Abstract
Eukaryotic RNA polymerase III (RNAP III) transcribes tRNA genes and short interspersed elements that have internal promoters consisting of A- and B-blocks. The B-block binding subunit of the transcription initiation factor TFIIIC binds to the B-block. The mobile bacterial insertion sequence (IS) 1 contains a RNAP III promoter-like sequence, which stimulates bacterial transcription along with the bacterial ArtA protein. Here, the DNA-binding ability of ArtA was examined in vitro using a simple, newly developed method. Various DNA fragments, including RNAP III promoter fragments, were separately incubated with purified ArtA, and then loaded onto a polyacrylamide gel. Since DNAs bound by ArtA remain in the gel wells during electrophoresis, SDS was added into the wells at the electrophoresis halfway point. It was hypothesized that SDS would dissociate the DNA–ArtA complexes in the wells, and then the DNAs would begin to migrate. In fact, new bands appeared in all of the lanes at similar intensities, indicating that ArtA binds nonspecifically to DNA. Therefore, labeled wild-type RNAP III promoter fragments were incubated with either the unlabeled wild-type or mutant fragments and ArtA, and electrophoresed. The B-block(-like) sequences of IS1, a human Alu element, and an anuran tRNA gene were important for binding to ArtA. Additionally, in silico analyses revealed the presence of the RNAP III promoter-like structures in the IS1 isoforms and the IS3 family elements. These results suggest the presence of parts of the RNAP III transcription machinery in bacteria, and might imply that its prototype existed in the common ancestor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial and archaeal genes are transcribed by their inherent single RNA polymerases (Murakami and Darst 2003; Hirata and Murakami 2009). In eukaryotes there are RNA polymerases (RNAPs) I, II, and III, which transcribe different kinds of genes: RNAP I transcribes genes for ribosomal RNAs, except 5S rRNA; RNAP II synthesizes precursors of mRNAs, as well as most snRNAs and microRNAs; and RNAP III catalyzes the transcription of DNA to produce 5S rRNA, tRNAs and other small RNAs, such as short interspersed elements (SINEs) (Archambault and Friesen 1993; White 2011). It is thought that the principal transcription machinery diverged with the evolution of the host organism. Although the archaeal RNAP is closely related to eukaryotic RNAP II (Hirata and Murakami 2009), in silico analyses detected the presence of the Rpc34 subunit of eukaryotic RNAP III in archaea (Blombach et al. 2009). Rpc34 is a key determinant in RNAP III recruitment by the preinitiation complex (Brun et al. 1997). In addition, the B-block binding subunit of the transcription initiation factor TFIIIC for RNAP III is found in the archaeal and bacterial translated protein databases (Matsutani 2014).
The B-block binding subunit of TFIIIC interacts with the type II promoters of eukaryotic tRNA genes and SINEs (Schramm and Hernandez 2002). These promoters are inside the genes and consist of two short nucleotide sequences called A- and B-blocks (Galli et al. 1981; Schramm and Hernandez 2002). The A- and B-block nucleotide sequences are conserved in various eukaryotic organisms and, interestingly, present also in bacterial tRNA genes (Galli et al. 1981; Matsutani 2004). B-block binding subunits contain helix-turn-helix (HTH) motif regions of about 75 amino acids (aa), which probably interact with the type II promoters. Their HTH regions are characteristic of the family of B-block TFIIIC motifs in the Pfam database (http://pfam.xfam.org/family/PF04182). Prokaryotic B-block binding subunits containing a B-block TFIIIC motif are about 100 aa in length, and larger eukaryotic subunits (more than 1000 aa in length) are suggested to be constructed by the repeated duplications of B-block TFIIIC over long periods of evolutionary time (Matsutani 2014).
Bacterial IS1 is a DNA fragment of 768 bp that moves from one site to another site in the genome (for a review see Mahillon and Chandler 1998). There are two tandem, out-of-frame, partially overlapping genes (insA and B′-insB) inside IS1, and the single mRNA is synthesized from the promoter in the left terminal inverted repeat of IS1 (Mahillon and Chandler 1998). IS1 is reported to have a eukaryotic RNAP III promoter-like sequence in insA (Matsutani 2007). The region containing the RNAP III promoter-like sequence acts as a cis-element to stimulate transcription from a bacterial promoter located upstream of the region (Matsutani 2005). The RNAP III promoter region of the Alu element, which is a human SINE, also stimulates bacterial transcription, similar to the cis-element region of IS1, and it is suggested that parts of the RNAP III transcription machinery may functionally exist in bacteria (Matsutani 2007). The presence of the bacterial artA gene increases the transcriptional stimulation along with the cis-element region of IS1 (Matsutani 2005). The ArtA protein is 104 aa in length, and the purified ArtA protein was shown to bind specifically to the RNAP III promoter-like sequence region of IS1 using a nitrocellulose filter binding assay (Matsutani 2012). However, the results of gel shift assays are not clear (Matsutani 2012). Because of the formation of large DNA–protein complexes in the reaction mixtures, DNAs remained at the top of the polyacrylamide gel (bottoms of the wells) when voltage was applied, and no shifted bands were observed. Thus, an agarose gel, not a polyacrylamide gel, had to be used to allow the complexes to enter the gel matrix. The bands that appeared in the agarose gel were weak and indistinct (Matsutani 2012). ArtA has the intrinsic ability to bind to DNA nonspecifically (Matsutani 2012). Previously identified DNA-binding motifs are not detected in ArtA (Matsutani 2012), and significant similarities are not found between ArtA and the B-block binding subunits on the amino acid sequence level (Matsutani 2014).
The gene expression of IS1 is unique (Mahillon and Chandler 1998). A ribosomal frameshift occurs at the slippery sequence, which is present in the crossover region between the insA end region and B′ region of B′-insB, and this frameshift is essential for transposase production. The bacterial IS3 family is the largest and most widespread family of IS elements (https://www-is.biotoul.fr//). Most of the IS3 family elements are in the range of 1200–1500 bp in length, and contain two tandem, out-of-frame, partially overlapping genes, as seen in IS1. Ribosomal frameshifts occur in some of the IS3 family elements, generating fusion proteins derived from two genes (Mahillon and Chandler 1998).
In this paper, the DNA-binding property of the bacterial ArtA protein is analyzed in vitro. To this end, a new assay method that allows the protein to easily aggregate in the reaction mixture was developed. The assay demonstrated that the RNAP III promoter-like sequence in the bacterial IS1 is important for the binding between ArtA and IS1. Similar results were obtained using the RNAP III promoter sequences of the human Alu element and the anuran tRNA gene. Moreover, whether the isoforms of IS1 and the IS3 family elements contained RNAP III promoter-like sequences was examined in silico.
Materials and methods
Plasmids
The plasmids used in this study were pSAM267, pSAM285, pSAM299, pSAM296 (Matsutani 2005), pSAM351, pSAM354, pSAM355 (Matsutani 2007), pQE70 (Qiagen), and pSAM520 (unpublished, see the next section). Construction of pSAM901 was as follows: the Xenopus laevis tRNALue gene (bp 9–99 of J01015) was artificially synthesized, including the addition of a XbaI site (TCTAGA) at the 5′ end and a BamHI (GGATCC) site at the 3′ site. Then, the XbaI-BamHI fragment containing the tRNA gene was cloned into the XbaI and BamHI sites of pUC57. Construction of pSAM902 and pSAM903 was identical to that of pSAM901, but they lack bp 20–30 and bp 74–84, respectively, of J01015. Gene synthesis and cloning were performed by GENEWIZ Inc., Japan.
Procedures to examine the DNA-binding ability of ArtA by adding SDS during electrophoresis of the ArtA-DNA mixtures (gel dissociation assays)
DNA fragments were PCR-amplified using the plasmids and primers shown in Fig. 1. Primer sequences were previously reported (Matsutani 2012). The nucleotide sequence of the M7 primer was 5′-CGAAAGGGGGATGTGCTGCA-3′ (bp 100–81 in the lacZ of V00296). Production of the IS1IRL and pQE-4 fragments was as described in Matsutani (2012). The pQE-3 and pQE-5 fragments were amplified from pQE70, and their nucleotide sequences correspond to bp 2696–3206 and bp 2696–218 of pQE70, respectively (primer sequences will be provided upon request). The 3′-rpoA fragment, which was amplified from pSAM520, consists of bp 185–252 of pUC18 (L08752), bp 1205–894 of rpoA (J01685), and bp 287–372 of pUC18. The construction of pSAM520 and the primers used for PCR amplification will be provided upon request. The expression and purification of the His-tagged ArtA protein were as described in Matsutani (2012), and the protein was used for assays.
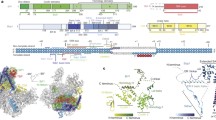
Structures of the parent (wild-type) and mutant plasmids used for PCR amplification. The amplified regions are shown as thick lines below the structures, and the PCR products were used for binding assays with the bacterial ArtA protein. Next to the parent plasmid structures, bp positions and nucleotide sequences of the RNAP III promoter’s A- and B-blocks are shown. For mutants, nucleotides substituted for the entire A- and B-block sequences or deleted bp positions are indicated. Primers used for PCR amplification and the sizes of the PCR products are shown on the right side. Asterisks indicate the positions of 6FAM. a IS1 (V00609) internal region containing a RNAP III promoter-like sequence. b Human Alu element (M13479) RNAP III promoter region (Perez-Stable et al. 1984), and bp position 1 in this figure corresponds to bp position 8 in M13479. c RNAP III promoter region of the X. laevis tRNALeu gene (J01015), and bp position 1 in this figure corresponds to bp position 13 in J01015. d Comparison of the RNAP III promoter(-like) sequences in IS1, Alu, and X. laevis tRNALeu gene with the consensus sequence. In pSAM296, the substitution generates the stretch of GGATCCACCGC in which ACCGC is from bp 191–195 in IS1. Compared with the B-block-like sequence in wild-type IS1, this stretch seems more functional as the B-block because of the better positioning of the conserved nucleotides (Matsutani 2007)
Binding assays of ArtA to single DNA fragments were carried out as follows: 0.2 µg of ArtA (15 pmol) was incubated for 10 min at 27 °C in 20 µl of binding buffer 1 (150 mM KCl, 10 mM MgCl2, 0.1 mM EDTA, 0.5 mM DTT, 0.1 mg/ml bovine serum albumin, 11.5 % glycerol, and 25 mM Tris–HCl pH 7.5). Then, 1.5 pmol of the DNA fragment was added, and the incubation was continued for 20 min. For electrophoresis, a 4 % polyacrylamide gel was prepared with 30 % Acrylamide/Bis (29:1) solution and TAE buffer (Sambrook et al. 1989). The gel size was 10 × 10 × 0.1 cm, and the width of each well was 4 mm. A total of 5 µl of 5X loading buffer [50 % glycerol and 0.2 % bromophenol blue (BPB) in 5X TAE buffer] was added to the reaction mixture. The mixture was divided into two aliquots, which were loaded into two adjacent wells of the gel. Electrophoresis began at 10 V/cm. When the BPB moved ~5 cm from the top of the gel, the voltage application was stopped and 20 µl of the elution buffer 1 [1 % (wt/vol) SDS and 10 % glycerol in 1X TAE buffer] was gently added to one of the wells filling with the running buffer. The running buffer was not at all removed from the wells before addition of the SDS solution. Voltage was reapplied at 1 V/cm for 30 min and then increased to 10 V/cm. Electrophoresis was stopped when the BPB had moved to the bottom of the gel. The gel was stained in a 0.5 µg/ml ethidium bromide solution and photographed.
Competition experiments were carried out using the same procedure as described just above, but 1.5 pmol of the 6FAM-labeled DNA fragment was added to each of the reaction mixtures together with 1.5 pmol of the unlabeled competitor DNA fragment. Loading buffer without BPB was used, and the entire volume of each mixture was loaded into a well. 0.3 pmol of probe DNAs were loaded as size markers. After electrophoresis, gels were scanned using a FLA-3000 fluorescent image analyzer (Fuji Photo Film). The band signals were quantified using the ImageJ software (Rasband 1997–2015).
Nitrocellulose filter binding assay
Production of the lacZ fragment was shown in Matsutani (2012). The AluAB366, IS1IRL, and pQE-4 fragments were also used. The nitrocellulose filter binding assay was almost the same as described in Matsutani (2012). Briefly, DNA fragments (about 4–5 pmol each) were mixed and incubated with 0.6 µg of ArtA (46 pmol) for 10 min at 27 °C in 100 µl of binding buffer 2 (100 mM KCl, 10 mM MgCl2, 0.1 mM EDTA, 0.5 mM DTT, 0.1 mg/ml bovine serum albumin, 17 % glycerol, and 25 mM Tris–HCl pH 7.5). Heparin (Sigma) was then added at 10 µg/ml, and the incubation was continued for 10 min. The samples were filtered through nitrocellulose filters (NC45, Whatman), which were washed three times with 1 ml of binding buffer 2. DNA fragments retained on the filter were eluted by soaking in 1 ml of elution buffer 2 [0.2 % (wt/vol) SDS and 25 mM Tris–HCl pH 7.5] for 30 min with shaking. The eluted DNA fragments were ethanol-precipitated and electrophoresed in 5 % polyacrylamide gels.
In silico analyses
Programs used to compare nucleotide sequences were as follows: BLASTN on the NCBI website at http://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&BLAST_SPEC=&LINK_LOC=blasttab&LAST_PAGE=blastn (Johnson et al. 2008); BLAST-Two-Sequences on the NCBI website at http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=MegaBlast&PROGRAM=blastn&BLAST_PROGRAMS=megaBlast&PAGE_TYPE=BlastSearch&BLAST_SPEC=blast2seq&QUERY=&SUBJECTS= (Johnson et al. 2008); and Clustal Omega on the EBI website at http://www.ebi.ac.uk/Tools/msa/clustalo/ (Sievers et al. 2011). BLASTN was conducted using the default setting. BLAST-Two-Sequences was performed using the default setting except that the word size was 7. The alignment parameters in Clustal Omega were used at the default settings.
Results
A new simple method to examine the DNA-binding ability of the ArtA protein
Although ArtA has been shown to bind specifically to the fragment containing the RNAP III promoter-like sequence in IS1 using nitrocellulose filter binding assays, the result of a gel shift assay using an agarose gel was not clear (Matsutani 2012). It was necessary to confirm that ArtA binds specifically to the RNAP III promoter-like sequence in IS1. When DNA is incubated with ArtA in the reaction mixture and electrophoresed in gel shift assay, there is always a portion that stays at the top of the polyacrylamide gel (bottom of the well). The remaining DNA seems to form a large complex with ArtA. Thus, there was a separation of the possible DNA–protein complex from the reaction mixture containing unbound free DNA. I hypothesized that this phenomenon could be used by adding a detergent like SDS to the wells of the polyacrylamide gel after the reaction mixtures were electrophoresed for a while. The SDS would dissociate the putative DNA–ArtA complexes remaining at the bottoms of the wells, and then the DNA fragments freed from ArtA would enter the gel matrix. These late migrating DNAs represent the DNAs bound by ArtA in the reaction mixtures.
First, I examined whether a band of DNA appeared at the appropriate position in the lane after the addition of SDS to the well halfway through electrophoresis. The IS1AB361 fragment, which contains the RNAP III promoter-like sequence (Fig. 1a), was incubated with or without ArtA. The reaction mixtures were electrophoresed as described in “Materials and Methods”. As shown in Fig. 2, when ArtA was not added to the reaction mixture, there were no additional bands, only the IS1AB361 band [indicated as IS1AB361(free) in Fig. 2], in both cases with and without SDS. When the reaction was carried out with ArtA and SDS was not added during electrophoresis, DNA bands appeared at the top of the gel (bottom of the well) and at the position of the free IS1AB361 DNA (Fig. 2). The band at the top of the gel appeared to represent the large complex of ArtA and DNA, and the other band was the DNA that did not associate with ArtA in the reaction mixture. When SDS was added to the well halfway through electrophoresis, the band at the top of the gel disappeared and a band appeared in the middle region of the lane (Fig. 2). The position of this band corresponded to that of IS1AB361 DNA that was loaded in an unused well as a size marker, concurrent with SDS loading (Fig. 2). This result shows that the complex of ArtA and IS1AB361 was detained at the bottom of the well and dissociated by SDS. The IS1AB361 molecules that were freed from ArtA entered the gel matrix to be electrophoresed properly. A similar result was obtained using IS1AB315 instead of IS1AB361 (Fig. 2).
Migration of the DNA fragments present in the binding reaction mixtures through the polyacrylamide gels. Each of the DNA fragments was incubated with the bacterial ArtA protein, the mixture was divided into two aliquots, and the samples were loaded to the two adjacent wells of a 4 % polyacrylamide gel. Halfway through electrophoresis, an SDS solution was added to one of the two adjacent wells. For the DNA fragments of IS1AB361 and IS1AB315, the same procedure was also followed without ArtA, as controls. Concurrently with SDS addition, the same DNA fragments as used in the binding reactions were loaded to empty lanes as size markers (shown as fragment names parenthesized above lanes). DNA bands that appeared after SDS addition are indicated as “fragment name (eluted)” in the figure. The “fragment name (free)” in the figure represents the band of the free DNA that did not associate with ArtA in the reaction mixture
ArtA can interact nonspecifically with DNA (Matsutani 2012). Therefore, similar assays were performed with the DNA fragments not containing or associated with the RNAP III promoter. The DNA fragments used were IS1IRL, pQE-4, pQE-3, 3′-rpoA, and pQE-5 (“Materials and Methods”). All of the results obtained were similar to those obtained with IS1AB361 and IS1AB315, which contain RNAP III promoter-like sequences (Fig. 2): When SDS was not added, a DNA band appeared at the top of the gel (bottom of the well) together with a band at the appropriate position for the size of the DNA fragment. However, with the addition of SDS, the band at the top of the gel disappeared and a band at the appropriate position for the size of the DNA fragment, corresponding to the DNA fragment size marker, appeared. ArtA associated with the DNA fragments irrelevant of the RNAP III promoter. In the three independent experiments of Fig. 2, clear differences in the quantities of ArtA-bound DNAs could not be visually found between the fragments containing a RNAP III promoter-like sequence and its irrelevant fragments.
Quantitative analyses of the DNA bands were not conducted here. The nucleotide sequences of IS1AB361, IS1AB315, and AluAB366 are (almost) entirely different from those of the other fragments in Fig. 2 (“Materials and Methods”). Therefore, if quantification was performed and differences of band intensity were found between the eluted DNA fragments, the results do not reflect only the effect of the presence or absence of the RNAP III promoter. There might be different effects of the non-specific binding of ArtA to various DNAs in the experiments of Fig. 2. These experiments showed qualitatively that SDS worked well in the gel wells for the complexes between ArtA and various DNAs. Only in the case of pQE-5, a DNA band still clearly appeared in the well of the SDS-added lane together with a new band in the middle of the lane (Fig. 2). This is because the pQE-5 PCR product sample contained a large DNA fragment(s) with pQE-5 itself (data not shown, but as a related data see the well of the marker lane of 3′-rpoA and pQE-5 and compare with the assay sample lanes of 3′-rpoA). Although the same volumes from the identical assay samples were loaded to pairs of lanes (see “Materials and Methods”), intensities of the free DNAs of IS1IRL, pQE-3, and pQE-5 were clearly different between the SDS-added lanes and no SDS lanes (Fig. 2). In addition, independent experiments sometimes showed intensity patterns of the bands different from those in Fig. 2 (data not shown). These are probably due to the difficulty of precise pipetting of the heterogeneous reaction mixtures containing large protein-DNA aggregates. I say here that the bands of SDS-eluted DNAs nevertheless always appeared clearly and intensely (Fig. 2).
One may think that part of the DNA–protein complexes were removed or flushed from the wells when the SDS solution was added into the wells which were filling with the running buffer (“Materials and Methods”). However, it is clear that the DNA–protein complexes in the wells were strongly retained at the bottoms of the wells (top of the gel). There were always intense bands at the bottoms of the wells of the no SDS lanes when ArtA was used (Fig. 2). After electrophoresis run, glass plates were detached from the tank, and acrylamide gels were peeled from the plates for staining. If the DNA–protein complexes were floating in the running buffer in the wells, they would be flushed out in these steps and no (or weak) bands should be at the bottoms of the wells (top of the gel). Hereafter, in this study, the procedure to detect the DNA bound by ArtA on a gel using SDS is called the gel dissociation assay. It should be noted, however, that with the self-aggregation of ArtA in the reaction mixtures, DNA fragments might be accidentally incorporated into the aggregates.
The B-block-like sequence is important for the binding between ArtA and bacterial IS1
To examine the specific binding ability of ArtA in the gel dissociation assay, and also to exclude the possibility of accidental incorporation of DNA into the ArtA aggregate in the assay, competition experiments were performed. 6FAM-labeled IS1AB361 was PCR-amplified from pSAM267 using the M7 and 6FAM-labeled QE3 primers (Fig. 1a). Unlabeled wild-type and mutant fragments were prepared from pSAM267, pSAM285, pSAM299, and pSAM296 using the M7 and QE3 primers (Fig. 1a): pSAM285, pSAM299, and pSAM296 are identical to pSAM267 except that pSAM285 contains an A-block mutation, and pSAM299 and pSAM296 contain B-block mutations. It has been demonstrated that the lacZ expression levels of pSAM285 and pSAM299 are ~threefold and twofold lower than that of pSAM267, respectively, and that the lacZ expression level of pSAM296 is 1.5-fold higher than that of pSAM267 (Matsutani 2007). The 6FAM-labeled IS1AB361 and each of the unlabeled IS1AB361 and mutants were incubated with ArtA. A binding reaction using only 6FAM-labeled IS1AB361 was also carried out. To exclude the influence of possible imprecise pipetting (see the preceding section), the entire volume of each of the reaction mixtures was loaded to one lane and electrophoresed (“Materials and Methods”). SDS was added to all of the sample lanes halfway through electrophoresis. The gel was scanned using a FLA3000 and the 6FAM-labeled IS1AB361 DNA was visualized. As shown in Fig. 3a, when unlabeled IS1AB361 (wild-type) was added as a competitor to the reaction mixture, the band signal of 6FAM-labeled IS1AB361 eluted by SDS was weak compared with that in the reaction without the competitor. When unlabeled IS1AB361 lacking the A-block-like sequence (synthesized from pSAM285 and named IS1AB361↓A in Fig. 1a) was added to the reaction mixture, the band signal of 6FAM-labeled IS1AB361 eluted by SDS showed a similar intensity compared with the signal produced when unlabeled wild-type IS1AB361 was used as a competitor (Fig. 3a). On the other hand, when unlabeled IS1AB361 lacking the B-block-like sequence (synthesized from pSAM299 and named IS1AB361↓B in Fig. 1a) was added to the reaction mixture, the band signal of 6FAM-labeled IS1AB361 eluted by SDS appeared a little more intensely than the signals produced when wild-type IS1AB361 and IS1AB361↓A were used as competitors (Fig. 3a). Moreover, when unlabeled IS1AB361 containing the B-block sequence mutation, which results in the higher expression level of the downstream gene (synthesized from pSAM296 and named IS1AB361↑B in Fig. 1a), was used, the band signal of 6FAM-labeled IS1AB361 eluted by SDS was weaker than those using wild-type IS1AB361 and IS1AB361↓A (Fig. 3a). Quantification of the results from the independent competition experiments was carried out (“Materials and Methods”). Ratios of signal amounts of the eluted DNAs in the lanes with mutant competitors to that with the wild-type competitor were as follows: +↓A, 1.03 ± 0.11; +↓B, 1.17 ± 0.04; and +↑B, 0.78 ± 0.11 (see Supplementary Fig. 1Ab). These results show that the B-block-like sequence is important for binding to ArtA. When competitor DNAs were used, the total amounts of DNA in the reaction mixtures increased twofold compared with the mixture without a competitor. This probably results in an increase in the nonspecific binding of ArtA to DNA, and thus the band signals with competitors may have been weaker than without competitors. If molar excesses of competitor DNAs to the probe DNA were used in the competition assays, the signal intensities of the SDS-eluted probe DNAs would become weaker, resulting in unclear differences between them. Alternatively, competition assays were performed using 0.75 pmol of the probe DNA, which was one-half of the original amount. However, the band intensities of eluted DNAs became weak, generating less clear differences between them (data not shown). Note again that as shown in Fig. 3a, there was a clear difference between the band intensities of eluted DNAs when IS1AB361↓B and IS1AB361↑B were used as competitors.
Binding assays of ArtA to the RNAP III promoter(-like) sequences and their mutants. Each of the 6FAM-labeled DNA fragments containing wild-type sequences and the unlabeled competitor fragment containing the wild-type or mutated sequence were mixed and incubated with ArtA (“Materials and methods”). As controls, assays without the protein were performed. Halfway through electrophoresis, an SDS solution was added into the wells. Concurrent with SDS addition, the labeled DNA fragments were loaded to empty lanes as size markers. Gels were scanned by FLA3000. Band signals that appeared after SDS addition are indicated as “eluted” in the figure. The “free” in the figure represents the band signal from the free DNA that did not associate with ArtA in the reaction mixture. a IS1 fragment containing the RNAP III promoter-like sequence. b Human Alu RNAP III promoter fragment. c RNAP III promoter fragment of the X. laevis tRNALue gene. d Binding activity of ArtA to each of the wild-type and mutant RNAP III promoters of the Alu element. 1.5 pmol of labeled AluAB366, and unlabeled AluAB366, AluAB366ΔA, and AluAB366ΔB were separately used in reactions, and the entire volume of each reaction mixture was loaded into a well. SDS solution was added halfway through the electrophoresis. 0.8 pmol of unlabeled AluAB366 was used as size markers. DNA bands were visualized by ethidium bromide staining and UV photography
Signals in all of the wells of the Fig. 3a gel, including the wells of marker lanes, were also quantified using ImageJ. While the reaction mixtures containing 1.5 pmol of the 6FAM-labeled DNA was loaded to the wells, 0.3 pmol of the probe was loaded to the marker lanes (“Materials and Methods”). Nevertheless, there were significant amounts of signals in the wells of the marker lanes compared with the assay sample lanes (Fig. 3a; Supplementary Fig. 1Aa). There was a band signal also in the well of the sample lane to which the reaction mixture without ArtA had been added. The signal intensity was almost at the same level as those in wells of the assay sample lanes with ArtA (Fig. 3a; Supplementary Fig. 1Aa). Although the scanner cannot exactly measure the signals at the edge of the gel because of the measurement system, the signal intensities in wells of the sample lanes with ArtA fluctuated within about ±10 % of that in the well of no ArtA sample lane (Supplementary Fig. 1Aa). These results clearly show that a small amount of longer DNA fragments were present in the PCR product sample. Signals in the wells of assay sample lanes do not derive from the ArtA-probe complex and can be ignored. In addition, the band intensities of free DNAs in the lanes with competitors were quantified, and these bands were shown to have almost the same amounts of signals (Supplementary Fig. 1Ac). Differences of the signal amounts between the eluted DNAs in the lanes with competitors were much smaller compared with the level of the signal amounts of free DNAs (within the error range in the signal amount level of the free DNAs, data not shown). Therefore, differences of the signal amounts between the eluted DNAs could not be correlated with those of the signal amounts between the free DNAs in the same lanes.
The B-block sequence in the RNAP III promoter of the human Alu element is important for binding to ArtA
To examine specific binding ability of ArtA to the RNAP III promoter in the human Alu element, nitrocellulose filter binding assays were carried out. Two mixtures of the PCR products were prepared: fragments of IS1IRL (478 bp), AluAB366 (366 bp), and pQE-4 (300 bp); and fragments of lacZ (639 bp), AluAB366, and pQE-4 (Fig. 1b; Matsutani 2012). Each of the mixtures was incubated with ArtA, the samples were filtered, and the DNA fragments that were bound by ArtA and retained on the filters were recovered. Heparin was added to the reaction mixtures to decrease nonspecific binding of ArtA to DNA (“Materials and Methods”; Matsutani 2012). Figure 4 shows the PCR products in the mixtures before filtration, and the DNA fragments retained on the filter after filtration. When the mixture of IS1IRL, AluAB366, and pQE-4 was used, the AluAB366 fragment was retained on the filter (Fig. 4a), showing that ArtA binds specifically to the fragment that contains the RNAP III promoter in the human Alu element. Also when the mixture of lacZ, AluAB366, and pQE-4 was used, the AluAB366 fragment was retained on the filter (Fig. 4b).
Results of nitrocellulose filter binding assays. In each gel, the mixture of PCR products before filtration (left lane) and the DNA fragment retained on the membrane after filtration (right lane) are shown. Small aliquots of the PCR product mixtures prior to incubation with ArtA were taken and electrophoresed with the retained fragments for exact comparisons. a DNA fragments of IS1IRL, AluAB366, and pQE-4. b DNA fragments of lacZ, AluAB366, and pQE-4
To examine which region of the Alu promoter is important for the binding to ArtA, AluAB366 was first confirmed to be bound by ArtA in the gel dissociation assay. AluAB366 and ArtA were incubated together, divided into two aliquots, and loaded into two adjacent wells of a polyacrylamide gel. As shown in Fig. 2, when SDS was not added, a DNA band appeared at the top of the gel (bottom of the well) together with a band at the appropriate position for the DNA fragment in the same lane. However, with the addition of SDS halfway through the electrophoresis, the band at the top of the gel disappeared and a band at the position corresponding to that of AluAB366AB, which was loaded in another well at the same time that SDS was added, appeared. This result was similar to those for the IS1 fragments containing a RNAP III promoter-like sequence and other DNA fragments not associated with the RNAP III promoter.
Next, a competition experiment was performed. The 6FAM-labeled AluAB366 was PCR-amplified from pSAM351 using the M7 and 6FAM-labeled QE3 primers (Fig. 1b). The unlabeled wild-type and mutant fragments were prepared from pSAM351, pSAM354, and pSAM355 using the M7 and QE3 primers (Fig. 1b). pSAM354 and pSAM355 are identical to pSAM351 except they lack bp 1–15 and bp 76–87, respectively, of Alu. These regions contain the entire A- and B-block sequences. It has been demonstrated that the lacZ expression levels of pSAM354 and pSAM355 are about 3.5-fold and twofold lower than that of pSAM351, respectively (Matsutani 2007). The 6FAM-labeled AluAB366 and each of the unlabeled AluAB366 and mutant fragments were mixed and incubated with ArtA in the binding buffer. A binding reaction using only 6FAM-labeled AluAB366 was also performed. The entire volumes of these reaction mixtures were loaded into the wells of a polyacrylamide gel and electrophoresed. SDS solution was added to all of the sample wells halfway through the electrophoresis. As shown in Fig. 3b, when unlabeled AluAB366 (wild-type) was used as a competitor, the band signal of 6FAM-labeled AluAB366 eluted by SDS was weak compared with the signal without the competitor. When unlabeled AluAB366 lacking the A-block sequence (named AluAB366ΔA in Fig. 1b) was added to the reaction mixture, the band signal of 6FAM-labeled AluAB366 eluted by SDS was a little weaker than the signal produced when AluAB366 (wild-type) was used as a competitor (Fig. 3b). When unlabeled AluAB366 lacking the B-block sequence (named AluAB366ΔB in Fig. 1b) was added to the reaction mixture, the band signal of 6FAM-labeled AluAB366 eluted by SDS was more intense than the signals produced when AluAB366 (wild-type) and AluAB366ΔA were used (Fig. 3b). Quantification of the results from the independent competition experiments was carried out, and ratios of signal amounts of the eluted DNAs in the lanes with mutant competitors to that with the wild-type competitor were as follows: +ΔA, 0.84 ± 0.11; and +ΔB, 1.25 ± 0.08 (see Supplementary Fig. 1Ba). These results show that the B-block sequence in the Alu RNAP III promoter is important for the binding to ArtA. The band intensities of free DNAs in the lanes with competitors were quantified, and these bands were shown to have almost the same amounts of signals (Supplementary Fig. 1Bb). The differences of the signal amounts between the eluted DNAs in the lanes with competitors were much smaller compared with the level of the signal amounts of free DNAs (within the error range in the signal amount level of the free DNAs, Supplementary Fig. 1Bc).
The DNA fragments of 6FAM-labeled AluAB366, and unlabeled AluAB366, AluAB366ΔA, and AluAB366ΔB, were separately mixed with ArtA in the binding buffer, and gel dissociation assays were carried out to examine whether specific binding was observed (Fig. 3d). Quantification of the band intensities of the eluted DNAs was performed using the result of Fig. 3d and those from independent experiments, and there were no significant differences (Supplementary Fig. 1D). The competition experiment was essential. Additionally, the 6FAM labeling of DNA did not influence the binding of ArtA to DNA (Fig. 3d; Supplementary Fig. 1D).
The B-block sequence in the RNAP III promoter of the Xenopus tRNA gene is also important for binding to ArtA
The X. laevis tRNALeu gene was previously used for the locational mapping of the RNAP III promoter, and this biochemical study revealed that the RNAP III promoter consisted of two sequence blocks (now called A- and B-blocks) (Galli et al. 1981). To examine the specific binding ability of ArtA to the RNAP III promoter sequences of the tRNA genes, the X. laevis tRNALeu gene was used as a representative. The wild-type tRNA gene and the gene lacking either the A- or B-block sequence were artificially synthesized and cloned into pUC57, generating pSAM901, pSAM902, and pSAM903 (“Materials and Methods”). The 6FAM-labeled tRNA AB252 fragment was PCR-amplified from pSAM901 using the 6FAM-labeled M5 and RV2 primers (Fig. 1c). The unlabeled tRNA AB252 and mutant fragments were prepared from pSAM901, pSAM902, and pSAM903 using the M5 and QE3 primers (Fig. 1c). pSAM902 and pSAM903 are identical to pSAM901 except they lack bp 8–18 and bp 62–72, respectively, of the tRNALeu gene, which correspond to the entire A- and B-block sequences, respectively. The 6FAM-labeled tRNA AB252 and each of the unlabeled tRNA AB252 and mutant fragments were mixed and incubated with ArtA. A binding reaction using only 6FAM-labeled tRNA AB252 was also performed. The reaction mixtures were electrophoresed as described in “Materials and Methods”. As shown in Fig. 3c, when unlabeled tRNA AB252 (wild-type) was used as a competitor, the band signal of 6FAM-labeled tRNA AB252 eluted by SDS was weak compared with that without the competitor. When unlabeled tRNA AB252 lacking the A-block sequence (named tRNA AB252ΔA in Fig. 1c) was used as a competitor, the band signal of 6FAM-labeled tRNA AB252 eluted by SDS showed a similar intensity to the result when tRNA AB252 (wild-type) was used as a competitor (Fig. 3c). When unlabeled tRNA AB252 lacking the B-block sequence (named tRNA AB252ΔB in Fig. 1c) was used as a competitor, the band signal of 6FAM-labeled tRNA AB252 eluted by SDS was more intense than the signals produced when wild-type and ΔA fragments were used as competitors (Fig. 3c). Quantification of the results from the independent competition experiments was carried out, and ratios of signal amounts of the eluted DNAs in the lanes with mutant competitors to that with the wild-type competitor were as follows: +ΔA, 0.90 ± 0.13; and +ΔB, 1.34 ± 0.05 (Supplementary Fig. 1Ca). These results show that the B-block of the X. laevis tRNALeu promoter is important for the binding to ArtA. The band intensities of free DNAs in the lanes with competitors were quantified, and these bands were shown to have almost the same amounts of signals (Supplementary Fig. 1Cb). The differences of the signal amounts between the eluted DNAs in the lanes with competitors were much smaller compared with the level of the signal amounts of the free DNAs (within the error range in the signal amount level of the free DNAs, data not shown).
RNAP III promoter-like structure in the bacterial IS1 family
There are nucleotide sequence variants of IS1 in enterobacteria (Mahillon and Chandler 1998), called isoIS1s (IS1 isoforms). Although most isoIS1s exhibit sequence divergences lower than 10 % at the nucleotide level in the IS1 family, the isoIS1s of NuXi (called also IS1N) (J01737, bp 18–783) and IS1H (NC_017635, bp c4390877–4390114) are significantly different from the others (45–47 % divergence at the nucleotide level) (Mahillon and Chandler 1998). However, NuXi and IS1H are similar to each other (20 % divergence at the nucleotide level), and no other distinct members in the IS1 family were discovered using BLASTN in this study (data not shown).
The RNAP III promoter-like sequence has been found in IS1R (V00609), which is the IS1 in the multiple drug resistance plasmid R100. IS1R has been used as a representative of the IS1 family of elements in various studies (Mahillon and Chandler 1998). The isoIS1s which are less divergent from IS1R (Ohtsubo et al. 1984), have A- and B-block-like sequences (data not shown). Whether NuXi and IS1H contain a RNAP III promoter-like sequence was examined. When the nucleotide sequences of IS1R and NuXi were compared using BLAST-Two-Sequences, the dot plot view of the alignments showed a long discrete line along the matrix’s main diagonal (Fig. 5a). One of its component short lines was from the alignment between the IS1R bp 179–192 and the NuXi bp 189–202 (E-value of 0.026) (Fig. 5a, b). This IS1R region contains the B-block-like sequence, indicating that the sequence was conserved in NuXi. A similar region to the A-block-like sequence of IS1R was not detected in NuXi (Fig. 5a). In BLAST-Two-Sequences using IS1R and IS1H, there were no alignments with the A- and B-block-like sequences of IS1R (data not shown). However, the B-block-like sequence found in NuXi (bp 190–200) was conserved in IS1H, with 10 of the 11 nucleotides corresponding to the IS1H bp 173–183 (data not shown).
Comparison of the nucleotide sequence of IS1 with that of the isoIS1 element NuXi by BLAST-Two-Sequences. a A dot plot view of the alignments. The positions of the A- and B-block-like sequences of IS1 are shown on the horizontal axis representing the IS1 sequence. The length of NuXi shown in the figure was taken from the database page of J01737 (the sequence in the database contains 17 bp and 20 bp of the host sequence flanking the 5′ and 3′ ends of NuXi, respectively). E-values are given near the lines representing the relevant alignments. b The alignment between the B-block-like sequence region of IS1 and the NuXi sequence. The B-block-like sequence of IS1 is underlined. The alignment corresponds to the shortest line segment from the long discrete line along the matrix’s main diagonal in the left figure
RNAP III promoter-like structure in the bacterial IS3 family
The gene organization of IS1 is similar to those of the IS3 family elements (Fig. 6a), and some IS3 family elements employ ribosomal frameshifting for the expression of gene fusion products, like IS1 (Mahillon and Chandler 1998). Whether the RNAP III promoter sequence is conserved in the IS3 family was investigated. However, the nucleotide sequences of the IS3 family elements are highly divergent when seen as a whole. In fact, conserved regions could not be found in the genes corresponding to the insA of IS1 when a large number of the IS3 family elements were compared using Clustal Omega (data not shown). The IS3 family elements are divided mainly into five groups, IS407, IS2, IS3, IS51, and IS150, based on similarities in the deduced amino acid sequences of the elements’ two genes (Mahillon and Chandler 1998). To find the conserved DNA regions in the IS3 family elements, the following grouping procedure was used: Nucleotide sequences of the intra-group ISs were aligned using Clustal Omega, and then obtained alignments were compared with one another. When the alignments of the intra-group ISs were constructed, minor elements divergent from the groups’ majorities at the nucleotide level were excluded.
Conserved DNA regions found in the IS3 family elements. a Organization of the typical IS3 family element. Left and right terminal inverted repeats (IRL and IRR) and two genes (genes I and II) in the element are shown. Gene II has the relative reading frame at the 5′ end (shown as a dotted line), which overlaps with gene I. Positions of the two regions conserved in the IS3, IS51, and IS407 groups are also shown. b Clustal Omega alignments of the nucleotide sequences of the regions conserved in the three IS groups. Underlined (or bold) sequences in the alignments have similarities of seven (or more than eight) out of the 11 consensus nucleotides of the A- and B-blocks, which include “nn”. Although IS1236 and IS600 belong to the IS3 group, they were added to the IS51 and IS407 groups in Clustal Omega, respectively, to determine the regions that also have similarities with the IS3 group elements (see the text). Nucleotides conserved between alignments are linked by vertical dotted lines. Bacterial host species in which the IS elements were originally found are as follows: IS199, S. mutans; ISL1, L. casei; IS1206, Corynebacterium glutamicum; IS911, Shigella dysenteriae; IS222, Pseudomonas aeruginosa; IS1500, Leptospira interrogans; IS1236, Acinetobacter calcoaceticus; IS600, Shigella sonnei; IS1133, Erwinia amylovora; IS1137, Mycobacterium smegmatis; IS986, Mycobacterium tuberculosis; IS1372, Streptomyces lividans; IS3411, E. coli; IS629, Shigella sonnei; IS401, Pseudomonas cepacia; IS1416, Burkholderia glumae; ISPbe1, Paracoccus bengalensis; IS1400, Pantoea ananatis; ISRm6, Rhizobium meliloti; IS511, Caulobacter crescentus; IS1389, Xanthomonas campestris; IS1222, Rahnella aquatilis; IS407, Pseudomonas cepacia; and IS476, Xanthomonas campestris. IS1372 is at bp 58–1361 in U50076, and IS401 is at bp 130–1445 in L09108. c Comparisons of A- and B-block-like sequences. The sequences shown in bold in the above b were aligned
From the IS3 group, IS199 (L23843), ISL1 (X02734), IS1206 (X69104), IS911 (X17613), IS222 (U00100), IS1500 (U13012), IS1236 (U03772), IS600 (X05952), and IS1133 (Z12167) were selected for alignment (see IS Finder at https://www-is.biotoul.fr//). As shown in Supplementary Fig. 2, two conserved regions were found in the internal regions of the genes corresponding to the insA of IS1.
From the IS51 group, IS1137 (X70913), IS986 (X52471), IS1372 (U50076, bp 58–1361), IS3411 (M19532), IS629 (X51586), IS401 (L09108, bp 130–1445), and IS1416 (D88443) were used (IS Finder at https://www-is.biotoul.fr//). In the alignment, common nucleotides were dispersed throughout the entire sequences, and a local similarity was not found (data not shown). However, when aligned together with IS600, which is a member of the IS3 group, two possible conserved regions corresponding to those detected in the IS3 group were revealed (Supplementary Fig. 3).
From the IS407 group, ISPbe1 (GQ871936), IS1400 (NZ_CAEJ01000173), ISR1 (X06616), ISRm6 (X95567), IS511 (U39501), IS1389 (AF108355), IS1222 (AY528232), IS407 (M82980), and IS476 (M28557) were used (IS Finder at https://www-is.biotoul.fr//). In the alignment, common nucleotides were dispersed throughout the entire sequences, and a local similarity was not found, as with the IS51 group elements (data not shown). However, two possible conserved regions corresponding to those detected in the IS3 group were revealed when aligned together with IS1236, which is a member of the IS3 group (Supplementary Fig. 4).
Conserved regions could not be found in the IS2 and IS150 groups. As an example, IS2 (V00610), IS426 (X56562), ISRm1 (X56563), IS1312 (U19148), and ISpRM2011-1 (AF126537) were used from the IS2 group (IS Finder at https://www-is.biotoul.fr//). When the nucleotide sequences of the five members were aligned, common nucleotides were dispersed throughout the entire sequences, and a local similarity was not found (data not shown). Even when aligned with the IS elements in the other groups, conserved regions were not revealed (data not shown). From the IS150 group, as an example, ISKpnI (AF345899), IS1397 (X92970), IS150 (X07037), IS1296 (U61140, bp 2903–4387), ISEnfa3 (AF469486), and IS1223 (U09558) were used (IS Finder at https://www-is.biotoul.fr//). In the alignments of the six elements in the IS150 group together with the IS elements in the other groups, there were no conserved regions, as with the IS2 group (data not shown).
The two conserved regions found in the alignments of the IS3, IS51, and IS407 groups are shown in Fig. 6 (named region A and region B). In these regions, some of the nucleotides conserved in each of the alignments were conserved in the other alignments (shown by vertical dotted lines in Fig. 6b). This result shows that these two regions are commonly conserved in the three groups of the IS3 family. The distances between regions A and B were ~50 bp in all of the groups (Supplementary Figs. 2, 3, and 4), and this feature was similar to the split structure of the RNAP III promoter. When the A- and B-block-like sequences were manually searched for in regions A and B of the IS3, IS51, and IS407 groups, they were found in many IS elements (underlined or shown in bold in the alignments in Fig. 6b). These results suggest the presence of the RNAP III promoter sequence in the IS3 family. B-block-like sequence was more frequently found than A-block-like sequence in the conserved regions (Fig. 6c).
Discussion
In this study, a new assay method to examine the DNA-binding ability of proteins was developed. This assay method can be applied for proteins that rapidly aggregate in the binding buffer. The expression of the artA gene is very low, and for the purification of the ArtA protein a codon-optimized artA was synthesized (Wu and Ippen-Ihler 1989; Matsutani 2012). Although it worked, the expression level is still weak and even the weakly expressed protein forms firm inclusion bodies. Purification is carried out under denatured conditions, and ArtA is refolded in a dialysis tube using a stepwise decrease in the concentration of urea (Matsutani 2012). The final dialysis buffer, which is also the storage buffer, contains 1 M KCl, 30 % glycerol, and no urea (Matsutani 2012). If the concentrations of KCl and glycerol are decreased, ArtA rapidly precipitates (data not shown). On the other hand, for in vitro investigations, ArtA has to be used in assay buffers with low concentrations of KCl and glycerol. Protein aggregation frequently becomes a barrier to the investigation of the proteins’ interactions and other functions in vitro, and many agents that promote protein solubility have been reported (Bondos and Bicknell 2003). Nevertheless, there are still difficulties in controlling protein aggregation. In gel shift assays, probe signals sometimes appear in the wells at the top of the gel instead of the shifted bands in the lanes. The simple assay method developed here can solve that problem and clearly demonstrate the presence or absence of DNA-binding ability of the protein which rapidly aggregates in the reaction buffer.
Using the new method, it was demonstrated that the B-block-like sequence in the bacterial IS1 and the B-block sequences of the eukaryotic RNAP III promoters are important for binding to ArtA. These results suggest that the B-block-like sequence in IS1 is a relative of the B-block sequence of the RNAP III promoter. At the same time, ArtA is suggested to be a relative of the B-block binding subunit of eukaryotic TFIIIC. It has been previously demonstrated by photocrosslinking that the yeast B-block binding subunit binds to the B-block sequence (Bartholomew et al. 1990). Although in silico analyses have not found a HTH motif or a similarity to B-block_TFIIIC in ArtA, improved programs may detect it in the near future. Bacterial proteins characterized as members of the B-block_TFIIC family are found in limited species (Matsutani 2014), even though genome sequencing has been completed in many bacteria. There is the possibility of a high-level sequence divergence of the motif (Matsutani 2014).
The isoIS1s and many IS3 family elements contained the RNAP III promoter-like structures, including IS1R. This result indicates a similarity between the IS1 and IS3 families in addition to their characteristic features, and they might have the same origin. Finding the RNAP III promoter-like structure in many IS3 family elements suggests the broad presence of the structure in bacteria. While the IS1 family of elements are present mainly in enteric bacteria like Escherichia coli and Shigella, the distribution of the IS3 family of elements, in which the RNAP III promoter-like structure was detected, was widespread among various bacterial species (see the caption of Fig. 6b). There are proteins confirmed to be members of the B-block_TFIIIC family in Streptococcus dysgalactiae, Streptococcus urinalis, and Streptococcus ictaluri (Matsutani 2014), and IS199, shown in Fig. 6b, has been found in the same genus of bacteria (Streptococcus mutans; Macrina et al. 1996). Also, there is a B-block_TFIIIC family protein in Lactobacillus gasseri (Matsutani 2014), and ISL1, shown in Fig. 6b, is an element in Lactobacillus casei (Shimizu-Kadota et al. 1985). Additionally, the artA gene was originally found in the F factor of E. coli (Wu and Ippen-Ihler 1989), and now its variants have been detected in various E. coli strains by in silico analysis (data not shown).
If ArtA binds specifically to the B-block-like sequence in IS1 and the B-block sequences of the RNAP III promoters, parts of the eukaryotic RNAP III transcription machinery should be present in bacteria. In the archaeal translated protein database, there are many proteins annotated as B-block_TFIIIC (Matsutani 2014). Such proteins are also present in some bacterial species (see above paragraph; Matsutani 2014). The prototype of the RNAP III transcription machinery might have existed in the common ancestor of eukaryotes and prokaryotes.
References
Archambault J, Friesen JD (1993) Genetics of eukaryotic RNA polymerases I, II, and III. Microbiol Rev 57:703–724
Bartholomew B, Kassavetis GA, Braun BR, Geiduschek EP (1990) The subunit structure of Saccharomyces cerevisiae transcription factor IIIC probed with a novel photocrosslinking reagent. EMBO J 9:2197–2205
Blombach F, Makarova KS, Marrero J, Siebers B, Koonin EV, van der Oost J (2009) Identification of an ortholog of the eukaryotic RNA polymerase III subunit RPC34 in Crenarchaeota and Thaumarchaeota suggests specialization of RNA polymerases for coding and non-coding RNAs in Archaea. Biol Direct 4:39
Bondos SE, Bicknell A (2003) Detection and prevention of protein aggregation before, during, and after purification. Anal Biochem 316:223–231
Brun I, Sentenac A, Werner M (1997) Dual role of the C34 subunit of RNA polymerase III in transcription initiation. EMBO J 16(18):5730–5741
Galli G, Hofstetter H, Birnstiel ML (1981) Two conserved sequence blocks within eukaryotic tRNA genes are major promoter elements. Nature 294:626–631
Hirata A, Murakami KS (2009) Archaeal RNA polymerase. Curr Opin Struct Biol 19:724–731
Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL (2008) NCBI BLAST: a better web interface. Nucleic Acids Res 36:W5–W9
Macrina FL, Jones KR, Laloi P (1996) Characterization of IS199 from Streptococcus mutans V403. Plasmid 36:9–18
Mahillon J, Chandler M (1998) Insertion sequences. Microbiol Mol Biol Rev 2:725–774
Matsutani S (2004) Similarities in transcription factor IIIC subunits that bind to the posterior regions of internal promoters for RNA polymerase III. BMC Evol Biol 4:26
Matsutani S (2005) The internal sequence of IS1 stimulates RNA synthesis from the IS1 own and exogenous promoters. J Biol Syst 13:313–329
Matsutani S (2007) Possible presence and role of the promoter sequence for eukaryotic RNA polymerase III in bacteria. Genetica 131:127–134
Matsutani S (2012) Bacterial ArtA protein specifically binds to the internal region of IS1 in vitro. Adv Biosci Biotechnol 3:869–875
Matsutani S (2014) Evolution of the B-block binding subunit of TFIIIC that binds to the internal promoter for RNA Polymerase III. Int J Evol Biol 2014:609865
Murakami KS, Darst SA (2003) Bacterial RNA polymerases: the wholo story. Curr Opin Struct Biol 13:31–39
Ohtsubo E, Ohtsubo H, Doroszkiewicz W, Nyman K, Allen D, Davison D (1984) An evolutionary analysis of iso-IS1 elements from Escherichia coli and Shigella strains. J Gen Appl Microbiol 30:359–376
Perez-Stable C, Ayres TM, Shen C-KJ (1984) Distinctive sequence organization and functional programming of an Alu repeat promoter. Proc Natl Acad Sci USA 81:5291–5295
Rasband WS (1997–2015) ImageJ. http://imagej.nihs.gov/ij/
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular coloning. Cold Spring Harbor Laboratory Press, New York
Schramm L, Hernandez N (2002) Recruitment of RNA polymerase III to its target promoters. Genes Dev 16:2593–2620
Shimizu-Kadota M, Kiwaki M, Hirokawa H, Tsuchida N (1985) ISL1: a new transposable element in Lactobacillus casei. Mol Gen Genet 200:193–198
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539
White RJ (2011) Transcription by RNA polymerase III: more complex than we thought. Nat Rev Genet 12:459–463
Wu JH, Ippen-Ihler K (1989) Nucleotide sequence of traQ and adjacent loci in the Escherichia coli K-12 F-plasmid transfer operon. J Bacteriol 171:213–221
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10709_2016_9905_MOESM1_ESM.pptx
Supplementary Fig. 1 ImageJ plots of the gel images in Fig. 3. Boxed regions were plotted in the directions of arrows, and the areas under the peaks were measured. All of the areas are shown as the ratios to the area of the peak at the left end (or the upper left) in each of the plot figures. The average values from independent experiments are shown in brackets below the plot figures. The original raw images were used for quantification of the fluorescent probe signals. A IS1 fragment containing the RNAP III promoter-like sequence. 0.3 pmol of the 6FAM-labeled IS1AB361was loaded to the marker lanes while reaction mixtures containing 1.5 pmol of the probe were loaded to the assay sample lanes. B Human Alu RNAP III promoter fragment. The image was cropped to show only the lanes with competitor DNAs. C RNAP III promoter fragment of the X. laevis tRNALue gene. The image was cropped to show only the lanes with competitor DNAs. D Quantified binding activity of ArtA to each of the wild-type and mutant RNAP III promoters of the Alu element (PPTX 2225 kb)
10709_2016_9905_MOESM2_ESM.pptx
Supplementary Fig. 2 The Clustal Omega alignment of nucleotide sequences of the elements belonging to the IS3 group (PPTX 64 kb)
10709_2016_9905_MOESM3_ESM.pptx
Supplementary Fig. 3 The Clustal Omega alignment of nucleotide sequences of the elements belonging to the IS51 group and IS600. IS1372 is at bp 58–1361 in U50076, and IS401 is at bp 130–1445 in L09108 (PPTX 74 kb)
10709_2016_9905_MOESM4_ESM.pptx
Supplementary Fig. 4 The Clustal Omega alignment of nucleotide sequences of the elements belonging to the IS407 group and IS1236 (PPTX 75 kb)
Rights and permissions
About this article
Cite this article
Matsutani, S. Possible interaction between the bacterial transcription factor ArtA and the eukaryotic RNA polymerase III promoter. Genetica 144, 361–374 (2016). https://doi.org/10.1007/s10709-016-9905-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-016-9905-2