Abstract
Forest conversion to agriculture and grassland has been widespread in south-eastern Mexico. The productivity, functioning and carbon dynamics of secondary forests growing after abandonment of agricultural fields are expected to differ from those of primary forests. This study analysed whether forest age and seasonal variations affect the amount and temporal distribution of litterfall and associated nutrient transfer. Litterfall was measured across a chronosequence of semi-evergreen tropical forest in Calakmul, Yucatan peninsula, Mexico, and an index was created to evaluate the effect of land use intensity on litterfall collected in 16 stands from October 2012 to September 2014. Total litterfall ranged from 5.2 ± 0.6 to 7.1 ± 0.3 Mg ha−1 year−1 and peaked in secondary forest aged 10–20 years. Leaves contributed 84–91 % of total litterfall. The associated transfer of carbon ranged from 2.3 ± 0.3 to 3.2 ± 0.1 Mg ha−1 year−1 and of nitrogen from 62 ± 7 to 84 ± 4 kg ha−1 year−1. Carbon and nutrient accumulation in the organic horizon (Oa) increased significantly with forest age. However, carbon in mineral soil (down to 0.30 m depth) did not increase over time. Peaks in monthly litterfall coincided with the dry season, with higher peaks in a year with lower rainfall in the dry season. Peaks were also higher in secondary forests than in primary forests, due to changes in species composition. Higher land use intensity reduced carbon and nutrient transfer through litter in regenerating secondary forests. Longer-term research is required to analyse the climate sensitivity of litter dynamics in these tropical forest frontiers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Secondary and primary forests in the tropics are undergoing rapid change in their function, composition and carbon cycling because of different types and degrees of human intervention (Brown and Lugo 1990; Malhi 2012; Aryal et al. 2014). In southern Mexico, most forests were converted to extensive pasture and agricultural fields during the last decades of the twentieth century, due to large-scale incentives for animal production and agriculture (De Jong et al. 2000; Turner et al. 2004; Aryal et al. 2012). These anthropogenic interventions created a landscape composed of secondary forests at various stages of succession, mixed with pasture land and patches of slash-and-burn agriculture and a few patches of remaining primary forest (Ochoa-Gaona et al. 2007; Rueda 2010). Slash-and-burn agriculture is still one of the major land uses for Mayan farmers in south-eastern Mexico (Schmook et al. 2013). One of the key challenges for modern ecologists is to understand the patterns, processes and pathways of carbon cycling in transitional forests at fine spatial and temporal scales (Thuille and Schulze 2006; Malhi 2012), as lack of understanding of forest functioning can lead to inappropriate management and governance strategies for these transitional forest frontiers in the tropics (Román-Dañobeytia et al. 2014).
Litterfall is one of the main nutrient cycling processes in forest ecosystems (Cuevas and Medina 1986; Takyu et al. 2003; Dent et al. 2006; Negash and Starr 2013) and an important pathway of carbon and energy transfer from vegetation to soil (Bray and Gorham 1964; Vogt et al. 1986; Zhou et al. 2014). Litter production is an important part of net primary production (NPP), i.e. the net amount of carbon captured by plants through photosynthesis (Melillo et al. 1993), and represents a link between carbon capture through photosynthesis and emission through litter decomposition (Meentemeyer et al. 1982). As most of the leaf, flower and fruit production in the sub-humid tropics is recycled every year, quantification of litterfall is important for understanding the productivity, phenology, carbon dynamics and capacity of forest ecosystems to recover from human and natural disturbances (Ewel 1976; Vitousek 1984; de Jong 2013). Litterfall studies can also provide the ability to detect synchronies between biological and meteorological cycles (Chapin and Eviner 2005). A better understanding of the temporal patterns and processes of forest litterfall dynamics is needed as a basis for modelling responses of forest ecosystems to climate change (Martinez-Yrizar and Sarukhan 1990; Thuille and Schulze 2006; Scheer et al. 2011).
Globally, studies examining litterfall and forest production have been reported (Ewel 1976; Chapin and Eviner 2005; Scheer et al. 2011; Zhou et al. 2014). However, the pattern of litterfall and associated carbon and nutrient flows during the successional stages and the effect of land use history in tropical secondary forest ecosystems are still not well understood. Therefore this study measured litterfall during 2 years and calculated associated nutrient fluxes in a chronosequence of tropical secondary and primary forests. The starting hypothesises were that: (1) annual litter production increases rapidly with forest age to reach a peak in early stages of succession, as leaf area index recovers rapidly in early stages of succession (Brown and Lugo 1990; Feldpausch et al. 2005); (2) seasonal variation in litterfall is associated with seasonal variation in rainfall; and (3) forest stands with more intensive land use before the abandonment have lower litter production in any particular successional stage than stands with a less intensive previous land use.
Methods
Study sites
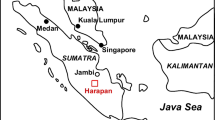
The study was conducted at two locations around Calakmul Biosphere Reserve, which is situated in the south of Yucatan Peninsula, Mexico. Sampling was conducted in forests in the communities (ejidos) of El Carmen II and Cristóbal Colon, in Calakmul municipality (Fig. 1). The region is composed of rolling limestone hills and ridges of karstic origin that range from 100 to 380 m above sea level (Bautista et al. 2011). The dominant soil types in the region are rendzic leptosols and vertisols (Bautista et al. 2011). The region is characterised by a sub-humid tropical climate (García 1973; Xuluc-Tolosa et al. 2003), with mean annual precipitation of about 1000 mm (a major proportion of which falls from June to October; Fig. 2) and mean annual temperature of about 26 °C. Medium-sized semi-evergreen tropical forest is the dominant forest type in the region (Pérez-Salicrup 2004; Rzedowski 2006). These are forests with trees reaching 15–30 m in height at maturity, which lose about 25–50 % of their leaves during the dry season (Martínez and Galindo-Leal 2002; Román-Dañobeytia et al. 2014). Portions of the primary forests have been converted to slash-and-burn agriculture, creating a mosaic of agricultural land mixed with secondary forests in various stages of development (Table 1) and patches of primary forest.
Litter sampling
Forest stands in four different phases of succession [young, medium and advanced secondary forests (YSF, MSF and ASF, respectively) and primary forests (PF)] were selected for the litterfall observations (Table 1). A total of 16 monitoring plots were established (four plots in each successional stage). Age refers to the number of years after abandonment of cultivation.
Litterfall was collected at fortnightly intervals over 2 years (from October 2012 to September 2014) using 12 circular litter traps of 0.5 m2 each (Cuevas and Medina 1986; Takyu et al. 2003), placed in each plot. All the traps were placed at a height of about 1 m above the ground surface around the plots, in which other experiments were also taking place (Aryal et al. 2014). The litter samples collected were placed in paper bags and transported to the laboratory for processing. The samples were oven-dried at 70 °C for 3 days to obtain stable dry weight and separated as follows: leaves, twigs and cortex, fruits and flowers and a residue group. These components were weighed separately and the carbon (C), nitrogen (N), phosphorus (P) and potassium (K) contents of subsamples of each component were determined in order to evaluate the nutrient flux associated with litterfall.
Forest floor litter samples (O horizon) and mineral soil samples down to 0.30 m depth (0–0.10, 0.11–0.20 and 0.21–0.30 m separately) were collected from four random locations in each plot, using standard procedures (Aryal et al. 2014). Forest floor litter was separated visually into three classes (Oi, Oe and Oa horizons) as the carbon fraction of the forest floor litter diminishes during the fragmentation and decomposition process (Orihuela-Belmonte et al. 2013). Newly fallen (relatively intact) litter was classified as Oi, fragmented (recognisable parts of components in the process of decomposition) litter as Oe and humus (components completely decomposed) as the Oa horizon.
Carbon in litter and soil samples was determined with a Shimadzu A500 organic carbon analyser (Shimadzu 2001) and N with the semi-micro Kjeldahl method (Bremner and Mulvaney 1982). Available P was determined with Olsen´s method of extraction with sodium bicarbonate (Olsen 1954) and exchangeable K by atomic-absorption spectrophotometry (David 1960). The amount of C and nutrient stocks per hectare was estimated using the nutrient fractions obtained from the laboratory analysis. The C and nutrient stocks (kg ha−1 or Mg ha−1) were calculated separately for litterfall, forest floor litter mass and soil to 0.30 m depth.
Annual aboveground primary productivity (ANPP) was estimated as current annual increment in above-ground biomass (AGB) of live trees between 2011 and 2012 (Aryal et al. 2014) plus mean annual litterfall (AL, measured from October 2012 to September 2014). A land use intensity (LUI) index (Eq. 1) and a soil quality (SQ) index were developed to analyse the effect and order of importance of these predictor variables for the litter production, forest floor litter mass and soil organic carbon. The LUI index was calculated following (Young 1997):
where C is cultivation years in a slash-and-burn cultivation cycle and F is fallow years after each cultivation period. Information on the land use history was obtained through landowner interviews.
The SQ index was calculated for each soil layer by summing the scaled values of different soil fertility parameters, taking into account the critical ranges that affect plant nutrient uptake compared with the ranges obtained from soil sample analysis (Table 2). The SQ index was also used to test whether soil quality affected the successional pattern of litter production, accumulation and aboveground primary productivity.
The seasonal variation in litterfall was analysed using Repeated Measures Analysis of Variance (ANOVA) with the data from 24 sampling months as repeated measures. The Tukey HSD (ρ = 0.05) test for homogeneity was used to verify significant differences in annual litterfall and nutrient data due to successional phases. Stepwise multiple regression analyses were performed to evaluate the effects of age, slash-and-burn cultivation intensity and soil quality on annual litter production, aboveground primary productivity, litter accumulation and SOC content.
Results
Litterfall and nutrient transfer at different successional phases
Mean annual litterfall ranged from 5.2 to 7.1 Mg ha−1 and was highest in ASF, followed by MSF. Leaves were the main component of the litterfall, comprising about 90 % in secondary forests and 84 % in primary forests, while small branches (twigs <10 mm in diameter and stem cortex) comprised 5–6 % in secondary forests and 10 % in primary forests. The highest twig fall was observed in primary forests, while there were no significant differences in fall for reproductive parts (flowers and fruits) (Table 3). The concentrations of C, N, P, and K in the litterfall components were similar across the successional stages and therefore the nutrient transfer from vegetation to soil followed the same pattern as litterfall production, with higher transfers observed in MSF and ASF than in YSF and PF. Total nutrient transfer varied between 2.3 ± 0.3–3.2 ± 0.1 Mg C, 62 ± 7–84 ± 4 kg N, 1.3 ± 0.2–1.8 ± 0.2 kg P and 36 ± 4–49 ± 2 kg K per hectare and year (Table 3). There was a significant negative correlation between litterfall distribution and monthly precipitation (r = 0.63, p = 0.02), indicating that litterfall was significantly higher in drier months and lower in rainy months, whereas there was no significant correlation between litterfall distribution and ambient temperature variation (r = 0.16, ρ = 0.14).
It was apparent from the results that total aboveground net primary productivity (ANPP) peaked at around 10 years of forest age and then decreased gradually over time (Fig. 3). Biomass accumulation was initially higher than litter production, but then slowed down gradually and fell below litter production at between 10 and 20 years of age. As net biomass accumulation (growth + recruitment − mortality of trees) decreases to close to zero in primary forests, ANPP in primary forests is mainly due to annual litter production. In YSF, the contribution of aboveground biomass increment to ANPP was higher than that of litter production (Fig. 3).
Total litter mass in the organic horizon (O) increased gradually with age and was highest in primary forests, mainly due to the gradual increase in the humus layer (Oa horizon). There were no significant differences between successional phases in the Oi and Oe horizons. Primary forests accumulated about 12 ± 1 Mg ha−1 of litter mass, while young secondary forests accumulated about 7 ± 0.8 Mg ha−1. There was a similar gradual increase in the total amount of CNPK in forest floor organic mass along the age gradient, as the nutrient content in litter horizons did not differ among successional stages. Primary forests accumulated about 3.6 Mg C ha−1, 162 kg N ha−1, 3 kg P ha−1 and 44 kg K ha−1 in litter mass (Table 4).
The amount of soil organic carbon showed no significant differences between the different phases of forest growth. The top horizon, 0–0.10 m depth, accumulated more carbon than deeper horizons in all phases. The average accumulated soil organic carbon amount ranged from 31 to 42 Mg ha−1 in the 0–0.10 m layer, 17–22 Mg ha−1 in the 0.11–0.20 m layer and 5–9 Mg ha−1 in the 0.21–0.30 m layer (Table 5). The slightly higher content of P and K in YSF could be the result of residual elements released from biomass burning before cultivation, which are reduced during early succession due to plant uptake and losses, but recover slowly afterwards due to the input from litterfall in later phases of succession (Table 5).
Seasonal pattern of litterfall
Monthly variation of litterfall showed a uni-modal pattern of litterfall, with a high peak during February and March in the first year and a lower peak from March to May in the second year (Fig. 4a, b). The 2-month (February–March) litterfall peak in the first year supplied between 65 and 80 % of total leaf litterfall in the first year, whereas the 3-month (March–May) peak in the second year only corresponded to between 44 and 54 % of litterfall in the second year. The high peak in the first year coincided with a very dry period between November 2012 and March 2013, whereas the much lower peak in the second year corresponded to higher rainfall between November 2013 and March 2014 (Fig. 4a, b). Monthly leaf litterfall ranged from 31 ± 2 kg to 3418 ± 544 kg ha−1 in the first year and from 196 ± 31 to 1513 ± 143 kg ha−1 in the second year (Figs. 5a, 6a). The highest monthly litterfall was observed in MSF during February 2012 and the lowest in YSF during June 2012. Monthly leaf litterfall was inversely correlated to monthly rainfall. Since the monthly variation in average ambient temperature and photoperiod did not vary greatly during the year, no significant relationship was observed between these variables and leaf litterfall. Unlike leaf fall, reproductive parts (flowers + fruits) fell mostly during December and April (Figs. 5b, 6b) as April is the flowering month (Ochoa-Gaona et al. 2008), whereas the high production in December might have resulted from an increased fruit production. The December production was higher in MSF and ASF than the April production, whereas in YSF and PF the production of reproductive parts was similar in these months (Figs. 5b, 6b). Twig fall was highest during February and March with peak values observed in primary forests (Figs. 5c, 6c).
a Monthly leaf litterfall (kg ha−1 year−1), Wilks’ lambda = 0.0042, F(44, 128.2) = 9.24, p = 0.000; b fruits and flower fall, Wilks’ lambda = 0.0989, F(44, 128.2) = 2.42, p = 0.000; and c twig fall, Wilks’ lambda = 0.0587, F(44, 128.2) = 3.25, p = 000; in four different phases of forest succession, measured from October 2012 to September 2013. YSF young secondary forests, MSF medium secondary forests, ASF advanced secondary forests, PF primary forests. Vertical bars denote 95 % confidence interval
a Monthly leaf litterfall (kg ha−1 year−1), Wilks’ lambda = 0.0053, F(44, 128.2 = 8.55, p = 0.000; b fruits and flower fall, Wilks’ lambda = 0.1212, F(44, 128.2) = 2.14, p = 0.001; and c twig fall, Wilks’ lambda = 0.1888, F(44, 128.2) = 1.59, p = 0.023; in four different phases of forest succession, measured from October 2013 to September 2014. YSF young secondary forests, MSF medium secondary forests, ASF advanced secondary forests, PF primary forest. Vertical bars denote 95 % confidence interval
Effect of forest age, land use intensity and soil quality on annual litter production and associated carbon transfer
Multivariate regression analysis showed that forest age and land use intensity (LUI) were significant predictors of annual litterfall (together explaining 59 % of the variations observed) and of aboveground net primary productivity (explaining 71 % of the variations). The soil quality was not a significant predictor (Table 6). Only forest age explained 36 % of the variance in litter mass accumulation on the forest floor. Forest age and LUI had no significant relationship with the soil organic carbon accumulation, but the SQ index appeared correlated to SOC in the 0.30 m topsoil (Table 6).
Discussion
Annual litter production and accumulation
The quantity of annual litter production recorded in this study was within the reported range in similar forest ecosystems (Table 7). In this respect, the medium-aged secondary forests seemed to be more productive than primary forests in terms of litter production. However, the leaf production in early secondary forests was already similar to that in primary forests, indicating that the associated leaf area index (LAI) increased rapidly to its maximum levels, as reported by Brown and Lugo (1990). The seasonal pattern of litterfall differed between the two study years, due to differences in seasonal rainfall pattern. This observation is in line with a regional study carried out by Chave et al. (2010) who found a positive correlation between seasonality in leaf litterfall and rainfall.
The amount of litter accumulation and nutrient return to soil depends not only on litter production, but also on the rate of litter decomposition (Dent et al. 2006; Wang et al. 2007). Litter decomposition is a function of environmental conditions, species composition, the presence of micro- and macro-organisms and substrate quality (Dickinson 2012; Keiser et al. 2013; Norris et al. 2013). Studies carried out in the region reported a faster litter decomposition in early successional species than for older secondary and primary forest species (Read and Lawrence 2003; Xuluc-Tolosa et al. 2003; Bejarano et al. 2014). The gradual accumulation of forest floor litter with increasing forest age observed in the present study can thus be explained by the higher litterfall and lower leaf decomposition rates in older secondary forests. The gradual increase in twig fall and its slower rate of decomposition may be another important factor explaining the accumulation of forest floor litter mass (Harmon et al. 1995).
Seasonality of litterfall and species assemblages of secondary forests
Previous studies in the study region have shown that there is a gradual shift in species composition and dominance during the successional stages of the forest that grows back after the abandonment of agricultural land (Ochoa-Gaona et al. 2007; Aryal et al. 2014; Chazdon 2014). Our findings also indicate that secondary forests differ from primary forests in species composition, which may influence their phenological characteristics. The characteristic species in MSF and ASF are more deciduous or undefined (deciduous or evergreen, depending on the local conditions), whereas those in YSF and PF are more dominated by evergreen species (see Table 1). This could explain the higher peaks of litterfall of MSF and ASF during the dry season of the first year compared with YSF and PF, with about 70 % of the annual litterfall in the first year concentrated in two dry months, in contrast to 55 % in YSF and primary forests (Fig. 4). In the second study year the peaks were more similar in all successional stages, representing about 40–50 % of the total annual litterfall. We attribute this variation to a combination of the differences in species composition and the annual rainfall pattern, which showed a larger dry spell in the first year. Previous studies have reported that the peaks in litterfall ranges from 25 to 100 % during the dry season in the Yucatan peninsula as a whole (Lawrence 2005; Cuba et al. 2013) but in semi-evergreen forests similar to those at the site of the study, the reported range varies from 25 to 50 % (Martínez and Galindo-Leal 2002). Our case indicates that in very dry periods, such as the first year of the present study, the peak may increase up to about 70 % of total annual litterfall in secondary forests.
The change in forest phenology in the secondary forests may be explained by changes in environmental conditions associated with land conversion favouring the establishment of more deciduous species. Another reason may be the reduced availability of seeds and propagules of original primary forest species and the higher reproduction capacity of early successional tree species (Moheno 2008). Although such changes from pioneer to persistent and late successional species are considered natural in tropical forest succession, it may take more than a century for the secondary forests to regain the original composition and dominance of primary forest species (Aryal et al. 2014). An increased proportion of deciduous species in secondary vegetation increases understory insolation and susceptibility to forest fires, leading to potential emissions of sequestered carbon to the atmosphere (Chapin III et al. 2011; Cuba et al. 2013). Changes in seasonal phenology and species composition of Yucatan forests can also negatively affect the habitat of forest fauna such as the white-lipped peccary (Tayassu pecari) by changing food sources and appropriate shelter (Reyna-Hurtado et al. 2009). Such changes in floristic composition, structure, productivity and functioning of forest ecosystems after the land use change may adversely affect conservation efforts in transitional tropical forests if not considered in policy issues (Román-Dañobeytia et al. 2014).
Predictor variables of litterfall and forest productivity
Forest age and LUI were found to be significant predictors of annual litter production and aboveground net primary productivity. The effect of fire, land use intensification, logging and other disturbances were reported as important for carbon exchange in mid-latitude forests (Ochoa-Gaona et al. 2007; Bradford et al. 2008; Aryal et al. 2014). A study on a tropical secondary forest chronosequence in Puerto Rico reported that site quality has a significant effect on litter production and decomposition (Ostertag et al. 2008). In the present study, site quality was only significantly correlated to soil organic carbon (SOC) content, indicating that higher quality soils may hold more organic carbon. The soil quality index, which combines various soil parameters as one independent variable, was also not correlated to litter production, indicating that soil characteristics do not influence the amount of litter produced per year. This contradicts other studies, which report higher litterfall in more fertile soil (Balvanera and Aguirre 2006; Ostertag et al. 2008). However, early recovery of annual litterfall rate by secondary forests indicate that the high nutrient input from vegetation to soil via litterfall in the early stages of succession contributes to higher nutrient uptake and faster growth in medium-aged secondary forests, as observed in our plots. However, litter decomposition in early successional species is faster than in late successional species (Xuluc-Tolosa et al. 2003), which might be the reason why soil quality did not show an improvement over time in our study.
It is interesting to note that LUI did not play a significant role for SOC content along the successional gradient. However, the plots that were used more intensively before the fallow period were less productive in terms of forest litter and ANPP.
In both study years, litterfall peaks were observed in drier months, confirming findings in other studies in the dry or sub-humid tropics (Moraes et al. 1999; Martius et al. 2004). The driest months coincided with the highest litterfall in both years, particularly in secondary forests. Since the peak litterfall characteristics of these dry tropical forests are highly linked to the temporal distribution of rainfall, changes in rainfall pattern, such as more prolonged droughts, can be expected to have significant effects on the functioning of forest ecosystems and associated ecosystem services.
Conclusions and recommendations
This study portrayed the litter carbon and nutrient transfer trajectory of successional forests and showed the effect of pre-abandonment cultivation intensity on carbon cycling in tropical forest ecosystems. It was found that secondary forests recovered the litter production capacity of primary forest already in early stages of succession. It was also found that carbon and nutrient accumulation in forest floor litter (O horizon) increased gradually with forest age. Older secondary forests seemed to contain more deciduous species than early secondary forests and primary forests. Forest governance strategies in Mexico usually consider only structural parameters of forests and not forest composition. This study shows the importance of taking into consideration the dynamic processes and functioning of forest succession, when designing forest governance strategies in Mexico. Future studies should include litter decomposition experiments to better understand the total nutrient flow and carbon balance of tropical forests. Detailed analysis of species composition and forest structure data, such as leaf area index, could also help to interpret the results.
References
Aryal DR, Geissen V, Ponce-Mendoza A et al (2012) Water quality under intensive banana production and extensive pastureland in tropical Mexico. J Plant Nutr Soil Sci 175:553–559
Aryal DR, De Jong BH, Ochoa-Gaona S et al (2014) Carbon stocks and changes in tropical secondary forests of southern Mexico. Agric Ecosyst Environ 195:220–230
Balvanera P, Aguirre E (2006) Tree diversity, environmental heterogeneity, and productivity in a Mexican Tropical Dry Forest. Biotropica 38:479–491
Bautista F, Palacio-Aponte G, Quintana P, Zinck JA (2011) Spatial distribution and development of soils in tropical karst areas from the Peninsula of Yucatan, Mexico. Driv Forces Glob Pedodiversity 135:308–321. doi:10.1016/j.geomorph.2011.02.014
Bejarano M, Crosby MM, Parra V et al (2014) Precipitation regime and nitrogen addition effects on leaf litter decomposition in tropical dry forests. Biotropica 46:415–424
Bradford JB, Birdsey RA, Joyce LA, Ryan MG (2008) Tree age, disturbance history, and carbon stocks and fluxes in subalpine Rocky Mountain forests. Glob Change Biol 14:2882–2897
Bray JR, Gorham E (1964) Litter production in forests of the world. In: Cragg JB (ed) Advances in ecological research. Academic Press, London, pp 101–157
Bremner JM, Mulvaney CS (1982) Nitrogen total. Methods of soil analysis part 2. Chemical and microbiological properties. Agron Monogr 9(2):595–624
Brown S, Lugo AE (1990) Tropical secondary forests. J Trop Ecol 6:1–32. doi:10.2307/2559366
Chapin FS III, Eviner VT (2005) Primary production. Biogeochemistry 8:215
Chapin FS III, Chapin MC, Matson PA, Vitousek P (2011) Principles of terrestrial ecosystem ecology. Springer, Berlin
Chave J, Navarette D, Almeida S, Álvarez E, Aragão LEOC, Bonal D, Châtelet P, Silva-Esperjo JE, Goret JY, von Hildebrand P, Jiménez E, Patiño S, Peñuela MC, Ol Phillips, Stevenson P, Malhi Y (2010) Regional and seasonal patterns of litterfall in tropical South America. Biogeosciences 7:43–55
Chazdon RL (2014) Second growth: the promise of tropical forest regeneration in an age of deforestation. University of Chicago Press, Chicago
Cuba N, Rogan J, Christman Z et al (2013) Modelling dry season deciduousness in Mexican Yucatán forest using MODIS EVI data (2000–2011). GIScience Remote Sens 50:26–49
Cuevas E, Medina E (1986) Nutrient dynamics within Amazonian forest ecosystems. Oecologia 68:466–472
David DJ (1960) The determination of exchangeable sodium, potassium, calcium and magnesium in soils by atomic-absorption spectrophotometry. Analyst 85:495–503
De Jong BHJ (2013) Spatial distribution of biomass and links to reported disturbances in tropical lowland forests of southern Mexico. Carbon Manag 4:601–615
De Jong BH, Ochoa-Gaona S, Castillo-Santiago MA et al (2000) Carbon flux and patterns of land-use/land-cover change in the Selva Lacandona, Mexico. AMBIO J Hum Environ 29:504–511
Dent DH, Bagchi R, Robinson D et al (2006) Nutrient fluxes via litterfall and leaf litter decomposition vary across a gradient of soil nutrient supply in a lowland tropical rain forest. Plant Soil 288:197–215
Dickinson CH (2012) Biology of plant litter decomposition. Elsevier, Amsterdam
Doran JW, Parkin TB (1994) Defining and assessing soil quality. In Doran et al. (eds) Defining soil quality for a sustainable environment. Soil Science Society of America special publication no. 35, Madison, pp 3–21
Ewel JJ (1976) Litter fall and leaf decomposition in a tropical forest succession in eastern Guatemala. J Ecol 64:293–308
Feldpausch TR, Riha SJ, Fernandes ECM, Wandelli EV (2005) Development of forest structure and leaf area in secondary forests regenerating on abandoned pastures in Central Amazônia. Earth Interactions 9, Paper 6, p 22
García E (1973) Modificaciones al Sistema de Clasificación Climática de Köppen. Instituto de Geografía, UNAM, México D.F
Harmon ME, Whigham DF, Sexton J, Olmsted I (1995) Decomposition and mass of woody detritus in the dry tropical forests of the northeastern Yucatan Peninsula, Mexico. Biotropica 27:305–316
Keiser AD, Knoepp JD, Bradford MA (2013) Microbial communities may modify how litter quality affects potential decomposition rates as tree species migrate. Plant Soil 372:167–176
Landon JR (2014) Booker tropical soil manual: a handbook for soil survey and agricultural land evaluation in the tropics and subtropics. Routledge
Lawrence D (2005) Regional-scale variation in litter production and seasonality in tropical dry forests of Southern Mexico1. Biotropica 37:561–570
Malhi Y (2012) The productivity, metabolism and carbon cycle of tropical forest vegetation. J Ecol 100:65–75
Martínez E, Galindo-Leal C (2002) La vegetación de Calakmul, Campeche, México: clasificación, descripción y distribución. Bol Soc Botánica México 71:7–32
Martinez-Yrizar A, Sarukhan J (1990) Litterfall patterns in a tropical deciduous forest in Mexico over a five-year period. J Trop Ecol 6:433–444
Martius C, Höfer H, Garcia MV et al (2004) Litter fall, litter stocks and decomposition rates in rainforest and agroforestry sites in central Amazonia. Nutr Cycl Agroecosystems 68:137–154
Medina E, Cuevas E (1996) Biomass production and accumulation in nutrient-limited rain forest: implications for responses to global change. In Gash et al. (eds) Amazonian Deforestation and Climate. Chichester, pp 221–239
Meentemeyer V, Box EO, Thompson R (1982) World patterns and amounts of terrestrial plant litter production. Bioscience 32:125–128. doi:10.2307/1308565
Melillo JM, McGuire AD, Kicklighter DW et al (1993) Global climate change and terrestrial net primary production. Nature 363:234–240
Moheno MB (2008) Forest recovery and management options in the Yucatan Peninsula, Mexico. ProQuest, Ann Arbor
Montagnini F, Ramstad K, Sancho F (1993) Litterfall, litter decomposition and the use of mulch of four indigenous tree species in the Atlantic lowlands of Costa Rica. Agrofor Syst 23:39–61
Moraes R, Dellitti WBC, Struffaldi Y (1999) Litterfall and litter nutrient content in two Brazilian Tropical Forests. Braz J Bot 22:09–16
Negash M, Starr M (2013) Litterfall production and associated carbon and nitrogen fluxes of seven woody species grown in indigenous agroforestry systems in the south-eastern Rift Valley escarpment of Ethiopia. Nutr Cycl Agroecosystems 97:29–41
Norris MD, Avis PG, Reich PB, Hobbie SE (2013) Positive feedbacks between decomposition and soil nitrogen availability along fertility gradients. Plant Soil 367:347–361
Ochoa-Gaona S, Hernández-Vázquez F, De Jong BHJ, Gurri-García FD (2007) Pérdida de diversidad florística ante un gradiente de intensificación del sistema agrícola de roza-tumba-quema: un estudio de caso en la Selva Lacandona, Chiapas, México. Bol Soc Botánica México 81:67–82
Ochoa-Gaona S, Pérez Hernández I, De Jong BH (2008) Fenología reproductiva de las especies arbóreas del bosque tropical de Tenosique, Tabasco, México. Rev Biol Trop 56:657–673
Olsen SR (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. United States Department of Agriculture, Washington. Circular 939. https://ia801703.us.archive.org/17/items/estimationofavai939olse/estimationofavai939olse.pdf
Orihuela-Belmonte DE, De Jong BHJ, Mendoza-Vega J, Van der Wal J, Paz-Pellat F, Soto-Pinto L, Flamenco-Sandoval A (2013) Carbon stocks and accumulation rates in tropical secondary forests at the scale of community, landscape and forest type. Agric Ecosyst Environ 171:72–84
Ostertag R, Marín-Spiotta E, Silver WL, Schulten J (2008) Litterfall and decomposition in relation to soil carbon pools along a secondary forest chronosequence in Puerto Rico. Ecosystems 11:701–714. doi:10.2307/40296320
Pérez-Salicrup D (2004) Forest types and their implications. In: Turner BL, Geoghegan J, Foster DR (eds) Integrated land change science and tropical deforestation in the southern Yucatán. Final fontiers. Oxford University Press, UK, pp 63–80
Read L, Lawrence D (2003) Litter nutrient dynamics during succession in dry tropical forests of the Yucatan: regional and seasonal effects. Ecosystems 6:747–761
Reyna-Hurtado R, Rojas-Flores E, Tanner GW (2009) Home range and habitat preferences of white-lipped peccaries (Tayassu pecari) in Calakmul, Campeche, Mexico. J Mammal 90:1199–1209
Rivera Vázquez R, Soto Pinto L, Núñez Colín CA et al (2013) Producción y tasa de descomposición de hojarasca en Acahuales de selva caducifolia en Chiapas. Rev Mex Cienc For 4:20–30
Román-Dañobeytia FJ, Levy-Tacher SI, Macario-Mendoza P, Zúñiga-Morales J (2014) Redefining Secondary forests in the Mexican forest code: implications for management, restoration, and conservation. Forests 5:978–991
Rueda X (2010) Understanding deforestation in the southern Yucatán: insights from a sub-regional, multi-temporal analysis. Reg Environ Change 10:175–189
Rzedowski J (2006) Vegetación de México. 1ra. Edición digital, Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, México, 504 pp. http://www.biodiversidad.gob.mx/publicaciones/librosDig/pdf/VegetacionMxPort.pdf
Scheer MB, Gatti G, Wisniewski C (2011) Nutrient fluxes in litterfall of a secondary successional alluvial rain forest in Southern Brazil. Rev Biol Trop 59:1869–1882
Schmook B, van Vliet N, Radel C et al (2013) Persistence of Swidden cultivation in the face of globalization: a case study from communities in Calakmul, Mexico. Hum Ecol 41:93–107
Shimadzu (2001) TOC-V CPH/CPN total organic carbon analyzer user’s manual. Shimadzu Corp. Process Environ. Instrum. Div, Kyoto, Japan
Takyu M, Aiba S-I, Kitayama K (2003) Changes in biomass, productivity and decomposition along topographical gradients under different geological conditions in tropical lower montane forests on Mount Kinabalu, Borneo. Oecologia 134:397–404
Thuille A, Schulze E-D (2006) Carbon dynamics in successional and afforested spruce stands in Thuringia and the Alps. Glob Change Biol 12:325–342
Turner BL, Geoghegan JM, Foster DR (eds) (2004) Integrated land-change science and tropical deforestation in the southern Yucatán: final frontiers. Oxford University Press, Oxford, New York
Vitousek PM (1984) Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65:285–298
Vogt KA, Grier CC, Vogt DJ (1986) Production, turnover, and nutrient dynamics of above-and belowground detritus of world forests. Adv Ecol Res 15:303–377
Wang Q, Wang S, Fan B, Yu X (2007) Litter production, leaf litter decomposition and nutrient return in Cunninghamia lanceolata plantations in south China: effect of planting conifers with broadleaved species. Plant Soil 297:201–211
Whigham DF, Zugasty-Towle P, Cabrera-Cano E, O'neill J, Ley E (1990) The effecto of annual variation in precipitation on growth and litter production in a tropical dry forest in the Yucatan of Mexico. Tropic Ecol 32:23–34
Xuluc-Tolosa FJ, Vester HFM, Ramırez-Marcial N et al (2003) Leaf litter decomposition of tree species in three successional phases of tropical dry secondary forest in Campeche, Mexico. For Ecol Manag 174:401–412
Young A (1997) Agroforestry for soil management. CAB international Wallingford, BPCC Wheatons Ltd, Exeter
Zhou G, Guan L, Wei X, Zhang D. Zhang Q. Yan J, Wen D, Liu J, Liu S, Huang Z, Kong G, Mo J, Yu Q (2007) Litterfall production along successional and altitudinal gradients of subtropical monsoon evergreen broadleaved forests in Guangdong, China. Plant Ecol 188:77–89
Zhou Y, Su J, Janssens IA et al (2014) Fine root and litterfall dynamics of three Korean pine (Pinus koraiensis) forests along an altitudinal gradient. Plant Soil 374:19–32
Acknowledgments
This study is part of the doctoral thesis of the first author. We thank three anonymous reviewers for their very valuable comments on earlier versions of the paper. We also thank Mary McAfee for the editing of the text. Consejo Nacional de Ciencia y Tecnología (CONACyT) Mexico provided a scholarship for the first author. Additional financial assistance was received from the US Forest Service through federal grant 12-IJ-11242306-054. Ejido members in Cristobal Colon and El Carmen II allowed us to establish experimental plots on their land and made the commitment to not change these during the experiment, for which we are very grateful. Regular litter collection and drying in the field was performed by Antonio Ramirez and Demetrio Alvarez, whom we thank. ECOSUR provided financial support for field and laboratory work. Soil and litter mass samples were analysed in the soil fertility and environmental chemistry laboratory of Colegio de Posgraduados in Montecillo, Mexico. We also thank Eduardo Martinez, Noel Gonzalez, Edith Orihuela, Mirna Canul, Isidra Pérez, Victoria Hernandez and Beatriz Peña for their cooperation in field and laboratory work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aryal, D.R., De Jong, B.H.J., Ochoa-Gaona, S. et al. Successional and seasonal variation in litterfall and associated nutrient transfer in semi-evergreen tropical forests of SE Mexico. Nutr Cycl Agroecosyst 103, 45–60 (2015). https://doi.org/10.1007/s10705-015-9719-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-015-9719-0