Abstract
Identifying how variations in functional traits arise is important to predicting the effects of such variations within communities and ecosystems. Here, we evaluated the effects of kin-discrimination on the uptake of soil resources and leaf traits in seedlings of Fagus crenata (Fagaceae). We grew F. crenata seedlings either alone or paired with a sibling or non-sibling, and examined the total biomass, shoot-to-root ratio, leaf traits (chlorophyll content, leaf mass per area, and total contents of phenolic compounds and condensed tannins), and uptake of soil nitrogen, phosphor, and water. In all neighbour treatments, seedlings grew similarly and had similar shoot-to-root ratios. Chlorophyll content and leaf mass per area were higher in plants grown with non-siblings than in those grown alone or with siblings. The total content of phenolic compounds was highest in single seedlings and lowest in non-siblings. Soil moisture was lowest and thus water uptake was highest in plants grown with non-siblings. Our findings suggest that differences in the intensity of competition for soil resources based on kin-discrimination result in differences in leaf traits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant functional traits affect ecosystem productivity (Weigelt et al. 2008), soil carbon storage (De Deyn et al. 2008), trophic interactions (Walters 2011), and species coexistence (Kraft et al. 2015). In addition to variations in functional traits among species, variations within species are a key component of the diversity of functional traits within population (Siefert et al. 2015), because they have large effects on ecological communities and ecosystem productivity (Des Roches et al. 2018; Koricheva and Hayes 2018). Therefore, identifying how variations in functional traits arise is important in predicting the effects of such variations in species within communities and ecosystems.
Genetic variation is one key factor underlying trait variations within species (Maddox and Root 1990; Crutsinger et al. 2006; Lamit et al. 2015). For example, genetic variations in the leaf defense traits (Whitham et al. 2006) and in the plant architecture (Crutsinger et al. 2014) underlie the diversity of arthropod and soil microbe communities. Although the effects of plant genetic diversity on biological communities have been examined in many studies (Whitham et al. 2012; Koricheva and Hayes 2018), evidence comes primarily from experiments without belowground interactions among plants. As the expression of plant functional traits is influenced by neighbouring plants through competition (Barton and Bowers 2006; Temperton et al. 2007; Broz et al. 2010; Yamawo 2015), such experiments limit the effects of plant–plant interactions on trait expression, and thus the effects of trait plasticity within communities.
Intraspecific competition is a primary mode of interaction in natural plant populations and is a fundamental aspect of population dynamics, evolution of dispersal strategies, life history, and the maintenance of genetic variation (Cheplick 1992; Rankin et al. 2007). Some plant species alter their resource allocation patterns or above- and below-ground architecture depending on whether encounter those of siblings or non-siblings (Chen et al. 2012; Crepy and Casal 2015; Yamawo et al. 2017). In the presence of conspecific non-siblings, plants can increase resource allocation to fine roots, which enables them to better compete for soil resources or inhibits growth of competitor plants (Dudley and File 2007; Biernaskie 2011; File et al. 2012; Dudley et al. 2013; Yamawo et al. 2017). Some species change their uptake of Nitrogen (N), Phosphor (P), Sulfur, Kalium, Calcium, and Magnesium (Mg) in response to their neighbour’s genotype, depending on whether it is kin or non-kin (Zhang et al. 2016; Li et al. 2018). Changes in root growth or resource allocation are the most common characteristic of kin-discrimination in plants (reviewed in Dudley et al. 2013), allowing plants to potentially minimise competitive ability with kin (Dudley 2015; Yamawo et al. 2017; but see Masclaux et al. 2010).
Differences in competition intensity may alter the expression of leaf traits. The compensatory continuum hypothesis predicts that plants should allocate more resources to defences when competition is low than when it is high (Karban and Myers 1989; Maschinski and Whitham 1989), because the cost of anti-herbivore defence traits is higher when competition is higher (Siemens et al. 2003; Cipollini 2007, 2010). In contrast, the defence stress benefit hypothesis predicts that plants should allocate more resources to defences when competition is high, when defences have additional beneficial functions such as allelopathy and associational defence (Inderjit and Del Moral 1997; Lankau and Strauss 2007), thereby reducing the costs of allocation to defence traits and promoting their expression under competition (Siemens et al. 2003; Cipollini 2007; Boege 2010). If plants growing with siblings invest more in defences than plants growing with non-siblings, this would support the compensatory continuum hypothesis. Conversely, if plants growing with non-siblings invest more in defences than plants growing with siblings, this would support the defence stress benefit hypothesis. Thus, kin-discrimination should produce variations in plant functional traits.
Fagus crenata (Fagaceae) is common in Japanese cool temperate forests. Because seed dispersal is strongly limited in this species, and seeds tend to germinate on the ground near the parent tree, seedlings often grow near kin (Hashizume et al. 1984). Mortality of F. crenata depends on both density and distance at both the seed (Tomita and Seiwa 2004) and seedling stages (Masaki et al. 2007). There are many competitive interactions in the first growing season, when seedling density is highest (Ichihara and Yamaji 2009); therefore, seedlings should express competitive responses in the first growing season. Here, we evaluated the effects of kin-discrimination on leaf traits with defensive functions. To estimate competition for soil resources, we measured resource uptake in seedlings in different neighbouring conditions: single, with siblings, and with non-siblings.
Materials and methods
Cultivation of Fagus crenata Blume seedlings
In September 2015, we collected 20 seeds per tree from two populations—12 trees in Kuroishi City (40°61′N, 140°71′E) and 8 trees in Nishi-meya City (40°57′N, 140°28′E)—both in Aomori Prefecture, northern Japan. Genotype of F. crenata is different between these populations (Fujii et al. 2002). Each tree was 10–12 m tall and was more than 2 km from any other sample tree. We refer to seeds collected from the same mother plant as ‘siblings’ and to seeds collected from different mother plants of different populations, with different genotypes, as ‘non-siblings’.
On 1 March 2016, we sowed seeds in 24 plastic containers (25 cm × 30 cm × 5 cm) that were filled with moist filter paper. The containers were kept in a growth chamber at 25 °C under a 12L:12D photoperiod for 2 weeks and watered every other day. On 9 April 2016, seedlings (5 cm in height) were selected randomly and planted (27 pairs of siblings, 24 random pairs of non-siblings) 4 cm apart in plastic pots (15 cm × 15 cm × 30 cm) containing 70% red soil and 30% humus. The seedling density reflects the natural conditions (H.T. unpublished). Each of 26 pots with a single seedling was divided in half by a plastic plate, and one seedling was planted in one half of the pot. The other half side of the pots were full of the soil but had no plant. At the beginning of the experiment, each seedling had both cotyledons and the first adult leaf expanded. We applied fertiliser (NPK = 8:8:8; APM Japan, Tokyo, Japan) once a month (4 g for pairs, 2 g for single seedlings) directly onto the soil surface around the stems. All pots received approximately 30% sunlight under a black shade cloth with a 1-mm mesh to simulate the 10% to 40% sunlight in the forest understory (H. T. unpublished). The pots were maintained for the first growing season (120 days) outdoors at Hirosaki University (40°58′N, 140°47′E) and watered every other day. All plants were then collected and dried at 50 °C for 10 days. Leaves, stems, and roots of each plant were weighed separately to a precision of 0.1 mg on an electronic balance (BP211D; Sartorius, Göttingen, Germany).
Leaf characteristics
Chlorophyll contents were measured with a chlorophyll meter (SPAD-502, Konica Minolta, Tokyo, Japan) on the last day of the experiment in the two youngest fully expanded leaves of each seedling. The average values were analysed. To measure leaf area, we scanned each dried leaf on an image scanner (PM-850A; Seiko Epson Corp., Suwa, Japan) and measured its area in photo-image analyser software (Scion Image; Scion Corp., Frederick, MD, USA). We calculated leaf mass per area (LMA) as leaf mass divided by leaf area.
We measured the contents of phenolic compounds and condensed tannins in leaves according to Feeny (1970) and Dudt and Shure (1994). Dried plant tissues were powdered in a mill. Total phenolics were extracted from 20 mg leaf powder in 10 mL 50% methanol for 1 h in an ultrasound bath at 40 °C. The concentration of phenolics (mg/g) was measured using the Folin–Ciocalteu method (Julkunen-Tiitto 1985). Condensed tannins were extracted from 50 mg dry leaves and were quantified by radial diffusion assay with tannic acid as a standard (Hagerman 1987).
Soil moisture and nutrients
Soil moisture was estimated monthly as in Yamawo et al. (2012). Soil nutrients were measured according to van Oorschot et al. (2000). To determine the levels of NH4–N and NO3–N, we sieved 50 mg of soil per pot and shook it in a shaker with 50 mL 50% methanol during 1 h; filtered the methanol through filter paper (Filter Paper Qualitative; Toyo Roshi Kaisha Ltd., Tokyo, Japan); and analysed the extract for mineral N levels as described by Joergensen and Brookes (1990). To measure the potential plant-available Phosphor (P), we extracted 1 g of dry soil per pot in a shaker with 50 mL of 0.04-M NH4F + 0.01-M HCl for 30 min. This method extracts a major portion of calcium phosphate, some aluminium and iron phosphates, and some organic-bound P. Extracts were filtered and held at 4 °C. After complexation with molybdate blue, PO43− was determined by extinction at 880 nm on a spectrophotometer.
Statistical analyses
All statistical analyses were performed in R v. 2.15.1 software (R Development Core Team 2015). Plant height, total biomass, LMA, chlorophyll content, and concentrations of tannins and phenolics in leaves were compared among neighbour treatments in generalised linear mixed-effects models (GLMMs) with a Gaussian distribution and identity link, including genotype (parent plant ID) as a random effect. Leaf number was compared among neighbour treatments in a GLMM with a Poisson distribution and log-link identity link, with genotype (parent plant ID) as a random effect. P values were corrected for false discovery rate. The relationship between leaf mass and leaf area was analysed by least-squares regression, because LMA is potentially affected by leaf size (Coleman et al. 1994). To investigate the difference in allometry between the shoot and root biomass between the two conditions, we analyzed these relationships using GLMMs with a Gaussian distribution and identity link, including the genotype as a random effect. Relationships among leaf traits were also analysed by least-squares regression. To investigate differences in allometry between the shoot and root biomass allocation patterns among neighbour treatments, we analysed these relationships using GLMMs with a Gaussian distribution and identity link, including genotype (parent plant ID) as a random effect. In the model, shoot biomass was treated as an independent variable, and root biomass and neighbour treatment were treated as dependent variables. To examine the significance of the explanatory variables, we conducted a likelihood-ratio test in the GLMM analyses. We also compared soil nutrients among neighbour treatments in GLMMs with a Gaussian distribution and identity link, including genotype (parent plant ID) as a random effect. P values were corrected for false discovery rate.
Results
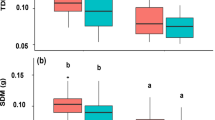
The siblings and non-siblings grown in pairs were significantly taller than single seedlings (Fig. 1b), but leaf number, whole plant biomass, and shoot-to-root allocation did not differ among the neighbour treatments (Figs. 1a, b, S1).
Growth and leaf characteristics of Fagus crenata seedlings in each experimental condition: single, siblings and non-siblings. a Number of leaves, b plant height, c plant biomass, d chlorophyll content (SPAD-value), e leaf mass per area, f condensed tannins and g total phenolics. Bars represent SE. Different letters denote significant differences (GLMM, P < 0.05)
The condensed tannin concentration was not affected by the presence or relatedness of neighbour plants (Fig. 1f). Leaf mass was not correlated with leaf area (r = 0.32, P = 0.42). The LMA and chlorophyll contents of plants grown with non-siblings were higher than those in the sibling or single treatment (Fig. 1d, e). Total phenolic concentration was highest in single seedlings and lowest in non-siblings (Fig. 1g) and was negatively correlated with chlorophyll content in all neighbour treatments, but was not correlated with LMA (Table 1).
Soil NO3− content was similar among neighbour treatments, but soil NH4+ and PO43− contents were significantly lower in the pots with paired siblings or non-siblings than in those with single seedlings (Table 2). Soil moisture was lower in the pots with paired non-siblings than in the other neighbour treatments (Table 2).
Discussion
LMA was significantly higher in plants grown with non-siblings than in those grown alone or with siblings, and soil moisture was lowest in pots with non-siblings. These results suggest that F. crenata can discriminate between sibling and non-sibling, as can other plant species (Dudley and File 2007; Chen et al. 2012; Crepy and Casal 2015; Yamawo et al. 2017). In addition, chlorophyll contents were highest and total phenolics were lowest in plants grown with non-siblings. These findings support our contention that differences in competition intensity based on kin-discrimination promote variations in functional traits within species.
Seedlings grown with either siblings or non-siblings had longer shoots than single plants had. Shoot elongation to avoid shading by a neighbour is common in many plant species (reviewed in Novoplansky 2009). Because F. crenata seedlings often establish on the forest floor under limited light, producing a taller shoot than the neighbours is an adaptive response to light competition.
Seedlings grown with siblings or non-siblings took up more NH4+ and PO43− than single seedlings (Table 2). Other plant species also took up more nutrients as an outcome of belowground competition (e.g., Zhang et al. 2015, 2016). These competitive responses, both above and below ground, were expressed irrespective of the genetic relatedness of a conspecific neighbour (Fig. 1b; Table 2).
Despite an increase in nutrient uptake in the presence of competing plants, shoot-to-root ratio did not differ between siblings and non-siblings neighbour treatments, as also reported in other plant species (Dudley et al. 2013; Yamawo 2015). Some tree species may take up nutrients via mycorrhizal fungi rather than directly through root hairs (Chen et al. 2016), more so those that host an ectomycorrhizal fungus, such as F. crenata does (Ishida et al. 2007). We considered that an increase in nutrient uptake in response to intraspecific competition in F. crenata depends on mycorrhizal symbioses.
The soil moisture content in pots with non-siblings was lower than that in pots with siblings and single plants, perhaps because of competition for soil water against non-siblings but not siblings. This response lends support to kin selection theory, which predicts altruism towards relatives (Hamilton 1964). This result supported previous studies in other species (Dudley et al. 2013).
Leaves of F. crenata grown with non-siblings had larger LMA than leaves of single plants and plants grown with siblings (Fig. 1e). Under water stress, the LMA of trees increases (e.g., Poorter et al. 2009) because the leaf cuticle thickens to minimize water loss or the mesophyll tissue thickens (Baird et al. 2017). Competition for water among non-sibling F. crenata seedlings may induce water stress and thus lead to a larger LMA in these plants than in the other neighbour treatments.
Leaves of F. crenata grown with non-siblings also had greater chlorophyll content than leaves of plants grown alone (Fig. 1d). Chlorophyll content can increase with increasing soil water availability because soil nutrient uptake depends on soil water uptake (Songsri et al. 2009). Soil water uptake was higher in non-siblings than in single plants and siblings, but there was no significant difference in soil nutrient uptake between siblings and non-siblings. Chlorophyll production depends on N, Mg, and other nutrients. Mg uptake, which we did not estimate, is affected by kin-discrimination (Zhang et al. 2016) and may have contributed to the increase in chlorophyll content of plants grown with non-siblings.
Total phenolics were decreased by the presence of non-siblings (Fig. 1g). Low soil water content promotes accumulation of phenolics in leaves (Garcia et al. 1987; Olson et al. 2009; Yamawo et al. 2012), but total phenolic concentration was highest in single trees and siblings, with higher soil moisture (Fig. 1g; Table 2), probably because of a trade-off between the production of chlorophyll and of phenolics, as suggested by their negative correlation over all neighbour treatments (Table 1). Therefore, our results are consistent with a growth–defence trade-off (Strauss et al. 2002; Walters 2011). The compensatory continuum hypothesis—that plants would allocate more to growth than to defence because the expression of defence traits is more costly under competitive conditions (Siemens et al. 2003; Cipollini 2007, 2010)—has received much empirical support (e.g., Cipollini and Bergelson 2001; Kurashige and Agrawal 2005). Our results also support the compensatory continuum hypothesis. The greater chlorophyll content in the presence of non-siblings reflects higher competition. The growth–defence trade-off is likely responsible for the variations in chlorophyll and phenolic concentrations among the neighbour treatments.
LMA is highly correlated with leaf toughness (Wright and Cannon 2001; Hanley et al. 2007), which provides defence against herbivores and microbes (Walters 2011). Phenolic compounds act as chemical defences against herbivores and fungi (Walters 2011). These variations in response to competition support the defence stress benefit hypothesis (that plants should allocate more resources to defences when competition is high; Karban and Myers 1989; Maschinski and Whitham 1989) and the compensatory continuum hypothesis (that plants should allocate more to defences when competition is low; Inderjit and Del Moral 1997; Lankau and Strauss 2007), respectively. Thus, the supported hypothesis may depend on types of defence traits such as chemical and physical and on their physiological relations with other traits such as chlorophyll content or growth.
Plants can discriminate neighbouring conspecific siblings from non-siblings through chemical and light cues perceived above- (Karban et al. 2013; Crepy and Casal 2015) and below-ground (Biedrzycki and Bais 2010; Yamawo et al. 2017) and potentially via mycorrhizal networks (File et al. 2012). Seedlings of F. crenata might discriminate the presence and relatedness of neighbouring individuals via these cues; detailed studies of kin-discrimination mechanisms in F. crenata are needed.
Many studies reported that the plant genetic diversity play crucial roles in determine the arthropod community (Crutsinger et al. 2006; Johnson et al. 2006; Koricheva and Hayes 2018). It is well known that the herbivory is determined by leaf defence traits such as chemical and physical (Walters 2011; Yamawo et al. 2014; Barbour et al. 2015). The variations in leaf traits resulted from kin-discrimination may contribute to increase in diversity of the arthropod community.
In conclusion, we demonstrated that (1) F. crenata seedlings compete both above- and belowground with siblings and non-siblings; (2) non-siblings compete for soil water more than siblings; (3) competition for soil water induces variation in leaf traits. These findings suggest that kin-discrimination underlies the variations in plant functional traits. Such interactions should be common in Japanese cool temperate forests, where F. crenata is a dominant species. Whereas our experiment continued for 120 days, seedlings growing in forests interact for many years (Akaji et al. 2016), so the effects observed in this study may be underestimated. Long-term investigations of the effects of genetic spatial structure within tree populations would contribute to a better understanding of forest ecology.
References
Akaji Y, Miyazaki Y, Hirobe M, Makimoto T, Sakamoto K (2016) The relationship between seedling survival rates and their genetic relatedness to neighbouring conspecific adults. Plant Ecol 217:465–470
Baird AS, Anderegg LDL, Lacey ME, Hille Ris Lambers J, Van Volkenburgh E (2017) Comparative leaf growth strategies in response to low-water and low-light availability: variation in leaf physiology underlies variation in leaf mass per area in Populus tremuloides. Tree Physiol 37:1140–1150
Barbour MA, Rodriguez-Cabal MA, Wu ET, Julkunen-Tiitto R, Ritland CE, Miscampbell AE, Jules ES, Crutsinger GM (2015) Multiple plant traits shape the genetic basis of herbivore community assembly. Funct Ecol 29:995–1006
Barton KE, Bowers MD (2006) Neighbor species differentially alter resistance phenotypes in Plantago. Oecologia 150:442–452
Biedrzycki ML, Bais HP (2010) Kin recognition in plants: a mysterious behavior unsolved. J Exp Bot 61:4123–4128
Biernaskie JM (2011) Evidence for competition and cooperation among climbing plants. Proc R Sci Lond B 278:1989–1996
Boege K (2010) Induced responses to competition and herbivory: natural selection on multi-trait phenotypic plasticity. Ecology 91:2628–2637
Broz AK, Broeckling CD, De-la-Pena C, Lewis MR, Greene E, Callaway RM, Sumner LW, Vivanco JM (2010) Plant neighbor identity influences plant biochemistry and physiology related to defense. BMC Plant Biol 10:115
Chen JW, During HJ, Anten NPR (2012) Detect thy neighbor: identity recognition at the root level in plants. Plant Sci 195:157–167
Chen W, Koide RT, Adams TS, DeForest JL, Cheng L, Eissenstat DM (2016) Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc Natl Acad Sci USA 113:8741–8746
Cheplick GP (1992) Sibling competition in plants. J Ecol 80:567–575
Cipollini DF (2007) Consequences of the overproduction of methyl jasmonate on seed production, tolerance to defoliation and competitive effect and response of Arabidopsis thaliana. New Phytol 173:146–153
Cipollini DF (2010) Constitutive expression of methyl jasmonate-inducible responses delays reproduction and constrains fitness responses to nutrients in Arabidopsis thaliana. Evol Ecol 24:59–68
Cipollini DF, Bergelson J (2001) Plant density and nutrient availability constrain constitutive and wound-induced expression of trypsin inhibitors in Brassica napus. J Chem Ecol 27:593–610
Coleman JS, Mc Connaughay KDM, Ackerly DD (1994) Interpreting phenotypic variation in plants. Trends Ecol Evol 9:187–191
Crepy M, Casal JJ (2015) Photoreceptor-mediated kin recognition in plants. New Phytol 205:329–338
Crutsinger GM, Collins MD, Fordyce JA, Gompert Z, Nice CC, Sanders NJ (2006) Plant genotypic diversity predicts community structure and governs an ecosystem process. Science 313:966–968
Crutsinger GM, Roodriguez-Cabal MA, Roddy AB, Peay KG, Bastow JL, Kidder AG, Dawson TE, Fine PVA, Rudgers JA (2014) Genetic variation within a dominant shrub structures green and brown community assemblages. Ecology 95:387–398
De Deyn GB, Cornelissen JHC, Bardgett RD (2008) Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett 11:516–531
Des Roches S, Post DM, Turley NE, Bailey JK, Hendry AP, Kinnison MT, Palkovacs EP (2018) The ecological importance of intraspecific variation. Nat Ecol Evol 2:57–64
Development Core Team R (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Dudley SA (2015) Plant cooperation. AoB PLANTS 7:plv113
Dudley SA, File AL (2007) Kin recognition in an annual plant. Biol Lett 3:435–438
Dudley SA, Murphy GP, File AL (2013) Kin recognition and competition in plants. Funct Ecol 27:898–906
Dudt JF, Shure DJ (1994) The influence of light and nutrients on foliar phenolics and insect herbivory. Ecology 75:86–98
Feeny PP (1970) Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 51:565–581
File AL, Murphy GP, Dudley SA (2012) Fitness consequences of plants growing with siblings: reconciling kin selection, niche partitioning and competitive ability. Proc R Soc B 279:209–218
Fujii N, Tomaru N, Okuyama K, Koike T, Mikami T, Ueda K (2002) Chloroplast DNA phylogeography of Fagus crenata (Fagaceae). Plant Syst Evol 232:21–23
Garcia AL, Torrecillas AL, Leon A, Ruiz Sanchez MC (1987) Biochemical indicators of the water stress in corn seedlings. Biol Plant 29:45–48
Hagerman AN (1987) Radial diffusion method for determining tannin in plant extracts. J Chem Ecol 13:437–449
Hamilton WD (1964) The genetical evolution of social behavior II. J Theol Biol 7:1–52
Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM (2007) Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Evol Syst 8:157–178
Hashizume H, Sugawara M, Nagae Y, Higuchi M (1984) Production and dispersal of reproductive organs in seed stands of buna (Fagus crenata Blume) (I) production and dispersal of seeds. Bull Fac Agric Tottori Univ 36:35–42 (in Japanese with English summary)
Ichihara Y, Yamaji K (2009) Effect of light conditions on the resistance of current-year Fagus crenata seedlings against fungal pathogens causing damping-off in a natural beech forest: fungus isolation and histological and chemical resistance. J Chem Ecol 35:1077–1085
Inderjit, Del Moral R (1997) Is separating resource competition from allelopathy realistic? Bot Rev 63:221–230
Ishida AT, Nara K, Hogetsu T (2007) Host effects on ectomycorrhizal fungal communities: insight from eight host species in mixed conifer–broadleaf forests. New Phytol 174:430–440
Joergensen RG, Brookes PC (1990) Ninhydrin-reactive nitrogen measurements of microbial biomass in 0.5 M K2SO4 soil extracts. Soil Biol Biochem 22:1023–1027
Johnson MTJ, Lajeunesse MJ, Agrawal AA (2006) Additive and interactive effects of plant genotypic diversity on arthropod communities and plant fitness. Ecol Lett 9:24–34
Julkunen-Tiitto R (1985) Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem 33:213–217
Karban R, Myers JH (1989) Induced plant responses to herbivory. Annu Rev Ecol Syst 20:331–348
Karban R, Shiojiri K, Ishizaki S, Wetzel WC, Evans RY (2013) Kin recognition affects plant communication and defence. Proc R Soc B 280:20123062
Koricheva J, Hayes D (2018) The relative importance of plant intraspecific diversity in structuring arthropod communities: a meta-analysis. Funct Ecol 32:1704–1717
Kraft NJB, Godoy O, Levine JM (2015) Plant functional traits and the multidimensional nature of species coexistence. Proc Natl Acad Sci USA 112:797–802
Kurashige NS, Agrawal AA (2005) Phenotypic plasticity to light competition and herbivory in Chenopodium album (Chenopodiaceae). Am J Bot 92:21–26
Lamit LJ, Lau MK, Næsborg RR, Wojtowicz T, Gehring CA (2015) Genotype variation in bark texture drives lichen community assembly across multiple environments. Ecology 96:960–971
Lankau RA, Strauss SY (2007) Mutual feedbacks maintain both genetic and species diversity in a plant community. Science 317:1561–1563
Li J, Xu X, Liu Y (2018) Kin recognition in plants with distinct lifestyles: implications of biomass and nutrient niches. Plant Growth Regul 84:333–339
Maddox GD, Root RB (1990) Structure of the encounter between goldenrod (Solidago altissima) and its diverse insect fauna. Ecology 71:2115–2124
Masaki T, Osumi K, Takahashi K, Hoshizaki K, Matsune K, Suzuki W (2007) Effects of microenvironmental heterogeneity on the seed-to-seedling process and tree coexistence in a riparian forest. Ecol Res 22:724–734
Maschinski J, Whitham TG (1989) The continuum of plant responses to herbivory: the influence of plant association, nutrient availability and timing. Am Nat 134:1–19
Masclaux F, Hammond RL, Meunier J, Gouhier-Darimont C, Keller L, Reymond P (2010) Competitive ability not kinship affects growth of Arabidopsis thaliana accessions. New Phytol 185:322–331
Novoplansky A (2009) Picking battles wisely: plant behaviour under competition. Plant Cell Environ 31:726–741
Olson DM, Cortesero AM, Rains GC, Potter T, Lewis WJ (2009) Nitrogen and water affect direct and indirect plant systemic induced defense in cotton. Biol Control 49:239–244
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588
Rankin DJ, Bargum K, Kokko H (2007) The tragedy of the commons in evolutionary biology. Trends Ecol Evol 22:643–651
Siefert A, Violle C, Chalmandrier L, Albert CH, Taudiere A, Fajardo A, Aarssen LW, Baraloto C, Carlucci MB, Cianciaruso MV, Dantas VL, de Bello F, Duarte LDS, Fonseca CR, Freschet GT, Gaucherand S, Gross N, Hikosaka K, Jackson B, Jung V, Kamiyama C, Katabuchi M, Kembel SW, Kichenin K, Kraft NJ, Lagerstom A, Bagousse-Pinguet YL, Li Y, Mason N, Messier J, Nakashizuka T, Overton JM, Peltzer DA, Pérez-Ramos IM, Pillar VD, Prentice HC, Richardson S, Sasaki T, Schamp BS, Schöb C, Shipley B, Sundqvist M, Sykes MT, Vandewalle M, Wardle DA (2015) A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol Lett 18:1406–1419
Siemens DH, Lischke H, Maggiulli N, Schürch S, Roy BA (2003) Cost of resistance and tolerance under competition: the defense—stress benefit hypothesis. Evol Ecol 17:247–263
Songsri P, Jogloy S, Holbrook CC, Kesmala T, Vorasoot N, Akkasaeng C, Patanothai A (2009) Association of root, specific leaf area and SPAD chlorophyll meter reading to water use efficiency of peanut under different available soil water. Agric Water Manag 96:790–798
Strauss SY, Rudgers JA, Lau JA, Irwin RE (2002) Direct and ecological costs of resistance to herbivory. Trends Ecol Evol 17:278–285
Temperton VM, Mwangi PN, Scherer-Lorenzen M, Schmid B, Buchmann N (2007) Positive interactions between nitrogen-fixing legumes and four different neighbouring species in a biodiversity experiment. Oecologia 151:190–205
Tomita M, Seiwa K (2004) Influence of canopy tree phenology on understorey populations of Fagus crenata. J Veg Sci 15:379–388
van Oorschot M, van Gaalen N, Maltby E, Mockler N, Spink A, Verhoeven JTA (2000) Experimental manipulation of water levels in two French riverine grassland soils. Acta Oecol 21:49–62
Walters DR (2011) Plant defense: warding off attack by pathogens, herbivores and parasitic plants. Blackwell Publishing, Iowa
Weigelt A, Schumacher J, Roscher C, Schmid B (2008) Does biodiversity increase spatial stability in plant community biomass? Ecol Lett 11:338–347
Whitham TG, Bailey JK, Schweitzer JA, Shuster SM, Bangert RK, LeRoy CL, Lonsdorf EV, Allan GJ, DiFazio SP, Potts BM, Fischer DG, Gehring CA, Lindroth RL, Marks JC, Hart SC, Wimp GM, Wooley SC (2006) A framework for community and ecosystem genetics: from genes to ecosystems. Nat Rev Gene 7:510–523
Whitham TG, Gehring CA, Lamit LJ, Wojtowicz T, Evans LM, Keith AR, Smith DS (2012) Community specificity: life and afterlife effects of genes. Trends Plant Sci 17:271–281
Wright IJ, Cannon K (2001) Relationships between leaf lifespan and structural defences in a low-nutrient, sclerophyll flora. Funct Ecol 15:351–359
Yamawo A (2015) Relatedness of neighboring plants alters the expression of indirect defense traits in an extrafloral nectary-bearing plant. Evol Biol 42:12–19
Yamawo A, Hada Y, Suzuki N (2012) Variations in direct and indirect defences against herbivores on young plants of Mallotus japonicus in relation to soil moisture conditions. J Plant Res 125:71–76
Yamawo A, Tagawa J, Hada Y, Suzuki N (2014) Different combinations of multiple defence traits in an extrafloral nectary-bearing plant growing under various habitat conditions. J Ecol 102:238–247
Yamawo A, Sato M, Mukai H (2017) Experimental evidence for benefit of self discrimination in roots of a clonal plant. AoB Plants 9:plx049
Zhang D, Zhang C, Tang X, Li H, Zhang F, Rengel Z, Whalley WR, Davies WJ, Shen J (2015) Increased soil phosphorus availability induced by faba bean root exudation stimulates root growth and phosphorus uptake in neighbouring maize. New Phytol 209:823–831
Zhang L, Liu Q, Tian Y, Xu X, Ouyang H (2016) Kin selection or resource partitioning for growing with siblings, implications from measurements of nitrogen uptake. Plant Soil 398:79–86
Acknowledgements
Work was supported in part by JSPS Grant-in-Aid for Young Scientists (B) (Grant No. 15K18611, 18K19353 and 19H03295 to A.Y.). This study was partly supported by aid from the Nippon Life Foundation to A.Y. This work was supported by a fund from Nissei Foundation to A.Y.
Author information
Authors and Affiliations
Contributions
AY developed the core idea, designed the experiments. HT and AY carried out all experiments, and analysed the data. AY wrote the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takigahira, H., Yamawo, A. Competitive responses based on kin-discrimination underlie variations in leaf functional traits in Japanese beech (Fagus crenata) seedlings. Evol Ecol 33, 521–531 (2019). https://doi.org/10.1007/s10682-019-09990-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-019-09990-3