Abstract
The Oriental fruit fly, Batrocera dorsalis s.s. (Hendel) is one of the most destructive agricultural pests, belonging to a large group of difficult to distinguish morphologically species, referred as the B. dorsalis complex. We report here a cytogenetic analysis of two laboratory strains of the species and provide a photographic polytene chromosome map from larval salivary glands. The mitotic complement consists of six chromosome pairs including a heteromorphic sex (XX/XY) chromosome pair. Analysis of the polytene complement has shown a total of five polytene chromosomes (10 polytene arms) that correspond to the five autosomes. The most important landmarks of each polytene chromosome and characteristic asynapsis at a specific chromosomal region are presented and discussed. Chromosomal homology between B. dorsalis and Ceratitis capitata has been determined by comparing chromosome banding patterns. The detection of chromosome inversions in both B. dorsalis strains is shown and discussed. Our results show that the polytene maps presented here are suitable for cytogenetic analysis of this species and can be used for comparative studies among species of the Tephritidae family. They also provide a diagnostic tool that could accelerate species identification within the B. dorsalis complex and could shed light on the ongoing speciation in this complex. Polytene chromosome maps can facilitate the development of biological control methods and support the genome mapping project of the species that is currently in progress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Oriental fruit fly, Batrocera dorsalis s.s. (Hendel) is one of the most destructive pests of agriculture (Clarke et al. 2005). It is a polyphagous species attacking more than 170 types of fruits and vegetables among which are many of significant economic importance, as commercially grown fruits. The species is widespread in various countries of Southeast Asia and the Pacific regions (White and Elson-Harris 1992; Drew and Hancock 1994). Outside Asia, it is currently present on all major Hawaiian Islands after being accidentally introduced into Hawaii between the years 1944 and 1945 (Mau 2007). Several infestations have also been reported in California but these were eradicated between 1960 and 1997. While not established in Florida, Oriental fruit flies are occasionally trapped in this state (CDFA 2008). Given its polyphagy, dispersal capacity and the capability of adapting to new areas, the Oriental fruit fly has been recognized as key pest of Asia–Pacific regions and is listed as an important quarantine species in most countries (White and Elson-Harris 1992; Drew and Hancock 1994; Clarke et al. 2005).
B. dorsalis s.s. is a member of a large group of difficult to distinguish morphological species, referred as the B. dorsalis complex. The early works of Drew (1989) and Drew and Hancock (1994) have led to considerable focus on these species aimed at identifying the different entities. Up to now, seventy-five entities have been identified in this rapidly evolving complex species, which includes several species of major agricultural importance (Clarke et al. 2005). Hybridization between species within the complex has been reported or suspected based either on laboratory studies (McInnis et al. 1999; Tan 2000) or in nature (Wee and Tan 2005).
Incursions of flies of this complex into Australia, Oceania, Central America, Africa and continental United States have stimulated studies in applied and quarantine research during the last decade. Most of these studies were focused on the development of diagnostic tools for species recognition within the complex (Adsavakulchai et al. 1998; Muraji and Nakahara 2002; Jamnongluk et al. 2003; Lawson et al. 2003; Drew et al. 2008), quarantine (Nakahara et al. 2000; Follett and McQuate 2001) and eradication (Malavasi et al. 2000; Seewootuthum et al. 2000).
However, many of the available diagnostic keys, based mainly on morphological characters, are variable at the species level and suitable only for good quality adult specimens. Given these problems, efforts have focused on developing other more reliable diagnostic characters, particularly molecular tools. Despite their economic importance, knowledge at the genetic and molecular level has remained relatively limited.
At the cytological level, mitotic chromosome differences have helped to distinguish entities within the B. dorsalis complex, including B. dorsalis s.s. (Hunwattanakul and Baimai 1994; Baimai 1998; Baimai et al. 1995, 1999a, b, 2000). Several autosomal recessive mutations were described for B. dorsalis s.s., belonging to five linkage groups [reviewed in McCombs and Saul (1992)]. Efforts have also been made in developing molecular markers for species identification (Armstrong et al. 1997; Armstrong and Cameron 2000; Muraji and Nakahara 2001, 2002; Nakahara et al. 2001; Naeole and Haymer 2003) or for the analysis of the genetic structure of natural populations (Aketarawong et al. 2007; Chen and Ye 2008; Nakahara et al. 2008). Recently, the complete sequence of the mitochondrial genome has been published (Yu et al. 2007) providing a tool for species diagnosis and for population genetic analysis.
A hobo related element, hopper, has been isolated from a wild type strain of B. dorsalis s.s. (Handler and Gomez 1997) and a second hopper from a mutant strain, the B. dorsalis white eye strain, exhibiting features consistent with transpositional activity (Handler 2003). If these elements prove to be active they may provide tools for transposon-mediated transformation and mutagenesis. TEs have been characterized as ‘powerful facilitators of evolution’ (Oliver and Greene 2009) and the presence of the aforementioned elements in B. dorsalis may be associated with the rapid evolution processes in this species complex (Clarke et al. 2005).
Polytene chromosomes represent very important tools for the analysis of the genetic organization of the chromosomes and the genome as a whole (Zhimulev et al. 2004). They have been used in studies related to phylogenetic relationships among species (Carson and Yoon 1982; Lacovaara and Saura 1982; Lemeunier et al. 1986), as tools for distinguishing members of species complexes (Coluzzi et al. 1979) and contributed significantly to genome mapping projects [e.g. (Adams et al. 2000; Holt et al. 2002)]. Moreover, in C. capitata, the model species for insects of economic importance, polytene chromosomes have helped to improve the sterile insect technique (SIT) by supporting the development of genetic sexing strains (Robinson et al. 1999; Gariou-Papalexiou et al. 2002; Franz 2005).
Here we present the mitotic karyotype and salivary gland polytene chromosome maps of B. dorsalis s.s. (Hendel). We show that the quality of polytene chromosomes permits the cytogenetic analysis of the species and allows comparative studies to other Tephritid species, providing thus a genetic tool that would facilitate the recognition of members in the B. dorsalis complex. These polytene chromosome maps would support the development of genetic control techniques for the species. Last, but not least, they could support the genome mapping project of the species that is currently in progress (USDA, Agricultural Research Service).
Materials and methods
Bactroceradorsalis strains
A wild type strain and a translocation-based genetic sexing strain (GSS) of B. dorsalis, maintained at the Entomology Unit, FAO/IAEA Agriculture and Biotechnology Laboratory, Seibersdorf were used in this study. The wild type strain was derived from pupae send to Seibersdorf from the Institute of Radiation for Agricultural Development, Thailand. The GSS, constructed by McCombs and Saul (1995), was established in Seibersdorf from a strain that is being mass reared in Hawaii (McInnis personal communication). Adults of both strains were fed on a mixture (1:1:3) of yeast:wheat germ:sugar, respectively. Larvae were reared on artificial medium containing 28% wheat bran, 7% brewer’s yeast, 13% sugar, 0.28% sodium benzoate and 1.7% HCl.
Mitotic chromosome preparations
Spread chromosome preparations were made from brain ganglia of late third instar larvae (Zacharopoulou 1990). Brain tissue was dissected in saline solution (0.7% NaCl,) and transferred to hypotonic solution (1% sodium citrate) on a depression slide for at least 15 min and then fixed for 3 min in freshly prepared fixative (methanol:acetic acid 3:1) with several changes to ensure the complete removal of water. At the end of fixation, the fixative was removed and a small drop of 60% acetic acid was added. Working quickly under a Leitz stereoscope, the tissue was dispersed by repeated drawing the material up into a micropipette. The cell suspension was finally laid on a clean slide placed on a warm hotplate (40–45°C) for drying. Chromosomes were stained with 5% Giemsa in 10 mM phosphate buffer (pH 6.8). The technique described by Selivon and Perondini (1997) was used for C-banding.
Polytene chromosome preparations
Polytene chromosome preparations were made from well-fed third instar larvae (Zacharopoulou 1990). Larvae were dissected quickly in 45% acetic acid and salivary glands were transferred to 3 N HCl on a depressed slide for 1 min. Glands were fixed in glacial acetic acid:water:lactic acid (3:2:1, respectively) for about 5 min (until transparent) before being stained in lactoacetic orcein for 5–7 min. Before squashing, any excess stain was removed by washing the glands in lactoacetic acid. Chromosome slides were analyzed at 100× magnification using a Leitz phase contrast microscope. Well spread nuclei or isolated chromosomes were photographed using a CD camera.
Construction of photographic polytene maps
Photographs showing the best morphology for each chromosome region were selected. These regions were assembled using the Adobe Photoshop CS2 software to construct the composite photographic map for each chromosome.
Results
Mitotic chromosomes
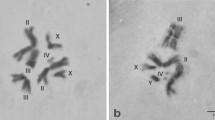
The analysis of metaphase spreads from the laboratory strains showed that both belong to the B. dorsalis s.s., a member of the B. dorsalis complex. The mitotic karyotype, referred to as form A, which, according to Baimai et al. (1995), represents the most ancestral form of the B. dorsalis complex and consists of five pairs of autosomes and one pair of heteromorphic sex chromosomes, XX in females and XY in males (Fig. 1). The autosomes can be differentiated by size and arm ratio and are labelled II to VI in descending order of size (Fig. 1b). The two longest (II, III) and the two shorter (V, VI) autosomes are submetacentric while chromosome IV is metacentric. The sex chromosomes (pair I) are the smallest of the set; the X is long while the Y is dot-like. The X chromosome has two constrictions, one in the middle that probably represents the centromere and a second one close to one tip (Fig. 1c). One arm is darkly stained following Giemsa and C-banding and seems to be totally heterochromatic. The opposite arm stains lighter and shares characteristics of both euchromatin and heterochromatin, as indicated by differences in the degree of staining and chromatid separation compared to autosomes (Fig. 1a, d). This is in agreement with all previously analysed Tephritid species (Ceratitis capitata, Bactrocera oleae, Bactrocera tryoni, Bactrocera cucurbitae and Anastrepha ludens). In all these species, the X chromosome is mostly heterochromatic and is not polytenised (see below). The Y chromosome is entirely heterochromatic as in all Tephritid species analysed so far.
Bactrocera dorsalis s.s. mitotic karyotype. Giemsa staining of mitotic metaphases from Bactrocera dorsalis s.s.a Male larva X and Y chromosomes are indicated. b–d Female larvae; c C-banding metaphase from a female larva. Asterisk indicates the secondary constriction of X chromosome; d the five autosomes and the X chromosome are shown and arrows indicate the centromeres
Polytene chromosomes
Polytene chromosomes of B. dorsalis s.s. are difficult material to work with due to characteristics that are common to other Tephritid species, such as extensive inter- and intra-ectopic pairing, frequent chromosome breaks and coiling and twisting of chromosomes. A large, high-density heterochromatic mass, in which the polytene elements are connected, was observed in polytene nuclei (Fig. 2). However, in most of the well spread nuclei this heterochromatic structure is split into several fragments probably due to the squashing procedure. In these cases, fragments of the heterochromatic mass are associated with individual chromosomes and/or groups of chromosomes (Supplementary Material, ESMs_1–3), indicating a loose “chromocenter”. This observation greatly facilitates the identification of the centromeric region of each chromosome, with the only exception being 3R that is usually found dissociated from the heterochromatic mass (Supplementary Material, ESMs_1–3).
B. dorsalis polytene nuclei contain five banded elements that correspond to the five autosomes of the mitotic karyotype (Supplementary Material, ESMs_1,2). No evidence of sex chromosome polytenization was observed after analysis of a significant number of chromosome preparations from both the wild type and the genetic sexing strain. This observation is consistent with previously analysed Tephritid species and further supports the heterochromatic nature of both sex chromosomes.
One of the most striking features of B. dorsalis polytene chromosomes, observed in both strains, is the high number of chromosome inversions (Supplementary Material, ESM_4). Most of them were found on the 2R chromosome arm. However, there is evidence that such rearrangements are distributed over most or even all polytene elements. The frequent asynapsis of the two homologous chromosomes of several polytene elements, observed in many preparations, could be attributed to heterozygous rearrangements (data not shown).
An additional feature observed in many preparations of both strains is the asynapsis of a specific chromosomal region on the right arm of chromosome 5 (Sections 73–74) (Supplementary Material, ESM_5). In some of these cases small deletions were detected as well as differences in the puffing patterns of the two homologous chromosomes in the asynaptic region.
The characteristic telomeric regions of each chromosome arm allowed them to be differentiated and identified (Supplementary Material, ESMs_1–3). In addition, many bands along each chromosome show a distinctive appearance providing landmarks for identification. Some of the telomere regions show ectopic pairing to other telomeres, a phenomenon also observed in other Tephritidae species.
The polytene chromosomes were numbered 2–6 to indicate homology to C. capitata, based on banding pattern similarity of specific chromosomal regions. This numbering does not imply any correlation to the mitotic chromosomes. B. dorsalis s.s polytene chromosome reference maps and their banding patterns comparison to C. capitata chromosome maps are presented in Figs. 3, 4, 5, 6, 7.
Reference map of Bactrocera dorsalis s.s. (Bd) chromosome 2 (Sections 1–20) and banding pattern comparison with Ceratitis capitata (Cc) chromosome 2 (C, centromere). Sections that can be used as diagnostic landmarks for B. dorsalis s.s. chromosome 2 (a–e) are underlined. Dot lines connecting the chromosomes indicate sections with similar banding pattern and arrows show their relative orientation to each other. One inversion relative to C. capitata is shown in 2L arm
Reference map of Bactrocera dorsalis s.s. (Bd) chromosome 3 (Sections 21–40) and banding pattern comparison with Ceratitis capitata (Cc) chromosome 3 (C, centromere). Sections that can be used as identification markers for B. dorsalis chromosome 3 are underlined. Dot lines between the chromosomes indicate sections with similar banding pattern and arrows show their relative orientation to each other. Three transpositions on B. dorsalis 3L with inverted orientation relative to C. capitata 3L arm are shown. Note the difference between the relative arm lengths of this chromosome in the two species
Reference map of Bactrocera dorsalis s.s. (Bd) chromosome 4 (Sections 41–60) and banding pattern comparison with Ceratitis capitata (Cc) chromosome 4 (C, centromere). Sections which permit the identification of the B. dorsalis chromosome 4 are underlined. Asterisk indicates the region that forms the Balbiani ring; the 4R telomere and the distal region (Sections 57–60) are the most prominent area of this chromosome arm. Dot lines connecting the chromosomes indicate sections with similar banding pattern and arrows show their relative orientation to each other. The 4L arm of B. dorsalis is mostly colinear with C. capitata 4L. One inversion was found in the middle of 4L arm as compared to C. capitata. In 4R, the similarity is limited to specific sections, as these in the telomere and distal regions (Sections 57–60) and in pericentromeric region (Section 52)
Reference map of Bactrocera dorsalis s.s. (Bd) chromosome 5 (Sections 61–80) and banding pattern comparison with Ceratitis capitata (Cc) chromosome 5 (C, centromere). The most important landmarks of B. dorsalis chromosome 5 are underlined. Dot lines connecting the chromosomes indicate sections with similar banding pattern and arrows show their relative orientation to each other. Banding pattern similarity of B. dorsalis and C. capitata chromosome 5 is remarkable. The B. dorsalis chromosome 5 differs by three transpositions in 5L arm, one of them with inverted orientation, one paracentric inversion in 5R and a paracentric one relative to C. capitata chromosome 5
Reference map of Bactrocera dorsalis s.s. (Bd) chromosome 6 (Sections 81–100) and banding pattern comparison with Ceratitis capitata chromosome 6 (C, centromere). Underlined sections show the chromosomal regions which can be used as identification markers of the B. dorsalis chromosome 6. Dot lines connecting the chromosomes of the two species indicate sections with similar banding pattern and arrows show their relative orientation to each other. One inversion in B. dorsalis 6L relative to C. capitata 6L arm is shown. Banding pattern similarity of the 6R tip and the distal regions (Sections 98–100) is obvious, although two transpositions are observed in B. dorsalis, in relation to C. capitata
Chromosome 2, sections 1–20 (Fig. 3)
Chromosome 2 is a long chromosome, with the left arm being longer than the right. The centromeric region of the two arms is always connected to the heterochromatic mass, allowing their identification (Supplementary Material, ESMs_1–3). The 2L arm exhibits numerous weak points, which, coupled with the frequent coiling, result in difficulties to follow the linear arrangement along the entire arm’s length (Supplementary Material, ESMs_1,2). Possibly, much of the coiling is due to chromosomal rearrangements. In fact, several inversions were detected; one of them is shown in Supplementary Material (ESM_4). However, the tip and the distal regions (Sections 1–3) have more constant and clear bands that contribute significantly to the identification of this arm. In contrast to 2L, 2R has a clear banding pattern along its length allowing easy identification (Fig. 3 and Supplementary Material, ESMs_1,2). Although the proximal region is easily recognised (Supplementary Material, ESM_3), the telomeric region shows some degree of variability. This variation seems to be correlated with the presence or absence of puffs at this end and/or with the inversion breakpoints that were detected in this region (Supplementary Material, ESM_4). Characteristic regions that can be used as identification landmarks are underlined in Fig. 3.
A limited similarity was found with the C. capitata chromosome 2, restricted mainly to telomeric and pericentromeric regions. One inversion was found in 2L arm relative to C. capitata 2L chromosome arm (Fig. 3).
Chromosome 3, sections 21–40 (Fig. 4)
It is the most “difficult” chromosome of the set to analyse, especially 3R. Very few nuclei were found to include this arm and even in these cases it was broken or dissociated from the heterochromatic mass (Supplementary Material, ESMs_1,2). 3L has a better banding pattern across its length and is usually connected to the heterochromatic mass (Supplementary Material, ESMs_2,3). The most prominent chromosomal regions that can be used as identification markers are shown in Fig. 4.
Comparison of B. dorsalis and C. capitata chromosome 3 revealed an extensive similarity of banding pattern for 3L. The two arms differ by three transpositions, all of them in reverted orientation relative to C. capitata. We did not find a sufficient banding pattern affinity between B. dorsalis 3R and C. capitata 3R, with the exception of the tips. In addition, a prominent difference between the relative arm lengths of this chromosome in the two species was found (Fig. 4).
Chromosome 4, sections 41–60 (Fig. 5)
Chromosome 4 is a feasible polytene element to identify. The centromere is obvious by its heterochromatic nature and its frequent association with the heterochromatic mass (Supplementary Material, ESMs_2,3). The tips and the pericentromeric regions of both arms are identified by their characteristic banding patterns. The left arm is longer than the right. The most important landmarks of this element are underlined and shown in Fig. 5.
Homology of this chromosome with C. capitata chromosome 4 is supported by the extensive similarity of banding patterns between the two species. One inversion in the middle of 4L was found as compared to C. capitata 4L arm (Fig. 5).
Chromosome 5, sections 61–80 (Fig. 6)
Chromosome 5 is the longest polytene element of the complement and 5L is significantly longer than 5R. The centromere is defined by a constriction, which is usually dissociated from the chromocenter and carries very little or no heterochromatic material (Supplementary Material, ESM_3). 5L has a distinctive banding pattern across most of its length and 5R exhibits the best banding pattern of the whole set (Fig. 6 and Supplementary Material, ESMs_1–3). The map of this chromosome, along with its landmarks is shown in Fig. 6.
The homology of chromosome 5 between B. dorsalis and C. capitata is apparent according to banding pattern similarities of the two chromosomes. Three transpositions, one of them in reverted orientation on the left arm, a paracentric inversion on 5R and a pericentric inversion of this chromosome relative to C. capitata are shown in Fig. 6.
Chromosome 6, sections 81–100 (Fig. 7)
Chromosome 6 is long with 6L being significantly longer than 6R, an analogous situation to chromosome 5. Both arms have a banding pattern of sufficient quality, permitting the identification of this chromosome. The 6R arm is fragile, exhibiting many weak points. Characteristic landmarks of this chromosome are underlined in Fig. 7.
The homology between chromosome 6 of B. dorsalis and C. capitata is obvious, based on their banding pattern similarities. The tips, as well as distal regions of B. dorsalis s.s. are matched to respective regions of C. capitata. One inversion on 6L and two transpositions on 6R relative to C. capitata are shown in Fig. 7.
Discussion
The first cytological data for B. dorsalis s.s. were reported by Hunwattanakul and Baimai (1994) and Baimai et al. (1995). They described a total of six pairs of mitotic chromosomes, including a heteromorphic sex chromosome pair; these data are consistent with our results. Four of the autosomes are submetacentric while one pair is metacentric. They are labelled from II to VI based on their size and arm ratio. The X chromosome is metacentric while the Y is a dot-like chromosome and totally heterochromatic. One arm of the metacentric X chromosome is totally heterochromatic which is in agreement with Baimai et al. (1995), while the opposite arm could be characterised as mostly heterochromatic contrary to a “totally euchromatic” arm as described by Baimai et al. (1995). Evidence that most of the X chromosome is heterochromatic derives from differences in the degree of Giemsa staining and chromatid separation as compared to the autosomes (Fig. 1). This, coupled with the absence of polytene elements corresponding to the sex chromosomes, further supports their heterochromatic nature (see below). This is in full agreement with previously analysed Tephritid species where both sex chromosomes are mostly heterochromatic and do not form polytene elements (Bedo 1987; Zacharopoulou 1990; Mavragani-Tsipidou et al. 1992; Zhao et al. 1998; Kounatidis et al. 2008; Garcia-Martinez et al. 2009).
The heterogametic karyotype (XY) was ascribed to the male. Male heterogamety in B. dorsalis s.s is supported by the fact that a Y-autosome translocation is inherited exclusively through the males (McCombs and Saul 1995). The same situation holds for all Tephritid species analysed so far. The exact structure of this translocation was also determined genetically and cytogenetically (unpublished data).
In B. dorsalis polytene nuclei, five banded polytene elements were found that correspond to the five autosomes, consistent with previous results for other Tephritid species. A large heterochromatic mass was observed in polytene nuclei to which the polytene elements are connected (Fig. 2). However, the splitting of this mass into several fragments associated to centromeric regions of chromosomes during slide preparation constitutes evidence of a “loose chromocenter”. A similar observation was made in B. oleae (Mavragani-Tsipidou et al. 1992), B. tryoni (Zhao et al. 1998) and Rhagoletis cerasi (Kounatidis et al. 2008). However, in C. capitata and B. cucurbitae the size and the density of the heterochromatic mass is different (Zacharopoulou 1990; Zacharopoulou et al. manuscript in preparation). This structure possibly represents under-replicated centromeric and pericentromeric chromosomal regions. The observed differences in the size and the structure of the heterochromatic mass possibly reflect differences in the amount of pericentromeric heterochromatin among these species.
Centromeres and pericentromeric regions along with telomeres and subtelomeric ones have long been recognized as dynamic regions of chromosome evolution due to their repetitive nature and are considered as ‘hotspots for the insertion or retention of repeat sequences’ (for review see Eichler and Sankoff 2003). Detectable differences in the amount and distribution of heterochromatin have been observed in diverse eukaryotic genera, including Dipteran genera such as Drosophila, Anopheles and Bactrocera. The differences in the amount of pericentromeric heterochromatin of mitotic chromosomes among two Bactrocera species complexes, B. dorsalis and B. tau (Walker), have been used as genetic markers for the identification of cryptic species (Hunwattanakul and Baimai 1994; Baimai 1998; Baimai et al. 1995, 1999a, b, 2000).
Comparison of banding patterns between B. dorsalis s.s. and C. capitata revealed extensive similarities supporting the proposed homology of the polytene chromosomes in these species. However, the 3R arm shows little chromosomal banding pattern affinity between the two species, restricted only to the tips. The chromosomal rearrangements observed so far between the two species involve paracentric inversions, within-arm transpositions and only one pericentric inversion on chromosome 5. It should be emphasized that the specific pericentric inversion has been detected in all Bactrocera species analyzed so far (Zhao et al. 1998) and B. cucurbitae (Zacharopoulou et al. manuscript in preparation). These observations support Muller’s hypothesis (1940) that karyotype evolution in Diptera has involved mainly with within-arm rearrangements, such as paracentric inversions and transpositions and very few translocations and pericentric inversions.
The asynapsis observed in the proximal region (Sections 73–74) of the 5R chromosome is interesting. It was found in all nuclei of a significant number of chromosome preparations of both strains. Asynapsis is frequently observed in hybrid chromosomes of inter- or intra-specific crosses in Dipteran species [e.g. (Machado et al. 2006; Cáceres et al. 2009)] and in the presence of heterozygous chromosome rearrangements (Lefevre 1976). Kounatidis et al. (2008) reported a high frequency of asynapsis distributed over several chromosomal regions of R. cerasi in four natural Greek populations. They suggested that short deficiencies or insertions of genetic material in one of the homologous chromosomes, undetectable by conventional cytology, could explain this asynapsis. They also discussed the possibility of gene transfer events between the Wolbachia and R. cerasi being associated with the asynaptic phenomena. Recently, Dunning Hotopp et al. (2007) confirmed gene transfer events from Wolbachia bacteria to genomes of four insects and four nematode species and in some cases it was shown that the inserted genes were transcriptionally active.
In B. dorsalis polytene chromosomes, at least one obvious deletion of a single band was detected (Supplementary Material, ESM_5). In many other cases additional differences between the aligned homologous chromosomes are visible (Supplementary Material, ESM_5) that could either be due to undetected (by the current cytological analysis) chromosomal rearrangements or to differences in gene activity, i.e. differential puffing activity between the two homologous chromosomes. B. dorsalis was shown to be infected by Wolbachia strains (Jamnongluk et al. 2002). Whether gene transfer events have occurred between Wolbachia and B. dorsalis could be further investigated and polytene chromosomes could facilitate such an analysis.
The most interesting finding during the analysis of the B. dorsalis s.s. polytene chromosomes is the detection of numerous chromosome inversions (Supplementary Material, ESM_4). It should be emphasized that naturally occurring chromosome rearrangements were never observed in B. oleae (Mavragani-Tsipidou et al. 1992; Mavragani–Tsipidou unpublished data) and B. tryoni (Zhao et al. 1998), neither in C. capitata (Zacharopoulou unpublished data). Especially in the case of C. capitata many different strains and populations were analyzed and no chromosome rearrangements were observed. Contrary to the last species, chromosomal inversions have been detected in another tephritid, Anastrepha fraterculus, which is supposed to be composed of an unknown number of cryptic species (Cáceres et al. 2009).
B. dorsalis chromosome inversions seem to be concentrated on the 4L and 2R chromosome arms (Supplementary Material, ESM_4). However, we have indications that inversions also exist in most and possibly all chromosomes. Evidence for that may be the frequent presence of asynaptic chromosomal regions, whole arm and/or partial asynapsis and specific pairing configurations of several regions. These phenomena were present in all nuclei of particular preparations (data not shown). Further studies are necessary, including the analysis of natural populations from different geographical regions, to clarify these data and estimate the frequency of the chromosome rearrangements in the species.
Chromosomal inversions were described in a variety of species, including mammals, but most are known from Diptera such as fruit flies (e.g. Drosophila) and Anopheline mosquitoes. In many groups of animals and plants chromosomal inversions are found as fixed differences between species and as polymorphisms within species. These differences have stimulated interest in the contribution of chromosomal rearrangements to speciation. In fact, for more than half a century they have been identified as being of great importance in adaptation and speciation (Dobzhansky 1970; Krimbas and Powell 1992; Coyne and Orr 2004; Hoffman and Rieseberg 2008). Current data suggest that inversions may contribute to species differentiation through the preservation of combination of alleles crucial to ecological adaptation by acting as suppressors of recombination. This mechanism may facilitate divergence among populations and eventually lead to species differentiation (Noor et al. 2001; 2007; Machado et al. 2002; Feder et al. 2003; Navarro and Barton 2003; Ayala and Coluzzi 2005; Machado et al. 2007; Kulathinal et al. 2008; Costantini et al. 2009). Elucidation of the causes of chromosomal inversions in natural populations will contribute to a better understanding of their role in speciation processes (Runcie and Noor 2009). Evidence currently supported by the availability of genome sequences in a number of different taxa, suggests that transposable elements are most likely associated with the generation of chromosomal rearrangements (Lyttle and Haymer 1992; Cáceres et al. 1999; Mathiopoulos et al. 1999; Evgen’ev et al. 2000; Cáceres et al. 2001; Richards et al. 2005), although an alternative mechanism has been proposed (Ranz et al. 2007). Transposable elements (TEs) are characterized as ‘powerful facilitators of genome evolution’, since they can induce a great variety of genetic changes, leading thus to diversity [reviewed in Oliver and Greene (2009)]. In accordance with the above suggestions, the presence of a possibly active transposable element (hopper) in B. dorsalis (Handler 2003) could be correlated with the inversions observed in the species.
Current evidence suggests that the B. dorsalis complex represents a rapidly evolving species complex (75 species have been described) and most of the species diversity has been generated during the past 1–2 million years. The observed hybridization between morphologically defined species within this complex could be consistent with incipient speciation in this complex, as observed in other species (della Torre et al. 2001; Guelbeogo et al. 2005; Cáceres et al. 2009). All the above and our cytogenetic analysis suggest that the B. dorsalis complex should be under species differentiation and, therefore, provide a useful model for the study of evolutionary phenomena. Accumulation of molecular and genetic data, which are in their infancy now, along with cytogenetic analysis of natural populations of this complex, could provide tools towards this direction.
Conclusion
The results of the current study show that B. dorsalis s.s. polytene chromosomes of sufficient quality, suitable for cytogenetic analysis of the species, can be prepared from larval salivary glands. Given that polytene chromosomes represent very important tools for the analysis of the genetic organization of the chromosomes and the genome as a whole, their availability in B. dorsalis s.s. could (1) facilitate species identification within the B. dorsalis complex currently relying mainly on morphological characters (2) clarify the phylogeny of Tephritids and (3) shed light on the species differentiation in this complex. Finally, polytene chromosome maps could also support the development of genetic control techniques and the genome-mapping project of the species.
References
Adams MD, Celniker SE, Holt RA et al (2000) The genome sequence of Drosophila melanogaster. Science 287:2185–2195
Adsavakulchai A, Baimai V, Prachyabrued W, Grote PJ, Lertlum S (1998) Morphometric study using wing image analysis for identification of the Bactrocera dorsalis complex (Diptera: Tephritidae). WWW J Biol 3:34–43
Aketarawong N, Bonizzoni M, Thanaphum S, Gomulski LM, Gasperi G, Malacrida AR, Gugliemino CR (2007) Inferences on the population structure and colonization process of the invasive oriental fruit fly, Bactrocera dorsalis (Hendel). Mol Ecol 16:3522–3532
Armstrong KF, Cameron CM (2000) Species identification of tephritids across a broad taxonomic range using ribosomal DNA. In: Tan KH (ed) Area-wide control of fruit flies and other insect pests. Penerbit Universiti Sains Malaysia, Penang, pp 703–710
Armstrong KF, Cameron CM, Frampton ER (1997) Fruit fly (Diptera: Tephritidae) species identification: a rapid molecular diagnostic technique for quarantine application. Bull Entomol Res 87:111–118
Ayala FJ, Coluzzi M (2005) Chromosome speciation: Humans, Drosophila and mosquitoes. PNAS 102:6535–6542
Baimai V (1998) Heterochromatin accumulation and karyotypic evolution in some dipteran insects. Zool Stud 37:75–88
Baimai V, Trinachartvanit W, Tigvattananont S, Grote PJ, Poramarcom R, Kijchalao U (1995) Metaphase karyotypes of fruit flies of Thailand. I. Five sibling species of the Bactrocera dorsalis complex. Genome 38:1015–1022
Baimai V, Phinchongsakuldit J, Tigvattananont S (1999a) Metaphase karyotypes of fruit flies of Thailand. IV. Evidence for six new species of the Bactrocera dorsalis complex. Cytologia 64:371–377
Baimai V, Phinchongsakuldit J, Trinachartvanit W (1999b) Metaphase karyotypes of fruit flies of Thailand (III). Six members of the Bactrocera dorsalis complex. Zool Stud 38:110–118
Baimai V, Sumrandee C, Tigvattananont S, Trinachartvanit W (2000) Metaphase karyotypes of fruit flies of Thailand. V. Cytotaxonomy of ten additional new species of the Bactrocera dorsalis complex. Cytologia 65:409–417
Bedo DG (1987) Polytene chromosome mapping in Ceratitis capitata (Diptera: Tephritidae). Genome 29:598–611
Cáceres M, Ranz JM, Barbadilla A, Long M, Ruiz A (1999) A transposable element mediated the generation of a Drosophila widespread chromosomal inversion. Science 285:415–418
Cáceres M, Puig M, Ruiz A (2001) Molecular characterization of two natural hotspots in the Drosophila buzzatii genome induced by transposon insertions. Genome Res 11:1353–1364
Cáceres C, Segura DF, Vera MT, Wornoayporn W, Cladera JL, Teal P, Sapountzis P, Bourtzis K, Zacharopoulou A, Robinson AS (2009) Incipient speciation revealed in Anastrepha fraterculus by studies on mating compatibility, sex pheromones, hybridisation and cytology. Biol J Linn Soc 97:152–165
Carson HL, Yoon JS (1982) Genetics and evolution of Hawaiian Drosophila. In: Ashburner M, Carson HL, Thompson JN Jr (eds) The genetics and biology of Drosophila. Academic Press, London, 3b, pp 296–344
CDFA (California Department of Food and Agricutlure) (2008) Oriental fruit fly. Pest detection/Emergency projects branch. http://www.cdfa.ca.gov/phpps/pdep/treatment/oriental_ff.html
Chen P, Ye H (2008) Relationship among five populations of Bactrocera dorsalis based on mitochondrial DNA sequences in western Yunnan. China J Appl Entomol 132:530–537
Clarke AR, Armstrong KF, Carmichel AE, Milne JR, Raghu S, Roderick GK, Yeates DK (2005) Invasive phytophagous pest arising through a recent tropical evolutionary radiation: the Bactrocera dorsalis complex of fruit flies. Annu Rev Entomol 50:293–319
Coluzzi M, Sabatini A, Petrarca V, DiDeco MA (1979) Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg 73:483–497
Costantini C, Ayala D, Guelbeogo WM, Pombi M, Some CY, Bassole IHN, Ose K, Fotsing J-M, Sagnon N, Fontenille D, Besansky NJ, Simard F (2009) Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae. BMC Ecol 9:16
Coyne JA, Orr HA (2004) Speciation. Sinauer Asociates, Sunderland MA
della Torre A, Fanello C, Akogbeto M, Dossou-yovo J, Favia G, Petrarca V, Coluzzi M (2001) Molecular evidence of incipient speciation within Anopheles gambiae s.s. in West Africa. Insect Mol Biol 10:9–18
Dobzhansky TG (1970) Genetics of the evolutionary process. Columbia University Press, New York
Drew RAI (1989) The tropical fruit flies (Diptera: Tephritidae: Dacinae) of the Australasian and Oceanian regions. Mem Qld Mus 26:521
Drew RAI, Hancock DL (1994) The Bactocera dorsalis complex of fruit flies (Diptera: Tephritidae: Dacinae) in Asia. Bull Entomol Res Suppl 2:1–68
Drew RAI, Raghu S, Halcoop P (2008) Bridging the morphological and biological species concepts: studies on the Bactrocera dorsalis (Hendel) complex (Diptera: Tephritidae: Dacinae) in South-East Asia. Biol J Linn Soc 93:217–226
Dunning Hotopp JC, Clark ME, Oliveira DCSG, Foster JM, Fisher P, Muñoz Torres MC, Giebel JD, Kumar N, Ishmael N, Wang S, Ingram J, Nene RV, Shepard J, Tomkins J, Richards S, Spiro DJ, Ghedin E, Slatko BE, Tettelin H, Werren JH (2007) Widespread lateral gene transfer form intracellular bacteria to multicellular eukaryotes. Science 317:1753–1756
Eichler EE, Sankoff F (2003) Structural dynamics of eukaryotic chromosome evolution. Science 301:793–797
Evgen’ev MB, Zelentsova H, Poluectova H, Lyozin GT, Veleikodvorskaja V, Pyatkov KI, Zhivotovsky LA, Kidwell MG (2000) Mobile elements and chromosomal evolution in the virilis group of Drosophila. PNAS 97:11337–11342
Feder JL, Roethele JB, Filchak K, Niedbalski J, Romero-Severson J (2003) Evidence for inversion polymorphism related to sympatric host race formation in the apple maggot fly, Rhagoletis pomonella. Genetics 163(3):939–953
Follett PA, McQuate GT (2001) Accelerated development of quarantine treatments for insects on poor hosts. J Econ Entomol 94:1005–1011
Franz G (2005) Genetic sexing strains in Mediterranean fruit fly, an example for other species amenable to large-scale rearing for the sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS (eds) Sterile insect technique: principles and practice in area-wide integrated pest management. Springer, Dordrecht, The Netherlands, pp 427–452
Garcia-Martinez V, Hernandez-Ortiz E, Zepeta-Cisneros CS, Robinson AS, Zacharopoulou A, Franz G (2009) Mitotic and polytene analysis in the Mexican fruit fly, Anastrepha ludens (Loew) (Diptera: Tephritidae). Genome 52:1–11
Gariou-Papalexiou A, Gourzi P, Delprat A, Kritikou D, Rapti K, Chrysanthakopoulou B, Mintzas A, Zacharopoulou A (2002) Polytene chromosomes as tools in the genetic analysis of the Mediterranean fruit fly, Ceratitis capitata. Genetica 116:59–71
Guelbeogo WM, Grushko O, Boccolini D, Ouédraogo PA, Besansky NJ, Sagnon NF, Costantini C (2005) Chromosomal evidence of incipient speciation in the Afrotropical malaria mosquito Anopheles funestus. Med Vet Entomol 19:46–458
Handler AM (2003) Isolation and analysis of a new hopper hAT transposon from the Bactrocera dorsalis white eye strain. Genetica 118:17–24
Handler AM, Gomez SP (1997) A new hobo, Ac, Tam3 transposable element, hopper, from Bactrocera dorsalis is distantly related to hobo and Ac. Gene 185:133–135
Hoffman AA, Rieseberg LH (2008) Revisiting the impact of inversions in evolution: from population genetic markers to drivers of adaptive shifts and speciation? Annu Rev Ecol Evol Syst 39:21–42
Holt RA, Subramanian GM, Halpern A, Sutton GG et al (2002) The genome sequence of the malaria mosquito Anopheles gambiae. Science 298:129–149
Hunwattanakul N, Baimai V (1994) Mitotic karyotypes of four species of fruit flies (Bactrocera) in Thailand. Kasetsart J (Nat Sci) 28:142–148
Jamnongluk W, Kittayapong P, Baimai V, O’Neill S (2002) Wolbachia infections of Tephritid fruit flies: molecular evidence for five distinct strains in a single host species. Curr Microb 45:255–260
Jamnongluk W, Baimai V, Kittayapong P (2003) Molecular evolution of tephritid fruit flies in the genus Bactrocera based on the cytochrome oxidase I gene. Genetica 119:19–25
Kounatidis I, Papadopoulos N, Bourtzis K, Mavragani-Tsipidou P (2008) Genetic and cytogenetic analysis of the fruit fly Rhagoletis cerasi (Diptera: Tephritidae). Genome 51:479–491
Krimbas CB, Powell JR (1992) Drosophila inversion polymorphism. CRC Press, Boca Raton, FL., USA
Kulathinal RJ, Stevison LS, Noor MAF (2008) The genomics of speciation in Drosophila: diversity, divergence, and introgression estimated using low-coverage genome sequencing. PLoS Genet 5(7):e1000550
Lacovaara S, Saura A (1982) Evolution and speciation in the Drosophila obscura group. In: Ashburner M, Carson HL, Thompson JN Jr (eds) The genetics and biology of Drosophila. Academic Press, London, 3b, pp 1–59
Lawson AE, McQuire DJ, Yeates DH, Drew RAI, Clarke AR (2003) Dorsalis: an interactive identification tool to fruit flies of the Bactrocera dorsalis complex. CDROM publication, Griffith Univ. Brisbane, Aust
Lefevre G (1976) A photographic representation and interpretation of the polytene chromosomes of Drosophila melanogaster salivary glands. In: Ashburner M, Novitski E (eds) The genetics and biology of Drosophila. Academic Press, London, 1a, pp 31–66
Lemeunier F, David JR, Tsakas L, Ashburner M (1986) The melanogaster species group. In: Ashburner M, Carson HL, Thopson JN (eds) The genetics and biology of Drosophila. Academic Press, London, 1e, pp 147–256
Lyttle TW, Haymer DS (1992) The role of the transposable element hobo in the origin of endemic inversions in wild populations of Drosophila melanogaster. Genetica 86:113–126
Machado CA, Kliman RM, Markert JA, Hey J (2002) Inferring the history of speciation from multilocus DNA sequence data: the case of Drosophila pseudoobscura and close relatives. Mol Biol Evol 19:472–488
Machado LPB, Madi-Ravazzi L, Tadei WJ (2006) Reproductive relationships and degree of synapsis in the polytene chromosomes of the Drosophila buzzatti species cluster. Braz J Biol 66:279–293
Machado CA, Matzkin LM, Reed LK, Markow TA (2007) Multilocus nuclear sequences reveal intra- and interspecific relationships among chromosomally polymorphic species of cactophilic Drosophila. Mol Ecol 16:3009–3024
Malavasi A, van Sauers-Muller A, Midgarden D, Kellman V, Didelot D, Caplong Ph, Ribeiro O (2000) Regional programme for the eradication of the Carambola fruit fly in South America. In: Tan KH (ed) Area-wide control of fruit flies and other insect pests. Penerbit Universiti Sains Malaysia, Penang, pp 395–399
Mathiopoulos KD, della Torre A, Santolamazza F, Predazzi V, Petrarca V, Coluzzi M (1999) Are chromosomal inversions induced by transposable elements? A paradigm from the malaria mosquito Anopheles gambiae. Parassitol 41:106–129
Mau MFA (2007) Bactrocera dorsalis (Hendel). Crop knowledge master. http://www.extento.hawaii.edu/Kbase/Crop/Type/bactro_d.htm
Mavragani-Tsipidou P, Karamanlidou G, Zacharopoulou A, Koliais S, Kastritsis C (1992) Mitotic and polytene chromosome analysis in Dacus oleae (Diptera: Tephritidae). Genome 35:373–378
McCombs SD, Saul SH (1992) Linkage analysis of genetic markers in the oriental fruit fly. In: McPheron BA, Steck GJ (eds) Fruit fly pests: a world assessment and management. St. Lucie Press, Fl, pp 231–235
McCombs SD, Saul SH (1995) Translocation based genetic sexing system for the oriental fruit fly (Diptera-Tephritidae) based on pupal color dimorphism. Ann Entomol Soc Am 88:695–698
McInnis DO, Rendon P, Jang E, van Sauers-Muller A, Sugayama R, Malavasi A (1999) Interspecific mating of introduced, sterile Bactrocera dorsalis with wild B. carambolae (Diptera: Tephritidae) in Suriname: a potential case for cross-species sterile insect technique. Ann Entomol Soc Am 92:758–765
Muller HJ (1940) Bearings of the Drosophila work on systematics. In: Huxley JS (ed) The new systematics. Oxford University Press (Clarendon), London and New York, pp 185–268
Muraji M, Nakahara S (2001) Phylogenetic relationships among fruit flies, Bactrocera (Diptera, Tephritidae), based on mitochondrial rDNA sequences. Insect Mol Biol 10:549–559
Muraji M, Nakahara S (2002) Discrimination among pest species of Bactrocera (Diptera: Tephritidae) based on PCR-RFLP of the mitochondrial DNA. Appl Entomol Zool 37:437–446
Naeole CKM, Haymer DS (2003) Use of oligonucleotide arrays for molecular taxonomic studies of closely related species in the oriental fruit fly complex. Mol Ecol Notes 3:662–665
Nakahara S, Tsuchiya T, Sato M, Masaki M, Kaneda M (2000) Research on infestation to many kinds of plants by the pests of quarantine importance. Bactrocera dorsalis complex. Res Bull Plant Prot Serv Jpn 36:53–56
Nakahara S, Kato H, Kaneda M, Sugimoto T, Muraji M (2001) Identification of Bactrocera dorsalis complex species (Diptera: Tephritidae) by PCR-RFLP analysis. II. A study of genetic variation in B. dorsalis complex (Philippines population) and B. dorsalis (Taiwan population). Res Bull Plant Prot Serv Jpn 37:69–73
Nakahara S, Kobashigawa Y, Muraji M (2008) Genetic variation among and within populations of the Oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae), detected by PCR-RFLP of the mitochondrion control region. Appl Entomol Zool 43:457–465
Navarro A, Barton NH (2003) Chromosomal speciation and molecular divergence—accelerated evolution in rearranged chromosomes. Science 300:321–324
Noor MAF, Gratos KL, Bertucci LA, Reiland J (2001) Chromosomal inversions and the reproductive isolation of species. PNAS 98:12084–12088
Noor MAF, Garfield DA, Schaeffer SW, Machado CA (2007) Divergence between the Drosophila pseudoobscura and D. persimilis genome sequences in relation to chromosomal inversions. Genetics 177:1417–1428
Oliver KR, Greene WK (2009) Transposable elements: powerful facilitators of evolution. Bioess 31:703–714
Ranz JM, Maurin D, Chan YS, Von Grotthuss M, Hillier LW, Roote J, Ashburner M, Bergman CM (2007) Principles of genome evolution in the Drosophila melanogaster species group. PLoS Biol 5e152:1369–1381
Richards S, Liu Y, Bettencourt BR, Hradecky P et al (2005) Comparative genome sequencing of Drosophila pseudoobscura: chromosomal, gene, and cis-element evolution. Genome Res 15:1–18
Robinson AS, Franz G, Fisher K (1999) Genetic sexing strains in the medfly, Ceratitis capitata: development, mass rearing and field application. Trends Entomol 2:81–104
Runcie DE, Noor MAF (2009) Sequence signatures of a recent chromosomal rearrangement in Drosophila mojavensis. Genetica 136:5–11
Seewootuthum SI, Permaloo S, Gungah B, Soonnoo AR, Alleck M (2000) Eradication of an exotic fruit fly from Mauritius. In: Tan KH (ed) Area-wide control of fruit flies and other insect pests. Penerbit Universiti Sains Malaysia, Penang, pp 389–394
Selivon D, Perondini ALP (1997) Evaluation of techniques for C and ASG banding of the mitotic chromosomes of Anastrepha species (Diptera: Tephritidae). Braz J Genet 20:651–653
Tan KH (2000) Behaviour and chemical ecology of Bactrocera flies. In: Tan KH (ed) Area-wide control of fruit flies and other insect pests. Penerbit Universiti Sains Malaysia, Pulau, Penang, pp 647–656
Wee SL, Tan KH (2005) Evidence of natural hybridization between two sympatric sibling species of Bactrocera dorsalis complex based on pheromone analysis. J Chem Ecol 31:845–858
White IM, Elson-Harris M (1992) Fruit flies of economic significance: their identification and bionomics. CAB International, Oxford, UK
Yu DJ, Xu L, Nardi F, Li JG, Zhang RJ (2007) The complete nucleotide sequence of the mitochondrial genome of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Gene 396:66–74
Zacharopoulou A (1990) Polytene chromosome maps in the medfly Ceratitis capitata. Genome 33:184–197
Zhao JT, Fommer M, Sved J, Zacharopoulou A (1998) Mitotic and polytene chromosome analyses in the Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae). Genome 41:510–526
Zhimulev IF, Belyaeva ES, Semeshin VF, Koryakov DE, Demakov SA, Demakova OV, Pokholkova GV, Andreyeva EN (2004) Polytene chromosomes: 70 years of genetic research. Int Rev Cytol 241:203–275
Acknowledgments
This work forms part of the Joint FAO/IAEA research programme for the development of improved control methodologies against fruit fly pest species. We would also like to thank the two anonymous reviewers for their significant comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zacharopoulou, A., Augustinos, A.A., Sayed, W.A.A. et al. Mitotic and polytene chromosomes analysis of the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). Genetica 139, 79–90 (2011). https://doi.org/10.1007/s10709-010-9495-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-010-9495-3