(1–2) linked Fuc, α-Glc, β(1–3) linked GlcNAc, β-Glc, and two β(1–4) linked Gal at δ 5.308, 5.219, 4.663, 4.697, 4.550 and 4.441, respectively. The spectrum had the characteristic H-5 and H-6 shifts of α(1–2) linked Fuc residue, at δ 4.219 and 1.227, respectively, and H-4 shift of β(1–4) linked Gal, which was substituted by α(1–3) linked GlcNAc, at δ 4.147. The spectrum had the NAc shift of β(1–3) linked GlcNAc at δ 2.038. From these observations, the oligosaccharide in RM-3 was characterized to be Fuc(α1–2)Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc (lacto-N-fucopentaose IV). This NMR pattern was essentially similar to the published data [21] for lacto-N-fucopentaose IV.
RM-2-1
The MALDI-TOF MS of the oligosaccharide in RM-2-1 had the MS ion at 1241 of [M + Na], indicating a monosaccharide composition of [Hex]4[HexNAc]2[deoxy Hex]1.
The 1H-NMR spectrum (chemical shifts in Table 1) of the fraction RM-2-1 had the anomeric shifts of α(1–2) linked Fuc, α-Glc, β(1–3) linked GlcNAc and β-Glc, at δ 5.310, 5.218, 4.696 and 4.663, respectively. The spectrum had the H-1 shifts of β(1–4) linked Gal at δ 4.549, 4.469, 4.435 and 4.431. It could be assigned that the shift at δ 4.549 arose from H-1 of β(1–4)Gal, which was substituted by α(1–2) linked Fuc. From the shift intensity, it was speculated that the shifts at δ 4.435 and 4.431 were resolved into two shifts due to the effect of α and β anomers at the reducing end. The spectrum had the characteristic chemical shifts of H-5 and H-6 of α(1–2) linked Fuc at δ 4.220 and 1.227, respectively, and two H-4 shifts at δ 4.154 and 4.145 of β(1–4) linked Gal, which were substituted by β(1–3) linked GlcNAc residues. The spectrum had two NAc shifts of β(1–3) linked GlcNAc at δ 2.037 and 2.030. It was concluded from the shift intensity that the shift at δ 4.696 arosed from two resonances of H-1 of β(1–3) linked GlcNAc residues. From these observations, the oligosaccharide in RM-2-1 was characterized to be Fuc(α1–2)Gal(β1–4)GlcNAc(β1–3)Gal(β1–3)GlcNAc(β1–3)Gal(β1–4)Glc (fucosyl paralacto-N-neohexaose).
RM-2-2
The MALDI-TOF MS of the oligosaccharide in RM-2-2 had the MS ion at 1364 of [M + Na], indicating a monosaccharide composition of [Hex]4[HexNAc]2[deoxy Hex]2.
The 1H-NMR spectrum (chemical shifts in Table 1) of the fraction RM-2-2 had the anomeric shifts of α(1–2) linked Fuc, α-Glc, β(1–3) linked GlcNAc, β-Glc, β(1–6) linked GlcNAc and three of β(1–4) linked Gal at δ 5.309, 5.217, 4.697, 4.663, 4.595, 4.549, 4.539 and 4.431, respectively. The H-1 shifts at δ 4.549 and 4.539 arose from H-1 of β(1–4) linked Gal, which were substituted by α(1–2) linked Fuc. The spectrum had the characteristic H-5 and H-6 shifts of α(1–2) linked Fuc at δ 4.222 and 1.229, respectively. The spectrum had the NAc shifts of β(1–3) and β(1–6) linked GlcNAc at δ 2.037 and 2.064, respectively, and H-4 shift of β(1–4) linked Gal, which was substituted by β(1–3) linked GlcNAc, at δ 4.138. From the signal intensities, the shifts at δ 5.309 and 4.222 corresponded to two protons of H-1 and H-5 of α(1–2) linked Fuc, and the shift at δ 1.229 corresponded to six protons of H-6 of α(1–2) linked Fuc. From these observations, the oligosaccharide in RM-2-2 was characterized to be Fuc(α1–2)Gal(β1–4)GlcNAc(β1–3)[Fuc(α1–2)Gal(β1–4)GlcNAc(β1–6)]Gal(1–4)Glc (difucosyl lacto-N-neohexaose).
RM-2-3 and RM-2-4
The 1H-NMR spectrum of fraction RM-2-3 had the anomeric shifts of α(1–2) linked Fuc, α-Glc, β(1–3) linked GlcNAc, β-Glc and β(1–6) linked GlcNAc at δ 5.310, 5.217, 4.693, 4.665 and 4.595, respectively. The spectrum had the H-1 shifts of β(1–4) linked Gal, which were substituted by α(1–2) linked Fuc at δ 4.563 and 4.534, and H-1 of other β(1–4) linked Gal at δ 4.465 and 4.427. The spectrum had H-5 and H-6 shifts of α(1–2) linked Fuc at δ 4.221 and 1.228, respectively, H-4 of β(1–4) linked Gal, which were substituted by β(1–3) linked GlcNAc, at δ 4.139, and NAc of β(1–3) and β(1–6) linked GlcNAc at δ 2.037 and 2.066, respectively. However, the structure of the oligosaccharide in this fraction could not be characterized in this study, because the signal intensity of each anomer shift was not clearly estimated due to complexity of the spectrum.
RM-2-4
As the 1H-NMR spectrum of fraction RM-2-4 was similar to that of RM-2-3, it was thought that these were resolved due to the α and β anomers of the same saccharide during the HPLC.
RM-1-1-1
The 1H-NMR spectrum of fraction RM-1-1-1 had the anomeric shifts of α(1–2) linked Fuc, α-Glc, β(1–3) linked GlcNAc, β-Glc and two β(1–6) linked GlcNAc at δ 5.308, 5.218, 4.696, 4.663, and 4.600 and 4.597, respectively. The spectrum had the H-1 shifts of β(1–4) linked Gal, which were substituted by α(1–2) linked Fuc, at δ 4.564, 4.547 and 4.536, and H-1 shifts of other β(1–4) linked Gal at δ 4.460 and 4.428. The spectrum had H-5 and H-6 of α(1–2) linked Fuc at δ 4.217 and 1.228, respectively. The spectrum had the NAc shifts of β(1–3) linked GlcNAc at δ 2.041 and 2.030, and β(1–6) linked GlcNAc at 2.067, and H-4 shift of β(1–4) linked Gal, which were substituted by β(1–3) linked GlcNAc, at δ 4.129. However, the structure of the oligosaccharide in this fraction could not be characterized in this study, because the signal intensity of each anomer shift was not clearly estimated due to complexity of the spectrum.
RM-1-1-2
As the clear 1H-NMR spectrum of the fraction RM-1-1-2 was not obtained, the oligosaccharide in this fraction was not characterized in this study.
The characterized structures of RM-2-1, RM-2-2, RM-3, RM-4 and RM-5 are shown in Fig. 5.
Characterization of acidic saccharides of raccoon milk
The components in the second peak obtained in the ion exchange chromatography (see Fig. 3), designated as RM-1-2, were further separated by HPLC with an Amide-80 column, as shown in Fig. 4. The separated oligosaccharides of RM-1-2-3, RM-1-2-4, RM-1-2-9 and RM-1-2-10 were characterized by 1H-NMR and MALDI TOF mass spectroscopies.
RM-1-2-3
The MALDI-TOF MS of the oligosaccharide in RM-1-2-3 had the MS ion at 2462 of [M + 2 K-H], indicating a monosaccharide composition of [Neu5Ac]1[Hex]6[HexNAc]4[deoxy Hex]2.
The 1H-NMR spectrum (Fig. 6a, chemical shifts in Table 2) had the characteristic H-3 axial and equatorial signals of α(2–3) linked Neu5Ac at δ 1.799 and 2.755, respectively, and H-3 of β(1–4) linked Gal, which was substituted by α(2–3) linked Gal, at δ 4.126, showing the presence of Neu5Ac(α2–3)Gal unit. The spectrum had the anomeric resonances of α(1–2) linked Fuc, α-Glc, β(1–3) linked GlcNAc, β-Glc, two β(1–6) linked GlcNAc, and five β(1–4) linked Gal at δ 5.309, 5.218, 4.693, 4.665, 4.627 and 4.594, and 4.562, 4.550, 4.521, 4.463 and 4.428, respectively. The H-1 resonances of β(1–4) linked Gal at δ 4.562, 4.550 and 4.521 arose from the β(1–4) linked Gal, which were substituted by α(2–3) linked Neu5Ac or α(1–2) linked Fuc, while those at δ 4.463 and 4.428 arose from the β(1–4) linked Gal, which were substituted by β(1–3) linked GlcNAc residues. The H-4 resonance at δ 4.140 arose from the β(1–4) linked Gal, which was substituted by β(1–3) linked GlcNAc at OH-3. The spectrum had the characteristic H-5 and H-6 of α(1–2) linked Fuc at δ 4.220 and 1.228, respectively. It was speculated that the NAc resonances at δ 2.067, 2.065, 2.057 and 2.053 arose from β(1–6) linked GlcNAc, while those at δ 2.036 and 2.030 arose from β(1–3) linked GlcNAc and α(2–3) linked Neu5Ac, respectively. From these observations, the possible structure of the oligosaccharide in fraction RM-1-2-3 is shown Fig. 7.
1H-NMR spectra of the oligosaccharides in (a) RM-1-2-3 and (b) RM-1-2-4 isolated from raccoon milk by HPLC (see Fig. 4). The spectra were obtained in D2O at 600 MHz with a Varian INOVA spectrometer operated at 293.1 K. Chemical shifts are expressed relative to internal 3-(trimethylsilyl)-1-propane sulfuric acid sodium salt
RM-1-2-4
The MALDI-TOF MS of the oligosaccharide in RM-1-2-4 had the MS ion at 2462 of [M + 2 K-H], indicating a monosaccharide composition of [Neu5Ac]1[Hex]6[HexNAc]4[deoxy Hex]2.
The 1H-NMR spectrum (Fig. 6b, chemical shifts in Table 2) had the H-3 axial and equatorial signals of α(2–6) linked Neu5Ac at δ 1.723 and 2.666, respectively. The spectrum had the H-1 resonances of α(1–2) linked Fuc, α-Glc, two β(1–3) linked GlcNAc, β-Glc, β(1–6) linked GlcNAc and six β(1–4) linked Gal at δ 5.309, 5.218, 4.719 and 4.696, 4.665, 4.596, and 4.565, 4.541, 4.535, 4.463, 4.453 and 4.429, respectively. The H-1 resonances of β(1–4) linked Gal at δ 4.565, 4.541 and 4.535 arose from the β(1–4) linked Gal, which were substituted by α(1–2) linked Fuc, while those at δ 4.463, 4.453 and 4.429 arose from the β(1–4) linked Gal, which were substituted by α(2–6) linked Neu5Ac or β(1–3) linked GlcNAc. The H-4 resonance of β(1–4) linked Gal at δ 4.146 arose from the β(1–4) linked Gal, which was substituted by β(1–3) linked GlcNAc at OH-3. The spectrum had the H-5 of α(1–2) linked Fuc at δ 4.221 and H-6 of that at δ 1.226 and 1.229. It was speculated that the NAc resonances at δ 2.069 and 2.066 arose from β(1–6) linked GlcNAc, those at δ 2.052 and 2.038 from β(1–3) linked GlcNAc and that at δ 2.028 from α(2–6) linked Neu5Ac. From these observations, the possible structure of the oligosaccharide in fraction RM-1-2-4 is shown Fig. 7.
RM-1-2-9
The MALDI-TOF MS of the oligosaccharide in RM-1-2-9 has the MS ion at 3338 of [M + 2 K-H], indicating a monosaccharide composition of [Neu5Ac]1[Hex]8[HexNAc]6[deoxy Hex]3.
The spectrum (chemical shifts in Table 3) had the H-3 axial and equatorial resonances of α(2–3) linked Neu5Ac at δ 1.800 and 2.755, respectively. The spectrum had the H-1 resonances of α(1–2) linked Fuc, α-Glc, β(1–3) linked GlcNAc, β-Glc, two β(1–6) linked GlcNAc, and five β(1–4) linked Gal at δ 5.310, 5.222, 4.694, 4.666, 4.636 and 4.596, and 4.563, 4.550, 4.536, 4.452 and 4.428, respectively. The H-1 resonances of β(1–4) linked Gal at δ 4.563, 4.550 and 4.536 arose from the β(1–4) linked Gal, which were substituted by α(2–3) linked Neu5Ac or α(1–2) linked Fuc, while those at δ 4.452 and 4.428 arose from the β(1–4) linked Gal, which were substituted by β(1–3) linked GlcNAc. The H-4 shift of β(1–4) linked Gal at δ 4.137 arose from the β(1–4) linked Gal, which was substituted by β(1–3) linked GlcNAc at OH-3. The spectrum had the H-5 and H-6 resonances of α(1–2) linked Fuc at δ 4.220 and 1.227, respectively. The NAc shifts at δ 2.068, 2.037 and 2.030 were assigned to those of β(1–6) linked GlcNAc, β(1–3) linked GlcNAc and α(2–3) linked Neu5Ac, respectively. From these observations, the possible structure of the oligosaccharide in fraction RM-1-2-9 is shown in Fig. 7.
RM-1-2-10
The MALDI-TOF MS of the oligosaccharide in RM-1-2-10 has the MS ion at 3338 of [M + 2 K-H], indicating a monosaccharide composition of [Neu5Ac]1[Hex]8[HexNAc]6[deoxy Hex]3.
The spectrum (chemical shifts in Table 3) had the H-3 axial and equatorial resonances of α(2–6) linked Neu5Ac at δ 1.722 and 2.667, respectively. The spectrum had the H-1 resonances of α(1–2) linked Fuc, α-Glc, β(1–3) linked GlcNAc, β-Glc, two β(1–6) linked GlcNAc and five β(1–4) linked Gal at δ 5.310, 5.224, 4.694, 4.667, 4.637 and 4.596 and 4.563, 4.550, 4.535, 4.452 and 4.429, respectively. The H-1 resonances of β(1–4) linked Gal at δ 4.563, 4.550 and 4.535 arose from the β(1–4) linked Gal, which were substituted by α(1–2) linked Fuc, while those at δ 4.452 and 4.429 arose from the β(1–4) linked Gal, which were substituted by α(2–6) linked Neu5Ac or β(1–3) linked GlcNAc. The H-4 shift of β(1–4) linked Gal at δ 4.136 arose from the β(1–4) linked Gal, which was substituted by β(1–3) linked GlcNAc at OH-3. The spectrum had the H-5 and H-6 of α(1–2) linked Fuc at δ 4.220 and 1.226, respectively. The NAc shift at δ 2.068 was assigned to β(1–6) linked GlcNAc, those at δ 2.052 and 2.038 were to β(1–3) linked GlcNAc, and that at δ 2.028 was to α(2–6) linked Neu5Ac. From these observations, the possible structure of the oligosaccharide in fraction RM-1-2-10 is shown in Fig. 7.
As the clear 1H-NMR spectra were not obtained, RM-1-2-1, RM-1-2-2, RM-1-2-5, RM-1-2-6, RM-1-2-7, RM-1-2-8, RM-1-2-11 and RM-1-2-12 were not characterized in this study.
Discussion
This study found that lactose was no more than a minor saccharide in raccoon milk, as in the milks of other species of Arctoidea including bears [9,10,11,12,13,14], seals [15,16,17,18], mink [21], striped skunk [20] and whitenosed coati [19]. The predominant saccharides in raccoon milk were found to be larger oligosaccharides, designated as RM-1 (Fig. 1); in addition to these saccharides, neutral oligosaccharides, whose core units are lacto-N-neotetraose (Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc, lacto-N-neohexaose (Gal(β1–4)GlcNAc(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc) or para lacto-N-neohexaose (Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc), were found in raccoon milk.
Previous studies had shown that the core units of milk oligosaccharides of bears [9,10,11, 13], seals [15,16,17], mink [21] and striped skunk [20] are lacto-N-neotetraose and lacto-N-neohexaose, as in those of raccoon milk. In addition, it has been shown that the milks of bearded and hooded seals contain oligosaccharides which have poly N-acetyllactosamine (Gal(β1–4)GlcNAc) attached to lacto-N-neohexaose [18]. This is also similar to that of raccoon, in which the acidic milk oligosaccharides such as RM-1-2-3, RM-1-2-4, RM-1-2-9 and RM-1-2-10 contained such structures. It is noteworthy that all oligosaccharide identified in milk of the Arctoidea contain the type II (Gal(β1–4)GlcNAc) but not type I (Gal(β1–3)GlcNAc) unit.
It is noteworthy that in Arctoidea, the non-reducing end units of their milk oligosaccharides vary depending on the species. The milk oligosaccharides of raccoon and hooded seal contain only H antigen (Fuc(α1–2)Gal) [15, 18], while those of whitenosed coati and mink contain H antigen or α-Gal epitope (Gal(α1–3)Gal(β1–4)Glc(NAc)) [19, 21]. In this study the α-Gal epitope was not found in raccoon milk oligosaccharides, in contrast to milk oligosaccharides of the whitenosed coati, which belongs to the same family. In contrast to raccoon milk saccharides, those of other Arctoidea such as the Japanese black bear contain B antigen (Gal(α1–3)[Fuc(α1–2)Gal] or α-Gal epitope [10, 13], while those of polar bear contain A antigen (GalNAc(α1–3)[Fuc(α1–2)]Gal) in addition to B antigen or α-Gal epitope [11]. Furthermore striped skunk milk contains A-tetrasaccharide (GalNAc(α1–3)[Fuc(α1–2)]Gal(β1–4)Glc and Galili pentasaccharide (Gal(α1–3)Gal(β1–4)GlcNAc(β1–3)Gal(β1–4)Glc [20] and it is noteworthy that Lewis x (Gal(β1–4)[Fuc(α1–3)]GlcNAc) is contained in the milk oligosaccharides of bears [7]. These were not found in raccoon milk oligosaccharides. However, raccoon milk oligosaccharides resemble those of other Arctoidea acidic milk oligosaccharides in that the only sialic acid is Neu5Ac.
It has been shown that in marsupials the milk carbohydrate components undergo changes in their composition as a function of lactation duration [28] On the other hand, there are only few data concerning changes in oligosaccharide composition in the milk of Arctoidea species during lactation. The only such data, in which the profile of lactose and milk oligosaccharides were compared in the same animal at different lactation periods, were obtained in milk collected at 37, 61 and 91 days lactation from a Japanese black bear, showing that any change is likely to be rather small during these periods (see Fig. 1 of reference 10) [10]. In addition, the profile of lactose and milk oligosaccharides was compared between two different polar bears in milk collected at 4 and 27 months post partum, showing that the change is small (see Fig. 1 of reference 11) [11]. From these observations, one may assume that any changes in milk carbohydrate composition are likely to be small in Arctoidea during lactation, compared with that of marsupials.
As mentioned above, oligosaccharides predominate over lactose in milk of the raccoon. It has been hypothesized that predominance of milk oligosaccharides over lactose is related to a low expression of α-lactalbumin within lactating mammary glands [4, 6]. It is well known that lactose is biosynthesized by co-action of β4galactosyyltransferase 1 with α-lactalbumin in the Golgi apparatus within the epithelial cells of the lactating glands, and that the milk oligosaccharides are synthesized by the action of several glycolsyltransferases acting on lactose as a substrate [4, 6]. If the biosynthesis of lactose is slow as a result of low expression of α-lactalbumin, lactose could not accumulate because most of it would be utilized for the formation of milk oligosaccharides.
The ratio of milk oligosaccharides to lactose was estimated to be 32:1 in raccoon milk, based on the profile of gel filtration of the carbohydrate extracts (see Fig. 1). It can be assumed that the ratio may vary between several lactation periods. On the other hand, we estimated the ratio of milk oligosaccharides in milk of several Carnivora species from our previous publication to be as follows; 7:1 in milk of striped skunk at 20~48 days post partum [20], 52:1 in milk of Japanese black bear milk at 37 days post partum [10], 5:1 in milk of mink at 15 days post partum [21], 2:1 in milk of white-nosed coati at 17 days post partum [19], 13:1 in milk of polar bear at 27 months post partum [11], 10:1 in milk of giant panda at 13 days post partum [14], 4:1 in milk of high arctic harbor seal [16], 1:6 in milk of house dog at 13 days post partum [22], 1:1 in colostrum of spotted hyena at 2 days post partum [24], 1:2 in milk of African lion at 127 days post partum [25], 1:1 in colostrum of clouded leopard at 1 day post partum [25]. Based on these estimations, we suggest that the predominance of milk oligosaccharides over lactose is common to milk of Arctoidea species even at different stages of lactation, while lactose is a dominant saccharide in milk of the house dog and of Felidae species.
As mentioned, milk oligosaccharides predominate over lactose in the milk of bears and seals. In these species the neonates depend mainly on milk lipids as an energy source for their development, and milk carbohydrates are not significant in this regard. In bears the neonate’s dependence on milk fat but not carbohydrate is related to the fact that the mothers usually feed their neonates while hibernating, thus having to produce milk without any access to food. This milk, by necessity, is very energy dense, containing a high concentration of fat. Similarly, seal mothers suckle their neonates only while on land, where they cannot feed, and their milk is very high in fat. However, the question of why milk oligosaccharides predominate over lactose in the milk of other species such as raccoon, mink, skunk and whitenosed coati, and why this phenomenon appears to be restricted to Arctoidea, is still difficult to answer. In these species the milk oligosaccharides may perform the same functions, i.e. act as receptor analogs and as prebiotics etc., as in humans.
References
Jenness, R.E., Regehr, E.A., Sloan, R.E.: Comparative studies of milks. II. Dialyzable carbohydrates. Comp. Biochem. Physiol. 13, 339–352 (1964)
Urashima, T., Saito, T., Nakamura, T., Messer, M.: Oligosaccharides of milk and colostrum in non-human mammals. Glycoconj. J. 18, 357–371 (2001)
Urashima, T., Messer, M., Oftedal, O.T.: Oligosaccharides in the milk of other mammals. In: McGuire, M., McGuire, M., Bode, L. (eds.) Prebiotics and probiotics in human milk, pp. 45–139. Academic Press, Amsterdam (2016)
Messer, M., Urashima, T.: Evolution of milk oligosaccharides and lactose. Trends Glycosci. Glycotechnol. 14, 153–176 (2002)
Urashima, T., Fukuda, K., Kitaoka, M., Ohnishi, M., Terabayashi, T., Kobata, A.: Milk oligosaccharides. In: Gordon, N.S. (ed.) Oligosaccharides, Properties and Applications, pp. 1–58. Nova Science, New York (2011)
Urashima, T., Fukuda, K., Messer, M.: Evolution of milk oligosaccharides and lactose: a hypothesis. Animal. 6, 369–374 (2012)
Urashima, T., Messer, M., Oftedal, O.T.: Comparative biochemistry and evolution of milk oligosaccharides of monotremes, marsupials, and eutherians. In: Pontarotti, P. (ed.) Evolutionary Biology: Genome Evolution, Speciation, Coevolution and Origin of Life, pp. 3–33. Springer, Swizerland (2014)
Urashima, T., Messer, M.: Evolution of milk oligosaccharides and their function in monotremes and marsupials. In: Pontarotti, P. (ed.) Evolutionary Biology: Self/Nonself Evolution, Species and Complex Traits Evolution, Methods and Concepts, pp. 237–256. Springer, Swizerland (2017)
Urashima, T., Kusaka, Y., Nakamura, T., Saito, T., Maeda, N., Messer, M.: Chemical characterization of milk oligosaccharides of the brown bear, Ursus arctos yesoensis. Biochim. Biophys. Acta. 1334, 247–255 (1997)
Urashima, T., Sumiyoshi, W., Nakamura, T., Arai, I., Saito, T., Komatsu, T., Tsubota, T.: Chemical characterization of milk oligosaccharides of the Japanese black bear, Ursus thibetanus japonicus. Biochim. Biophys. Acta. 1472, 290–306 (1999)
Urashima, T., Yamashita, T., Nakamura, T., Arai, I., Saito, T., Derocher, A.E., Wiig, O.: Chemical characterization of milk oligosaccharides of the polar bear, Ursus maritimus. Biochim. Biophys. Acta. 1475, 395–408 (2000)
Urashima, T., Nagata, H., Nakamura, T., Arai, I., Saito, T., Imazu, K., Hayashi, T., Derocher, A.E., Wiig, O.: Differences in oligosaccharides pattern of a sample of polar bear colostrum and mid-lactation milk. Comp. Biochem. Physiol. B136, 887–896 (2003)
Urashima, T., Nakamura, T., Teramoto, K., Arai, I., Saito, T., Komatsu, T., Tsubota, T.: Chemical characterization of sialyl oligosaccharides in milk of the Japanese black bear, Ursus thibetanus japonicas. Comp. Biochem. Physiol. 139B, 587–595 (2004)
Nakamura, T., Urashima, T., Mizukami, T., Fukushima, M., Arai, I., Senshu, T., Imazu, K., Nakao, T., Saito, T., Ye, Z., Zuo, H., Wu, K.: Composition and oligosaccharides of a milk sample of the giant panda, Ailuropoda melanoleuca. Comp. Biochem. Physiol. B135, 439–448 (2003)
Urashima, T., Arita, M., Yoshida, M., Nakamura, T., Arai, I., Saito, T., Arnould, J.P.Y., Kovacs, K.M., Lydersen, C.: Chemical characterization of the oligosaccharides in hooded seal. (Cystophora cristata) and Australian fur seal (Arctocephalus pusillus doriferus) milk. Comp. Biochem. Physiol. B128, 307–323 (2001)
Urashima, T., Nakamura, T., Yamaguchi, K., Munakata, J., Arai, I., Saito, T., Lydersen, C., Kovacs, K.M.: Chemical characterization of the oligosaccharides in milk of high Arctic harbour seal (Phoca vitulina vitulina). Comp. Biochem. Physiol. A135, 549–563 (2003)
Urashima, T., Nakamura, T., Nakagawa, D., Noda, M., Arai, I., Saito, T., Lydersen, C., Kovacs, K.M.: Characterization of oligosaccharides in milk of bearded seal (Erignathus barbatus). Comp. Biochem. Physiol. B138, 1–18 (2004)
Kinoshita, M., Ohta, H., Higaki, K., Kojima, Y., Urashima, T., Nakajima, K., Suzuki, M., Kovacs, K.M., Lydersen, C., Hayakawa, T., Kakehi, K.: Structural characterization of multi-branched oligosaccharides from seal milk by combination of off-line HPLC-MALDI-TOF MS and sequential exoglycosidase digestion. Anal. Biochem. 388, 242–253 (2009)
Urashima, T., Yamamoto, M., Nakamura, T., Arai, I., Saito, T., Namiki, M., Yamaoka, K., Kawahara, K.: Chemical characterisation of the oligosaccharides in a sample of milk of a white-nosed coati, Nasua narica (Procyonidae: Carnivora). Comp. Biochem. Physiol. A123, 187–193 (1999)
Taufik, E., Sekii, N., Senda, A., Fukuda, K., Saito, T., Eisert, R., Oftedal, O.T., Urashima, T.: Neutral and acidic milk oligosaccharides of the striped skunk (Mephitidae: Mephitis mephitis). Anim. Sci. J. 84, 569–578 (2013)
Urashima, T., Nakamura, T., Ikeda, A., Asakuma, S., Arai, I., Saito, T., Oftedal, O.T.: Characterization of oligosaccharides in milk of a mink, Mustela vison. Comp. Biochem. Physiol. A142, 461–471 (2005)
Bubb, W.A., Urashima, T., Kohso, K., Nakamura, T., Arai, I., Saito, T.: Occurrence of an unusual lactose sulfate in dog milk. Carbohydr. Res. 318, 123–128 (1999)
Rostami, M.S., Benet, T., Spears, J., Reynolds, A., Satyaraj, E., Sprenger, N., Austin, S.: Milk oligosaccharides over time of lactation from different dog breeds. PLoS One. e99824 (2014)
Uemura, Y., Takahashi, S., Senda, A., Fukuda, K., Saito, T., Oftedal, O.T., Urashima, T.: Chemical characterization of milk oligosaccharides of a spotted hyena (crocuta crocuta). Comp. Biochem. Physiol. A152, 158–161 (2009)
Senda, A., Hatakeyama, E., Kobayashi, R., Fukuda, K., Uemura, Y., Saito, T., Packer, C., Oftedal, O.T., Urashima, T.: Chemical characterization of milk oligosaccharides of an African lion (Panthera leo) and a clouded leopard (Neofelis nebulosa). Anim. Sci. J. 81, 687–693 (2010)
Dubois, M., Gill, K.A., Hamilton, J.K., Roberts, P.A., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956)
Jourdian, G.W., Dean, L., Roseman, S.: The sialic acid XI. A periodate – resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J. Biol. Chem. 256, 430–435 (1971)
Green, B., Merchant, J.C.: The composition of marsupial milk. In: Tyndale Biscoe, C.H., Janssens, P.A. (eds.) The developing marsupial, pp. 41–54. Springer-Verlag, Berlin (1988)
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (C), grant No. 16 K08003, from the Japan Society for the Promotion of Science, Sports and Culture (Japan), and a grant from Yotsuba Milk Products Co (Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Urashima, T., Yamaguchi, E., Ohshima, T. et al. Chemical structures of oligosaccharides in milk of the raccoon (Procyon lotor). Glycoconj J 35, 275–286 (2018). https://doi.org/10.1007/s10719-018-9821-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-018-9821-z