Abstract
Although gonadotrophins are major regulators of ovarian function in teleosts and other vertebrates, accumulating evidence indicates that the growth hormone (GH)-insulin-like growth factor (IGF) axis also plays an important role in fish reproduction. As a first step to understand the physiological role of the GH-IGF system in the ovarian development of starry flounder (Platichthys stellatus), the expression profiles of GH and IGF messenger RNAs (mRNAs) and plasma GH, IGF-I, estradiol-17β (E2), and testosterone (T) levels during the ovarian development were investigated. The developmental stages of ovaries were divided into five stages (II, III, IV, V, and VI) by histological analysis. The hepatosomatic index (HSI) and gonadosomatic index (GSI) values increased and peaked at stage IV and stage V, respectively, and then declined at stage VI. Pituitary GH mRNA levels decreased sharply at stage III and raised to top level at stage VI. The hepatic IGF-I mRNA levels ascended to maximum value at stage V and then declined significantly at stage VI. However, the hepatic IGF-II mRNA levels remained stable and increased significantly at stage VI. In contrast, the ovarian IGF-I mRNA levels increased gradually and peaked at stage VI. The ovarian IGF-II mRNA levels were initially stable and increased significantly at stage V until the top level at stage VI. Consistent with the pituitary GH mRNA levels, plasma GH levels reduced sharply at stage III and remained depressed until stage V and then raised remarkably at stage VI. Plasma IGF-I level peaked at stage V and then declined to initial level. Plasma E2 level peaked at stage IV and then dramatically descended to the basal level. Plasma T level peaked at stage V and then declined significantly back to the basal level. Based on statistical analysis, significant positive correlations between hepatic IGF-I mRNA and GSI, ovarian IGF-II mRNA and hepatic IGF-II mRNA, ovarian IGF-I mRNA and ovarian IGF-II mRNA, and plasma IGF-I and plasma T were observed, respectively. These results suggest that the GH-IGF system may be involved in the ovarian development of starry flounder; GH and IGFs appear to play distinct roles in the regulation of the ovarian development in paracrine/autocrine manners. These findings extend our knowledge of the roles of the GH-IGF axis on reproduction regulation in fish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that the growth of vertebrates including fish is primarily controlled by the growth hormone (GH)-insulin-like growth factor (IGF) system. The GH-IGF system of fish is particularly complex due to genome duplication in their evolution, resulting in multiple forms of GH, GH receptor (GHR), and IGF (Reindl and Sheridan 2012; Wang et al. 2015). The classical view of the GH-IGF system holds that GH released from pituitary gland stimulates synthesis and secretion of IGF-I principally from the liver through binding to GHRs and IGF-I, in turn, stimulates cell growth and differentiation in a variety of target tissues via distinct IGF receptors (Reindl and Sheridan 2012; Wood et al. 2005). The regulation of growth has focused on the production and release of GH from pituitary gland, and the intracellular signaling mechanisms mediating multifactorial neuroendocrine control of GH secretion have been well demonstrated (Canosa et al. 2007; Chang et al. 2012; Gahete et al. 2009; Wang et al. 2014). Moreover, the IGF system clearly plays an essential role in the growth regulation of vertebrates (Beckman 2011; Moriyama et al. 2000; Wood et al. 2005).
In addition to being involved in growth regulation, there is accumulating evidence that the GH-IGF system also plays an important role in fish reproduction (Bevers and Izadyar 2002; Hull and Harvey 2014; Reinecke 2010; Silva et al. 2009; Wood et al. 2005). Specific localization of GHR messenger RNA (mRNA) in tilapia ovary was identified, and the GHR mRNA level was significantly greater in the immature oocytes, as compared with matured oocytes (Kajimura et al. 2004). Similarly, the localization of IGF-I receptor was reported in ovarian follicle of gilthead sea bream (Perrot et al. 2000) and sterlet (Wuertz et al. 2007). Moreover, expression of IGF-I receptor in the ovaries of sterlet elevated at the onset of vitellogenesis (Wuertz et al. 2007). Both IGF-I mRNA and IGF-I immunoreactivity were demonstrated to be present in the ovary of several fish species, including tilapia (Berishvili et al. 2006; Schmid et al. 1999), sea bream (Kagawa et al. 1995; Perrot et al. 2000), and sterlet (Wuertz et al. 2007). In addition, IGF-II mRNA and IGF-II immunoreactivity occurred only in granulosa cells of late follicle stages in tilapia (Schmid et al. 1999). Recently, a novel IGF subtype, IGF-III, was discovered in fish and the expression of IGF-III mRNA was gonad specific (Wang et al. 2008). Further investigations showed that IGF-III was involved in gonadal development (Baroiller et al. 2014; Berishvili et al. 2010) and oocyte maturation (Li et al. 2015; Li et al. 2011). Moreover, GH could stimulate ovarian steroidogenesis by increasing the aromatase activity in spotted seatrout (Singh and Thomas 1993) and GH had a direct modulatory effect on gonadotropin-stimulated steroid production in goldfish (Van der Kraak et al. 1990). On the other hand, IGF-I also played a role in regulation of ovarian steroidogenesis in coho salmon (Maestro et al. 1997) and white perch (Weber et al. 2007), and both IGF-I and IGF-II induced oocyte maturation in red sea bream (Kagawa et al. 1994) and southern flounder (Picha et al. 2012). In addition, IGF-I and IGF-II increased germinal vesicle breakdown (GVBD), a marker for resumption of meiosis, in oocytes of striped bass (Weber and Sullivan 2000) and southern flounder (Picha et al. 2012). Collectively, the previously mentioned studies indicate that GH-IGF axis appears to play a crucial role in the formation, differentiation, and function of fish ovary (Lubzens et al. 2010).
Starry flounder (Platichthys stellatus), which belongs to the order Pleuronectiformes, is an economically important marine fish species and a popular table fish in East Asia due to its tasty flesh. In addition, starry flounder features an asynchronous ovarian development, and oocytes at different developmental stages are simultaneously present in a single ovary, thus providing a unique model for reproductive biology study. In our previous studies, the full-length complementary DNAs (cDNAs) encoding GH, IGF-I, and IGF-II from starry flounder were isolated and their spatial expression pattern in adult organs and temporal expression profile throughout embryonic development were examined (Xu et al. 2015; Zang et al. 2014). However, the mode of actions of GH-IGF axis in the ovarian development in this species remains unknown. Moreover, the natural spawning of this species still cannot be attained under artificial conditions; the underlying mechanisms for gonadal growth and reproduction should be clarified urgently, which is beneficial for sustainable development of farming industry. Accordingly, the aims of the present study were (1) to characterize the ovarian development of starry flounder and (2) to examine the changes of GH and IGF mRNAs along with their peptides during the ovarian development, as the first step to understand the role of the GH-IGF axis in the reproduction regulation of starry flounder.
Materials and methods
Experimental fish
All of the animal experiments were approved by the Animal Care and Use Committee of Chinese Academy of Fishery Sciences. Sexually matured female starry flounder (total length [TL] = 34.1 ± 0.5 cm and body weight [BW] = 868.4 ± 49.7 g) were sampled from a local flatfish hatchery in Qingdao, China. The fish were reared in an indoor concrete tank (25 m3 in volume) supplied with flow-through seawater (water exchange rate 400 % per day; salinity 27–31 ppt; pH 7.8–8.2). The seawater was sand filtered and decanted prior to tank supply. The fish were fed to satiation twice daily with a commercial dry diet (Shengsuo Aquafeed Co., Ltd., Yantai, China) at a rate of 2–3 % of BW. All of the fish were exposed to natural photothermal regimes, and water temperature ranged from 10 to 26 °C as seasons changed.
Histological analysis of ovarian development
Four females were sampled at monthly intervals between January 2013 and August 2013. All fish were anesthetized with MS222 (180 mg/L) and measured, blood was drawn from the caudal vein, and the plasma obtained by centrifugation at 10,000 rpm (4 °C) for 5 min and stored at −30 °C until analysis. Following being sacrificed by decapitation, the ovaries and livers were removed and weighed to calculate the gonadosomatic index (GSI = [gonad weight / (BW − Viscera weight)] × 100) and the hepatosomatic index (HSI = [liver weight / (BW − Viscera weight)] × 100). In order to verify the developmental stages of ovaries, histological analysis of the ovaries was performed as previously described (Shi et al. 2015). Briefly, the ovaries were transferred to 70 % ethanol after being fixed for 24 h in Bouin’s solution. Following paraffin embedding, the ovaries were serially sectioned at 5 μm and stained with hematoxylin and eosin (H&E). The ovarian developmental stages were identified under light microscopy (Leica DM500, Germany). The maturity of the females with the asynchronous-type ovary was classified according to the most advanced type of oocytes found in the ovary as described in previous studies (Garcia-Lopez et al. 2007; Shi et al. 2015), and five phases of ovarian development were identified, as follows: previtellogenesis (stage II), vitellogenesis (stage III), late vitellogenesis (stage IV), maturation (stage V), and post ovulation (stage VI).
RNA extraction, reverse transcription, and real-time quantitative PCR
Pituitaries, livers, and ovaries were collected from fish at different ovarian developmental stages following anesthetization and decapitation. The tissues were immediately snap-frozen in liquid nitrogen and stored at −80 °C until RNA extraction. Total RNAs were extracted using RNAiso reagent (Takara, Dalian, China) according to the manufacturer’s instructions. The purity and yield of RNA were assessed by a NanoDrop 2000C spectrophotometer (Thermo Scientific, USA) with 260:280 ratios of 1.8–2.0. The integrity of RNA was determined on 1 % agarose gel electrophoresis with ethidium bromide (EB) staining. Subsequently, cDNA synthesis and real-time quantitative PCR were performed as previously described (Xu et al. 2015). In brief, the first-strand cDNA was synthesized with PrimeScript First-Strand cDNA Synthesis Kit (Takara, Dalian, China) according to the manufacturer’s protocol. Gene expression levels were determined with real-time quantitative PCR assay. The primers used in this study are listed in Table 1. No amplification was observed with nontemplate as a negative control to confirm that the reagents were not contaminated. The specificity of the primers was evaluated by the melting curve analysis, and the PCR products were determined on 2 % agarose gel electrophoresis with EB staining. Five-fold serial dilutions of standards containing the qPCR amplicon were conducted to estimate PCR efficiency as previously described (Schmittgen and Livak 2008). Beta-actin was used as the internal reference, and beta-actin levels remained stable throughout the study. The relative gene expression levels were normalized to beta-actin levels and calculated by the comparative Ct method (Schmittgen and Livak 2008).
Assay of plasma hormones
The plasma levels of E2 and T were measured using a validated radioimmunoassay, as described elsewhere (Xu et al. 2012). The assay detection limits were 1.8 pg/mL for E2 and 22 pg/mL for T, respectively. The intra- and inter-assay coefficients of variation were 1.9 and 7.4 % for E2 and 2.1 and 7.7 % for T, respectively. The plasma levels of GH and IGF-I were measured by commercially available ELISA kits (Cusabio, Wuhan, China) according to the manufacturer’s protocols. The assay detection limits were 312.5 pg/mL for GH and 31.25 pg/mL for IGF-I, respectively. The intra- and inter-assay coefficients of variation were typically less than 15 % for GH and IGF-I, respectively.
Statistical analysis
All data were expressed as mean ± SEM and analyzed by one-way ANOVA followed by Duncan’s multiple comparison test using SPSS 16.0 software (Chicago, IL, USA). Levels of correlation were determined by calculating the Pearson correlation coefficient (r). Statistical significance was considered as p value of <0.05.
Results
Characterization of the ovarian development of starry flounder
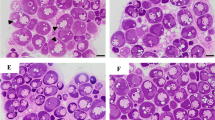
The ovary developmental stages were systematically determined by histological analysis. The ovarian development of starry flounder is asynchronous, and oocytes of different developmental stages can be simultaneously found in the same ovary. As shown in Fig. 1, five phases of ovarian development were identified: previtellogenesis (stage II, Fig. 1a), vitellogenesis (stage III, Fig. 1b), late vitellogenesis (stage IV, Fig. 1c), maturation (stage V, Fig. 1d), and post ovulation (stage VI, Fig. 1e).
Representative photomicrographs of the ovarian development of starry flounder. a Previtellogenesis, ovary at stage II; b vitellogenesis, ovary at stage III; c late vitellogenesis, ovary at stage IV; d maturation, ovary at stage V; and e post ovulation, ovary at stage VI. YP yolk plates, FM follicle membrane
Changes in GSI and HSI during ovarian development
As shown in Fig. 2a, GSI was low at stages II and III and increased progressively to peak level at stage V. Thereafter, GSI declined post ovulation (stage VI). HSI increased gradually with the ovarian development and peaked at stage IV and then declined gradually to the initial level at stage VI (Fig. 2b).
Gene expression of GH and IGF mRNAs during ovarian development
As shown in Fig. 3a, the pituitary GH mRNA levels decreased sharply at stage III and remained depressed until stage V and raised to top level after spawning (stage VI). The hepatic IGF-I mRNA levels ascended progressively to the maximal value at stage V and then declined significantly post ovulation (stage VI) (Fig. 3b). However, hepatic IGF-II mRNA levels remained stable during the ovarian development and increased significantly after spawning (stage VI) (Fig. 3c). In contrast, the ovarian IGF-I mRNA levels increased gradually with ovarian development and peaked at stage VI (Fig. 3d). The ovarian IGF-II mRNA levels were initially stable and increased significantly at stage V until the top level at stage VI (Fig. 3e).
Relative gene expression levels of pituitary GH mRNA (a), hepatic IGF-I (b), and IGF-II (c) as well as ovarian IGF-I (d) and IGF-II (e) mRNAs at different ovarian developmental stages of starry flounder. Each bar represents the mean ± SEM (n = 4). Groups with different letters are significantly different from each other (p < 0.05)
Changes in plasma levels of hormones during ovarian development
We evaluated the plasma levels of GH, IGF-I, E2, and T at various ovarian stages. Consistent with the pituitary GH mRNA levels, plasma GH concentration reduced sharply at stage III and remained depressed until stage V and then raised remarkably post spawning (stage VI) (Fig. 4a). Plasma IGF-I concentration increased progressively, peaked at stage V, and then declined to initial level (Fig. 4b). Plasma E2 concentration remained low at stages II and III, raised rapidly to the top level at stage IV, and then descended gradually to the basal level (Fig. 4c). Plasma T concentration remained stable before stage IV, raised sharply to the maximum level at stage V, and then significantly reduced back to the basal level at stage VI (Fig. 4d).
Correlation analysis among reproductive parameters
As shown in Table 2, there was a significant positive correlation between hepatic IGF-I mRNA and GSI, between ovarian IGF-II mRNA and hepatic IGF-II mRNA, between ovarian IGF-I mRNA and ovarian IGF-II mRNA, and between plasma IGF-I and plasma T, respectively. Other correlations were not significant though there was a strong correlation among some factors.
Discussion
The present study characterized the ovarian development of starry flounder and analyzed the expression profiles of GH and IGF mRNAs as well as plasma hormone levels during the ovarian development of starry flounder. Our data suggest that the GH-IGF system plays an important role in the ovarian development of starry flounder.
Starry flounder displayed an asynchronous ovarian development, and oocytes of different developmental stages could be simultaneously found, suggesting that starry flounder was a multiple batch spawner. On one hand, GSI is an important indicator of gonad development in fish. In our study, GSI in starry flounder began to increase significantly at stage IV and reached the highest value at stage V and then declined sharply after spawning, displaying a relation with ovarian development. The variation of GSI was consistent with those of Korean spotted sea bass (Lee and Yang 2002), amberjack (Micale et al. 1999), and Atlantic cod (Dahle et al. 2003). On the other hand, HSI is a good indicator of liver energy content (Dahle et al. 2003). Starry flounder HSI increased continuously and peaked at stage IV, which is likely to reflect vitellogenesis in the liver and preparation for ovarian development. The decrease of HSI at stage V suggested that vitellogenin stored in the liver had been transferred to gonad, and the decline of HSI after spawning (stage VI) was associated with the reduction of metabolic activity in the liver (Maddock and Burton 1998; Sol et al. 1998).
A strong positive correlation of pituitary GH mRNA levels with plasma GH levels was found in our study. Both pituitary GH mRNA levels and plasma GH levels remained depressed during the ovarian development and regained higher levels after spawning, implying that GH had no direct effects on ovarian development. In agreement with our hypothesis, treatment of previtellogenic ovarian fragments from short-finned eel with recombinant human IGF-I resulted in increased oocyte diameters, whereas no such effect was seen after treatment with GH (Lokman et al. 2007). In contrast, GH seems to control ovarian development via regulating expression of IGF-I in the liver or ovary (Duan et al. 1993; Duguay et al. 1994; Lokman et al. 2007). The GH treatment in the prereproductive ovary of sea bream induced a significant increase of IGF-I expression (Gioacchini et al. 2005). In rainbow trout, IGF-I mRNA levels increased in the liver and other extra-hepatic tissues in response to recombinant bovine somatotropin treatment (Biga et al. 2004). In addition, the binding capacity of GH to GHR present in the ovary of rainbow trout increased during the ovarian development (Gomez et al. 1999). Based on the previously mentioned studies, we hypothesized that a low concentration of plasma GH was associated with the inhibition of somatic growth, while an ovary-specific increase of the binding capacity of GHR contributed to the ovarian development of starry flounder.
Both IGF-I and IGF-II mRNAs were detected in the liver and ovary at various stages of ovarian development of starry flounder, which was in line with previous reports in tilapia (Berishvili et al. 2006; Schmid et al. 1999) and gilthead sea bream (Perrot et al. 2000). A strong positive correlation of hepatic IGF-I mRNA levels with plasma IGF-I levels was observed in this study. Both hepatic IGF-I mRNA levels and plasma IGF-I levels increased gradually, reaching the maximal level at stage V and then dropped after spawning, suggesting that IGF-I mainly participated in vitellogenesis and oocyte maturation in starry flounder. However, hepatic IGF-II mRNA levels remained constant during the ovarian development, implying that IGF-II derived from the liver may not be involved in the ovarian development. Consistent with previous studies in rainbow trout (Bobe et al. 2003; Bobe et al. 2004), ovarian levels of IGF-I and IGF-II mRNAs increased with ovarian development, suggesting that locally produced IGFs play important roles in the paracrine/autocrine regulation of ovarian development. Moreover, the significant correlation between ovarian IGF-I mRNA levels and IGF-II mRNA levels was observed, which may indicate a cooperative function relationship between IGF-I and IGF-II in ovarian development of starry flounder.
Sex steroid hormones are important regulators of sexual maturation (Lubzens et al. 2010). E2 controls the synthesis of vitellogenin and vitelline envelope proteins in the liver (Davis et al. 2007; Larsson et al. 2002). In this study, peak levels of E2 appeared around the start of spawning period and a subsequent decrease during spawning was observed, which was in accordance with a number of periodic spawners (Harmin et al. 1995; Johnson et al. 1991; Scott et al. 1998). T also plays a role in the synthesis of vitellogenin (Sundararaj et al. 1982). A significant positive correlation between serum T levels and serum IGF-I levels was observed in our study, and the increase of sex steroid hormone levels might be associated with the enhancement of IGF-I levels. IGF-I promoted E2 production by stimulating aromatase activity and cytochrome P450 aromatase gene expression in the ovarian fragments of red sea bream (Kagawa et al. 2003). Similarly, IGF-I increased T and E2 production in ovarian fragments of white perch in vitro (Weber et al. 2007). Furthermore, the plasma IGF-I levels positively correlated with plasma levels of E2 and T in chum salmon (Onuma et al. 2009). Collectively, these data suggest that IGF-I plays an essential role in steroidogenesis during vitellogenic period. The role of IGF-II in steroidogenesis awaits further studies in teleosts.
In summary, we characterized the ovarian development of starry flounder and analyzed the expression profiles of GH and IGF mRNAs as well as the plasma hormone levels during the ovarian development of starry flounder. GSI increased during the course of ovarian development and reached a maximum during spawning in starry flounder, while HSI arrived at the peak level before spawning. These changes were associated with changes in the gene expression of GH and IGF mRNAs along with their peptides and sex steroids. These results indicate that paracrine/autocrine actions of the GH/IGF-I axis play an important role in the development of the ovary in starry flounder.
References
Baroiller JF, D’Cotta H, Shved N, Berishvili G, Toguyeni A, Fostier A, Eppler E, Reinecke M (2014) Oestrogen and insulin-like growth factors during the reproduction and growth of the tilapia Oreochromis niloticus and their interactions. Gen Comp Endocrinol 205:142–150
Beckman BR (2011) Perspectives on concordant and discordant relations between insulin-like growth factor 1 (IGF1) and growth in fishes. Gen Comp Endocrinol 170:233–252
Berishvili G, Baroiller JF, Eppler E, Reinecke M (2010) Insulin-like growth factor-3 (IGF-3) in male and female gonads of the tilapia: development and regulation of gene expression by growth hormone (GH) and 17alpha-ethinylestradiol (EE2). Gen Comp Endocrinol 167:128–134
Berishvili G, D’Cotta H, Baroiller JF, Segner H, Reinecke M (2006) Differential expression of IGF-I mRNA and peptide in the male and female gonad during early development of a bony fish, the tilapia Oreochromis niloticus. Gen Comp Endocrinol 146:204–210
Bevers MM, Izadyar F (2002) Role of growth hormone and growth hormone receptor in oocyte maturation. Mol Cell Endocrinol 197:173–178
Biga PR, Schelling GT, Hardy RW, Cain KD, Overturf K, Ott TL (2004) The effects of recombinant bovine somatotropin (rbST) on tissue IGF-I, IGF-I receptor, and GH mRNA levels in rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol 135:324–333
Bobe J, Maugars G, Nguyen T, Rime H, Jalabert B (2003) Rainbow trout follicular maturational competence acquisition is associated with an increased expression of follicle stimulating hormone receptor and insulin-like growth factor 2 messenger RNAs. Mol Reprod Dev 66:46–53
Bobe J, Nguyen T, Jalabert B (2004) Targeted gene expression profiling in the rainbow trout (Oncorhynchus mykiss) ovary during maturational competence acquisition and oocyte maturation. Biol Reprod 71:73–82
Canosa LF, Chang JP, Peter RE (2007) Neuroendocrine control of growth hormone in fish. Gen Comp Endocrinol 151:1–26
Chang JP, Habibi HR, Yu Y, Moussavi M, Grey CL, Pemberton JG (2012) Calcium and other signalling pathways in neuroendocrine regulation of somatotroph functions. Cell Calcium 51:240–252
Dahle R, Taranger GL, Karlsen O, Kjesbu OS, Norberg B (2003) Gonadal development and associated changes in liver size and sexual steroids during the reproductive cycle of captive male and female Atlantic cod (Gadus morhua L.). Comp Biochem Physiol A Mol Integr Physiol 136:641–653
Davis LK, Hiramatsu N, Hiramatsu K, Reading BJ, Matsubara T, Hara A, Sullivan CV, Pierce AL, Hirano T, Grau EG (2007) Induction of three vitellogenins by 17beta-estradiol with concurrent inhibition of the growth hormone-insulin-like growth factor 1 axis in a euryhaline teleost, the tilapia (Oreochromis mossambicus). Biol Reprod 77:614–625
Duan C, Duguay SJ, Plisetskaya EM (1993) Insulin-like growth factor I (IGF-I) mRNA expression in coho salmon, Oncorhynchus kisutch: tissue distribution and effects of growth hormone/prolactin family proteins. Fish Physiol Biochem 11:371–379
Duguay SJ, Swanson P, Dickhoff WW (1994) Differential expression and hormonal regulation of alternatively spliced IGF-I mRNA transcripts in salmon. J Mol Endocrinol 12:25–37
Gahete MD, Duran-Prado M, Luque RM, Martinez-Fuentes AJ, Quintero A, Gutierrez-Pascual E, Cordoba-Chacon J, Malagon MM, Gracia-Navarro F, Castano JP (2009) Understanding the multifactorial control of growth hormone release by somatotropes: lessons from comparative endocrinology. Ann N Y Acad Sci 1163:137–153
Garcia-Lopez A, Couto E, Canario AV, Sarasquete C, Martinez-Rodriguez G (2007) Ovarian development and plasma sex steroid levels in cultured female Senegalese sole Solea senegalensis. Comp Biochem Physiol A Mol Integr Physiol 146:342–354
Gioacchini G, Cardinali M, Maradonna F, Funkenstein B, Mosconi G, Carnevali O (2005) Hormonal control of the IGF system in the sea bream ovary. Ann N Y Acad Sci 1040:320–322
Gomez JM, Mourot B, Fostier A, Le Gac F (1999) Growth hormone receptors in ovary and liver during gametogenesis in female rainbow trout (Oncorhynchus mykiss). J Reprod Fertil 115:275–285
Harmin S, Crim L, Wiegand M (1995) Plasma sex steroid profiles and the seasonal reproductive cycle in male and female winter flounder, Pleuronectes americanus. Mar Biol 121:601–610
Hull KL, Harvey S (2014) Growth hormone and reproduction: a review of endocrine and autocrine/paracrine interactions. Int J Endocrinol 2014:234014
Johnson LL, Casillas E, Myers MS, Rhodes LD, Olson OP (1991) Patterns of oocyte development and related changes in plasma 17-β estradiol, vitellogenin, and plasma chemistry in English sole Parophrys vetulus Girard. J Exp Mar Biol Ecol 152:161–185
Kagawa H, Gen K, Okuzawa K, Tanaka H (2003) Effects of luteinizing hormone and follicle-stimulating hormone and insulin-like growth factor-I on aromatase activity and P450 aromatase gene expression in the ovarian follicles of red seabream, Pagrus major. Biol Reprod 68:1562–1568
Kagawa H, Kobayashi M, Hasegawa Y, Aida K (1994) Insulin and insulin-like growth factors I and II induce final maturation of oocytes of red seabream, Pagrus major, in vitro. Gen Comp Endocrinol 95:293–300
Kagawa H, Moriyama S, Kawauchi H (1995) Immunocytochemical localization of IGF-I in the ovary of the red seabream, Pagrus major. Gen Comp Endocrinol 99:307–315
Kajimura S, Kawaguchi N, Kaneko T, Kawazoe I, Hirano T, Visitacion N, Grau EG, Aida K (2004) Identification of the growth hormone receptor in an advanced teleost, the tilapia (Oreochromis mossambicus) with special reference to its distinct expression pattern in the ovary. J Endocrinol 181:65–76
Larsson DG, Mayer I, Hyllner SJ, Forlin L (2002) Seasonal variations of vitelline envelope proteins, vitellogenin, and sex steroids in male and female eelpout (Zoarces viviparus). Gen Comp Endocrinol 125:184–196
Lee W-K, Yang S-W (2002) Relationship between ovarian development and serum levels of gonadal steroid hormones, and induction of oocyte maturation and ovulation in the cultured female Korean spotted sea bass Lateolabrax maculatus (Jeom-nong-eo). Aquaculture 207:169–183
Li J, Chu L, Sun X, Liu Y, Cheng CH (2015) IGFs mediate the action of LH on oocyte maturation in zebrafish. Mol Endocrinol 29:373–383
Li J, Liu Z, Wang D, Cheng CH (2011) Insulin-like growth factor 3 is involved in oocyte maturation in zebrafish. Biol Reprod 84:476–486
Lokman PM, George KA, Divers SL, Algie M, Young G (2007) 11-Ketotestosterone and IGF-I increase the size of previtellogenic oocytes from shortfinned eel, Anguilla australis, in vitro. Reproduction 133:955–967
Lubzens E, Young G, Bobe J, Cerda J (2010) Oogenesis in teleosts: how eggs are formed. Gen Comp Endocrinol 165:367–389
Maddock D, Burton M (1998) Gross and histological observations of ovarian development and related condition changes in American plaice. J Fish Biol 53:928–944
Maestro MA, Planas JV, Moriyama S, Gutierrez J, Planas J, Swanson P (1997) Ovarian receptors for insulin and insulin-like growth factor I (IGF-I) and effects of IGF-I on steroid production by isolated follicular layers of the preovulatory coho salmon ovarian follicle. Gen Comp Endocrinol 106:189–201
Micale V, Maricchiolo G, Genovese L (1999) The reproductive biology of the amberjack, Seriola dumerilii (Risso 1810). I. Oocyte development in captivity. Aquac Res 30:349–355
Moriyama S, Ayson FG, Kawauchi H (2000) Growth regulation by insulin-like growth factor-I in fish. Biosci Biotechnol Biochem 64:1553–1562
Onuma TA, Makino K, Ban M, Ando H, Fukuwaka MA, Azumaya T, Swanson P, Urano A (2009) Elevation of the plasma level of insulin-like growth factor-I with reproductive maturation prior to initiation of spawning migration of chum salmon. Ann N Y Acad Sci 1163:497–500
Perrot V, Moiseeva EB, Gozes Y, Chan SJ, Funkenstein B (2000) Insulin-like growth factor receptors and their ligands in gonads of a hermaphroditic species, the gilthead seabream (Sparus aurata): expression and cellular localization. Biol Reprod 63:229–241
Picha ME, Shi B, Thomas P (2012) Dual role of IGF-II in oocyte maturation in southern flounder Paralichthys lethostigma: up-regulation of mPRalpha and resumption of meiosis. Gen Comp Endocrinol 177:220–230
Reindl KM, Sheridan MA (2012) Peripheral regulation of the growth hormone-insulin-like growth factor system in fish and other vertebrates. Comp Biochem Physiol A Mol Integr Physiol 163:231–245
Reinecke M (2010) Insulin-like growth factors and fish reproduction. Biol Reprod 82:656–661
Schmid AC, Naf E, Kloas W, Reinecke M (1999) Insulin-like growth factor-I and -II in the ovary of a bony fish, Oreochromis mossambicus, the tilapia: in situ hybridisation, immunohistochemical localisation, Northern blot and cDNA sequences. Mol Cell Endocrinol 156:141–149
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Scott P, Witthames P, Turner R, Canario AV (1998) Plasma concentrations of ovarian steroids in relation to oocyte final maturation and ovulation in female plaice sampled at sea. J Fish Biol 52:128–145
Shi B, Liu X, Xu Y, Wang S (2015) Molecular characterization of three gonadotropin subunits and their expression patterns during ovarian maturation in Cynoglossus semilaevis. Int J Mol Sci 16:2767–2793
Silva JR, Figueiredo JR, van den Hurk R (2009) Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 71:1193–1208
Singh H, Thomas P (1993) Mechanism of stimulatory action of growth hormone on ovarian steroidogenesis in spotted seatrout, Cynoscion nebulosus. Gen Comp Endocrinol 89:341–353
Sol SY, Olson O, Lomax D, Johnson LL (1998) Gonadal development and associated changes in plasma reproductive steroids in English sole, Pleuronectes vetulus, from Puget sound, Washington. Fishery Bulletin-National Oceanic and Atmospheric Administration 96:859–870
Sundararaj BI, Goswami SV, Lamba VJ (1982) Role of testosterone, estradiol-17β, and cortisol during vitellogenin synthesis in the catfish, Heteropneustes fossilis (Bloch). Gen Comp Endocrinol 48:390–397
Van der Kraak G, Rosenblum PM, Peter RE (1990) Growth hormone-dependent potentiation of gonadotropin-stimulated steroid production by ovarian follicles of the goldfish. Gen Comp Endocrinol 79:233–239
Wang B, Jia J, Yang G, Qin J, Zhang C, Zhang Q, Sun C, Li W (2015) In vitro effects of somatostatin on the growth hormone-insulin-like growth factor axis in orange-spotted grouper (Epinephelus coioides). Gen Comp Endocrinol. doi:10.1016/j.ygcen.2015.1010.1014
Wang B, Qin C, Zhang C, Jia J, Sun C, Li W (2014) Differential involvement of signaling pathways in the regulation of growth hormone release by somatostatin and growth hormone-releasing hormone in orange-spotted grouper (Epinephelus coioides). Mol Cell Endocrinol 382:851–859
Wang D-S, Jiao B, Hu C, Huang X, Liu Z, Cheng CH (2008) Discovery of a gonad-specific IGF subtype in teleost. Biochem Biophys Res Commun 367:336–341
Weber GM, Moore AB, Sullivan CV (2007) In vitro actions of insulin-like growth factor-I on ovarian follicle maturation in white perch (Morone americana). Gen Comp Endocrinol 151:180–187
Weber GM, Sullivan CV (2000) Effects of insulin-like growth factor-I on in vitro final oocyte maturation and ovarian steroidogenesis in striped bass, Morone saxatilis. Biol Reprod 63:1049–1057
Wood AW, Duan C, Bern HA (2005) Insulin-like growth factor signaling in fish. Int Rev Cytol 243:215–285
Wuertz S, Gessner J, Kirschbaum F, Kloas W (2007) Expression of IGF-I and IGF-I receptor in male and female sterlet, Acipenser ruthenus—evidence for an important role in gonad maturation. Comp Biochem Physiol A Mol Integr Physiol 147:223–230
Xu Y, Zang K, Liu X, Shi B, Li C, Shi X (2015) Insulin-like growth factors I and II in starry flounder (Platichthys stellatus): molecular cloning and differential expression during embryonic development. Fish Physiol Biochem 41:139–152
Xu YJ, Liu XZ, Liao MJ, Wang HP, Wang QY (2012) Molecular cloning and differential expression of three GnRH genes during ovarian maturation of spotted halibut, Verasper variegatus. J Exp Zool A Ecol Genet Physiol 317:434–446
Zang K, Liu X, Xu Y, Zhang K, Shi B, Shi X, Li C (2014) Molecular cloning and in vitro expression of growth hormone gene from Platichthys stellatus. J Fish China 38:1230–1242
Acknowledgments
This work was supported by grants from the National High Technology Research and Development Program of China (2012AA10A413) and the China Agriculture Research System (CARS-50).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All of the animal experiments were approved by the Animal Care and Use Committee of Chinese Academy of Fishery Sciences.
Rights and permissions
About this article
Cite this article
Xu, Y., Wang, B., Liu, X. et al. Evidences for involvement of growth hormone and insulin-like growth factor in ovarian development of starry flounder (Platichthys stellatus). Fish Physiol Biochem 43, 527–537 (2017). https://doi.org/10.1007/s10695-016-0307-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0307-2