Abstract
Structural characterizations of marsupial milk oligosaccharides have been performed in four species to date: the tammar wallaby (Macropus eugenii), the red kangaroo (Macropus rufus), the koala (Phascolarctos cinereus) and the common brushtail possum (Trichosurus vulpecula). To clarify the homology and heterogeneity of milk oligosaccharides among marsupials, the oligosaccharides in the carbohydrate fraction of eastern quoll milk were characterized in this study. Neutral and acidic oligosaccharides were separated and characterized by 1H-nuclear magnetic resonance spectroscopy and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. The structures of the neutral oligosaccharides were Gal(β1–3)Gal(β1–4)Glc (3’-galactosyllactose), Gal(β1–3)Gal(β1–3)Gal(β1–4)Glc (3”,3’-digalactosyllactose), Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (lacto-N-novopentaose I), Gal(β1–3)Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (galactosyl lacto-N-novopentaose I), Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–3)Gal(β1–4)Glc (galactosyl lacto-N-novopentaose II), Gal(β1–3)[Gal(β1–3)Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (galactosyl lacto-N-novopentaose III) and Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (lacto-N-novooctaose). The structures of the acidic oligosaccharides detected are Neu5Ac(α2–3)Gal(β1–4)Glc (3’-sialyllactose), Gal(β1–3)(O-3-sulfate)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (lacto-N-novopentaose I sulfate a), Gal(β1–3)[Gal(β1–4)(O-3-sulfate)GlcNAc(β1–6)]Gal(β1–4)Glc (lacto-N-novopentaose I sulfate b), Neu5Ac(α2–3)Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (sialyl lacto-N-novopentaose a), Gal(β1–3)[Neu5Ac(α2–3)Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (sialyl lacto-N-novopentaose c), Neu5Ac(α2–3) Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc, and Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc with an α(2–3) Neu5Ac linked to β(1–4)Gal residue of either branch of Gal(β1–4)GlcNAc(β1–6) units. The most predominant oligosaccharides in the carbohydrate fraction of mid-lactation milk were found to be lacto-N-novopentaose I and lacto-N-novooctaose, i.e., branched oligosaccharides that contain N-acetylglucosamine. The predominance of these branched oligosaccharides, rather than of a series of linear β(1–3) linked galacto oligosaccharides, appears to be the main feature of the eastern quoll milk oligosaccharides that differentiates them from those of the tammar wallaby and the brushtail possum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammalian milk or colostrum contains from a trace to over 10 % of carbohydrate in which the disaccharide lactose (Gal(β1–4)Glc) usually predominates over lower concentrations of a variety of oligosaccharides: these mostly have a lactose unit at their reducing ends [1, 2]. In the milk of monotremes, marsupials and some Arctoidea species of Carnivora (ursids, mustelids, pinnipeds), however, oligosaccharides usually predominate over free lactose [2, 3]. Among marsupial species, oligosaccharides have been characterized in the tammar wallaby [4–9], red kangaroo [10], koala [11] and brushtail possum [12]. The neutral oligosaccharides of milk of the tammar wallaby have been isolated and are characterized by the presence of a major series of galactosyllactoses ranging from Gal(β1–3)Gal(β1–4)Glc to Gal(β1–3)Gal(β1–3)Gal(β1–3)Gal(β1–3)Gal(β1–4)Glc [5, 6] and a minor series of branched oligosaccharides containing β(1–6) linked GlcNAc including Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (lacto-N-novopentaose I) [7, 8]. The acidic oligosaccharides of milk of the red kangaroo were found to contain non reducing N-acetylneuraminic acid or sulfate at OH-3 of non reducing Gal residues whose core structures were similar to the core structures of the neutral milk oligosaccharides of the tammar wallaby [10]. Most of the neutral and acidic milk oligosaccharides of the brushtail possum were found to be similar to the neutral oligosaccharides of the tammar wallaby and the acidic oligosaccharides of the red kangaroo, respectively [12]. Some of the neutral and acidic milk oligosaccharides of the koala are similar to those of the tammar wallaby and red kangaroo, but koala milk carbohydrate uniquely contains two fucosyl oligosaccharides, viz. fucosyl lacto-N-novopentaose I and fucosyl sialyl lacto-N-novopentaose I [11].
Other than those, changes during lactation in the milk carbohydrates of the carnivorous eastern quoll (“native cat”), Dasyurus viverrinus, were studied by thin layer chromatography. The results suggested that some of these carbohydrates were similar to tri- to penta-saccharides found in tammar wallaby milk [13], but the details of their chemical structures have not as yet been investigated. In this study we have characterized the neutral and acidic milk oligosaccharides of the eastern quoll.
Materials and methods
Milk carbohydrate sample and chemicals
Milk samples were collected from a colony of eastern quolls at the Division of Wildlife and Rangelands Research, CSIRO, Canberra. All the females had given birth in mid-June of 1978 or 1979 [13]. The sample used in this investigation originated from a study on changes in milk carbohydrates during lactation in the eastern quoll [13]. For more detailed analysis the carbohydrates in milk collected from 7 to 11 weeks post partum, i.e., about mid-way through lactation [13], were extracted as described by Messer and Mossop [14] and freeze-dried. The freeze-dried fractions were stored in sealed tubes at −20 °C for about 35 years, prior to analysis; these were combined to a total of 150 mg for this study.
Gal(β1–3)Gal(β1–4)Glc (3’-galactosyllactose) and Gal(β1–3)Gal(β1–3)Gal(β1–4)Glc (3’,3”-digalactosyllactose) were isolated from tammar milk [5, 6], while Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (lacto-N-novopentaose I), Gal(β1–3)Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (galactosyl lacto-N-novopentaose I) and Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–3)Gal(β1–4)Glc (galactosyl lacto-N-novopentaose II) were isolated from the carbohydrate fraction of brushtail possum milk [12].
Neutral oligosaccharides
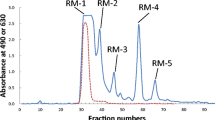
Of the carbohydrate fraction of eastern quoll milk, 75 mg were dissolved in 2 mL of water and the solution passed through a BioGel P-2 column (<45 μm, 2.5 × 100 cm, Bio-Rad Laboratories, Hercules, CA) that had been calibrated with 2 mg of each galactose (monosaccharide), lactose (disaccharide), and raffinose (trisaccharide). The gel was washed with 0.1 M HCl and 0.1 M NaOH before use. Elution was done with distilled water at a flow rate of 15 mL/h, and fractions of 5 mL were collected. Aliquots (0.5 mL) of each fraction were analyzed for hexose with phenol – H2SO4 [15] and for sialic acid with periodate-resorcinol [16]. Peak fractions were pooled as shown in Fig. 1a and freeze-dried. The saccharides in the peak fractions, DV-1 to DV-7 (see Fig. 1a) were checked by thin layer chromatography (TLC) using acetone/2-propanol/0.1 mol lactic acid (2:2:1, v/v/v) as a developing solvent. Detection of the spots was done by spraying with 5 % H2SO4 in ethanol and heating. Gel filtration was performed twice, each with 75 mg of the carbohydrate fraction, and the corresponding peak fractions were combined.
Gel chromatogram (a) of the carbohydrate fraction from the eastern quoll milk on a BioGel P-2 column (2.5 × 100 cm), and anion exchange chromatography (b) of DV-1 on a DEAE-Sephadex A-50 column (2.0 × 35 cm). Each chromatography condition is shown in the Methods. a Each fraction was monitored by the phenol-H2SO4 method at 490 nm (solid line) and the periodate-resorcinol method at 630 nm (dotted line). b Each fraction was monitored by the phenol-H2SO4 method
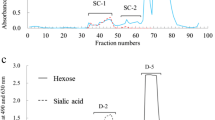
The components in DV-5, DV-6 and DV-7 were characterized by 1H-NMR spectroscopy. Those in DV-2—DV-4 were subjected to high performance liquid chromatography (HPLC) (chromatograms in Fig. 2a, b and c). The Hitachi 7000 series HPLC system (Tokyo) consisted of an autosampler L-7200, a column oven L-7300, a pump L-7100, and an evaporation light scattering detector SEDEX-75 with a system controller D-7100. The HPLC stationary phase was a 7 μm Hypercarb column (100 × 4.6 mm i.d.; Thermo Fisher Scientific), and the mobile phase was acetonitrile in distilled water run at 40 °C. The LC gradient was delivered at 1.0 mL/min and consisted of an initial linear increase from 5 to 30 % acetonitrile over 80 min. The oligosaccharides in the separated fractions were pooled, lyophilized and characterized by 1H-NMR spectroscopy and MALDI-TOF mass spectrometry.
High performance liquid chromatography of the neutral oligosaccharide fractions a DV-4, b DV-3 and c DV-2 separated from the carbohydrate fraction of eastern quoll milk by gel chromatography (see Fig. 1a), and the acidic oligosaccharide fraction d DV-1-2 separated from DV-1 fraction by anion exchange chromatography (see Fig. 1b). Each HPLC condition was shown in the Methods
Acidic oligosaccharides
The components in peak DV-1 of the gel chromatogram (see Fig. 1a) that reacted positively with both periodate-resorcinol (630 nm) and phenol-H2SO4 (490 nm) were dissolved in 2 mL of 50 mmol/L Tris hydroxyaminomethane-HCl buffer solution (pH 8.7) and subjected to anion exchange chromatography on a DEAE-Sephadex A-50 column (2.0 × 35 cm; GE Healthcare, Uppsalla, Sweden) that was equilibrated and eluted with the same solution. Elution was done at a flow rate of 15 mL/h and fractions were analyzed for hexose using phenol-H2SO4 method [13]. Figure 1b shows that the ion exchange chromatography had separated the DV-1 fraction into two peaks. The components in the peak designated DV-1-2 were pooled, lyophilized, dissolved in 2 mL of water, and passed through a column (2.0 × 35 cm) of BioGel P-2 to remove salts, as described above.
The components in DV-1-2 were subjected to HPLC on a TSK gel Amide-80 column (4.6 × 250 mm, Pore size 80 Å, particle size 5 μm; Tosoh, Japan (chromatogram in Fig. 2d). The mobile phase was 50 % and 80 % (vol/vol) acetonitrile in 15 mmol/L potassium phosphate buffer (pH 5.2). Elution was done using a linear gradient of acetonitrile from 80 to 50 % at 60 °C at a flow rate of 1 mL/min. The eluates were monitored by measuring the absorbance at 195 nm. The peaks designated as DV-1-2-1 to DV-1-2-17 (Fig. 2d) were each pooled, concentrated by rotary evaporation, and subjected to 1H-NMR spectroscopy and MALDI-TOF mass spectrometry to determine their structures.
1H-NMR spectroscopy
Nuclear magnetic resonance spectra were recorded in D2O (99.96 atom D%; Aldrich, Milwaukee, WI) at 500 or 600 MHz for 1H-NMR with a JEOL ECP-500 Fourier transform-NMR (Jeol, Tokyo, Japan) or a Varian INOVA 600 spectrometer (Varian Inc, Palo Alto, CA) operated at 293.1 K. Chemical shifts are expressed as change relative to internal 3-(trimethylsilyl)-1-propane sulfuric acid, sodium salt, but measured by reference to internal acetone (δ = 2.225).
Mass spectrometry
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was performed on the oligosaccharide fractions, using an Autoflex II TOF/TOF mass spectrometer (Brucker Daltonics, Bremen, Germany). Lyophilized oligosaccharide fractions were dissolved in 5 μL of milli-Q water. The oligosaccharide solution was mixed with an equal volume of 10 mg/mL. SDHB (Brucker Daltonics), which is a mixture of 2,5-dihydrobenzoic acid and 2-hydroxy-5-methoxybenzoic acid, saturated in milli-Q water, spotted on a MTP 384 target plate ground steel TF (Bruker Daltonics), and dried. Mass spectra were obtained using a pre-installed method, RP_0-2 kDa (a reflector positive ion mode focusing on the mass range up to 2 kDa). Peptide calibration standard II (Bruker Daltonics) was used for external calibration of the mass spectrometer.
Results
Characterization of neutral saccharides
The crude carbohydrate fraction (total 150 mg) from eastern quoll milk separated into several peaks, designated DV-1 to DV-7, during gel filtration on BioGel P-2. Since the components in DV-2 to DV-7 (Fig. 1a) did not react positively with periodate – resorcinol they were considered to be neutral oligosaccharides. Figure 1a shows that of the six peaks containing neutral oligosaccharides, the most prominent were DV-2 and DV-4. The components in DV-2 to DV-4 were subjected to HPLC using a Hypercarb column, as shown in Fig. 2a, b and c. The resulting peaks were designated as DV-2-1 to DV-2-12, DV-3-1 to DV-3-8 and DV-4-1 to DV-4-4. The separated peak components obtained by gel filtration and HPLC were characterized by 1H-NMR and MALDI-TOF MS spectra.
DV-7, DV-6, DV-5, DV-4-2 and DV-4-3, DV-3-1 and DV-3-2, and DV-3-3 and DV-3-6
From the agreement of 1H-NMR spectra (chemical shifts in Supplemental Table 1 and Supplemental Table 2) with those of authentic saccharides as well as the published data, the oligosaccharides in these fractions were characterized to be as follows; lactose (DV-7), 3’-galactosyllactose (DV-6), 3”,3’-digalactosyllactose (DV-5), lacto-N-novopentaose I (DV-4-2 and DV-4-3), galactosyl lacto-N-novopentaose II (DV-3-1 and DV-3-2), and galactosyl lacto-N-novopentaose I (DV-3-3 and DV-3-6) (see Table 1).
DV-3-5
The MALDI-TOF MS of the oligosaccharide in DV-3-5 had the MS ions at 1070 and 1054 of [M + K] and [M + Na], respectively, showing its monosaccharide composition to be [Hex]5[HexNAc]1.
The oligosaccharide in this fraction was characterized by comparison of its 1H-NMR spectrum with the spectra of DV-3-1 and DV-3-3. The spectrum of DV-3-5 (Fig. 3, chemical shifts in Supplemental Table 2) had the H-1 shifts of α-Glc, β-Glc, β(1–6) linked GlcNAc, external β(1–3) linked Gal, and two β(1–4) linked Gal at δ 5.225, 4.671, 4.651 and 4.644, 4.613, 4.532 and 4.500. The intensity of the shift at δ 4.613 corresponded to two protons, indicating the presence of two external β(1–3) linked Gal residues. The H-1 shift of one β(1–4) linked Gal at δ 4.532 shifted down field, compared with the H-1 shift of β(1–4) linked Gal of DV-3-3 at δ 4.472, showing that this residue was substituted by β(1–3) linked Gal. The absence of an H-1 shift at δ 4.68 indicated the absence of an internal β(1–3) linked Gal residue. The NAc shift at δ 2.063 showed that the β(1–6) GlcNAc residue was linked to a β(1–4) linked Gal but not to a β(1–3) linked Gal residue because if the β(1–6) GlcNAc residue was linked to a β(1–3) linked Gal, the NAc shift would have been at δ 2.044 as in DV-3-1. The shifts at δ 4.182 and 4.198 were assigned to H-4 of two β(1–4) linked Gal, which were substituted by β(1–3) linked Gal at OH-3. From these observations, the saccharide in DV-3-5 was characterized to be Gal(β1–3)[Gal(β1–3)Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (galactosyl lacto-N-novopentaose III).
1H-NMR spectrum of the oligosaccharide in DV-3-5 isolated from eastern quoll milk carbohydrate by HPLC (Fig. 2b) and the characterized structure. The spectrum was obtained in D2O at 600 MHz with a Varian INOVA spectrometer operated at 293.1 K. Chemical shifts are expressed relative to internal 3-(trimethylsilyl)-1-propane sulfuric acid, sodium salt
DV-2-1, DV-2-2
As the 1H-NMR patterns of DV-2-1 and DV-2-2 were identical, it was concluded that these two peaks contained the same saccharides. The MALDI-TOF MS of the oligosaccharides in both of DV-2-1 and DV-2-2 had the MS ions at 1435 of [M + K] and 1419 of [M + Na], showing its monosaccharide composition to be that of an octasaccharide, [Hex]6[HexNAc]2.
The 1H-NMR spectrum of DV-2-1 (Fig. 4, chemical shifts in Supplemental Table 2) had two NAc shifts of β(1–6) linked GlcNAc at δ 2.067 and 2.043, showing the presence of β(1–6)GlcNAc linked to β(1–4) linked Gal and to β(1–3) linked Gal, respectively. The spectrum had the H-1 shifts of α-Glc, internal β(1–3) linked Gal, β-Glc, β(1–6) linked GlcNAc, external β(1–3) linked Gal, β(1–6) linked GlcNAc, and two β(1–4) linked Gal at δ 5.225, 4.687, 4.672, 4.664 and 4.656, 4.616, 4.594, 4.500 and 4.475, respectively. The shifts at δ 4.664 and 4.656 were assigned to the H-1 of β(1–6)GlcNAc linked to β(1–4) linked Gal, while the H-1 at δ 4.594 was assigned to the H-1 of β(1–6)GlcNAc linked to β(1–3) linked Gal. The signal intensity of the shift at δ 4.475 of H-1 of β(1–4) linked Gal corresponded to two chemical shifts, showing the presence of two β(1–4) linked Gal in addition to another β(1–4) linked Gal, whose H-1 shift arose from δ 4.500; thus the spectrum indicated the presence of three β(1–4) linked Gal residues. The shift at δ 4.172 was assigned to H-4 of β(1–3) linked Gal which was substituted at OH-3, while the shift at δ 4.157 was assigned to H-4 of β(1–4) linked Gal substituted at OH-3. From these observations, the oligosaccharides in DV-2-1 and DV-2-2 were characterized to be Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc, named as lacto-N-novooctaose.
1H-NMR spectrum of oligosaccharide in DV-2-1 isolated from eastern quoll milk carbohydrate by HPLC (Fig. 2c) and the characterized structure. The spectrum was obtained in D2O at 500 MHz with a JEOL ECP-500 Fourier transform-NMR spectrometer operated at 293.1 K
The alternative possibility that the oligosaccharide in these fractions was Gal(β1–4)GlcNAc(β1–6)Gal(β1–3)Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc seems unlikely because the β6N-acetylglycosaminyltransferase found in lactating mammary glands of the tammar wallaby transfers GlcNAc to a penultimate Gal but not to a non reducing Gal. The presence of lacto-N-novopentaose I, combined with the absence of Gal(β1–4)GlcNAc(β1–6)Gal(β1–4)Glc in eastern quoll milk, supports our hypothesis that the eastern quoll transferase has the same substrate specificity as that of the tammar wallaby, transferring GlcNAc to a penultimate Gal but not to a non reducing Gal of the linear unit.
DV-3-4
From the 1H-NMR and MALDI-TOF MS results it was concluded that this fraction was a mixture of the same saccharides as those in DV-3-5 and DV-2-1.
DV-3-7
From the 1H-NMR and MALDI-TOF MS, it was concluded that this fraction was a mixture of the saccharide found in DV-2-1 (lacto-N-novooctaose) as well as other unknown constituents with many unassigned chemical shifts.
DV-2-3
The data from 1H-NMR and MALDI-TOF MS indicated that this fraction contained a major component, Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc as well as another minor saccharide. As this fraction was not clearly resolved from DV-2-2 in the HPLC, it was concluded that it contained the same oligosaccharide as in DV-2-1 and DV-2-2. i.e., lacto-N-novooctaose.
DV-2-5, DV-2-6, DV-2-7. DV-2-8
The MALDI-TOF MS of the oligosaccharides in DV-2-5 to DV-2-8 had the MS ions at 1581 of [M + Na] as each major ion, showing the monosaccharide composition of a major saccharide in these fractions as [Hex]7[HexNAc]2. From the 1H-NMR spectra of DV-2-5 to DV-2-8, it was concluded that the major saccharide in each fraction contained a β(1–3) linked Gal additional to the structure in DV-2-1. This β(1–3) Gal residue could be linked either to the linear unit or to one of the branched units of Gal(β1–4)GlcNAc(β1–6).
DV-2-9 and DV-2-10
The MALDI-TOF MS of the oligosaccharides in DV-2-9 and DV-2-10 had the MS ions at 1946 of [M + Na] as one of the ions, showing the monosaccharide composition of a saccharide in these fractions as [Hex]8[HexNAc]3. From the 1H-NMR as well as the MS, it was thought that one oligosaccharide in each fraction contained an additional Gal(β1–4)GlcNAc(β1–6) unit and a β(1–3) linked Gal linked to the structure in DV-2-1.
DV-2-11 and DV-2-12
The MALDI-TOF MS of the oligosaccharides in DV-2-11 and DV-2-12 had the MS ions of 1743 of [M + Na] as each major ion, showing the monosaccharide composition of a major saccharide in these fractions to be [Hex]8[HexNAc]2. From the 1H-NMR as well as the MS, it was concluded that the major saccharide in each fraction contained two β(1–3) linked Gal residues additional to the structure in DV-2-1.
Characterization of acidic oligosaccharides
Fraction DV-1 separated into two unadsorbed peaks during ion exchange chromatography, as shown in Fig. 1b. The first peak designated as DV-1-1, was thought to contain a mixture of high molecular weight neutral oligosaccharides which were not investigated in this study. The components in the second peak, designated as DV-1-2, were further separated by HPLC, as shown in Fig. 2d. The separated oligosaccharides were characterized by 1H-NMR spectroscopy and MALDI TOF mass spectrometry.
DV-1-2-2, DV-1-2-4 and DV-1-2-6
The 1H-NMR spectra of fractions DV-1-2-4 and DV-1-2-6 showed that both contained each of two oligosaccharides. From the agreement of 1H-NMR spectra of DV-1-2-2, DV-1-2-4 and DV-1-2-6 (chemical shifts in Supplemental Table 3) with those of authentic saccharides as well as the published data, the oligosaccharides in these fractions were characterized to be as follows; 3’-sialyllactose (DV-1-2-2), lacto-N-novopentaose I sulfate a (DV-1-2-4-1), lacto-N-novopentaose I sulfate b (DV-1-2-4-2), sialyl lacto-N-novopentaose a (DV-1-2-6-1) and sialyl lacto-N-novopentaose c (DV-1-2-6-2) (see Table 1).
DV-1-2-10
The MALDI-TOF MS of the oligosaccharides in DV-1-2-10 had the MS ions at 1726 and 1764 of [M + K] and [M + 2 K-H], respectively, indicating a monosaccharide composition of [Neu5Ac]1[Hex]6[HexNAc]2.
The oligosaccharides in this fraction were characterized by comparing their 1H-NMR spectra (Fig. 5, chemical shifts in Supplemental Table 4) with that of DV-2-1. The spectrum had H-3 axial, H-3 equiatorial and NAc shifts at δ 1.801, 2.754 and 2.029, respectively, and H-3 of β-Gal, which was substituted by α(2–3) linked Neu5Ac, at δ 4.119 indicating the presence of an α(2–3) linked Neu5Ac. The spectrum had two NAc shifts of β(1–6) linked GlcNAc at 2.062 and 2.043, showing the presence of both a β(1–6)GlcNAc linked to β(1–4) linked Gal and a β(1–3) linked Gal, respectively. The spectrum had the H-1 shifts of α-Glc, internal β(1–3) linked Gal, β-Glc, β(1–6) linked Gal, external β(1–3) linked Gal, β(1–6) linked GlcNAc, and three β(1–4) linked Gal at δ 5.225, 4.687, 4.669, 4.652 and 4.644, 4.617, 4.593, 4.554, 4.500 and 4.474, respectively. The shifts at δ 4.652 and 4.644 were assigned to H-1 of β(1–6)GlcNAc linked to a β(1–4) linked Gal, while the H-1 at δ 4.593 was assigned to that of β(1–6)GlcNAc linked to a β(1–3) linked Gal. The H-1 shift of β(1–4) linked Gal at δ 4.554 shifted down field when compared with that at δ 4.474, due to the attachment of α(2–3) linked Neu5Ac. The line broadening of the shift of internal β(1–3) linked Gal at δ 4.687 showed that this corresponded to both external β(1–3) linked Gal of Gal(β1–3)Gal(β1–3) and Neu5Ac(α2–3)Gal(β1–3) units. The shift at δ 4.171 was assigned to H-4 of β(1–3) linked Gal, which was substituted at OH-3, while the shift at δ 4.155 was assigned to H-4 of β(1–4) linked Gal, which was substituted at OH-3. From these observations, the oligosaccharides in DV-1-2-10 were characterized to be Neu5Ac(α2–3)Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc (DV-1-2-10-1), and Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–3)[Gal(β1–4)GlcNAc(β1–6)]Gal(β1–4)Glc with an α(2–3) Neu5Ac linked to β(1–4)Gal residue of either branch of Gal(β1–4)GlcNAc(β1–6) units (DV-1-2-10-2).
1H-NMR spectrum of oligosaccharide in DV-1-2-10 isolated from eastern quoll milk carbohydrate by HPLC (Fig. 2d) and the characterized structures. The spectrum was obtained in D2O at 500 MHz with a JEOL ECP-500 Fourier transform-NMR spectrometer operated at 293.1 K
DV-1-2-1
The 1H-NMR showed that the component in DV-1-2-1 fraction was not a saccharide.
DV-1-2-3, DV-1-2-5, DV-1-2-7, DV-1-2-8, DV-1-2-9, DV-1-2-11, DV-1-2-12, DV-1-2-13, DV-1-2-14
Because of the low signal intensity in the NMR spectra, the components in these fractions could not be characterized.
Discussion
The only previous study on milk carbohydrates of the eastern quoll [13] showed that, as in the tammar wallaby and other marsupials, there are marked quantitative as well as qualitative changes during the course of lactation. The results of TLC and gel filtration had indicated that between 4 and 8 weeks post partum the milk carbohydrates consisted mainly of small neutral saccharides, of which a pentasaccharide was the most prominent. By 12 weeks, however, there was a marked decrease in these small oligosaccharides and an increase in larger ones.
The present investigation was done on a sample which had been pooled from these obtained at 7 to 11 weeks post partum [13], in order to provide enough material for detailed analysis. The results obtained with this pooled sample during gel filtration on BioGel P-2 (Fig. 1a) are in accord with those previously obtained at 8 weeks post partum using Sephadex G-25 [13, Fig. 1a], in that in both studies the most prominent constituent was a pentasaccharide. Our pooled sample yielded, in addition, a prominent peak (DV-2, Fig. 1a), containing larger oligosaccharides which were presumably similar to those that appeared in eastern quoll milk at about 10 to 12 weeks post partum [13, Fig. 1b].
The structures of the eastern quoll neutral and acidic milk oligosaccharides characterized in this study are shown in Table 1 and also in the upper parts of Figs. 3, 4 and 5. It can be seen that the large linear β(1–3) linked galacto oligosaccharides, such as Gal(β1–3)Gal(β1–3)Gal(β1–3)Gal(β1–4)Glc and Gal(β1–3)Gal(β1–3)Gal(β1–3)Gal(β1–3)Gal(β1–4)Glc, which have been identified in the milks of tammar wallaby [5, 6] and brushtail possum [12], were not detected in the eastern quoll milk carbohydrate fraction. In contrast we found that the most dominant oligosaccharide in milk of the eastern quoll is a branched saccharide, lacto-N-novopentaose I. This saccharide had also been identified in tammar wallaby and brushtail possum milks, in which it is one of a minor series of oligosaccharides that contain β(1–6) linked GlcNAc, including galactosyl lacto-N-novopentaose I [5–8, 12]. From the ratio of the peak height of DV-4 to the total peak heights of DV-3 to DV-7, plus two peaks of DV-2 in Fig. 1a, we estimate that the abundance of lacto-N-novopentaose I in the eastern quoll neutral oligosaccharide fraction was about 23 %. This is in contrast to that of the brushtail possum neutral milk oligosaccharides, in which lacto-N-novopentaose I could be estimated to constitute about 7.3 % [12]. The abundances of the pentasaccharide Gal(β1–3)Gal(β1–3)Gal(β1–3)Gal(β1–4)Glc (BP-3), the tetrasaccharide Gal(β1–3)Gal(β1–3)Gal(β1–4)Glc (BP-4) and the trisaccharide Gal(β1–3)Gal(β1–4)Glc (BP-5) in the neutral oligosaccharide fraction of the brushtail possum milk were about 24, 22 and 18 %, respectively [12], whereas in the eastern quoll milk neutral oligosaccharides those of the tetra (DV-5) and trisaccharide (DV-6) were only about 4.9 and 11 %, respectively. Furthermore, the abundance of lacto-N-novooctaose in the neutral oligosaccharide fraction of the eastern quoll milk could be estimated to be about 23 %, assuming that this octasaccharide constituted most of the second peak of DV-2.
The suggested biosynthetic pathways for the neutral milk oligosaccharides of marsupials, including those of the eastern quoll characterized in this study, are shown in Fig. 6. The activities of β3 and β4galactosyltransferases as well as of β6N-acetylglucosaminyltransferase have been found in lactating mammary glands of the tammar wallaby [17, 18]. Based on our results we hypothesize that the activity of β3galactosyltransferase is higher in lactating mammary glands of the tammar wallaby and the brushtail possum than that in those of the eastern quoll, while the activity of β6N-acetylglucosaminyltransferase is likely to be higher in those of the eastern quoll than in those of the tammar wallaby and brushtail possum. These suggestions would explain the differences between the milk carbohydrate compositions of the eastern quoll and the tammar wallaby or brushtail possum.
At present it is unclear to what extent the above-mentioned differences between milk oligosaccharide composition among marsupials can be explained in terms of phylogeny. It may be worth noting, however, that the herbivorous tammar wallaby and brushtail possum (Order Diprotodontis) are more closely related to each other than either is to the carnivorous eastern quoll (Order Dasyuromorphia) and it is tempting to speculate that differences between the milk oligosaccharides of the tammar wallaby and brushtail possum on the one hand, and those of the eastern quoll on the other, reflect these phylogenic relationships. However, support for such speculations would require further studies on the milk oligosaccharides of other marsupial species such as the numbat and Tasmanian devil (Dasyuromorphia) and the bilby and bandicoots (Paramelemorphia).
Galactosyl lacto-N-novopentaose III appears to be a novel component of marsupial milk oligosaccharides. Its presence suggests that the β3galactosyltransferase of lactating marsupial mammary glands catalyses the attachment of a β(1–3) linked Gal not only to the non reducing β(1–3/4) linked Gal residue of the linear saccharide but also to that of the branched saccharide. Although this hexasaccharide has not been detected in the milks of other marsupial species, it may occur in these milks at low concentrations. Lacto-N-novooctaose, which contains two β(1–6) linked GlcNAc residues, is also a novel oligosaccharide, although this type has been found in high molecular weight monosialyl and disialyl oligosaccharide fractions of tammar wallaby milk [9]. Again it is possible that this saccharide occurs at low concentrations in other marsupial milks, including those of tammar wallaby and brushtail possum. The dominance of this oligosaccharide in milk of the eastern quoll could be due to higher activity of β6N-acetylglucosaminyltransferase activity in the eastern quoll mammary glands, a suggestion which is consistent with the previous finding that the carbohydrate fraction of eastern quoll milk contains about four times more glucosamine (presumably derived from N-acetylglucosamine) than that of tammar wallaby milk [13]. It seems likely that, in contrast to the milk oligosaccharides of the tammar wallaby and brushtail possum, those of the eastern quoll are dominated by a major series of branched N-acetylglucosamine-containing saccharides, with linear β(1–3) linked saccharides constituting a minor series.
The core units of the eastern quoll milk acidic oligosaccharides are lactose, lacto-N-novopentaose I and lacto-N-novooctaose. This is in accord with these two saccharides being the predominant neutral oligosaccharides in this milk. All sialyl oligosaccharides characterized in this study contained α(2–3) linked Neu5Ac but no α(2–6) linked Neu5Ac. This is in contrast to the sialyl milk oligosaccharides of brushtail possum and red kangaroo which contained α(2–6) linked Neu5Ac as well as α(2–3) linked Neu5Ac residues. The absence of α(2–6) linked Neu5Ac could be caused by absence of α6sialyltransferase activity in the lactating mammary glands of the eastern quoll; this suggestion, however, is subject to confirmation from characterization of the other unidentified acidic oligosaccharides. It should be noted that, with respect to acidic oligosaccharides, we focused in this study only on monosialyl/monosulfated oligosaccharides, even though it can be assumed that disialyl and polysialyloligosaccharides were present in the adsorbed fraction during ion exchange chromatography; in a previous study [9], disialyl oligosaccharides were found in the adsorbed fraction during ion exchange chromatography of the carbohydrate fraction of tammar wallaby.
References
Jenness, R.E., Regehr, E.A., Sloan, R.E.: Comparative studies of milks. II. Dialyzable carbohydrates. Comp. Biochem. Physiol. 13, 339–352 (1964)
Urashima, T., Asakuma, S., Messer, M.: Milk oligosaccharides. In: Kamehring, J.P.Y. (ed.) Comprehensive glycoscience, pp. 695–724. Elsevier, Amsterdam (2007)
Ursshima, T., Saito, T., Nakamura, T., Messer, M.: Oligosaccharides of milk and colostrum in non-human mammals. Glycoconj. J. 18, 357–371 (2001)
Messer, M., Green, B.: Milk carbohydrates of marsupials II. Quantitative and qualitative changes in milk carbohydrates during lactation in the Tammar Wallaby (Macropus eugenii). Aust. J. Biol. Sci. 32, 519–531 (1979)
Messer, M., Trifonoff, E., Stern, W., Collins, J.G., Bradbury, J.H.: Structure of a marsupial milk trisaccharide. Carbohydr. Res. 83, 327–334 (1980)
Collins, J.G., Bradbury, J.H., Trifonoff, E., Messer, M.: Structures of four new oligosaccharides from marsupial milk, determined mainly by 13C-NMR spectroscopy. Carbohydr. Res. 92, 136–140 (1981)
Messer, M., Trifonoff, E., Collins, J.G., Brudbury, J.H.: Structure of a branched tetrasaccharide from marsupial milk. Carbohydr. Res. 102, 316–320 (1982)
Bradbury, J.H., Collins, J.G., Jenkins, G.A., Trifonoff, E., Messer, M.: 13C-NMR study of the structures of two branched oligosaccharides from marsupial milk. Carbohydr. Res. 122, 327–331 (1983)
Urashima, T., Saito, T., Tsuji, Y., Taneda, Y., Takasawa, T., Messer, M.: Chemical characterization of sialyl oligosaccharides isolated from tammar wallaby (Macropus eugenii) milk. Biochim. Biophys. Acta 1200, 64–72 (1994)
Anraku, T., Fukuda, K., Saito, T., Messer, M., Urashima, T.: Chemical characterization of acidic oligosaccharides in milk of the red kangaroo (Macropus rufus). Glycoconj. J. 29, 147–156 (2012)
Urashima, T., Taufik, E., Fukuda, R., Nakamura, T., Fukuda, K., Saito, T., Messer, M.: Chemical characterization of milk oligosaccharides of the koala (Phascolarctos cinereus). Glycoconj. J. 30, 801–811 (2013)
Urashima, T., Fujita, S., Fukuda, K., Nakamura, T., Saito, T., Cowan, P., Messer, M.: Chemical characterization of milk oligosaccharides of the common brushtail possum (Trichosurus vulpecula). Glycoconj. J. 31, 387–399 (2014)
Messer, M., FitzGerald, P.A., Merchant, J.C., Green, B.: Changes in milk carbohydrates during lactation in the eastern quoll, Dasyurus vivieerinus (Marsupialia). Comp. Biochem. Physiol. B88(4), 1083–1086 (1987)
Messer, M., Mossop, G.S.: Milk carbohydrates of marsupials I. Partial separation and characterization of neutral milk oligosaccharides of the Eastern Grey Kangaroo. Aust. J. Biol. Sci. 30, 379–388 (1977)
Dubois, M., Gills, K.A., Hamilton, J.K., Roberts, P.A., Smith, F.: Colorimetric method for determinetion of sugars and related substances. Anal. Chem. 28, 350–356 (1956)
Jourdian, G.W., Dean, L., Roseman, S.: The sialic acids XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J. Biol. Chem. 256, 430–435 (1971)
Urashima, T., Messer, M., Bubb, W.A.: Biosynthesis of marsupial milk oligosaccharides II: characterization of a β6-N-acetylglucosaminyltransferase in lactating mammary glands of the tammar wallaby, Macrupus eugenii. Biochim. Biophys. Acta 1117, 223–231 (1992)
Messer, M., Nicholas, K.R.: Biosynthesis of marsupial milk oligosaccharides: characterization and developmental changes of two galactosyltransferases in lactating mammry glands of the tammar wallaby, Macrupus eugenii. Biochim. Biophys. Acta 1077, 79–85 (1991)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
(PDF 27 kb)
Supplemental Table 2
(PDF 22 kb)
Supplemental Table 3
(PDF 25 kb)
Supplemental Table 4
(PDF 26 kb)
Rights and permissions
About this article
Cite this article
Urashima, T., Sun, Y., Fukuda, K. et al. Chemical characterization of milk oligosaccharides of the eastern quoll (Dasyurus viverrinus). Glycoconj J 32, 361–370 (2015). https://doi.org/10.1007/s10719-015-9600-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-015-9600-z