Abstract
Arbuscular mycorrhizal (AM) fungi (AMF) establish beneficial symbioses with the roots of the majority of land plants, including major food crops. The susceptibility of sunflower (Helianthus annuus) to AMF was studied in 26 genotypes—nine wild accessions, 11 cultivars and six inbred lines—by assessing mycorrhizal root colonization in individual plants, with the aim of gaining insights into the genetic control of this trait. The analysis of genetic diversity among sunflower wild accessions, cultivars, and inbred lines, performed by retrotransposon display (multilocus fingerprinting), showed large variability among the analysed genotypes, with wild accessions more variable than domesticated genotypes. Wild accessions were also more susceptible to mycorrhizal colonization than cultivars. Nevertheless, analyses of inbred lines revealed a low repeatability value of the mycorrhizal colonization trait, suggesting the absence of a clearcut genetic control; variability should therefore mostly reflect environmental effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizal (AM) fungi (AMF, Glomeromycota) establish beneficial symbioses with the roots of the majority of land plants, including the most important food crops, from cereals to legumes, vegetables and fruit trees (Smith and Read 2008). AMF are the essential elements of soil fertility, plant nutrition and productivity, facilitating soil mineral nutrient uptake by means of an extensive extra-radical network of fungal hyphae spreading from colonized roots into the soil (Avio et al. 2006). Several microcosm experiments showed that AMF may protect plants from biotic and abiotic stresses, such as fungal pathogens, drought and salinity (Augé 2001; Evelin et al. 2009; Sikes et al. 2009) and provide key agroecosystem services, including soil aggregation and carbon sequestration (Gianinazzi et al. 2010). Nevertheless, data on AMF relevance in extensive cropping systems are still inconsistent. For example, Ryan and Kirkegaard (2012) reported the absence of positive impacts of mycorrhizal colonization on crop growth and yield, while other authors found that AMF benefits may depend on early and extensive root colonization, especially in short season crops (Bittman et al. 2006; Njeru et al. 2014). Although the relationship between colonization rate and yield increase is still unresolved (Hetrick et al. 1996, Ryan and Kirkegaard 2012; Kirkegaard and Ryan 2014; Leiser et al. 2015), two meta-analyses showed a positive role of AM fungal colonization on plant growth (Lekberg and Koide 2005; Lehmann et al. 2012).
Plant breeding for improving mycorrhizal colonization depends on the availability of varieties with a range of genetic variation for this trait, which has been poorly investigated so far. Many authors reported a great variability in susceptibility to AMF—assessed by colonised root length measurements—among and within a few plant species, which may be ascribed to plant genotype, soil fertility, root weight and fibrousness, P use efficiency and symbiont identity (Koide and Schreiner 1992; Giovannetti and Gianinazzi-Pearson 1994; Smith et al. 2009).
Different breeding strategies may lead to different responses in mycorrhizal colonization in crop species and varieties (Toth et al. 1984, 1990; Parke and Kaeppler 2000). On the basis of a few investigated genotypes, some authors suggested that modern high-yielding varieties, selected for the optimal performance in high fertility soils, may have reduced their capacity to respond to AMF, compared with old ones (Hetrick et al. 1992; Zhu et al. 2001). Though, other data showed no loss of AM colonization ability in newer lines (An et al. 2010; Leiser et al. 2015). Overall, a meta-analysis of 410 trials found that in cultivars released after 1900 mycorrhizal colonization was 30 %, compared with 40 % in older cultivars and landraces (Lehmann et al. 2012).
Differences in AM fungal colonization were found among wheat genotypes differing in ploidy level, geographic origin, nutrient use efficiency and year of variety release (Yücel et al. 2009; Azcon and Ocampo 1981; Hetrick et al. 1992; Graham and Abbott 2000; Yao et al. 2001; Zhu et al. 2001). A recent study, reporting a significant variation in mycorrhizal colonization among a small number of modern cultivars of durum wheat, stressed the need to screen more genotypes to assess the genetic variability of this trait (Singh et al. 2012). Unfortunately, no clear-cut relationship between the ability of a plant species to be colonised by AMF and its genotype has been detected so far. Nevertheless, the evaluation of variation in symbiosis establishment among cultivars is important for the breeding of new genotypes, since the level of colonization may modulate the cost/benefit balance of AM symbiosis (Sawers et al. 2008).
Sunflower (Helianthus annuus L.) is an important industrial crop worldwide. Modern sunflower cultivars, collected primarily by native Americans, are most close to wild sunflower populations in the eastern regions of North America (Harter et al. 2004), although a more recent study has shown an earlier presence of domesticated sunflower in Mexico, suggesting that another domestication event occurred in this area (Lentz et al. 2008). Modern sunflower breeding began in Russia in the 19th century using relatively few American genotypes imported into Europe by early Spanish explorers (Putt 1978), as shown by pedigree analysis of XXth century cultivars (Cheres and Knapp 1998). Even in North America, the original area of sunflower domestication, modern breeding started using early Russian cultivars (Semelczi-Kovacs 1975). This may have determined uniformity, at least for some genes, even in cultivars of very different origin.
Sunflower mycorrhizal status has not been adequately investigated, as most experiments studied growth responses and P nutrition (Thompson 1987; Chandrashekara et al. 1995) and tolerance to heavy metals (Ultra et al. 2007; Ker and Charest 2010). To the best of our knowledge, no information is available on the variability of AM fungal root colonization among sunflower genotypes.
In the present work we investigated the mycorrhizal status of sunflower by screening a collection of genetically different genotypes. To this aim, we first assessed the genetic variability among sunflower wild accessions, cultivars, and inbred lines using a multilocus fingerprinting. The occurrence and level of mycorrhizal colonization of the screened genetically different sunflower genotypes was determined using the fast-colonising Funneliformis mosseae, a generalist and globally distributed AM fungal symbiont (Avio et al. 2009; Turrini and Giovannetti 2012). The experiments were carried out in two different experimental years/seasons. The data obtained allowed the identification of sunflower genotypes with different susceptibility to mycorrhizal colonization, which will be utilized for specific crossings in order to gain further insight into the genetic control of colonization level.
Materials and methods
Plant and fungal materials
The sunflower genotypes used in the reported experiments are listed in Table 1. Genotypes were chosen according to their origin: wild accessions were collected in different states in the USA, one per state; one cultivar was selected per country. Cultivars were randomly chosen from countries where sunflower is a major crop, and represent a reliable sample of genetic diversity in the domesticated materials of this species. Wild accessions and cultivars were obtained from USDA, ARS, National Genetic Resources Program, USA (ARS-GRIN); experimental inbreds from USDA and from the Department of Agriculture, Food, and Environment of University of Pisa, Italy (DAFE). Further data on analyzed wild and cultivated genotypes can be found at National Germplasm Resources Laboratory homepage (http://www.ars-grin.gov/npgs/searchgrin.html).
The AM fungal isolate used was Funneliformis mosseae (T. H. Nicolson & Gerd.) C. Walker & A. Schüßler comb. nov., isolate IMA1. Inoculum was obtained from pot-cultures maintained in the collection of DAFE Microbiology Laboratories. Such pots, containing a mixture (1:1 by volume) of soil and a calcinated clay (OILDRI Chicago, IL, USA), were inoculated with a crude inoculum containing mycorrhizal roots, spores and extra-radical mycelium, sown with Trifolium alexandrinum and Medicago sativa and maintained for 6 months. At harvest, the shoots were excised and discarded whilst the substrate and roots cut in ca. 1-cm fragments were mixed to form a homogenous crude inoculum mixture, to be used for sunflower inoculation.
Plant DNA isolation
The DNA was isolated with Nucleospin Plant Isolation kit (Macherey–Nagel) using C1 lysis buffer, which is based on the CTAB procedure. DNA was purified by RNaseA treatment. The genomic DNA was dissolved with 1× TE (1 mM EDTA, 10 mMTris–HCl, pH 8.0) solution at 55 °C. DNA was quantified using spectrophotometric analyses and DNA quality was assessed by visualization after gel electrophoresis. For fingerprinting, genomic DNA was isolated from leaflets of pools of five seedlings, an approach allowing evaluation of variability among wild accessions or open pollinated varieties independently from variation in single individuals.
Inter-retrotransposon amplified polymorphism (IRAP) analysis
H. annuus long-terminal-repeat (LTR) sequences used in these experiments are those identified by Vukich et al. (2009a, b) and confirmed by Buti et al. (2013). Primers were designed using OLIGO 4.0 software (Rychlik and Rhoads 1989) and were used in the combinations reported in Table 2 onto genomic DNAs from the 26 sunflower genotypes as templates. PCR reactions for IRAP analyses were performed as in Vukich et al. (2009a) in a 20 μl reaction mixture containing: 20 ng genomic DNA, 1x PCR buffer (80 mM Tris–HCl, 20 mM (NH4)2SO4, 0.02 % w/v Tween-20), 2 mM MgCl2, 200 nM each primer, 200 μM each dNTP, 1U Thermostable DNA polymerase, FIREPol (Solis BioDyne). After an initial denaturing step at 95 °C for 3 min, thermocycling was performed at 95 °C for 20 s, 55 °C for 60 s and 72 °C for 60 s, for 30 cycles, final extension at 72 °C for 5 min.
The PCR products were separated by electrophoresis at 60 V for 8 h in a 1.7 % agarose gel (RESolute Wide Range, BIOzym). Gels were stained with Gel RED (Biotium), scanned using a FLA-5100 imaging system (Fuji Photo Film GmbH., Germany) and photographed with a Canon PSA700. Each electrophoresis was repeated three times and fingerprints were scored to prepare binary matrices (Kalendar and Schulman 2006).
Polymorphisms were employed for analyses of genetic variability among wild accessions, cultivars, and inbreds. IRAP bands were interpreted as (1) for presence or (0) absence, assuming that each band represents a single locus. Non-reproducible bands were very rare and were excluded from the analyses along with weak bands. Three independent matrices (among wild accessions, among cultivars, among inbreds) were prepared. Jaccard’s (1908) genetic similarity index was used to calculate genetic dissimilarity, employing the software NTSYS (Rohlf 2000). Given two genotypes, A and B, M11 represents the total number of bands where they both have a value of 1, M01 represents the total number of bands whose values are 0 in A and 1 in B, M10 represents the total number of bands whose values are 1 in A and 0 in B. The Jaccard’s similarity index, JS, is given as:
The dissimilarity index, JD is calculated as:
The average JD was calculated keeping separate data obtained from each group of genotypes. One-way ANOVA, Tukey’s tests and correlation statistics were performed using GraphPad Prism software.
Sunflower seedling inoculation and growth
Sunflower seedlings were pre-germinated on moistened filter paper for 5 days, then transplanted into pots containing turf substrate (Hochmoor Hortus, TERFLOR Capriolo BS, Italy) mixed with AM fungal inoculum (15 % by volume). The turf was not sterilised as a preliminary experiment showed the absence of naturally occurring AMF. The plantlets were maintained in the greenhouse under natural daylight conditions (750 μm−2 s−1, maximal photon flux density), with air temperature maintained at 17–29 °C, and relative humidity from 55 to 90 % for 35 days in Experiment 1 (that included all genotypes listed in Table 1 and was established on October 2013) and 45 days in Experiment 2 [that included ten selected genotypes, i.e. the six inbreds, two highly divergent wild accessions (WA and MS) and two highly divergent cultivars (Karlik and Colliguay) and was established on April 2014]. At harvest, the root systems were removed from the pots, washed with tap water and stained for mycorrhizal colonization. Five replicate plants per genotype were used.
Mycorrhizal assessment
Mycorrhizal colonization was assessed by clearing roots with 10 % KOH in a 80 °C water bath for 15 min and staining with Trypan blue in lactic acid (0.05 %) after 10 min in 2 % aqueous HCl. Percentages of AM fungal root colonization were estimated under a dissecting microscope (Wild, Leica, Milano, Italy) at ×25 or ×40 magnification by the gridline intersect method (Giovannetti and Mosse 1980). Samples of colonised roots were mounted on slides and observed at magnification of ×125 and ×500 under a Polyvar light microscope for assessing the occurrence of arbuscules and intracellular structures (Reichert-Jung, Vienna, Austria).
Mycorrhizal colonization data were arcsine transformed before subjecting them to analysis of variance (ANOVA). Correlation analyses and ANOVA were performed using Graph-Pad software. The occurrence of significant differences among genotypes was established performing the Tukey test, separately for accessions, cultivars, and inbreds.
Results and discussion
Analysis of genetic diversity
H. annuus wild accessions, cultivars, and inbreds were analysed for genetic diversity using molecular markers based on retrotransposon display. This fingerprinting method was chosen because large eukaryotic genomes are filled with transposable elements, especially retrotransposons, which transpose by a “copy and paste” mechanism, i.e. by replicating themselves and inserting the replicate into a new locus in the genome, so producing genetic variability (Schulman et al. 2004). The ubiquity, abundance, dispersion, and dynamism of retrotransposons in plant genomes, including the sunflower genome (Natali et al. 2013), have made them excellent sources of molecular markers (Schulman et al. 2004; Kalendar and Schulman 2006). In particular, LTR-retrotransposons, i.e. elements flanked by long terminal repeat sequences, can be conveniently used to produce molecular fingerprinting by PCR, with primers designed onto LTRs. The IRAP protocol (see Kalendar and Schulman 2006) can detect genomic loci bounded by retrotransposon LTRs if elements lie close enough to be amplified by a thermostable polymerase. These multilocus markers have been shown to be suitable to evaluate genetic diversity in many crop species, including sunflowers (Vukich et al. 2009a). Primers designed on putative LTRs of the sunflower SURE retroelement (Vukich et al. 2009a) produced a large number of bands indicating the repetitiveness of the related retrotransposons and the large variability in their insertion sites. Nearly identical patterns were obtained in three independent experiments. However, the rare non-reproducible bands were excluded from subsequent analyses.
A total of 71 bands among nine H. annuus wild accessions were scored (Table 3), of which 69 were polymorphic. Among 11 cultivated genotypes of H. annuus, 21 out of 39 bands were polymorphic. The percentages of polymorphic bands were lower compared to wild accessions (Table 3). As expected, a lower number of IRAP bands were scored among inbreds, although all showing polymorphic patterns (Table 3).
The Jaccard’s Dissimilarity Indices between wild accessions, cultivars and inbreds are reported in Fig. 1. The average Jaccard’s Dissimilarity Index was calculated for all groups of genotypes (Table 3). In all groups, high mean values (i.e., higher than 0.5) were measured. Wild accessions showed the highest Jaccard’s Dissimilarity index, significantly higher than cultivars or inbreds.
Triangular matrices with Jaccard’s Dissimilarity Indices between the wild accessions, the cultivars, and the inbreds used in the experiments, calculated on data obtained IRAP fingerprints produced by three primer combinations. Genotypes codes are listed in Table 1
Overall, molecular analyses showed a large genetic variability among the selected genotypes. Genetic variability among groups (wild accessions, cultivars, and inbreds) relies on genetic differences within such groups. In fact, wild accessions represent populations of heterozygous individuals, cultivars are also (at least partially) heterozygous, although gene pools are obviously smaller than those of wild accessions. Finally, inbreds are homozygous and all individuals within a line are genetically identical. The larger variability among wild accessions than cultivars confirm previous results (Vukich et al. 2009a) and, at least in part, it is related to sunflower breeding history: modern sunflower cultivars largely derive from a relatively limited number of genotypes that were introduced from North America into Europe, where they were subjected to selection and breeding (Putt 1978).
Mycorrhizal colonization variability among sunflower genotypes
Overall, mycorrhizal colonization showed large and continuous variation among the different wild accessions, cultivars and inbreds of sunflower tested. Detailed observations on stained roots showed that mycorrhizal colonization was established after appressoria formation by the fungal symbiont, which produced many intercellular hyphae and developed dense patches of arbuscules in contiguous cortical root cells (Fig. 2). Intercellular and intracellular vesicles were also found. Such a colonization pattern, which was observed in all sunflower genotypes, is typical of the Arum-type, one of the two classes of arbuscular mycorrhizas described by Gallaud (1905), widely distributed among herbaceous plant species, including the family of Asteraceae and characterized by rapid spread of the fungus via the apoplastic space between cortical cells of the root parenchyma (Smith and Smith 1997).
Light micrographs showing Arum-type colonization pattern in cortex of sunflower (Helianthus annuus) roots by Funneliformis mosseae. Roots are stained with Trypan blue to reveal mycorrhizal structures. a Dense patches of arbuscules in contiguous cortical root cells of Washington wild accession, bar = 130 μm; b sparse root colonization of Karlik cultivar, with rare arbuscules and vesicles, bar = 130 μm; c dense colonization of Texas wild accession, showing intercellular hyphae running along the longitudinal root axis and forming many arbuscules and vesicles, bar = 90 μm; d detail of arbuscules formed within adjacent root cells, showing dichotomous branching of hyphae, bar = 25 μm
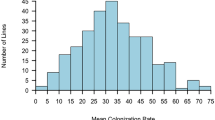
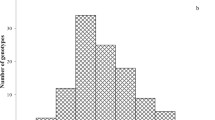
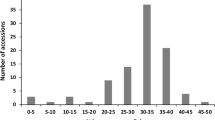
Although mycorrhizal colonization occurred in all analysed sunflower genotypes, the percent of individual root colonization varied among genotypes from 8.6 to 78.7 % in cultivars and from 24.5 to 91.4 % in wild accessions, in Experiment 1. Comparing all wild plants to domesticated ones, the mean percentage of root colonization was higher (p < 0.01) in wild accessions than in cultivars (Fig. 3). To gain a further insight into genetic variability in sunflower susceptibility to AMF, the analysis of mycorrhizal colonization was performed separately within each group of sunflower genotypes, as wild accessions, cultivars, and inbreds were genetically different with regard to heterozygosis and to the number of alleles in the population (Fig. 4). Our data do not allow the detection of significant differences among wild accessions, suggesting that mycorrhizal colonization among wild plants of different geographical origin is substantially uniform. Significant differences could be found between cultivars (the Chilean, Argentinian, and Indian cultivars vs. the Spanish variety) and between inbreds (EF2, 383, and GB vs. 821), probably as an effect of selection during the breeding.
Distribution of plants of wild accessions, cultivars, and inbreds, with regard to percentage of root mycorrhizal colonization. Genotypes codes are listed in Table 1. For each genotype, the mean (horizontal bar; ±SE) is reported. Keeping separate each group of genotypes, those indicated by different letters are significantly different (p < 0.05) according to Tukey’s test
These results support the hypothesis proposed for common wheat, that breeding programs could have produced varieties with a reduced mycorrhizal colonization compared to landraces. Hetrick et al. (1992, 1995, 1996) described genetic variation in the response to AMF among wheat cultivars developed at different times and proposed that development of new cultivars adapted to highly fertilized soil may have resulted in selection against genotypes that interact with, or respond to, AMF. Indeed, AMF may occasionally decrease plant growth when P availability is not limiting (Graham and Abbott 2000), since the cost of maintaining mycorrhizae exceeds the benefit to the host in such a case. Hence, it is presumable that selection under adequate fertilizer has selected for genotypes with lower root colonization levels.
The view that breeding programs on highly fertilized soils have lead to selection for reduced mycorrhizal performance has found some confirmation (Rao et al. 1990; Kaeppler et al. 2000; Tawaraya 2003; Zhu et al. 2001). However, analysis of colonization in eight wild accessions and two tomato cultivars proved that some modern varieties were more susceptible to AMF than wild accessions (Bryla and Koide 1990), while no differences were found between wild and cultivated oat (Koide et al. 1988). Evaluating numerous maize genotypes, An et al. (2010) demonstrated that AM fungal root colonization varies with germplasm type and origin (country and location), and concluded that modern plant breeding programs do not necessarily lead to the suppression of colonization.
In sunflower, as proposed for wheat (Sawers et al. 2008), the selection in highly fertilized soil could have produced cultivars that may not have all the alleles necessary to support mycorrhizal association. Alternatively, selection might have increased the inherent genetic ability of developed cultivars to uptake nutrients in the absence of AMF, leading to the development of genotypes less susceptible to the symbiosis.
A genetic control of mycorrhizal colonization in sunflower?
To establish the repeatability of mycorrhizal colonization levels in sunflower, Experiment 2 was carried out using ten genotypes, i.e., the six inbreds, the two highly divergent wild accessions (WA and MS) and two highly divergent cultivars (Karlik and Colliguay). Although, in general, the percentages of root colonization were lower in the second experiment than in the first, concerning wild and cultivated genotypes, the different colonization levels were confirmed only between the two wild accessions (data not shown). This result suggests that environmental factors may play a major role in determining the susceptibility to mycorrhizal root colonization, as the two experiments were performed in October and April. On the other hand, it is important to note that individuals within wild accessions and within cultivars in the two experiments could be somewhat genetically different.
Such genetic differences did not occur among individuals belonging to one and the same inbred line. Consequently, inbred genotypes are the most suitable for evaluating the genetic component of this character, if any. When replicating an experiment, within-individual differences arising from temporary circumstances are entirely environmental in origin, caused by environmental differences between the two experiments. The between-individual component of variance arises from permanent circumstances and is partly environmental and partly genetic. The ratio of the between-individual component to the total phenotypic variance is called intraclass correlation (r) and is known as the repeatability of the character (Falconer 1981). The intraclass correlation between the percentage of root colonization of the six inbred in the 2 years is reported in Fig. 5. The correlation is not significant (r = 0.35, p = 0.499). This value (35 %) expresses the proportion of the variance of single measurements that is due to both genetic and permanent environmental differences between individuals and sets an upper limit to the degree of genetic determination and to the heritability of this trait. Even if this result was obtained analysing only six genotypes, this value indicates that, in our pool of inbreds, genetic effects on mycorrhizal colonization account from 0 to 35 % of the phenotypic variability of the character. Obviously, the heritability could be much less than the repeatability and at least 65 % of phenotypic variance is to be attributed to environmental and to gene-environmental interaction, that is, to non-heritable effects.
Relative percentages of mycorrhizal root colonization in plants of six inbred lines, in experiments carried on in 2013 and 2014. The correlation coefficient and the slope (±SE) of the putative regression line are reported. The origin represents the mean of percentages of colonization in experiments of 2013 and 2014
A low heritability value (0.13) compatible to the repeatability value observed in our experiments was reported by Kaeppler et al. (2000) for this trait in their set of maize recombinant inbred lines, indicating that fungal colonization levels are generally prevalently affected by environmental factors. As already proposed for maize and sorghum (Kaeppler et al. 2000; Leiser et al. 2015), we are currently performing genetic analyses of mycorrhizal colonization in sunflower, using segregating populations to fully define the genetic control of this trait.
Conclusions
We estimated AM fungal colonization in a sunflower germplasm collection, which was shown to be genetically highly variable by molecular analyses. In this set of genotypes, our data indicated that mycorrhizal root colonization in sunflowers shows continuous variation, i.e., it is a metric character determined by many genes. In our pool of inbreds, the observed variability seems to include a large environmental component, while the genetic component, if any, is very small.
Our work showed a trend towards a reduced root colonization level in domesticated plants compared with wild individuals. It can be supposed that, during sunflower breeding, this character has not been selected, probably because selection has been performed in soils in which P provided by AMF was not limiting. It is also possible that mycorrhizal colonization level in sunflower was not subjected to selection because of its low heritability, causing the reduction of colonization in some cultivars and, consequently, reducing the possibility of exploiting putative beneficial plant/fungus interactions.
Further research is in progress to estimate the additive gene component of AM fungal susceptibility in sunflower, by studying segregating populations obtained by crossing lines with different root colonization levels.
References
An GH, Kobayashi S, Enoki H, Sonobe K, Muraki M, Karasawa T, Ezawa T (2010) How does arbuscular mycorrhizal colonization vary with host plant genotype? An example based on maize (Zea mays) germplasms. Plant Soil 327:441–453
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Avio L, Pellegrino E, Bonari E, Giovannetti M (2006) Functional diversity of arbuscular mycorrhizal fungal isolates in relation to extraradical mycelia networks. New Phytol 172:347–357
Avio L, Cristani C, Strani P, Giovannetti M (2009) Genetic and phenotypic diversity of geographically different isolates of Glomus mosseae. Can J Microbiol 55:242–253
Azcon R, Ocampo JA (1981) Factors affecting the vesicular-arbuscular infection and mycorrhizal dependency of thirteen wheat cultivars. New Phytol 87:677–685
Bittman S, Kowalenko CG, Hunt DE, Forge TA, Wu X (2006) Starter phosphorus and broadcast nutrients on corn with contrasting colonization by mycorrhizae. Agron J 98:394–401
Bryla DR, Koide RT (1990) Role of mycorrhizal infection in the growth and reproduction of wild vs. cultivated plants. II. Eight wild accessions and two cultivars of Lycopersicon esculentum Mill. Oecologia 84:82–92
Buti M, Giordani T, Vukich M, Pugliesi C, Natali L, Cavallini A (2013) Retrotransposon-related genetic distance and hybrid performance in sunflower (Helianthus annuus L.). Euphytica 192:289–303
Chandrashekara CP, Patil VC, Sreenivasa MN (1995) VA-mycorrhiza mediated P effect on growth and yield of sunflower (Helianthus annuus L.) at different P levels. Plant Soil 176:325–328
Cheres MT, Knapp SJ (1998) Ancestral origins and genetic diversity of cultivated sunflower: analysis of the pedigrees of public germplasm. Crop Sci 38:1476–1482
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280
Falconer DS (1981) Introduction to quantitative genetics, 2nd edn. Longman, New York
Gallaud I (1905) Études sur les mycorrhizes endotrophes. Rev Gén Bot 17:5–48, 66–83, 123–136, 223–239, 313–325, 425–433, 479–500
Gianinazzi S, Gollotte A, Binet MN, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530
Giovannetti M, Gianinazzi-Pearson V (1994) Biodiversity in arbuscular mycorrhizal fungi. Mycol Res 98:705–715
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Graham JH, Abbott LK (2000) Wheat responses to aggressive and non-aggressive arbuscular mycorrhizal fungi. Plant Soil 220:207–218
Harter AV, Gardner KA, Falush D, Lentz DL, Bye RA, Rieseberg LH (2004) Origin of extant domesticated sunflowers in eastern North America. Nature 430:201–205
Hetrick BAD, Wilson GWT, Cox TS (1992) Mycorrhizal dependence of modern wheat varieties, landraces, and ancestors. Can J Bot 70:2032–2040
Hetrick BAD, Wilson GWT, Gill BS, Cox TS (1995) Chromosomal location of mycorrhizal responsive genes in wheat. Can J Bot 73:891–897
Hetrick BAD, Wilson GWT, Cox TS (1996) Mycorrhizal response in wheat cultivars: relationship to phosphorus. Can J Bot 74:19–25
Jaccard P (1908) Nouvelles recherches sur la distribution florale. Bull Soc Vaud Sci Nat 44:223–270
Kaeppler SM, Parke JL, Mueller SM, Senior L, Stuber C, Tracy WF (2000) Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci 40:358–364
Kalendar R, Schulman AH (2006) IRAP and REMAP for retrotransposon-based genotyping and fingerprinting. Nat Protoc 1:2478–2484
Ker K, Charest C (2010) Nickel remediation by AM-colonized sunflower. Mycorrhiza 20:399–406
Kirkegaard JA, Ryan MH (2014) Magnitude and mechanisms of persistent crop sequence effects on wheat. Field Crops Res 164:154–165
Koide RT, Schreiner RP (1992) Regulation of the vesicular-arbuscular mycorrhizal symbiosis. Annu Rev Plant Physiol Plant Mol Biol 43:557–581
Koide R, Li M, Lewis J, Irby C (1988) Role of mycorrhizal infection in the growth and reproduction of wild vs. cultivated plants. I. Wild vs. cultivated oats. Oecologia 77:537–543
Lehmann A, Barto EK, Powell JR, Rillig MC (2012) Mycorrhizal responsiveness trends in annual crop plants and their wild relatives—a meta-analysis on studies from 1981 to 2010. Plant Soil 355:231–250
Leiser W, Olatoye M, Rattunde HF, Neumann G, Weltzien E, Haussmann BG (2015) No need to breed for enhanced colonization by arbuscular mycorrhizal fungi to improve low-P adaptation of West African sorghums. Plant Soil. doi:10.1007/s11104-015-2437-1
Lekberg Y, Koide RT (2005) Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol 168:189–204
Lentz DL, Pohl MD, Alvarado JL, Tarighat S, Bye R (2008) Sunflower (Helianthus annuus L.) as a pre-Columbian domesticate in Mexico. Proc Natl Acad Sci USA 105:6232–6237
Natali L, Cossu RM, Barghini E, Giordani T, Buti M, Mascagni F, Morgante M, Gill N, Kane NC, Rieseberg L, Cavallini A (2013) The repetitive component of the sunflower genome as shown by different procedures for assembling next generation sequencing reads. BMC Genom 14:686
Njeru ME, Avio L, Sbrana C, Turrini A, Bocci G, Barberi P, Giovannetti M (2014) First evidence for a major cover crop effect on arbuscular mycorrhizal fungi and organic maize growth. Agron Sustain Dev 34:841–848
Parke JL, Kaeppler SW (2000) Effects of genetic differences among crop species and cultivars upon the arbuscular mycorrhizal symbiosis. In: Kapulnik Y, Douds DD Jr (eds) Arbuscular mycorrhizas: physiology and function. Kluwer Academic Publishers, Dordrecht, pp 131–146
Putt ED (1978) History and present world status. In: Carter J (ed) Sunflower science and technology. Am Soc Agron, Madison, pp 1–30
Rao PSK, Tilak BR, Arunachalam V (1990) Genetic variation for VA mycorrhiza-dependent phosphate mobilization in groundnut (Arachis hypogaea L.). Plant Soil 122:137–142
Rohlf FJ (2000) NTSys-pc: numerical taxonomy and multivariate analysis system, version 2.1. Exeter Software, New York
Ryan MH, Kirkegaard JA (2012) The agronomic relevance of arbuscular mycorrhizas in the fertility of Australian extensive cropping systems. Agric Ecosyst Environ 163:37–53
Rychlik W, Rhoads RE (1989) A computer program for choosing optimal oligonucleotides for filter hybridization, sequencing and in vitro amplification of DNA. Nucleic Acids Res 17:8543–8551
Sawers RJH, Gutjahr C, Paszkowski U (2008) Cereal mycorrhiza: an ancient symbiosis in modern agriculture. Trends Plant Sci 13:93–97
Schulman AH, Flavell AJ, Ellis TH (2004) The application of LTR retrotransposons as molecular markers in plants. Methods Mol Biol 260:145–173
Semelczi-Kovacs A (1975) Acclimatization and dissemination of the sunflower in Europe. Acta Ethnogr Acad Sci Hung 24:47–88
Sikes BA, Kottenie K, Klironomos JN (2009) Plant and fungal identity determines pathogen protection of plant roots by arbuscular mycorrhizas. J Ecol 97:1274–1280
Singh AK, Hamel C, DePauw RM, Knox RE (2012) Genetic variability in arbuscular mycorrhizal fungi compatibility supports the selection of durum wheat genotypes for enhancing soil ecological services and cropping systems in Canada. Can J Microbiol 58:293–302
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, London
Smith FA, Smith SE (1997) Structural diversity in (vesicular)-arbuscular mycorrhizal symbioses. New Phytol 137:373–388
Smith FA, Grace EJ, Smith SE (2009) More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. New Phytol 182:347–358
Tawaraya K (2003) Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci Plant Nutr 49:655–668
Thompson JP (1987) Decline of vesicular-arbuscular mycorrhizas in long fallow disorder of field crops and its expression in phosphorus deficiency in sunflower. Aust J Agric Res 38:847–867
Toth R, Page T, Castleberry R (1984) Differences in mycorrhizal colonization of maize selections for high and low ear leaf phosphorus. Crop Sci 24:994–997
Toth R, Toth D, Starke D (1990) Vesicular-arbuscular mycorrhizal colonization in Zea mays affected by breeding for resistance to fungal pathogens. Can J Bot 68:1039–1044
Turrini A, Giovannetti M (2012) Arbuscular mycorrhizal fungi in national parks, nature reserves and protected areas worldwide: a strategic perspective for their in situ conservation. Mycorrhiza 22:81–97
Ultra VU Jr, Tanaka S, Sakurai K, Iwasaki K (2007) Arbuscular mycorrhizal fungus (Glomus aggregatum) influences biotransformation of arsenic in the rhizosphere of sunflower (Helianthus annuus L.). Soil Sci Plant Nutr 53:499–508
Vukich M, Schulman AH, Giordani T, Natali L, Kalendar R, Cavallini A (2009a) Genetic variability in sunflower (Helianthus annuus L.) and in the Helianthus genus as assessed by retrotransposon-based molecular markers. Theor Appl Genet 119:1027–1038
Vukich M, Giordani T, Natali L, Cavallini A (2009b) Copia and Gypsy retrotransposons activity in sunflower (Helianthus annuus L.). BMC Plant Biol 9:150
Yao Q, Li X, Christie P (2001) Factors affecting arbuscular mycorrhizal dependency of wheat genotypes with different phosphorus efficiencies. J Plant Nutr 24:1409–1419
Yücel C, Özkan H, Ortaş I, Yağbasanlar T (2009) Screening of wild emmer wheat accessions (Triticum turgidum subsp. dicoccoides) for mycorrhizal dependency. Turk J Agric For 33:513–523
Zhu YG, Smith SE, Barritt AR, Smith FA (2001) Phosphorus (P) efficiencies and mycorrhizal responsiveness of old and modern wheat cultivars. Plant Soil 237:249–255
Acknowledgments
This work was funded by the University of Pisa (Fondi di Ateneo) and by C.N.R.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Turrini and T. Giordani contributed equally to this work.
Rights and permissions
About this article
Cite this article
Turrini, A., Giordani, T., Avio, L. et al. Large variation in mycorrhizal colonization among wild accessions, cultivars, and inbreds of sunflower (Helianthus annuus L.). Euphytica 207, 331–342 (2016). https://doi.org/10.1007/s10681-015-1546-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1546-5