Abstract
Heavy metal concentrations in black mussels (Mytilus galloprovincialis) collected from Cape Town Harbour were determined using energy dispersive X-ray fluorescence (EDXRF) and inductively coupled plasma-mass spectrometry (ICP-MS). EDXRF showed that tissue portions of the mussels contained K, Ca, Fe, Cu, Zn, Si, Sr, Al and Au, while the shell portion contained K, Ca, Fe, Cr, Zn, Si and Sr. In addition to these metals, EDXRF also revealed the presence of Al in the shells of the largest mussels. Highest concentrations of Cu and Zn were recorded in the tissues of the smallest mussels. Due to poorer detection limits of EDXRF, ultra-trace elements (Mn, Pb, As, Hg, V, Cr, Sn, Cd, Ni and Co) were determined in mussels using ICP-MS. The average metal concentrations found in the mussels are as follows; Pb (7.30 ± 0.67), Cd (1.98 ± 0.13), Hg (4.92 ± 0.60), As (6.94 ± 0.04), Sn (2.63 ± 0.13), Ni (1.88 ± 0.05), Cr (3.54 ± 0.05), V (4.17 ± 0.23), Co (0.74 ± 0.01) and Mn (35.20 ± 1.46). ANOVAs, Pearson correlation and principal component analysis (PCA) were employed in data analysis. The order of the abundance of metals in the mussels is Mn > Pb > As > Hg > V > Cr > Sn > Cd > Ni > Co. The average metal concentrations found in the mussels were higher than the permissible Food and Agriculture Organization (FAO) limits and other international guidelines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Sea bottom sediments are known to serve as a sink for heavy metals introduced to the ocean by river run-off, waste water discharge, and dry and wet atmospheric depositions. Therefore, benthic organisms are expected to be exposed to increased heavy metal contents (Forstner and Wittman 1981; Fergusson 1990; Fatoki and Mathabatha 2001). Mussels are used widely as bioindicators of heavy metal pollution in coastal areas because as filter-feeders they can accumulate different elements (Regoli 1998). Among other features for being suitable as bioindicators are suitable dimensions, easy identification and collection of organisms, accumulation of the elements to a degree suitable for measuring, and abundance in the ecosystem (Wagner and Boman 2004).
A wide variety of contaminants are well known to accumulate in mussel tissues. Thus, they are used widely to monitor metal pollution in the sea. Size variation and heavy metal contents in the shell have sometimes been shown to be important variables (Cevik and Damla 2008; Saavedra et al. 2004). Several species, including mussels, have been used as indicators of life exposure, based on the assumption that the metal content in soft tissue of mussels is related to metal concentrations in the corresponding environment (Pempkowiak et al. 1999; Bryan and Langston 1992; Cossa 1989; Abisil et al. 1996; Hummel et al. 1997).
Moreover, results of previous studies have shown metal bioavailability in marine sediments of Cape Town Harbour. The enrichment factors of Sn, Pb, Zn, Fe, Cd, Al and Hg revealed anthropogenic inputs of these metals into the marine environment. The enrichment factor of Cu was less than 1, and this suggests that its presence was largely due to natural causes. The results are indications of the contributions of heavy metals contained in the runoffs from domestic and urban drains, as well as the inflow of storm water. Ship repair activities appeared to constitute a major factor responsible for the higher metal contamination in the dockyards. In addition, industrial wastes and mining of metals also create a potential source of heavy metal pollution in the aquatic environment (Turkmen and Ciminli 2007; Cumgum and Ünlü 1994; Lee and Stuebing 1990).
Heavy metal discharges to the marine environment are of great concern worldwide, as they have a major ecological significance due to their toxicity and accumulative behaviour. Metals such as iron, copper, zinc and manganese are essential, since they play an important role in biological systems, whereas mercury, lead, and cadmium are non-essential elements, as they are toxic, even in trace amounts (Matta et al. 1999). In addition, the essential metals may also have toxic effects when they are ingested to excess. Therefore, the presence of high concentrations of heavy metals in the environment presents a potential danger to human health due to their extreme toxicity. Metal body loads of aquatic biota are often measured and used to evaluate ecological risks and potential sublethal effects (Rainbow 1995; De Astudillo et al. 2005). Thus, people consuming seafood would be exposed to these metals with a potential danger to their health. For this reason, accurate monitoring of metal concentrations in marine mussels plays an important role. The trace metal and heavy metal content in the mussels can serve as an indicator of the effects of the mixtures of potential pollutants in the marine environment. However, no studies have been carried out in the study area on the heavy metal content in the mussels. It is therefore imperative to carry out research on the pollutant concentrations in the tissues as well as the shells of the mussels in order to measure the level of bioaccumulation of these contaminants. The objective of this study is to determine the effects of heavy metal bioaccumulation by measuring the concentration of metals in black mussels (Mytilus galloprovincialis) from Cape Town Harbour and to compare the results of metal accumulation in shells, tissues and various sizes of mussels as well as in the water and sediment samples collected from this site.
2 Materials and methods
2.1 The study area
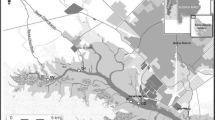
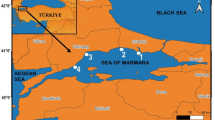
Cape Town Harbour was used as the study area. This harbour is one of the busiest ports in South Africa. It handles the largest amount of fresh fruit for export and has a major repair and maintenance facility which is used by several large fishing boats and the West African oil industry. The study area is shown in Fig. 1a while Fig. 1b represents the sampling points.
The harbour is located at 33°54′S, 18°26′E. The port has evolved greatly over the centuries and currently consists of several main components. The Ben Schoeman Dock is the largest outer dock of the port, where the container terminal is situated. The sediment samples collected at this site were very muddy. The Duncan Dock is the smallest and the older inner dock, containing the multipurpose and fruit terminals as well as the dry dock, repair quay and tanker basin. Both water and sediment samples at this site were very muddy and oily. The synchrolift dry dock is where the ships are lifted up for repair.
2.2 Reagents
MilliQ water 18.2 MΩ cm (Millipore, Bedford, MA, USA) deionising system was used for the preparation of solutions throughout the study. All reagents used were of analytical grade and were supplied by Merck and Sigma of South Africa. The polypropylene (PP), high density polyethylene (HPDE) bottles were prewashed with laboratory grade detergent followed by adequate rinsing with deionised water, and soaking in 0.1 M HNO3 (overnight) followed by thorough rinsing with deionised water. BCR-277R reference standard material (for trace elements in sediment) was purchased from the European Community Bureau of reference, IRMM, Belgium.
2.3 Sampling
Water, sediment and mussel samples were collected in August 2011 from Cape Town Harbour inside the harbour area at 33°54.367′, 18°25.370′E. The sampling site is called the Robinson dry dock. Water and sediment samples were collected in triplicate with the aid of a sampling boat, a Waveride DTC 787C (6.3-m cat hull) supplied by Stingray Marine, powered by Suzuki 90 hp 4-stroke engines and equipped with a Van Veen Grab sampler. Garmin GPS was used to obtain the sampling coordinates. The sediment samples were placed in plastic polyzip block bags in an ice chest and transported to the laboratory.
Water samples were collected in 1-L plastic containers which were initially washed with detergent and rinsed with distilled water. The containers were finally soaked in 10% nitric acid. The containers were then rinsed at least three times with MilliQ water. At the sampling sites, containers were rinsed three times with the water samples before being filled with the samples. The samples were preserved by adding concentrated HNO3 to each sample bottle and the pH adjusted to 2.0 by use of pH meter. The samples were stored in a refrigerator at about 4°C, before subsequent analysis. As samples may contain particulate or organic materials, pre-treatment in the form of digestion is required before analysis.
Different sizes of mussels (Mytilus galloprovincialis) were collected at polluted sites in Cape Town Harbour with the help of a sampling team from the Council for Scientific and Industrial Research (CSIR), University of Stellenbosch, South Africa.
2.4 Sample treatment
In the laboratory, sediment samples were spread on aluminium foils and air-dried at room temperature for a week. The dried sediment were ground using mortar and pestle, screened and sieved with a laboratory test sieve of size 500 μm, homogenised and finally stored at 4°C in a refrigerator prior to microwave acid digestion.
For water samples, 100-mL aliquots of the well-mixed sample were measured into a beaker. Then, 2 mL of concentrated HNO3 and 5 mL of concentrated HCl were added. The sample was covered with a ribbed watch glass or other suitable covers and heated on a steam bath, hot plate or other heating source at 90–95°C until the volume was reduced to 15–20 mL.
2.5 Determination of mercury in water samples
An amount of 1 ml of concentrated H2SO4 followed by 1 ml of 5% KMnO4 solution were added to 40 ml of the water sample. The mixture was boiled for about 1 min, cooled in an ice chest, transferred into a 50-ml standard flask and diluted to a volume of 50 ml. A set of blank samples were prepared in the same manner.
The mussel specimens were sorted with respect to their sizes as follows: Group A: >5 to >7 cm (large size); Group B: >5 to <7 cm (medium size); Group C: <5 cm (small size); Group D: whole mussel samples. Around 20 mussels were selected from each group. After the samples were sorted, they were dried in an oven at 105°C overnight before their soft tissues and shells were separated. Dried shells and tissues were ground into fine powders for 20 min using a Spex mill. Each powder was then sieved through a 500-µm mesh sieve. Forty milligrams of this powder were pressed into 13 mm diameter. Total digestion of mussel samples was performed with CEM MARS, model 240/50 microwave digestion system configured with MDR-1000/6 carousel TFM vessels. Triplicate samples of 0.5 g of each fine powdered sample were weighed into Teflon digestion vessels and 10 ml of 65% HNO3 was added before the samples were digested in the CEM MARS microwave oven. The temperature of each sample in the microwave oven was ramped to 200°C at a pressure of 800 psi for 25 min and kept at this temperature for a further 15 min. Each residue was diluted to 25.0 mL with deionised water (Bulut 2007; Pempkowiak et al. 1999). A triplicate of the blank solution was also subjected to microwave digestion.
2.6 Instrumentation
2.6.1 Analysis of non-toxic elements
Non-toxic elements (K, Ca, Fe, Cu, Zn, Si, Sr, Al) were analyzed using energy dispersive X-ray fluorescence technique (EDXRF) by employing the standard addition method in sample preparation. The mussel samples were stimulated by 55Fe and 241Am radioactive sources. To detect the radiation scattered from the sample, a Geometrically Optimized Large Area Drift Detector (GOLD) proprietary detector with 180,000 throughput cps having a resolution of <185 eV and 4 μs shaping time was used. A total of 4,096 channels of a multichannel analyzer (MCA) were employed for the data acquisition. In quantitative analysis, characteristic X-rays emitted by excited atoms of the sample were registered for 5,000 s (Cevik and Damla 2008).
2.6.2 Analysis of toxic elements
Due to the poorer detection limit of EDXRF, mussel samples were analyzed for toxic elements (Mn, Pb, As, Hg, V, Cr, Sn, Cd, Ni and Co) by inductively coupled plasma-mass spectrometry (ICP-MS) with an Agilent 7700 ICP-MS. The Agilent 7700 instrument was used with a Meinhart nebuliser and silica cyclonic with continuous nebulisation. The operating parameters were: plasma RF power: 1,550 W; sample depth: 8.0 mm; Carrier gas: 1.08 L/min; nebuliser pump: 0.10 rps; helium gas: 5.3 mL/min. ICP-MS and a Varian Liberty II ICP-AES were also used for determination of toxic elements in the water and sediment samples, respectively.
2.7 Quality assurance
All analyses were carried out in triplicate. The blank samples were analysed after every 10 measurements. Toxic and non-toxic elements were analysed by a Varian ICP-AES and Agilent 7700 ICP-MS, respectively. For quantification of the element of interest, the instruments were calibrated daily using NIST traceable standards. A quality control standard was analysed prior to the samples to verify the accuracy of the calibration standards, while control standards were used throughout the analysis to monitor accuracy and instrument drift. On the ICP-MS, internal standards were introduced continuously with the samples and standards to correct for drift due to high matrix load. Data acquisition and processing was software controlled and exported in Excel format. In order to check for the accuracy of the extraction procedure, reference sediment materials BCR-277R (for trace elements) were extracted using the above procedure and analysed in triplicate. These reference materials were purchased from the European Community Bureau of reference, IRMM, Belgium.
2.8 Recovery efficiency using the reference sediment materials
The recovery values obtained for Al, Si, Sn, Pb, Zn, Fe, Hg, Cd and Cu ranged between 92.05–101.01% for ICP-MS and 100.52–108.25% for ICP-AES with % deviation less than 10% in all cases. For the EDXRF, the recovery values ranged between 95 and 110%.
2.9 Statistical analyses
The results were statistically analysed using Statistical Analysis Software (SAS) 9 software (Cary, NC, USA). Pearson’s correlation was applied to evaluate the relationships between the variables and correlation coefficient with P ≤ 0.05 was regarded as significant and principal component analysis (PCA) was used to analyse the analytical data.
3 Results and discussion
The concentrations of elements in mg/kg in different sizes of M. galloprovincialis tissues and shells are shown in Table 1, while the concentrations recorded in group D (whole mussels) are shown in Table 2. EDXRF analysis showed the presence of K, Ca, Fe, Cu, Zn, Si, Sr, Al and Au in the tissue of the mussels while K, Ca, Fe, Cu, Zn, Si and Sr were found in all sizes of mussel shell. Al was additionally detected in the biggest size. Cu and Zn exhibited the highest concentration in tissue of small-sized mussels. While lowest concentrations were measured for Cu and Zn in the shell as shown in Fig. 3. This is an indication that Cu and Zn bioaccumulated more in the tissue than in the shell. High concentration levels for copper may come from agricultural use as many vineyards and orchards are situated along the harbour.
Standard two-dimension PCAs for seven non-toxic elements (K, Ca, Sr, Si, Fe, Cu and Zn) measured in M. galloprovincialis collected from Cape Town Harbour, South Africa (Figs. 2 and 3), were plotted on the axes. The distribution of K, Zn, Fe, and Cu components were highly concentrated in the soft tissue of the mussels while Ca, Al and Si were principally accumulated in the shell. PCA was also used to show the distribution of all the toxic and non-toxic elements analysed in the different sizes (Figs. 2, 3 and 4). Size has sometimes been shown to be an important variable. In this study, effects of size on metal contents in the mussels were investigated. Significant variation was obtained at 0.05 levels, the results confirming that both the non-toxic and toxic elements varies among the various sizes anjd bioaccumulates more in the largest size. The order of metal accumulation amongst the various sizes of mussels follows the order: C (large size) > B (medium size) > A (small size) as shown in Table 1. The results obtained with the PCA are in agreement with Pearson correlation analysis.
Percentage distributions of non-toxic elements were represented in Fig. 5 and of toxic elements in Fig. 6. In the shell, Ca recorded the highest accumulation (49%) while Si and Al had 16 and 5%, respectively. These results confirmed that Ca generally bioaccumulates more in the shell than in the tissue. The order of non-toxic element accumulation in the shell is Ca > Si > Al, while in the tissue, K recorded the highest percentage of 23%, while Fe, Cu and Zn recorded 4, 2 and 1%, respectively. High concentrations recorded for Fe in the mussels indicates a high impact of terrigenic particles, which are general rich in this metal. Similar results were found by Ugur et al. (2002) for M. galloprovincialis collected from the Aegean coast of Turkey. High concentration levels can be attributed to the fact that Fe occurs naturally in the environment and may come from different backgrounds. These results agreed with results obtained with the PCA. The PCA further allowed the classification of the metals into two main groups: Sn–Cd–As–Hg–Pb, and Mn–V–Cr–Co–Ni–Pb. While Sn, Cd, As, Hg and Pb appeared to bioaccumulate more in the tissue, V, Cr, Mn, Co, Ni and Pb tended to bioaccumulate significantly in the shell. This is in agreement with the results of Saavedra et al. (2004). From the data obtained using ICP-MS, the average metal concentrations found in the mussels are as follows: Pb (7.30 ± 0.67), Cd (1.98 ± 0.13), Hg (4.92 ± 0.60), As (6.94 ± 0.04), Sn (2.63 ± 0.13), Ni (1.88 ± 0.05), Cr (3.54 ± 0.05), V (4.17 ± 0.23), Co (0.74 ± 0.01) and Mn (35.20 ± 1.46) ppm. The order of the metal concentrations is Mn > Pb > As > Hg > V > Cr > Sn > Cd > Ni > Co. In comparison to the heavy metal concentrations in the mussels from the Black Sea, the concentrations measured in the mussels from Cape Town Harbour were higher. Similar results were, however, found for Mn and Cd. Moreover, a significant correlation was observed between Zn and Cu, and this finding is in agreement with Cevik and Damla (2008). Metal accumulation in the organs, the gills and the visceral mass of M. galloprovincialis are the most interesting from the ecotoxicological point of view. The distribution of metals among the various organs seems to depend on the species and on the metals. Serra et al. (1999) observed that the gills were the prefential organ for cadmium uptake in M. galloprovincialis. In this study, Cu and Cd concentration levels are higher in mussel tissues than in their shells and these results are in agreement with those obtained by Stanciu et al. (2004). The elevated concentrations for Cu, Cd and Zn may be attributed to traffic of tankers and large freighters, both kinds of boats which may bring metallic pollutants to the environment and become bioavailable to the mussels. These results are in agreement with Romeo et al. (2005) (Fig. 6).
Pearson correlation coefficients for metals in M. galloprovincialis collected from Cape Town Harbour are presented in Tables 3 and 4. Strong correlation (r > 0.8) was calculated between (Cu and K) and (Cu and Zn). High correlations (r > 0.9) were also calculated between (Zn and K), (Fe and K), (Ca and Sr), (Ca and Al) and (Zn and Al) which indicate strong associations between these metals. Al, Sr, K, Cu, Ca, Fe and Zn would most likely be of natural origin. High correlations (r > 0.9) were also calculated between (V and Cr, Mn, Ni, As, Pb), (Cr and Mn, Co, Ni, Pb), (Mn and Co, Ni, Pb), (Co and Ni, Pb), and (Pb and Ni, As) and also strong correlation coefficients (r > 0.8) were calculated between (As and V, Cr, Mn, Co, Ni) depicting a strong association, and hence would most probably be of anthropogenic origin. These findings are in agreement with the results obtained from the PCA. These results suggest that the correlated metals share a common accumulation process in tissues and shell of mussels. Tables 5 and 6 show the metal concentrations in the water and sediment samples from the same locations where mussels were collected. It is apparent from the tables that the detected Sn concentration in sediment samples is quite high.
The average metal concentrations levels in the mussels were compared with the national and international standards for metals in molluscs compiled by United Nations Environment Programme, International Atomic Energy Agency, Turkish Food Codex, European Commission and Food and Agriculture Organisation as shown in Table 7. The mean concentrations obtained for the metals studied were higher than those recorded in the literature. Cu, Fe and Zn concentrations found in the mussels samples are far higher than the permissible limits allowed in national and international guidelines.
Important heavy metal contamination of Cape Town Harbour could be from industrial and domestic sources, as well as from non-point inflows from the storm water/stream discharging from urban/industrial areas in and around the harbour. Nevertheless, it is necessary to step-up the regular monitoring and adequate control measures in order to ensure compliance with national and international regulations on the protection of the marine water system.
4 Conclusion
This study has further supported the importance of mussels as useful organisms for measuring the pollution levels of aquatic environment. Among other features for being suitable as bioindicators are suitable dimensions, easy identification and collection of organisms, accumulation of the elements to a degree suitable for measuring, abundance in the ecosystem. Thus, biological features of mussels may give vital information about water pollution. Several species, including mussels, have been used as indicators of life exposure, based on the assumption that metal contents in soft tissues of mussels are related to metal concentrations in the corresponding environment (Pempkowiak et al. 1999; Bryan and Langston 1992; Cossa 1989; Abisil et al. 1996; Hummel et al. 1997). The soft tissues accumulate metals more efficiently than the shells, although shells may also give information about pollution levels in the environment. In this study, Sn, Cd, As, Hg and Pb bioaccumulated more in tissues while V, Cr, Mn, Co, Ni, Pb bioaccumulated majorly in the shell depicting strong associations and hence would most probably be of anthropogenic origin. These findings are in agreement with the results obtained from the PCA. This study further revealed that the average metal concentrations levels in the mussel (Mytilus galloprovincialis) were higher than the national and international standards for metals in molluscs compiled by UNEP, IAEA, TFC, EC Directives and the FAO. It is therefore important that Cape Town Harbour requires regular monitoring in otherrder to meet national and international guidelines on the protection of the marine environment.
References
Abisil M, Berntssen M, Gerringa L (1996) The influence of sediment, food and organic ligands on the uptake of copper by sediment-dwelling bivalves. Aquat Toxicol 34:13–29
Bryan GW, Langston WJ (1992) Bioavailability, accumulation and effects of heavy metals is sediments with special reference to United Kingdom estuaries, a review. Environ Pollut 76:89–102
Bulut VN, Gundogdu A (2007) A multi-element solid-phase extraction method for trace metals determination in environmental samples on Amberlite XAD-2000. J Hazard Mater 146:155–163
Cevik U, Damla N (2008) Assessment of metal element concentrations in mussel (M. Galloprovincialis) in Eastern Black Sea, Turkey. J Hazard Mater 160:396–401
Cossa D (1989) A review of the use of Mytilus spp as quantitative indicator of cadmium and mercury contamination in coastal waters. Oceanol Acta 12:417–432
Cumgum B, Ünlü E (1994) Heavy metal pollution in water, sediment and fish from the Tigris River in Turkey. Chemosphere 29:111–116
De Astudillo LR, Yen IC, Bekele I (2005) Heavy metals in sediments, mussels and oysters from Trinidad and Venezuela. Rev Biol Trop 53:41–53
EC (European Commission) (2005) Commission Regulation (EC) No. 78/2005 of 19 January 2005 amending Regulation (EC) No. 466/2001 as regards heavy metals. L16:43–45
FAO (Food and Agriculture Organisation) (1983) Compilation of legal limits for hazardous substances in fish and fishery products’. FAO fishery circular (464):5–100
Fatoki O, Mathabatha S (2001) An assessment of heavy metal pollution in the East London and Port Elizabeth harbours. Water SA 27:233–240
Fergusson J (1990) The heavy elements: chemistry, environmental impact and health effects. Pergamon, Oxford, p 614
Forstner U, Wittman G (1981) Metal pollution in the aquatic environment. Springer, Berlin
Hummel H, Moderman R, Triquet CA, Rainglet F, Duijn Y, Herssevoost M et al (1997) A comparative study on the relation between copper and condition in marine bivalves and the relation with copper in the sediment. Aquat Toxicol 38:165–181
Lee Y, Stuebing R (1990) Heavy metal contamination in the River Toad, Bufo jux-tasper (inger), near a copper mine in East Malaysia. Bull Environ Contam Toxicol 45:272–279
Matta J, Milad M, Manger R, Tosteson t (1999) Heavy metals, lipid prexidation, and cigateratoxicity in liver of the Caribben barracuda (Sphyraena). Biol Trace Elem Res 70:69–79
Pempkowiak J, Sikora A, Biernacka A (1999) Speciation of heavy metals in marine sediments vs their bioaccumulation by mussels. Chemosphere 39:313–321
Rainbow P (1995) Biomonitoring of heavy metal availability in the marine environment. Mar Pollut Bull 31:183–192
Regoli F (1998) Metals and antioxidant enzymes in gills and digestive gland of the Mediterranean mussel M. Galloprovincialis. Arch Environ Contam Toxicol 34:48–63
Romeo M, Frasila C, Gnassia-Barelli M, Damiens G, Micu D, Mustata G (2005) Biomonitoring of trace metals in the Black Sea (Romania) using mussels Mytillus galloprovincialis. Water Res 39:596–604
Saavedra Y, Gonzalez A, Fernandez P, Blanco J (2004) The effect of size on trace metal levels in raft cultivated mussels (M. galloprovincialis). Sci Total Environ 384:115–124
Serra R, Isani G, Tratamontano G, Carpene E (1999) Seasonal dependence of cadmium accumulation and Cd-binding protein in Mytillus galloprovincialis exposed to cadmium. Comp Biochem Physiol 123C:165–174
Stanciu et al (2004) Analysis of heavy metals and organic pollutants from black sea mussels. Turkey Proc 221:1–3
TFC (Turkish Food Codex) (2002) Official Gazette (24885)
Turkmen M, Ciminli C (2007) Determination of metals in fish and mussel species by inductively coupled plasma-atomic emission spectrometry 103:670–675
Ugur A, Yener G, Basasari A (2002) Trace metals and Po, Pb concentrations in mussels (Mytillus galloprovincialis) consumed at western Anatolia. Appl Radiat Isot 257:565–571
UNEP (United Nations Environment Programme) (1985) Determination of total Hg in marine sediments and suspended solids by cold vapour AAS. Reference Methods for Marine Pollution Studies 26
Wagner A, Boman J (2004) Biomonitoring of trace elements in Vietnamese freshwater fish. Spectrochim Acta B 59:1125–1132
Wyse EJ (2003) Report on the world-wide intercomparison exercise for the determination of trace elements and methylmercury in fish homogenate IAEA-407, IAEA/AL/144 (IAEA/MEL/72). International Atomic Energy Agency, Monaco
Acknowledgments
The authors wish to thank the management of Cape Peninsula University of Technology, Cape Town, South Africa, for financial support. H.K. Okoro also acknowledges University of Ilorin, Ilorin, Nigeria, for a supplementation staff development award for his doctoral studies. Acknowledgment also goes to M. Spicer at the XRF laboratory in the University of Stellenbosch for her contribution during XRF analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fatoki, O.S., Okoro, H.K., Adekola, F.A. et al. Bioaccumulation of metals in black mussels (Mytilus galloprovincialis) in Cape Town Harbour, South Africa. Environmentalist 32, 48–57 (2012). https://doi.org/10.1007/s10669-011-9370-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10669-011-9370-5