Abstract
Dietary supplementation with a multi-strain probiotic containing Bacillus subtilis, Enterococcus faecium, Pediococcus acidilactici and Lactobacillus reuteri has been examined for its effects on growth performance, intestinal microbiota, non-specific immune response and antioxidant status of rainbow trout. Three groups of sub-adult trout were stocked into experimental tanks. A commercial diet was used as control, while the other two groups received diets supplemented with the multi-strain probiotic at levels of 1 and 5 g kg−1 diet. The fish were fed to apparent satiation three times daily for 8 weeks. Dietary probiotic at 1 g kg−1 diet improved (P < 0.05) growth and feed efficiency compared to control diet. Lactic acid bacteria loads were higher in probiotic fed fish at both inclusion levels compared to control; however, Enterobacteriaceae, Coliforms and Aeromonas spp. were similar between groups. Dietary probiotics decreased (P < 0.05) malondialdehyde formation on day 0 compared to control fish but not on day 5 of storage. Probiotics also increased (P < 0.05) the activity of glutathione-based enzymes. Serum lysozyme levels were similar among dietary treatments. Probiotic supplementation at 1 g kg−1 diet reduced serum nitric oxide levels compared to control. In conclusion, dietary probiotics at the level of 1 g kg−1 of diet exerted both a growth promoting and antioxidant protective activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The success of intensive aquaculture has been the direct result of improved genetics, nutrition and management, as well as maximizing disease control (Staykov et al. 2007). Manipulation and control of fish intestinal microbiota, which helps the digestive and absorptive process and protects the host against invading pathogens, have been identified as a critical point in aquaculture (Merrifield et al. 2010b). The increasing regulations and bans on the use of antibacterial drugs due to the continual emergence of antibiotic-resistant strains in both terrestrial or aquatic organisms insist on the need for novel approaches (Kesarcodi-Watson et al. 2008). Probiotics is one field commanding considerable attention in this line.
Probiotics are live, non-pathogenic bacteria or viable spores that may confer health benefits to the host through balance of their intestinal tract. Probiotics have been widely used in terrestrial animals to improve growth performance and feed conversion (Patterson et al. 1997; Mountzouris et al. 2007; Burr et al. 2005; Balcázar et al. 2006; Gómez and Balcázar 2008; Wang et al. 2008; Nayak 2010; Dimitroglou et al. 2011) and maintain a host-specific flora to reduce health risk in aquatic organisms (Gildberg et al. 1995; Nikoshelainen et al. 2001). The modes of actions of probiotics may include (1) maintaining normal intestinal microbiota by competitive exclusion, antagonism and promotion of beneficial microbes; (2) altering metabolism by increasing digestive enzyme activity and decreasing pathogen bacterial enzyme activity and ammonia production; (3) improving feed intake and digestion; and (4) neutralizing enterotoxins and stimulating the immune system (Jin et al. 1998).
The establishment, maintenance and stabilization of a beneficial gut microbiota and an optimized innate immune system are crucial to withstand pathogens. Probiotics based on B. subtilis, P. acidilactici and E. faecium have shown that can improve immunological response and growth performance and affect gut microbiota and mortality in rainbow trout (Merrifield et al. 2010b). The probiotic bacteria can dominate pathogenic bacteria by competitive exclusion, improving fish immune response and sometimes can also act as antiviral instrument (Kesarcodi-Watson et al. 2008). Most of the scientific studies carried out to evaluate the effect of probiotics on aquatic farmed animals, apart from demonstrating improvement in survival and growth of the host species have generally focused on nutrition, antagonism towards pathogens and immunity of the host (Merrifield et al. 2010b; Dimitroglou et al. 2011).
The objective of this study was to investigate the hypothesis that dietary inclusion of a combination of probiotic strains including B. subtilis, P. acidilactici, E. faecium and L. reuteri may have both growth-promoting effects and health benefits on growing rainbow trout and to evaluate the dietary effects of this combination of selected probiotic strains on performance, immune response, intestinal bacteria population and antioxidant status.
Materials and methods
Reagents and equipment
5,5′-Dithiobis-(2-nitrobenzoic acid) (DTNB), thiobarbituric acid (TBA), 1,1,3,3-tetraethoxypropane, (GR), (GSH), NADPH and Coomassie Brilliant Blue G-50, butylated hydroxytoluene (BHT) were purchased from Sigma (St. Louis, MO, USA). Sodium azide, tert-butyl-hydroxyperoxide (t-BHP) and bovine serum albumin and solid agar media were purchased from Fluka (Sigma-Aldrich, Taufkirchen, Germany) or Merck (Darmstadt, Germany). Potassium and sodium phosphate, Folin–Ciocalteau reagent and ethanol were purchased from Merck (Darmstadt, Germany). The spectrophotometer used was a Hitachi U-1900 model (Hitachi, Tokyo, Japan), tissue homogenizer was Ultraturrax IKA® T18 basic (IKA, Jacqvepagua, Brazil), and the centrifuge was a Centurion model by Scientific Ltd. Company (West Sussex, UK).
Fish and experimental protocol
The experiment was carried out at a commercial trout farm in Plastiras Lake, Karditsa, Thessaly, Greece. Groups of 54 sub-adult trout (113.0 ± 10.4 g) were randomly allocated into three different treatments with three replicates each. All subgroups were housed in separate fibreglass tanks containing aerated recirculating fresh water and reared at 23 ± 1 °C with a 12:12 h light:dark photoperiod. Water pH was maintained between 7.0 and 7.5, dissolved oxygen between 7.5 and 8.1 mg L−1. The trial was carried out under the regulations of local Public Veterinary Services and the Authorities of the Veterinary Faculty of University of Thessaly.
Experimental design, diets and performance
A commercial type diet was formulated to meet all nutrient requirements of the fish according to NRC (2011). The diet was in pelleted form and analysed according to the Weende system (Table 1). Control group was fed the basal diet, while the other groups received the basal diet supplemented with the same probiotic product at two corresponding levels; group 2: Pr5.0: AQUASTAR® Hatchery (BIOMIN®) at the level of 5 g kg−1 feed; group 3: Pr1.0: AQUASTAR® Hatchery (BIOMIN®) at the level of 1 g kg−1 feed; (BIOMIN GmbH, Herzogenburg, Austria). AQUASTAR® Hatchery (BIOMIN®) is a multi-strain probiotic product containing populations of Bacillus subtilis, Enterococcus faecium, Pediococcus acidilactici and Lactobacillus reuteri. The total concentration of bacteria in the AQUASTAR Hatchery product is 3 × 109 CFU g−1. Experimental feeds were verified for probiotic containing microorganisms at tested levels. Fish were fed to apparent satiation three times daily for 56 days. All fish were individually weighed on a weekly basis following a 14-h starvation period. Groups of fish at each time point were anaesthetized with phenoxyethanol (0.2 mL L). Fish body weight, fish size and feed intake were taken every week. Mortality was recorded daily. The experiment was set up as a complete randomized block design (CRB) using three replicates each. At the end of the trial (56 days), live weight gain and feed efficiency were obtained.
Sampling
Blood samples (6 fish/replicate) were collected from the caudal vein. Groups of fish were anaesthetized with phenoxyethanol (0.2 mL L). Blood samples left to clot for 12 h at 4 °C. Following centrifugation at 700 g for 30 min at 4 °C, the serum was removed, aliquoted and stored at −20 °C until use for the detection of lysozyme, reactive nitrogen intermediates, total complement and catalase activity. Six fish per replicate were sampled and stored frozen (−80 °C) for further tissue or intestinal content analysis.
Enumeration of intestinal microbiota
To determine the effect of the different dietary treatments on intestinal bacteria populations, intestinal samples from six fish per replicate were collected at the end of the experimental period. Fresh digesta samples from the posterior intestinal tract were taken for bacterial analyses. Digesta samples were serially diluted in 8.5 g L−1 sterile saline solution for enumeration of total aerobes, total anaerobes, lactic acid bacteria (LAB), Coliforms, Enterobacteriaceae and Aeromonas spp. by conventional microbiological techniques using selective agar media (Giannenas et al. 2012). All microbiological analyses were performed in duplicate, and the average values were used for statistical analysis. Results were expressed as base-10 logarithm colony-forming units per gram of ileal or caecal digesta.
Antioxidant status determination
To determine the effect of the different dietary treatments on antioxidant status, fish fillet samples from 18 fish per group were collected at the end of the experimental period and levels of glutathione peroxidase and malondialdehyde values were determined both on day 0 and day 5 at refrigerated storage according to Giannenas et al. (2011).
All excised tissues were assayed for the levels of malondialdehyde (MDA), glutathione S-transferase (GST) and glutathione reductase (GR) according to the procedures described below. All samples were immediately frozen at −80 °C after collection and were analysed within a month of collection. To assess the effect of dietary treatment on lipid oxidation of raw tissue during refrigerated storage, samples were thawed, wrapped in transparent oxygen-permeable polyvinyl chloride film (6,000–8,000 cm3/m2 × 24 h), placed in a non-illuminated refrigerated cabinet at 4 °C for 5 days and submitted to determination of antioxidant enzyme activities and lipid oxidation at 0 and 5 days of refrigerated storage of trout fillet.
Lipid peroxidation assay
Malondialdehyde (MDA) was used as a marker of lipid peroxidation. A quantity of 14 μL of butylated hydroxytoluene and 1,400 μL of a mixture of 3.75 g L−1 thiobarbituric acid and 9 g L−1 trichloroacetic acid in 0.25 N HCl was added to 100 μL of tissue homogenate; samples (0.5 g) were placed in tubes and homogenized. The samples were incubated at 100 °C in a water bath for 15 min, centrifuged at 13,000g for 5 min, and the absorbance of the supernatant was read at 532 nm on the spectrophotometer. MDA concentration in the samples was plotted against a reference curve made using known amounts of MDA and expressed as nmol mg−1 of protein (Buege and Aust 1978).
Assay of glutathione S-transferase (GST)
GST activity was measured after a GST reagent mixture was made, consisting of 50 mL of phosphate buffer, pH 6.5 (200 mL of 0.1 M potassium phosphate buffer with 55 mL of 0.144 M sodium phosphate buffer) and 2 mL of 20 mM CDNB (ethanol solution). A quantity of 800 μL of this reagent along with 100 μL of 5 mM GSH were mixed in a cuvette, and to this solution, 100 μL of 1:100 diluted sample was added. The cuvette was immediately inserted into a spectrophotometer, and absorbance rate was read at 340 nm for 3 min. GST activity was expressed as mmol min−1 mg−1 protein (extinction coefficient = 9.6).
Assay of glutathione reductase (GR)
GR activity was determined, in brief, by mixing 50 μL of 25 mM GSSG (Merck Darmstadt, Germany) and 10 μL of sample to 890 μL of 143 mM sodium phosphate buffer (pH 7.5) containing 1 mM EDTA. The reaction was initiated by adding 50 μL of 3 mM NADPH, and the absorbance decrease rate was recorded using a spectrophotometer at 340 nm for 3 min against blank containing all components except GSSG. GR activity was expressed as units/mg protein (extinction coefficient for NADPH = 0.00622 nmol−1 min mL, 1 unit = the amount of NADPH oxidized min mL−1).
Tissue protein determination
Proteins were determined by the method of Bradford (1976) using bovine serum albumin as a standard.
Detection of lysozyme, reactive nitrogen intermediates, total complement values and catalase activity in blood serum
Lysozyme activity was measured by a modified assay based on the lysis of the lysozyme-sensitive Gram positive bacterium Micrococcus lysodeikticus (Sigma-Aldrich, Athens, Greece) (Lie et al. 1989). Lyophilized bacteria (0.2 mg mL−1) suspended in 100 μL of 0.05 M sodium phosphate buffer, pH 6.2, were added to 100 μL of serially diluted serum, and the reduction in absorbance at 450 nm was measured after 5 and 15 min at 25 °C. One unit of lysozyme activity was defined as a reduction in absorbance of 0.001 min−1. Quantification of lysozyme activity in serum was obtained from a standard curve made with chicken egg white lysozyme (Sigma).
Nitrite oxide (NO) concentrations in blood were determined by the Griess reaction (Green et al. 1982). Briefly, 100 μL of each blood serum and 50 μL of 10 g L−1 sulphanilamide (Sigma) and 1 g L−1 N-naphthylethylene-diamine dihydrochloride (Sigma) in 1.25 % (v/v) H3PO4 were placed in 96-well plates for 10 min at room temperature in the dark. The optical density was determined using an ELISA reader (Dynatech MRX, West Sussex, England) at 550 nm. The molar concentration of nitrite in blood serum was determined from standard curves generated using known concentrations of NaNO2 (Sigma-Aldrich, Athens, Greece).
Total serum complement activity was determined under aseptic conditions according to the methodology of Staykov et al. (2007) regarding the classical complement pathway. In brief, each serum sample (100 mL) was first diluted with 100 mL veronal–Na buffer. In U-bottomed plates (Flow Laboratories UK), five other dilutions from each diluted serum were prepared in veronal–Na buffer. Buffer (100 mL) and sheep erythrocyte suspension sensitized with haemolytic antibodies (1 % v/v, 100 mL) were then added drop wise to each dilution, and the mixtures were incubated at 37 °C for 1 h. Optical density at 540 nm was then measured by use of a Sumal-PE2 ELISA reader. CPCA activity was expressed as CH50 units (CH50 units correspond to 50 % v/v of complement-induced haemolysis of applied erythrocytes).
Catalase activity was measured by an assay of hydrogen peroxide based on formation of its stable complex with ammonium molybdate (Goth 1991). In brief, 10 μL of serum incubated in 1 mL reaction mixture containing 65 mM hydrogen peroxide (Fluka) in 60 mM sodium phosphate buffer, pH 7.5 at room temperature for 4 min. The reaction was stopped with 1 mL of 32.4 mM ammonium molybdate, and the sample was measured in a spectrophotometer at 405 nm. The optical density found was plotted against a reference curve of known concentrations of hydrogen peroxide.
Statistical analysis
The experiment was set up as a complete randomized block design (CRB) using three replicates each. Performance data were analysed by a one-way ANOVA with initial body weight used as a covariate and the pen being the experimental unit. For data on antioxidant activity, bacteria loads and non-specific immune response, individual samples were considered to be nested within pens and data were analysed by a nested ANOVA; in addition, data on antioxidant activity were analysed by a two-way nested ANOVA with time and treatment being the experimental factors. As bacterial numbers were not normally distributed, they were log-transformed to create a normal distribution prior to analysis. Bacteria load means are presented on transformed basis. Levene’s test was performed to check homogeneity of variances, and Tukey’s test was carried out to assess any significant differences at a probability level (P) of 0.05 among the experimental groups. All data were subjected to analysis using the statistical package of SPSS version 17.00 for Windows (SPSS, Inc., Chicago, IL).
Results
Growth performance
Survival at the end of the experiment was high (about 98 %), and no significant differences were noted among other treatments (Table 2). Growth performance and feed utilisation of trout after 56 days of feeding on experimental diets are also presented in Table 2. A high growth performance was observed in all groups; fish biomass increased by over 100 %. Specific growth rate was found to be 1.52 for the control group, 1.56 for the Pr5.0 group and 1.71 for the Pr1.0 group that was significantly (P < 0.05) higher compared to the control group. The Pr1.0 group at the inclusion level of 1 g kg−1 significantly (P < 0.05) increased body weight gain in the test group compared to the control by an average of 11 % at day 49 and 56 of the experimental trial. The Pr5.0 group presented BW and BWG values that were intermediate among the control and the Pr1.0 group. Furthermore, the feed conversion ratio value was significantly (P < 0.05) better in Pr1.0 group than both control (+18.8 %) and Pr5.0 (+13.1 %) groups. The Pr5.0 group presented also a significantly (P < 0.05) better FCR value compared to the control group (+6.5 %).
Culturable intestinal microbiota
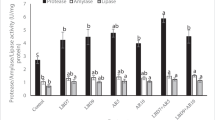
The present study included an investigation of intestinal bacteria populations of trout fed the probiotic products (Table 3). Total anaerobe counts were significantly (P < 0.05) lower in probiotic-supplemented group at the low inclusion level compared to control and that of high inclusion level. Table 3 also shows that LAB loads were 55 % higher in Pr1.0 and 115 % higher in Pr5.0 compared to control. The lactic acid production makes the microbial environment acidic, which may inhibit the growth of some harmful bacteria. However, other bacteria loads were similar among dietary treatments (Table 3).
Antioxidant status
Probiotic inclusion decreased significantly (P < 0.05) malondialdehyde formation on both day 0 and day 5 of refrigerated storage compared to control fish. The activity of glutathione-based enzymes (GR and GST) at both time points after slaughter was significantly higher in both probiotic-supplemented groups compared to control (P < 0.05) (Table 4). Glutathione (GSH), generated by GR, is an important cellular antioxidant; low levels of antioxidants, or inhibition of the antioxidant enzymes, cause oxidative stress and may damage or kill cells. The activity of GST contributes to the detoxification of poisonous compounds, which are conjugated to reduced glutathione and finally excreted from the body.
Blood parameters
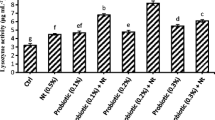
Levels of total complement concentrations as well as catalase activity were higher in the serum of trout fed with probiotic-supplemented diets compared to control (Table 5). Nitric oxide, a vasodilating factor, was lower in the serum of fish fed the probiotic with lower inclusion level, while lysozyme levels were similar among dietary treatments.
Discussion
The use of probiotics in practical diets for fish is a very topical concept in aquaculture (Burr et al. 2005), and new research efforts should be expended regarding the use of probiotics as potential growth and health promoters in healthy and challenged fish. In the present study, we demonstrated that probiotic dietary supplementation induced a significant enhancement on growth performance of trout with improved rate in feed conversion. Although there is no comparable data regarding these probiotic products in fish studies, it has been published that probiotic products containing lactic acid or Bacillus spp. bacteria improved the growth performance in different cultured fish (Faramarzi et al. 2011; Askarian et al. 2011; Aly et al. 2008). Even though the results seem to indicate a stimulatory effect of probiotic products on fish growth, gaps exist in the understanding of the mechanisms of action of probiotic products both in fish and terrestrial farm animals. Further experimental results might clarify whether specific components possess higher potency as appetite enhancers in fish. Additionally, future research in this area should concentrate on understanding the physiological mechanisms by which dietary probiotic products improve growth in trout and other farmed fish species.
The intestinal microbiota have been shown to be sensitive to dietary ingredients (Ringø et al. 2006a, b, 2008; Merrifield et al. 2009; Giannenas et al. 2011; 2012). Lactobacilli populations are currently a topic of great interest regarding fish gut microbiota (Ringø and Gatesoupe 1998; Balcázar et al. 2006; Gatesoupe 2008); certain Lactobacillus species have demonstrated positive effects on fish health and growth as probiotics (Balcázar et al. 2006; 2007; Panigrahi et al. 2007; Merrifield et al. 2010a) and yet some Lactobacillus species are known to cause disease (Eldar et al. 1999; Gatesoupe 2008). These findings suggest that dietary inclusion of probiotic products may affect the intestinal populations of trout; however, further work should be conducted with molecular-based analysis to identify specifically intestinal bacteria species that are affected the most and how these changes are related to trout’s health status or its ability to confront with a disease challenge.
Another objective of our study was to investigate whether the sustained consumption of probiotic products would affect the antioxidant status of trout muscle. We found that both lipid oxidative stability and glutathione-based enzyme activity were significantly improved at day 0, especially at the low inclusion level of probiotics. To our knowledge, very few studies with aquacultured species have reported probiotic effects on antioxidant defence and oxidative stress status.
There are a number of specific haematological parameters recognized as valuable tools for monitoring fish health and physiological responses to environmental stress (Schuett et al. 1997; Jawad et al. 2004). Svobodova et al. (1991) suggested that icthyo-haematology is useful in the assessment of feed composition, nutritional status in relation to environmental conditions affecting fish. Serum lysozyme activity and serum catalase activity, often used as indicators of stress (Wendelaar Bonga 1997), were also improved by the inclusion of probiotic products. Innate immunity factors such as nitric oxide and total complement are important indexes that show the ability of the host defence immune mechanisms in order to find out the influence of infection. Although we did not conduct any bacterial or parasitic challenge in trout, we found that probiotic products have the ability to alter nitric oxide levels and total complement production. Results of our study showed that preparations rich in lactic acid bacteria, enterococci and bacilli in trout diets could improve feed utilization and various antioxidant parameters.
The present study demonstrated that relatively low levels of probiotic-based feed additives had a positive effect on trout growth performance with apparent effects towards antioxidant defence and innate immunity status. The fact that feed conversion ratio values were significantly lower in fish fed probiotic-supplemented diets compared to control and feed intake was not significantly affected suggests that probiotics can exert growth-promoting activity. This faster fish growth would lead to improved production time; however, research is required with different important aquaculture species and longer time scales to fully evaluate the value of probiotic inclusion at industrial farming levels.
Conclusion
The results of the present study indicate that an inclusion of 1 g kg−1 feed of a multi-strain probiotic product containing B. subtilis, E. faecium, P. acidilactici, L. reuteri into a commercial type diet increases growth performance and health status. It also modulated intestinal microbial communities favouring LAB and affected non-specific immune response by decreasing NO serum levels. These findings suggest that aquaculture could further benefit by the dietary strategy to use probiotics in fish diets. Probiotic products could be also further tested in experimentally infected fish in order to evaluate their implications under stress conditions or infections.
References
Aly SM, Mohamed MF, John G (2008) Effect of probiotics on the survival, growth and challenge infection in Tilapia nilotica (Oreochromis niloticus). Aquac Res 39:647–656
AOAC (1995) Official methods of analysis of AOAC international. In: Agricultural chemicals; contaminants, drugs, vol 1. AOAC International, Arlington
Askarian F, Kousha A, Salma W, Ringø E (2011) The effect of lactic acid bacteria administration on growth, digestive enzyme activity and gut microbiota in Persian sturgeon (Acipenser persicus) and beluga (Huso huso) fry. Aquac Nutr 17:488–497
Balcázar JL, de Blas I, Ruiz-Zazuela I, Cunningham D, Vandrell D, Muzquiz JL (2006) The role of probiotics in aquaculture. Vet Microbiol 114:173–186
Balcázar JL, de Blas I, Ruiz-Zazuela I, Vandrell D, Gironés O, Muzquiz JL (2007) Enhancement of the immune response and protection induced by probiotic lactic acid bacteria against furunculosis in rainbow trout (Oncorhynchus mykiss). FEMS Immunol Med Microbiol 51:185–193
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Buege JA, Aust ST (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Burr G, Gatlin D III, Ricke S (2005) Microbial ecology of the gastrointestinal tract of fish and the potential application of prebiotics and probiotics in finfish aquaculture. J World Aquac Soc 36:425–436
Dimitroglou A, Merrifield DL, Carnevali O, Picchietti S, Avella M, Daniels S, Güroy D, Davies SJ (2011) Microbial manipulations to improve fish health and production—a Mediterranean perspective. Fish Shelfish Immunol 30:1–16
Eldar A, Goria M, Ghittino C, Zlotkin A, Bercovier H (1999) Biodiversity of Lactococcus garvieae strains isolated from fish in Europe, Asia, and Australia. Appl Environ Microbiol 65:1005–1008
Faramarzi M, Kiaalvandi S, Iranshahi F (2011) The effect of probiotics on growth performance and body composition of common carp (Cyprinus carpio). J Anim Vet Adv 10:2408–2413
Gatesoupe F-J (2008) The importance of lactic acid bacteria in fish farming: natural occurrence and probiotic treatments. J Mol Microbiol Biotechnol 14:107–114
Giannenas I, Tsalie E, Chronis E, Mavridis S, Tontis D, Kyriazakis I (2011) Consumption of Agaricus bisporus mushroom affects the performance, intestinal microflora composition and morphology, and antioxidant status of turkey poults. Anim Feed Sci Technol 165:218–229
Giannenas I, Triantafillou E, Stavrakakis S, Margaroni M, Mavridis S, Steiner T, Karagouni E (2012) Assessment of dietary supplementation with carvacrol or thymol containing feed additives on performance, intestinal microbiota and antioxidant status of rainbow trout (Oncorhynchus mykiss). Aquaculture 350:26–32
Gildberg A, Johansen A, Bogwald J (1995) Growth and survival of Atlantic salmon (Salmo salar) fry given diets supplemented with fish protein hydrolysate and lactic acid bacteria during a challenge trial with Aeromonas salmonicida. Aquaculture 138:23–34
Gómez GD, Balcázar JL (2008) A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol Med Microbiol 52:145–154
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–151
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum S (1982) Analysis of nitrate, nitrite, and (15 N) nitrate in biological fluids. Anal Biochem 126:131–138
Jawad LA, Al-Mukhtar MA, Ahmed HK (2004) The relationship between haematocrit and some biological parameters of the Indian Shad, Tenualosa ilisha (Family Clupeidae). Anim Biodivers Conserv 27:47–52
Jin LZ, Ho YW, Abdullah N, Jalaludin S (1998) Growth performance, intestinal microbial populations, and serum cholesterol of broilers fed diets containing Lactobacillus cultures. Poult Sci 77:1259–1265
Kesarcodi-Watson A, Kaspar H, Lategan ML, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274:1–14
Lie O, Evensen PI, Sorensen A, Froysadal E (1989) Study on lysozyme activity in some fish species. Dis Aquat Org 6:1–5
Merrifield DL, Burnard D, Bradley G, Davies SJ, Baker RTM (2009) Microbial community diversity associated with the intestinal mucosa of farmed rainbow trout (Oncoryhnchus mykiss Walbaum). Aquac Res 40:1064–1072
Merrifield DL, Bradley G, Baker RTM, Dimitroglou A, Davies SJ (2010a) Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) I. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria. Aquac Nutr 16:504–510
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RTM, Bogwald J, Castex M, Ringø E (2010b) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302:1–18
Mountzouris KC, Tsirtsikos P, Kalamara E, Nitsch S, Schatzmayr G, Fegeros K (2007) Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poult Sci 86:309–317
Nayak SK (2010) Probiotics and immunity. A fish perspective. Fish Shelfish Immunol 29:2–14
Nikoshelainen S, Ouwehand A, Salminen S, Bylund G (2001) Protection of rainbow trout (Oncorhynchus mykiss) from furunculosis by Lactobacillus rhamnosus. Aquaculture 198:229–236
NRC (2011) Nutrient requirements of fish and shrimp. In: National Research Council of the National Academies. Natl Acad. Press, Washington
Panigrahi A, Kiron V, Satoh S, Hirono I, Kobayashi T, Sugita H, Puangkaew J, Aoki T (2007) Immune modulation and expression of cytokine genes in rainbow trout (Oncorhynchus mykiss) upon probiotic feeding. Dev Comp Immunol 31:372–382
Patterson JA, Orban JI, Sutton AL, Richards GN (1997) Selective enrichment of bifidobacteria in the intestinal tract of broilers by thermally produced kestoses and effect on broiler performance. Poult Sci 76:497–500
Ringø E, Gatesoupe F-J (1998) Lactic acid bacteria in fish: a review. Aquaculture 160:177–203
Ringø E, Sperstad S, Myklebust R, Mayhew TM, Mjelde A, Melle W, Olsen RE (2006a) The effect of dietary krill supplementation on epithelium-associated bacteria in the hindgut of Atlantic salmon (Salmo salar L.): a microbial and electron microscopical study. Aquac Res 37(1644):1653
Ringø E, Sperstad S, Myklebust R, Refstie S, Krogdahl A (2006b) Characterisation of the microbiota associated with intestine of Atlantic cod (Gadus morhua L.). The effect of fish meal, standard soybean meal and a bioprocessed soybean meal. Aquaculture 261:829–841
Ringø E, Sperstad S, Kraugerud OF, Krogdahl Å (2008) Use of 16S rRNA gene sequencing analysis to characterize culturable intestinal bacteria in Atlantic salmon (Salmo salar) fed diets with cellulose or non-starch polysaccharides from soy. Aquac Res 39:1087–1100
Schuett DA, Lehmann J, Goerlich R, Hamers R (1997) Haematology of swordtail, Xiphiphorus helleri. 1: blood parameters and light microscopy of blood cells. J Appl Ichthyol 13:83–89
Staykov Y, Spring ZP, Denev ZS, Sweetman ZJ (2007) Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout (Oncorhynchus mykiss). Aquac Int 15:153–161
Svobodova Z, Fravda D, Palakova J (1991) Unified methods of hematological examination of fish. Research Institute of Fish Culture and Hydrobiology, Vodnany, p 331
Wang Y-B, Li J-R, Lin J (2008) Probiotics in aquaculture: challenges and outlook. Aquaculture 281:l1–l4
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Acknowledgments
The authors gratefully acknowledge Biomin GmbH, Herzogenburg, Austria, for donation of probiotic products and Messieurs J. and A. Missas for trout donation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Giannenas, I., Karamaligas, I., Margaroni, M. et al. Effect of dietary incorporation of a multi-strain probiotic on growth performance and health status in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 41, 119–128 (2015). https://doi.org/10.1007/s10695-014-0010-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-014-0010-0