Abstract
The current research aimed to assay the role of dietary supplementation of Lactobacillus delbrueckii subsp. bulgaricus (LDB) and Asparagus officinalis L. (AR) root in a single form or combination (as synbiotics) on growth performance, digestive enzyme activities, gut lactic acid bacteria (LAB), and multi-biomarkers against crowding stress in rainbow trout. During a 60-day feeding trial, rainbow trout (initial weight, 35.48 ± 0.34 g) were subjected to seven experimental diets including CG (control group without LDB and/or AR inclusion), LDB7 (1 × 107 CFU/g CG), LDB9 (1 × 109 CFU/g CG), AR5 (5 g/kg CG), AR10 (10 g/kg CG), LDB7 + AR5 (1 × 107 CFU/g CG + 5 g/kg CG), and LDB9 + AR10 (1 × 109 CFU/g CG + 10 g/kg CG). After 60 days, all supplemented diets with LDB and/or AR significantly improved growth performance, feed utilization, and digestive protease enzyme. Moreover, these feed additives could mitigate the stress-related effects on serum immune responses, hematocrit percentage (%), hemoglobin value, white blood cell and neutrophil counts, glutathione peroxidase and superoxide dismutase activities, malondialdehyde content, and cortisol and glucose value, as well as alkaline phosphatase and aspartate aminotransferase levels (P < 0.05). The highest red blood cell count was obtained in the fish of the LDB7 + AR5 group at both experimental times (before and after stress). Dietary supplementation of synbiotics markedly improved amylase and lipase activities and total bacterial count (TBC) (P < 0.05). Serum catalase activity was significantly improved in response to diets supplemented with LDB7, AR5, and synbiotics at both times (P < 0.05). After stress, monocyte count in fish fed with dietary supplementation of LDB and/or AR except for the LDB7 group was significantly higher than that in the control group (P < 0.05). Although all dietary supplements of LDB and/or AR exhibited beneficial effects on most tested parameters, synbiotic diets, especially LDB7 + AR5, provided higher efficiency on metabolic processes and microbial function. Furthermore, they strongly alleviated the negative effects of crowding stress on immune, hematological, antioxidant, and serum biochemical markers. Therefore, dietary AR5 + LDB7 is recommended for high-stocking density rainbow trout rearing systems and aquaculture operations such as fish transportation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rainbow trout (Oncorhynchus mykiss) is one of the commonly cultured species in inland water bodies (739,500 t in 2020; 1.6% of total) and accounts for the large volume of salmonid production (FAO 2022). In the past few years, the continuous reduction of available freshwater resources, deficiency of land, and the prospect of enhanced profitability have driven a shift in rainbow trout production towards intensive rearing systems (Mirghaed et al. 2018; Chekani et al. 2021). However, this culturing system is stressful for fish due to increased ammonia and carbon dioxide value, suspended particles, and oxygen fluctuations (Yousefi et al. 2023a, 2023b, 2023c, 2023d; Gabr et al. 2023). In this situation, increased stress hormones impair the host’s immune system and growth performance by suppressing the immune and respiratory systems and inhibiting the activity of thyroid hormones (Yousefi et al. 2019; Chekani et al. 2021). Moreover, crowding stress can result in the excessive production of reactive oxygen species (ROS), disrupting cellular integrity, and the leakage of liver enzymes into the blood circulation system (Mirghaed et al. 2018; Ibrahim et al. 2021).
One practical way to increase fish’s physiological capacity to withstand abnormal conditions is dietary administration with various biostimulants (Acar et al. 2019; Kesbiç et al. 2020; Pawar et al. 2023). Among them, probiotics, prebiotics, and their combination as synbiotics are some of the most profitable biostimulants (Sewaka et al. 2019; Yousefi et al. 2023a, 2023b, 2023c, 2023d). Several studies indicated that dietary supplementation of synbiotic can boost growth variables, digestive enzymes, and immune and antioxidant responses of the host (Kumar et al. 2018a, 2018b; Aftabgard et al. 2019; Yousefi et al. 2023a, 2023b, 2023c, 2023d). To the best of our knowledge, only a limited number of studies have explored synbiotic properties against stress in fish. Azimirad et al. (2016) assayed the effects of Pediococcus acidilactici and fructooligosaccharide on angelfish (Pterophyllum scalare) and found clear evidence of improvement in mitigating environmental stress including high salinity and low temperature. Therefore, more studies are needed to examine the potential of synbiotics in addressing various stresses, such as crowding, in rainbow trout.
Lactobacillus delbrueckii subsp. bulgaricus (LDB) isolated from yogurt is widely used in the fermentation process of animal and plant products (Aslim et al. 2007). In aquaculture, dietary supplementation of LDB could improve immune parameters in Barbus grypus (Mohammadian et al. 2016) and attenuated the toxic effects of lead (Pb) on the hematological and immune parameters of rainbow trout (Mohammadian et al. 2020). In addition, previous findings at the in vitro conditions showed that this strain produces compounds with high antioxidant activity, such as exopolysaccharide, which can act as stress relievers (Abedi et al. 2013; Khalil et al. 2022).
Asparagus officinalis root (AR) is a perennial and herbaceous plant and its roots are rich in natural prebiotic sources such as fructans, xylose, inulin, and arabinose (Guo et al. 2020; Zou et al. 2021). These polymers can be selectively employed as substrates for the production of valuable metabolites such as short-chain fatty acids (SCFAs) (Redondo-Cuenca et al. 2023). Zou et al. (2021) reported that asparagus powder increased propionic acid and butyric acid levels in mice’s gut by changing the gut microbial composition. Previous findings in aquaculture also showed that the combination of natural prebiotics and LAB such as Jerusalem artichoke + Lactobacillus rhamnosus (Sewaka et al. 2019) and gum Arabic + Lactobacillus helveticus (Yousefi et al. 2023a, 2023b, 2023c, 2023d) enhanced the growth and immune parameters of red tilapia (Oreochromis spp.) and common carp (Cyprinus carpio), respectively. Considering the multiple beneficial properties of pre-/pro- and synbiotics in fish, the present study was carried out to reveal another possible advantage of these biostimulants in reducing or neutralizing the negative effects of crowding stress on the physiological system of rainbow trout.
Material and methods
Preparation of feed additives
The L. delbrueckii subsp. bulgaricus bacterium DSM 20081, (LDB) was supplied from the Center of Genetic and Biological Reserves of Iran. The LDB was transferred to Man, Rogosa, Sharpe (MRS) culture medium and incubated in a shaking incubator at 37 °C for 48 h. Then, the cells were centrifuged at 4000 g for 15 min, washed three times using sterile phosphate-buffered saline (PBS), and resuspended in PBS. Next, the LDB was added to the basal diet to create probiotic-enriched diets (Mohammadian et al. 2016). The concentrations of 1 × 107 CFU/g and 1 × 109 CFU/g of diets were selected based on the favorable outcomes documented in previous studies (Mohammadian et al. 2016; Mohammadian et al. 2020). The number of bacterial cells was determined by the spectrophotometric method at 600 nm and confirmed by the poured-plate technique (Mohtashami et al. 2021). Asparagus roots were obtained from a reputable grocery store in Shahrekord city. Roots were washed, dried in the shade, and broken into smaller slices. A mill powdered these slices. AR powder was incorporated into the paste at concentrations of 5 and 10 g/kg based on studies (Sewaka et al. 2019; Yousefi et al. 2023a, 2023b, 2023c, 2023d).
Diet preparation
Seven experimental diets including CG (control group without LDB and/or AR inclusion), LDB7 (1 × 107 CFU/g CG), LDB9 (1 × 109 CFU/g CG), AR5 (5 g/kg CG), AR10 (10 g/kg CG), LDB7 + AR5 (1 × 107 CFU/g CG + 5 g/kg CG), and LDB9 + AR10 (1 × 109 CFU/g CG + 10 g/kg CG) were considered to conduct this research. The prepared ingredients were blended (Table 1) for 20 min. Then, 30% water was joined to the desired ingredients to form a paste. The pellets were made by passing the dough from a meat grinder. The obtained pellets were dried for 24 h and saved at 4 °C until use. Besides, the preparation of the treated diets was carried out by slowly adding the LDB suspension and AR to the other components in a drum mixer to obtain a homogeneous paste (Giri et al. 2013; Mohammadian et al. 2019a, 2019b). This was performed under sterile conditions. The biochemical composition of the diets was ascertained using standard methods (AOAC 1995). In order to prevent the reduction of the count of LDB in the pellets, experimental diets were prepared every 14 days. Besides, the count of LDB colonies in the respective diets was confirmed through culture on MRS medium (Yousefi et al. 2023a, 2023b, 2023c, 2023d) (Table 1).
Experimental procedure
A total of 900 rainbow trout juveniles were obtained from a local farm in Ardal city and transferred to our laboratory in Dehcheshmeh Village (Chahrmahal and Bakhtyari, Iran). Fish health was confirmed by examining swimming patterns and feeding behaviors, as well as the absence of disease signs or external injuries on the body surface. The fish were stocked in 1000-l tanks for 14 days to adapt to the new environmental conditions. During the adaption, the feeding of specimens was carried out using the control diet three times (7:30, 13:30, and 19:30) a day and based on ad libitum (Naderi Farsani et al. 2021). After elapsed adaption time, 840 fish (initial weight, 35.48 ± 0.34) with healthy physical and normal swimming and almost equal weights were selected and transferred to 21 tanks (3 tanks per group,40 animals per tank; 30 kg biomass/m3) (Mirghaed et al. 2018; Yousefi et al. 2023a, 2023b, 2023c, 2023d). The fish received their respective diets during the 60-day feeding trial. The water flow was 0.5 l/min*kg and aeration was maintained continuously to provide an acceptable level of oxygen. Besides, uneaten pellets and feces were removed from each tank daily through siphons. The water physicochemical parameters including temperature (15.2–16 °C), dissolved oxygen (7.7–8.1 mg/l), pH (7.7–7.8), and unionized ammonia nitrogen (0.002 mg/l) were recorded. During the feeding trial, the rearing density was always set at 30 kg/m3 through measuring the weight of the biomass. At the end of the feeding trial, the density of fish increased to 60 kg/m3 by reducing the water volume, subjecting them to 2 weeks of crowding stress (Mirghaed et al. 2018; Chekani et al. 2021; Yousefi et al. 2023a, 2023b, 2023c, 2023d). In this phase, feeding and other rearing practices were similar to the previous phase and water physicochemical parameters were as follows: temperature 15.5–16.2 °C, dissolved oxygen 6.3–7.25 mg/l, pH 7.65–7.75, and unionized ammonia nitrogen 0.006 mg/l.
Growth performance and sampling
After the completion of the rearing period in the first phase, specimens were fasted for 24 h. Then, all fish within each tank were weighed and counted to assess growth variables, survival rate, and feed utilization. The aforementioned parameters were calculated using the below listed formulas (Chekani et al. 2021).
where Nf is the fish number at the final trial feeding, Ni fish number at initial trial feeding, and d days.
Sampling
To obtain a blood sample, three fish were caught randomly from each experimental tank. The specimens were sedated using an anesthetic solution (eugenol, 100 mg/l, Yousefi et al. 2023a, 2023b, 2023c, 2023d), and their body surface was dried with tissue paper. Blood sampling from the caudal stem was carried out using standard 2-mm syringes. To measure the serum and hematology parameters, blood samples were aliquoted into the tubes containing the anticoagulant and the tubes without it, respectively. To obtain the serum, the blood samples that were not mixed with heparin were centrifuged (3000 g for 10 min at 4 °C). Then, the obtained supernatant was immediately stored at − 80 °C until the evaluation of serum enzymes and immune variables (Naderi Farsani et al. 2021). Moreover, three specimens from each replicate were humanly killed by a high concentration of the anesthetic solution (200 mg/l; Shekarabi et al. 2021) and dissected using sharp tools. The gut and liver organs were harvested to examine the digestive and antioxidant enzymes, respectively. Cleaned intestinal samples were homogenized with Tris-HCl buffer (pH 7.5, 50 mM). Enzyme source was obtained through centrifugation (10,000 g for 20 min at 4 °C) of the homogenates and saved at − 80 until subsequent tests. For the antioxidant enzyme assay, the liver was cut into small pieces. Then, tissues were homogenized using phosphate buffer and homogenizer. The homogenates were centrifuged at 10,000 g for 20 min at 4 °C and the supernatant was placed at − 80 °C until analysis (Haghparast et al. 2019; Yousefi et al. 2023a, 2023b, 2023c, 2023d).
Analysis
Digestive enzyme assay
To determine the total protease enzyme activity, 20 μl of enzyme source was mixed with 0.5 ml of 2% azocasein solution (50 mM Tris-HCl and pH 5.7), and then, the mixture was incubated at 25 °C for 10 min. The reaction was ended by pipetting 0.5 ml trichloroacetic acid as explained by Garcia-Carreno and Haard (1993). Then, the optical density (OD) of the supernatant was determined at 440 nm after centrifuging at 6500 g for 5 min. To estimate lipase activity, 7 μl of the enzyme source was blended with 86 μl of sodium cholate solution and 2.5 μl of methoxy ethanol solution. Afterward, 5.5 μl of para-nitrophenyl myristate was added to the reaction, and samples were incubated at 25 °C for 15 min. Finally, recording OD of samples was performed at 405 nm (Iijima et al. 1998). To measure alpha-amylase enzyme activity, 250 μl of 1% starch as substrate was mixed with 250 μl of enzyme source. After 3 min, 0.5 ml of dinitrosalicylic acid color reagent was pipetted to them followed by the reaction kept at 100 °C for 5 min. Then, 5 ml of distilled water was joined to the cocktail and the OD was read at a wavelength of 540 nm (Bernfeld 1955).
Hematology parameters
The count of white blood cells (WBC) and red blood cells (RBC) was computed based on the protocol explained by Blaxhall and Daisley (1973), using a hemocytometer counting chamber and a light microscope with × 40 magnification. Hemoglobin (HB) was measured using the cyanomethemoglobin method and recording OD of the supernatants at 540 nm (Řehulka 2000). The amount of hematocrit (HCT) was measured by the microhematocrit method (Řehulka 2000). The count of lymphocyte (LYM), neutrophile (NEU), and monocyte (MON) was calculated by Giemsa staining as described by Borges et al. (2004).
Serum immune responses
Lysozyme enzyme function in the serum sample was detected based on its ability in the lysis peptidoglycan wall of Micrococcus luteus (Sigma, St Louis, MO, USA) with turbidity method at 440 nm as suggested by Ellis (1990). The quantity of serum alternative complement activity (ACH50) was ascertained using its capability in sheep erythrocyte hemolysis, prepared in veronal buffer (gelatin, magnesium, and EGTA), as recommended by Yano (1992). In this protocol, the hemolysis rate of serum as ACH50 activity was detected at 420 nm. Ig content in the serum sample was determined using 12% PG (polyethylene glycol) as described by Siwicki and Anderson (1993). Serum acid phosphatase (ACP) level was computed by the method suggested by Andersch and Szczypinski (1947) employing p-nitrophenyl phosphate disodium salt as the substrate and recording the OD at 410 nm.
Hepatic antioxidant enzymes and MDA content
The hepatic antioxidant enzyme activity including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) was detected using available diagnostic commercial kits (ZellBio GmbH Co., Deutschland, Germany) following the manufacturer’s instruction. MDA level was found based on the reaction with thiobarbituric acid as previously explained by Naderi Farsani et al. (2021).
Serum biochemical parameters
The serum biochemical parameters were measured using the kits prepared by Pars Azmoon and with an autoanalyzer (Model: Alpha-Classic Autoanalyzer, Tajhizat Sanjesh). Cortisol level was estimated based on the ELISA method and using commercial kits (IBL Germany). The activity of alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine transaminase (ALT), and glucose value was computed using Pars Azmoon commercial kit (Tehran, Iran) and instructions recommended by the manufacturer’s protocol.
Statistical analysis
This project was performed in a fully randomized design with seven experimental groups, each with three replications. All data was analyzed via SPSS software version 20. Detecting normal distribution of data and homogeneity of variances was carried out using Kolmogorov-Smirnov and Levene tests, respectively. Growth performance, digestive enzymes, and gut microbiota data were analyzed using one-way ANOVA with Tukey’s post hoc test to ascertain significant discrepancies among experimental groups. The effects of crowding stress and supplements on hematology indices, immunological parameters, antioxidant enzymes, and biochemical variables were assayed by two-way ANOVA and Tukey’s post hoc analysis. A significance level of P < 0.05 was employed.
Results
Growth performance and feed utilization
The results obtained for growth performance, feed utilization, and survival rate are exhibited in Table 2. Dietary supplementation of LDB and/or AR significantly increased weight gain (WG), final weight (FW), and protein efficiency rate (PER) compared to the control group (P < 0.05); however, LDB7 + AR5 indicated higher levels than LDB9 treatment. Dietary supplementation of LDB and/or AR with a similar statistical trend increased the SGR compared to the control group (P < 0.05). Dietary administration of LDB and/or AR remarkably decreased FCR compared to basal diet; FCR value in LDB7 + AR5 was lower than that in LDB7 and LDB9 treatments (P < 0.05). Dietary LDB and/or AR supplementation induced no remarkable difference in fish SR and FI (feed intake) (P > 0.05) (Table 2).
Digestive enzyme activities
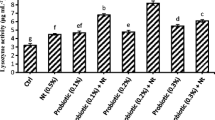
The results of the digestive enzyme activity analysis are depicted in Fig. 1. Dietary supplementation of LDB and/or LA significantly boosted protease activity compared to basal diet (P < 0.05); moreover, its activity in LDB7 + AR5 was higher than that in other supplemented groups except for AR5. Dietary supplementation of synbiotics remarkably increased amylase activity compared to the control group (P < 0.05). Besides, all diets containing LDB and/or LA except LDB9 significantly increased lipase activity compared to the control group (P < 0.05).
Digestive enzymes’ activity of rainbow trout fed with experimental diets (control, LDB7, LDB9, AR5, AR10, LDB7 + AR5, and LDB9 + AR10) for 60 days. Abbreviations: LDB7 (1 × 107 CFU/g CG), LDB9 (1 × 109 CFU/g CG), AR5 (5 g/kg CG), AR10 (10 g/kg CG), LDB7 + AR5 (1 × 107 CFU/g + 5 g/kg CG), and LDB9 + AR10 (1 × 109 CFU/g + 10 g/kg CG). Different letters above the bars indicate significant differences among the experimental groups. All data were analyzed with one-way ANOVA and values are presented as mean ± SD (n= 3)
Hematological indices
The interaction effects were found between dietary LDB and/or AR supplementation and crowding stress on hematological parameters including RBC (P = 0.037), HB (P = 0.008), HCT (P = 0.005), WBC (P = 0.036), LYM (P <0.001), MON (P = 0.030), and NEU (P = 0.01) (Table 3). Before stress, dietary supplementation of LDB7 + AR5 significantly increased RBC count compared to the control group (P < 0.05). After stress, RBC count was remarkably affected by crowding stress and its count in the control group was lower than that in AR5, AR10, LDB7 + AR5, and LDB9 + AR10 treatments (P < 0.05). Before stress, dietary LDB and/or AR supplementation induced no significant discrepancy in HB value (P > 0.05). After stress, dietary inclusion of LDB and/or AR significantly increased HB value (P < 0.05). Before stress, the rainbow trout fed with AR5, LDB7 + AR5, and LDB9 + AR10 diets presented a significant elevation in blood HCT, compared to the control group (P < 0.05). After stress, all LDB and/or AR-treated rainbow trout revealed a remarkable improvement in fish HCT compared to the control group (P < 0.05). All dietary LDB and/or AR supplementation significantly elevated WBC count, at both times (P < 0.05). Before stress, no significant difference was detected in the LYM count among the experimental groups (P > 0.05). After stress, the highest LYM count was found in the control group and exhibited a notable increase compared to the supplemented groups (P < 0.05). Before stress, no significant discrepancy was detected in fish MON count among experimental treatments (P > 0.05). After stress, fish supplemented with diets LDB and/or AR except LDB7 exhibited a significant difference in MON count compared to the control group (P < 0.05). Before stress, LDB9, AR5, LDB7 + AR5, and LDB9 + AR10 diets remarkably boosted NEU count compared to the control group (P < 0.05). After stress, all dietary supplementation of LDB and/or AR remarkably elevated NEU count compared to control treatment (P < 0.05) (Table 3).
Innate immune responses
The interaction effects were found between dietary LDB and/or AR supplementation and crowding stress on serum immune responses including lysozyme activity (P < 0.001), ACH50 activity (P = 0.002), Ig (P = 0.001), and ACP (P = 0.001) (Fig. 2). All dietary supplementation of LDB and/or AR significantly increased serum lysozyme activity, compared to basal diet, at both times. ACH50 activity of all dietary treatments was higher than that of the control group (P < 0.05); moreover, its highest activity was obtained in LDB7 + AR5, before and after stress. Serum Ig content in fish fed with diets containing LDB and/or AR was significantly higher than that in fish of the control group (P < 0.05), at both times; however, LDB7 + AR5 treatment had higher Ig value than LDB9 and AR10 treatments. Before stress, ACP activity was only affected by dietary LDB7 + AR5 (P < 0.05). After 14 days of stress procedure, all dietary LDB and/or AR supplementation significantly increased ACP activity compared to the control group (P < 0.05).
Serum lysozyme, ACH50, ACP activities, and Ig value in rainbow trout fed with experimental diets (control, LDB7, LDB9, AR5, AR10, LDB7 + AR5, and LDB9 + AR10) for 60 days and exposed to crowding stress for 14 days. Abbreviations: LDB7 (1 × 107 CFU/g CG), LDB9 (1 × 109 CFU/g CG), AR5 (5 g/kg CG), AR10 (10 g/kg CG), LDB7 + AR5 (1 × 107 CFU/g + 5 g/kg CG), LDB9 + AR10 (1 × 109 CFU/g + 10 g/kg CG), LYZ lysozyme activity, Ig total immunoglobulin level, ACH50 alternative complement activity, ACP acid phosphatase. Different letters above the bars indicate significant differences among the treatment combinations (dietary LDB or/ and AR × stress; Tukey test; n = 6)
Serum antioxidant parameters
The interaction effect was identified between dietary LDB and/or AR supplementation and crowding stress on hepatic antioxidant parameters including GPX activity (P < 0.001), CAT activity (P < 0.001), SOD activity (P = 0.003), and MDA content (P < 0.001) (Fig. 3). All dietary supplementation of LDB and/or AR significantly boosted hepatic GPX activity, and the highest value was obtained in LDB7 + AR5 treatment, at both times (P < 0.05). CAT activity was significantly improved in fish receiving LDB7, AR5, LDB7 + AR5, and LDB9 + AR10 diets, at both times (P < 0.05). Before stress, there was no significant discrepancy in SOD activity among the experimental group (P > 0.05). After stress, all dietary supplementation of LDB and/or AR with similar statistical patterns significantly boosted SOD activity (P < 0.05). MDA content remarkably decreased in fish fed with all supplemented diets at both times (P < 0.05).
Hepatic GPX (glutathione peroxidase) and CAT (catalase) values, SOD (superoxide dismutase) activities, and MDA (malondialdehyde) value (D) in rainbow trout fed with experimental diets (control, LDB7, LDB9, AR5, AR10, LDB7 + AR5, and LDB9 + AR10) for 60 days and exposed to crowding stress for 14 days. Abbreviations: LDB7 (1 × 107 CFU/g CG), LDB9 (1 × 109 CFU/g CG), AR5 (5 g/kg CG), AR10 (10 g/kg CG), LDB7 + AR5 (1 × 107 CFU/g + 5 g/kg CG), LDB9 + AR10 (1 × 109 CFU/g + 10 g/kg CG); GPX glutathione peroxidase, CAT catalase, MDA malondialdehyde, SOD superoxide dismutase. Different letters above the bars indicate significant differences among the treatment combinations (dietary LDB or/and AR × stress; Tukey test; n = 6)
Serum biochemical parameters
The interaction effects were detected between dietary LDB and/or AR supplementation and crowding stress on serum biochemical responses including ALT (P < 0.001), AST (P < 0.001) and ALP (P = 0.036) activities and cortisol (P < 0.001) and glucose (P < 0.001) levels. Before stress, dietary supplementation of LDB + AR (both combined forms) significantly decreased ALT activity (P < 0.05). After stress, LDB7, AR5, AR10, LDB7 + AR5, and LDB9 + AR10 diets significantly decreased ALT activity in stressed fish compared to the control group (P < 0.05). Before stress, AST activity remarkably decreased in supplemented groups, except for specimens of AR10 group (P < 0.05). After 14 crowding stress, all dietary LDB and/or AR supplementation significantly decreased AST activity compared to the control diet (P < 0.05). Before stress, ALP activity was only significantly affected in the LDB7 + AR5 group (P < 0.05). After stress, ALP activity in all stressed fish fed with diets containing LDB and/or AR was lower than that in stressed fish fed with an unsupplemented diet (P < 0.05). Before the stressor was introduced, the level of cortisol in the supplemented group with LDB7 + AR5 was lower than that in the control group (P < 0.05). After stress, all dietary supplementation of LDB and/or AR remarkably decreased fish cortisol levels compared to those in the control group (P < 0.05). Before stress, glucose values in fish fed with supplemented diets except for LDB9 and AR10 significantly decreased (P < 0.05). After stress, all dietary supplementation of LDB and/or AR significantly reduced serum glucose values compared to those in the control group (P < 0.05) (Table 4).
Discussion
Growth responses and feed efficiency indices are the main indicators of fish productivity (Acar et al. 2021; Niazi et al. 2023). In the current study, dietary inclusion of LDB or/and AR significantly boosted growth variables and feed efficiency; however; diets containing synbiotics exhibited better function. Similarly, the dietary inclusion of synbiotic including Jerusalem artichoke and L. rhamnosus significantly improved the growth performance and feed efficiency in red tilapia (Sewaka et al. 2019). Besides, Yousefi et al. (2023a, 2023b, 2023c, 2023d) reported that diet incorporated with the combination of gum arabic and L. helveticus remarkably improved WG, FW, FCR, and PER in common carp. An analysis of the nutritional data showed that the FCR and PER improved in the supplemented groups without a significant impact on the FI value across different treatments. The improvement of these two nutritional parameters reflects the increase in digestibility, nutrient absorption, and protein retention. In this study, the administration of synbiotic diets exerted strong effects on the level of digestive enzymes. In support, some studies have reported increased activity of digestive enzymes in fish fed with diets supplemented with synbiotics such as Aspergillus oryzae and β-glucan (Dawood et al. 2020a, 2020b) and fructooligosaccharide and Bacillus licheniformis in common carp (Cyprinus carpio; Yuan et al. 2022). It has been reported that AR can enhance the digestive tract functioning by interacting with the gut epithelium surface and mucus (Sakr 2022). Also, the bifidogenic properties of AR stimulate intestinal bacterial enzymatic activity (Redondo-Cuenca et al. 2023). Therefore, improving the digestive enzyme function was one of the main reasons for this study’s remarkable improvement of growth and nutritional variables in synbiotic treatments.
Blood parameters are mainly used to evaluate the effects of environmental stress and manipulated diets (Fazio 2019). Previous findings showed that environmental stress suppressed hematological parameters (Zhang et al. 2020a, 2020b; Ghafarifarsani et al. 2022). Earlier data reported that dietary synbiotics have great potential to improve blood components. The administration of fructooligosaccharide and B. subtilis remarkably elevated WBC, RBC count, and Hb in Labeo fimbriatus fry (Pawar et al. 2023). In common carp, dietary supplementation of L. helveticus and gum Arabic boosted the proliferation of WBC and phagocytic cells (i.e., neutrophil and monocyte) (Yousefi et al. 2023a, 2023b, 2023c, 2023d). In this study, synbiotics significantly reduced the effects of crowding stress on blood components among the processed diets. Supporting the total blood components may be due to inhibiting the release of stress hormones, preventing swelling and lysis of mature RBC and iron loss by ROS, strengthening the hematopoietic tissues, increasing heme synthesis, and improving migration of new phagocytic cells into the circulatory system (Bujjamma and Padmavathi 2018; Ibrahim et al. 2021).
Earlier studies indicated that fish exposed to stress could exhibit immune system dysfunction (Mirghaed et al. 2018). ACH50, lysozyme, ACP, and Ig are the main components of the first line of the body’s immune system against infections (Ellis 1999). In this study, the lowest level of these parameters was recorded in stressed fish of the control group. The reduction of immune responses in this treatment may be due to the release of corticosteroid hormones during stress and immunosuppression, a hypothesis that was confirmed in previous studies (Mirghaed et al. 2018; Chekani et al. 2021). Other findings showed that oxidative stress affects ACH50, LYZ, and ACP levels through damage to intestinal epithelial cells, hepatocytes, and phagocytosing cells (Moro-García et al. 2018). The decrease in Ig content may result from a reduction in the count of mature B lymphocytes and their inability to biosynthesize Ig subunits (Ercal et al. 2000). Based on available evidence, pro-/pre-/synbiotics can alter the biosynthesis rate of immunity proteins and increase their secretion into the blood (Ringø and Song 2016; Kumar et al. 2018a, 2018b). LAB mitigates immunosuppression via the secretion of extracellular enzymes, improving the intestinal structure, inhibiting cortisol, and metabolite generation with antistress properties such as folate (Hoseinifar et al. 2020). The effect of AR as a prebiotic on the immunological function of aquatic animals has not yet been investigated. However, other findings reported that prebiotics could influence fish immune system function by modulating the generation of the pro-inflammatory cytokines in gut mucosa or triggering interleukins (Patel and Goyal 2015; Naiel et al. 2022). It seems that LDB and AR have been able to mitigate or neutralize the effects of crowding stress on the immunological function of fish through diverse mechanisms.
Among the physiological parameters, the host antioxidant enzyme is of particular importance in aquaculture, when oxidative stress occurs (Shadegan and Banaee 2018). In this study, antioxidant capacity was decreased in the hepatic of fish exposed to crowding stress, and this reduction was greater in specimens fed with the unsupplemented diet. In addition, the increase in MDA level reflects the overproduction of ROS during crowding stress. Mirghaed et al. (2018) reported a reduction in the antioxidant capacity of rainbow trout subjected to crowding stress, as MDA level increased in this situation. Similar findings were reported by Chekani et al. (2021) in the same species. LAB strains including LDB could improve fish antioxidant capacity by generating metabolites with antioxidant properties such as exopolysaccharides (EPS), butyrate, glutathione (GSH), and folate (Hoseinifar et al., 2020). Moreover, LAB can directly secrete GSH in the intestine (Hoseinifar et al., 2020). Gabr et al. (2023) reported that Lactobacillus acidophilus supports the antioxidant capacity of common carp against crowding stress. Zhang et al. (2020a, 2020b) reported that AR contains prebiotics with antioxidant features such as fructan. Moreover, AR contains a high level of caffeic acid with strong antioxidant activity (Symes et al. 2018).
The endocrine system plays a key role in maintaining the homeostasis of the body and the animal’s coordination with successive changes in rearing conditions. The present results showed a significant increase in circulating cortisol and glucose levels. Mirghaed et al. (2018) and Yousefi et al. (2019) found a significant increase in cortisol and glucose levels in rainbow trout and common carp exposed to crowding stress, respectively. Our results showed that the oral administration of LDB or AR especially synbiotics had a high potential in inhibiting cortisol and glucose release and attenuating the negative effects of crowding stress on fish. Similarly, Gabr et al. (2023) reported that a diet containing L. acidophilus mitigated plasma and cortisol levels in common carp exposed to high density. Besides, oral administration of β-glucan significantly decreased plasma cortisol and glucose levels in Nile tilapia reared in high density (Dawood et al. 2020a, 2020b).
Liver enzyme activity is a biochemical marker to evaluate the health and integrity of hepatocyte cells. An increase in circulating AST, ALT, and ALP levels could be reflective of hepatocellular injury. Yousefi et al. (2019) reported an increase in AST, ALT, and ALP activity in common carp subjected to ambient ammonia toxicity. The same study identified the supportive role of dietary garlic in mitigating the effect of crowding stress on plasma enzymatic activities; these results are consistent with our findings. In addition, a decrease in the activity of ALP enzyme in rainbow trout fed with symbiotic (Lactobacillus fermentum and cinnamon) and cultured in high density was observed (Jasim et al. 2022), which is in line with our results. In this study, the decrease in the liver enzyme activity in the supplemented groups is probably due to the boosting of the antioxidant capacity and supporting cell integrity.
Conclusion
The results obtained in this study showed that dietary inclusion of LDB or/and AR improved the growth performance, gut LAB count, and the digestive protease activity; however, a significant improvement in amylase and lipase activities was only found in fish that were exposed to dietary synbiotics. Additionally, the study findings revealed that crowding stress negatively affects the health of fish through suppressing the immune system and oxidative stress and inhibition of corticosteroid secretion. While all dietary supplements of LDB or/and AR mitigated the effect of crowding stress on HB, HCT, WBC count, NEU, serum immune responses, SOD, GPX, MDA content, cortisol, glucose, ALP, and AST, it was proved that the diets containing synbiotic were more effective in improving these factors. Furthermore, synbiotic diets also showed enhanced effects on RBC and MON count and CAT at both times. Thus, synbiotics diets are suggested to be considered a strategy for mitigating the harmful effects of crowding stress when transporting rainbow trout over long distances or rearing them in recirculating culture systems.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Abedi D, Feizizadeh S, Akbari V, Jafarian-Dehkordi A (2013) In vitro anti-bacterial and anti-adherence effects of Lactobacillus delbrueckii subsp bulgaricus on Escherichia coli. Res Pharm Sci 8(4):260
Acar Ü, Kesbiç OS, İnanan BE, Yılmaz S (2019) Effects of dietary Bergamot (Citrus bergamia) peel oil on growth, haematology and immune response of European sea bass (Dicentrarchus labrax) juveniles. Aquacult Res 50(11):3305–3312
Acar Ü, Kesbiç OS, Yılmaz S, İnanan BE, Zemheri-Navruz F, Terzi F et al (2021) Effects of essential oil derived from the bitter orange (Citrus aurantium) on growth performance, histology and gene expression levels in common carp juveniles (Cyprinus carpio). Animals 11(5):1431
Aftabgard M, Salarzadeh A, Mohseni M, Bahri Shabanipour AH, Zorriehzahra MEJ (2019) The combined efficiency of dietary isomaltooligosaccharides and Bacillus spp. on the growth, hemato-serological, and intestinal microbiota indices of Caspian brown trout (Salmo trutta caspius Kessler, 1877). Probiotics Antimicrob Proteins 11:198–206
Andersch MA, Szczypinski AJ (1947) Use of p-nitrophenylphosphate as the substrate in determination of serum acid phosphatase. Am J Clin Pathol 17:571–574
AOAC (1995) Official methods of analysis of the Association of Official Analytical Chemists. Association of Official Analytical Chemists, Washington, DC, USA
Aslim B, Onal D, Beyatli Y (2007) Factors influencing autoaggregation and aggregation of Lactobacillus delbrueckii subsp. bulgaricus isolated from handmade yogurt. J Food Prot 70(1):223–227
Azimirad M, Meshkini S, Ahmadifard N, Hoseinifar SH (2016) The effects of feeding with synbiotic (Pediococcus acidilactici and fructooligosaccharide) enriched adult Artemia on skin mucus immune responses, stress resistance, intestinal microbiota and performance of angelfish (Pterophyllum scalare). Fish Shellfish Immunol 54:516–522
Bernfeld P (1955) Amylase. In: Colowick SP, Kaplan NO (eds) Methods in enzymology. Academic Press, New York, pp 149–158
Blaxhall PC, Daisley KW (1973) Routine haematological methods for use with fish blood. J Fish Biol 5(6):771–781
Borges A, Scotti LV, Siqueira DR, Jurinitz DF, Wassermann GF (2004) Hematologic and serum biochemical values for jundiá (Rhamdia quelen). Fish Physiol Biochem 30:21–25
Bujjamma P, Padmavathi P (2018) Effect of cadmium on Haematological changes in a freshwater catfish, Heteropneustes fossilis. Int J Zool Stud 3(1):132–141
Chekani R, Akrami R, Ghiasvand Z, Chitsaz H, Jorjani S (2021) Effect of dietary dehydrated lemon peel (Citrus limon) supplementation on growth, hemato-immunolological and antioxidant status of rainbow trout (Oncorhynchus mykiss) under exposure to crowding stress. Aquaculture 539:736597
Dawood MA, Eweedah NM, Moustafa EM, Shahin MG (2020a) Synbiotic effects of Aspergillus oryzae and β-glucan on growth and oxidative and immune responses of Nile tilapia, Oreochromis niloticus. Probiotics Antimicrob Proteins 12:172–183
Dawood MA, Metwally AES, El-Sharawy ME, Atta AM, Elbialy ZI, Abdel-Latif HM, Paray BA (2020b) The role of β-glucan in the growth, intestinal morphometry, and immune-related gene and heat shock protein expressions of Nile tilapia (Oreochromis niloticus) under different stocking densities. Aquaculture 523:735205
Ellis, A.E., 1990. Lysozyme assays. In: Stolen, J.S. (Ed.), Techniques in fish immunology.
Ellis AE (1999) Immunity to bacteria in fish. Fish Shellfish Immunol 9(4):291–308
Ercal N, Neal R, Treeratphan P, Lutz PM, Hammond TC, Dennery PA, Spitz DR (2000) A role for oxidative stress in suppressing serum immunoglobulin levels in lead-exposed Fisher 344 rats. Arch Environ Contam Toxicol 39:251–256
FAO. The state of world fisheries and aquaculture. Towards blue transformation. 2022. Available online: https://www.fao.org (accessed on 4 July 2022).
Fazio F (2019) Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture 500:237–242
Gabr GA, Ibrahim YS, Al-Shawi SG, Abosaooda M, Gupta J, Oudaha KH et al (2023) Single or combined consumption of resveratrol and the probiotic, Lactobacillus acidophilus attenuate the effects of crowding stress on growth, immune characteristics, and antioxidant defense in the common carp, (Cyprinus carpio). Aquacult Rep 29:101471
Garcia-Carreno FL, Haard NF (1993) Characterization of proteinase classes in Langostilla pleuroncodesplanipes and crayfish Pacifastacus astacus extracts. Food Biochem 17:97–113
Ghafarifarsani H, Hoseinifar SH, Aftabgard M, Van Doan H (2022) The improving role of savory (Satureja hortensis) essential oil for Caspian roach (Rutilus caspicus) fry: Growth, haematological, immunological, and antioxidant parameters and resistance to salinity stress. Aquaculture 548:737653
Giri SS, Sukumaran V, Oviya M (2013) Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol 34(2):660–666
Guo Q, Wang N, Liu H, Li Z, Lu L, Wang C (2020) The bioactive compounds and biological functions of Asparagus officinalis L.–a review. J Funct Foods 65:103727
Haghparast RJ, Moghanlou KS, Mohseni M, Imani A (2019) Effect of dietary soybean lecithin on fish performance, hemato-immunological parameters, lipid biochemistry, antioxidant status, digestive enzymes activity and intestinal histomorphometry of pre-spawning Caspian brown trout (Salmo trutta caspius). Fish Shellfish Immunol 91:50–57
Hoseinifar SH, Yousefi S, Van Doan H, Ashouri G, Gioacchini G, Maradonna F, Carnevali O (2020) Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev Fish Sci Aquacult 29(2):198–217
Ibrahim ATA, Banaee M, Sureda A (2021) Genotoxicity, oxidative stress, and biochemical biomarkers of exposure to green synthesized cadmium nanoparticles in Oreochromis niloticus (L.). Comp Biochem Physiol C Toxicol Pharmacol 242:108942
Iijima N, Tanaka S, Ota Y (1998) Purification and characterization of bile salt-activated lipase from the hepatopancreas of red sea bream, Pagrus major. Fish Physiol Biochem 18:59–69
Jasim SA, Hafsan H, Saleem HD, Kandeel M, Khudhair F, Yasin G et al (2022) The synergistic effects of the probiotic (Lactobacillus fermentum) and cinnamon, Cinnamomum sp. powder on growth performance, intestinal microbiota, immunity, antioxidant defence and resistance to Yersinia ruckeri infection in the rainbow trout (Oncorhynchus mykiss) under high rearing density. Aquacult Res 53(17):5957–5970
Kesbiç OS, Acar Ü, Yilmaz S, Aydin ÖD (2020) Effects of bergamot (Citrus bergamia) peel oil-supplemented diets on growth performance, haematology and serum biochemical parameters of Nile tilapia (Oreochromis niloticus). Fish Physiol Biochem 46(1):103–110
Khalil MA, Sonbol FI, Al-Madboly LA, Aboshady TA, Alqurashi AS, Ali SS (2022) Exploring the therapeutic potentials of exopolysaccharides derived from lactic acid bacteria and bifidobacteria: antioxidant, antitumor, and periodontal regeneration. Front Microbiol 13:803688
Kumar P, Jain KK, Sardar P (2018b) Effects of dietary synbiotic on innate immunity, antioxidant activity and disease resistance of Cirrhinus mrigala juveniles. Fish Shellfish Immunol 80:124–132
Kumar P, Jain KK, Sardar P, Jayant M, Tok NC (2018a) Effect of dietary synbiotic on growth performance, body composition, digestive enzyme activity and gut microbiota in Cirrhinus mrigala (Ham.) fingerlings. Aquacult Nutr 24(3):921–929
Mirghaed AT, Hoseini SM, Ghelichpour M (2018) Effects of dietary 1, 8-cineole supplementation on physiological, immunological and antioxidant responses to crowding stress in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 81:182–188
Mohammadian T, Alishahi M, Tabandeh MR, Ghorbanpoor M, Gharibi D, Tollabi M, Rohanizade S (2016) Probiotic effects of Lactobacillus plantarum and L. delbrueckii ssp. bulgaricus on some immune-related parameters in Barbus grypus. Aquacult Int 24:225–242
Mohammadian T, Dezfuly ZT, Motlagh RG, Jangaran-Nejad A, Hosseini SS, Khaj H, Alijani N (2020) Effect of encapsulated lactobacillus bulgaricus on innate immune system and hematological parameters in rainbow trout (Oncorhynchus mykiss), post-administration of Pb. Probiotics Antimicrob Proteins 12:375–388
Mohammadian T, Nasirpour M, Tabandeh MR, Heidary AA, Ghanei-Motlagh R, Hosseini SS (2019a) Administrations of autochthonous probiotics altered juvenile rainbow trout Oncorhynchus mykiss health status, growth performance and resistance to Lactococcus garvieae, an experimental infection. Fish Shellfish Immunol 86:269–279
Mohammadian T, Nasirpour M, Tabandeh MR, Mesbah M (2019b) Synbiotic effects of β-glucan, mannan oligosaccharide and Lactobacillus casei on growth performance, intestine enzymes activities, immune-hematological parameters and immune-related gene expression in common carp, Cyprinus carpio: an experimental infection with Aeromonas hydrophila. Aquaculture 511:634197
Mohtashami M, Mohamadi M, Azimi-Nezhad M, Saeidi J, Nia FF, Ghasemi A (2021) Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol Appl Biochem 68(6):1421–1431
Moro-García MA, Mayo JC, Sainz RM, Alonso-Arias R (2018) Influence of inflammation in the process of T lymphocyte differentiation: proliferative, metabolic, and oxidative changes. Front Immunol 9:339
Naderi Farsani, M., Meshkini, S., & Manaffar, R. (2021). Growth performance, immune response, antioxidant capacity and disease resistance against Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss) as influenced through singular or combined consumption of resveratrol and two-strain probiotics. Aquacult Nutr, 27(6), 2587-2599.
Naiel MA, Abd El-hameed SA, Arisha AH, Negm SS (2022) Gum Arabic-enriched diet modulates growth, antioxidant defenses, innate immune response, intestinal microbiota and immune related genes expression in tilapia fish. Aquaculture 556:738249
Niazi A, Mehrgan MS, Islami HR (2023) Optimizing turmeric and green tea fermentation with Lactobacillus brevis to enhance growth performance, digestive enzymes, and immunity in rainbow trout. Aquaculture:739962
Patel S, Goyal A (2015) Applications of natural polymer gum arabic: a review. Int J Food Prop 18(5):986–998
Pawar NA, Prakash C, Kohli MPS, Jamwal A, Dalvi RS, Devi BN et al (2023) Fructooligosaccharide and Bacillus subtilis synbiotic combination promoted disease resistance, but not growth performance, is additive in fish. Sci Rep 13(1):11345
Redondo-Cuenca A, García-Alonso A, Rodríguez-Arcos R, Castro I, Alba C, Rodríguez JM, Goni I (2023) Nutritional composition of green asparagus (Asparagus officinalis L.), edible part and by-products, and assessment of their effect on the growth of human gut-associated bacteria. Food Res Int 163:112284
Řehulka J (2000) Influence of astaxanthin on growth rate, condition, and some blood indices of rainbow trout, Oncorhynchus mykiss. Aquaculture 190(1-2):27–47
Ringø E, Song SK (2016) Application of dietary supplements (synbiotics and probiotics in combination with plant products and β-glucans) in aquaculture. Aquacult Nutr 22(1):4–24
Sakr EA (2022) Structural characterization and health benefits of a novel fructan produced by fermentation of an Asparagus sprengeri extract by Lactobacillus plantarum DMS 20174. Process Biochem 118:370–380
Sewaka M, Trullas C, Chotiko A, Rodkhum C, Chansue N, Boonanuntanasarn S, Pirarat N (2019) Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish Shellfish Immunol 86:260–268
Shadegan MR, Banaee M (2018) Effects of dimethoate alone and in combination with Bacilar fertilizer on oxidative stress in common carp, Cyprinus carpio. Chemosphere 208:101–107
Shekarabi SPH, Mostafavi ZS, Mehrgan MS, Islami HR (2021) Dietary supplementation with dandelion (Taraxacum officinale) flower extract provides immunostimulation and resistance against Streptococcus iniae infection in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 118:180–187
Siwicki, A., Anderson, D., 1993. Nonspecifc defense mechanisms assay in fsh: II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin level in serum. In: Siwicki, A., Anderson, D., Waluga, J. (Eds.), Fish disease diagnosis and prevention methods. Wydawnictwo Instytutu Rybactwa Srodladowego, Olsztyn, Poland, pp. 105–112.SOS Publication, Fair Haven, pp. 101–103.
Symes A, Shavandi A, Zhang H, Mohamed Ahmed IA, Al-Juhaimi FY, Bekhit AEDA (2018) Antioxidant activities and caffeic acid content in New Zealand Asparagus (Asparagus officinalis) roots extracts. Antioxidants 7(4):52
Yano T (1992) Assays of hemolytic complement activity. In: Stolen JS (ed) Techniques in fish immunology. SOS Publication, Fair Haven, pp 131–141
Yousefi M, Farsani MN, Ghafarifarsani H, Raeeszadeh M (2023a) Dietary Lactobacillus helveticus and gum Arabic improves growth indices, digestive enzyme activities, intestinal microbiota, innate immunological parameters, antioxidant capacity, and disease resistance in common carp. Fish Shellfish Immunol 135:108652
Yousefi M, Ghafarifarsani H, Raissy M, Yilmaz S, Vatnikov YA, Kulikov EV (2023c) Effects of dietary malic acid supplementation on growth performance, antioxidant and immunological parameters, and intestinal gene expressions in rainbow trout, Oncorhynchus mykiss. Aquaculture 563:738864
Yousefi M, Hoseini SM, Kulikov EV, Babichev NV, Bolshakova MV, Shopinskaya MI et al (2023b) Effects of dietary pomegranate peel supplementation on growth performance and biochemical responses of common carp, Cyprinus carpio, to chronic crowding stress. Aquac Rep 30:101532
Yousefi M, Hoseini SM, Vatnikov YA, Kulikov EV, Drukovsky SG (2019) Rosemary leaf powder improved growth performance, immune and antioxidant parameters, and crowding stress responses in common carp (Cyprinus carpio) fingerlings. Aquaculture 505:473–480
Yousefi M, Nedaei S, Farsani MN, Ghafarifarsani H, Zhang ML, Du ZY (2023d) Dietary chrysin supplementation improves growth performance, immune responses, antioxidant status, and resistance against crowding stress in rainbow trout. Aquac Rep 32:101708
Yuan X, Xu R, Qi Q, Xu M, Li B, Wang B, Zhang C (2022) Dietary fructooligosaccharide and Bacillus licheniformis on growth performance, digestive enzyme, immune indices, and antioxidant capacity of common carp (Cyprinus carpio). Reg Stud Mar Sci 56:102670
Zhang J, Li Z, Zhou L, Bao J, Xu J (2020a) The modifications of a fructan from Anemarrhena asphodeloides Bunge and their antioxidant activities. Int J Biol Macromol 164:4435–4443
Zhang M, Yin X, Li M, Wang R, Qian Y, Hong M (2020b) Effect of nitrite exposure on haematological status, oxidative stress, immune response and apoptosis in yellow catfish (Pelteobagrus fulvidraco). Comp Biochem Physiol C Toxicol Pharmacol 238:108867
Zou Y, Yu H, Zhang L, Ruan Z (2021) Dietary vegetable powders modulate immune homeostasis and intestinal microbiota in mice. Foods 11(1):27
Funding
This paper has been supported by the RUDN University Strategic Academic Leadership Program dedicated to Morteza Yousefi.
Author information
Authors and Affiliations
Contributions
MY: Did the Supervision, Conceptualization, Funding acquisition, Project administration and performed Writing - review & editing of the paper. MNF: Performed the Methodology, Conceptualization, Resources, Writing – original draft of the paper. AAK: Performed the Methodology. HG: Did the process of Conceptualization, Formal analysis and Methodology. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Competing interests
There is no conflict of interest to declare.
Consent to participate
Not applicable.
Consent for publication
All authors give consent for publication.
Additional information
Handling Editor: Brian Austin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yousefi, M., Farsani, M.N., Afzali-Kordmahalleh, A. et al. Effects of dietary synbiotic supplementation on growth performance, digestive enzyme activities, and physiological resistance against high stocking density in rainbow trout (Oncorhynchus mykiss). Aquacult Int 32, 3295–3315 (2024). https://doi.org/10.1007/s10499-023-01323-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01323-0