Abstract
The aim of this study was to investigate the accumulation of elements (Se, As, Hg, Cr, Cd, Pb, Cu, Zn, Ni, Mn, Al, Fe, Co and V) in some marine organisms [Eriphia verrucosa (Forskål, 1775), Solea solea (Linnaeus, 1758), Mytilus galloprovincialis Lamarck, 1819, and Ulva lactuca Linnaeus, 1753] collected from the northern Sea of Marmara (Büyükçekmece, Silivri, Tekirdağ, and Şarköy) in 2019. For this purpose, element accumulation was measured using ICP-MS (Inductively Couple Plasma-Mass Spectrometer). The lowest and highest element concentrations in marine organisms are Fe (0.00 –7499.78 µg g−1 dw); Pb (0.00–31.12 µg g−1 dw); Cd (0.00–9.93 µg g−1 dw); Cu (2.98–775.63 µg g−1 dw); Cr (0.24–26.19 µg g−1 dw); Mn (1.53–427.60 µg g−1 dw); Al (0.00– 13,788.4 µg g−1 dw); Ni (0.00–56.26 µg g−1 dw); Hg (0.03–3.37 µg g−1 dw); Zn (0.00–1554.23 µg g−1 dw); As (0.00– 1.84 µg g−1 dw); Se (0.00–0.04 µg g−1 dw); V (0.13–72.54 µg g−1 dw); and Co (0.00–10.98 µg g−1 dw), respectively. According to these element values, Pb, Cd and Hg values were found to be higher in mussels than in crabs and exceeded the limits of the Turkish Food Codex. This study shows that more caution should be exercised when consuming marine organisms that exceed the limits of the Turkish Food Codex.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution and its damages affect the marine ecosystem and the organisms living there. Marine ecosystems are constantly exposed to pollution due to increasing population and industrialization. If precautions are not taken, especially with regard to domestic and industrial waste, this may cause serious problems for marine ecosystems.
Heavy metals, among chemical substances, play a significant role in causing pollution in aquatic environments (Bettini et al. 2006). Metals are important pollutants that reach coastal areas through various pathways and contribute to environmental degradation. In addition, metals also enter the oceans as a result of human activities, through rivers and the atmosphere (Mason 2013). Today, the use of metals in industrial products has increased. As a result, people who use these essential materials (such as dental amalgam fillings, toothpaste, mouthwash, lead-based paint, shampoo, etc.) and those who consume aquatic products where elements bioaccumulate in their environment are gradually exposed to elemental toxicity. Since aquatic organisms interact with their environment, these chemicals can accumulate in the tissues of aquatic organisms and pass through higher trophic levels (Raposo et al. 2009). Essential (Cu, Fe, Zn) and non-essential elements (Hg, Cd, Pb) can have toxic effects when present at high levels in organisms (Odzak et al. 2000).
The Sea of Marmara is located between the continents of Asia and Europe which approximately 240 km in length, 70 km in width, 1390 m in maximum depth and with a surface area of 11.500 km2. It is connected to the Black Sea through the Bosphorus Strait and to the Aegean Sea through the Dardanelles Strait, forming a transition area between the Black Sea and the Mediterranean Sea (Beşiktepe et al. 1994). The Sea of Marmara has a two-layered water system due to the flow of low salinity (17.6‰) Black Sea waters through the upper current via the Bosphorus and high salinity (38‰) Mediterranean-origin saltwater through the lower current via the Dardanelles (Ullyot and Pektaş 1952; Yüce and Türker 1991). Between the two layers there is a halocline at a depth of about 25 m, which limits the exchange of oxygen between the surface and the lower waters. The upper layer, with a volume of about 230 km3, is renewed every 4–5 months, while the lower layer, with a volume of 3,378 km3, is renewed approximately every 6–7 years (Beşiktepe et al. 1993, 1994; Ünlüata et al. 1990).
The Sea of Marmara is constantly exposed to pollution due to the terrestrial inputs, the presence of many settlements and industrial facilities around it and the effects of maritime transport. It can be said that the Sea of Marmara, which can only breathe through two narrow straits (the Bosphorus and the Dardanelles), is struggling more and more with the increasing pollution load. This pollution can have a negative impact on the ecosystem. The recent mucilage event in the Sea of Marmara is a demonstration of this (Algan et al. 2004; Kayhan et al. 2006, 2007; Kurun et al. 2006, 2010; Okuş et al. 2007; Aktan et al. 2008; Tüfekçi et al. 2010; Aksu et al. 2011; Balkis et al. 2011; Acar et al. 2021; Balkis-Ozdelice et al. 2021; Ergul et al. 2021). The deteriorating condition of the Sea of Marmara is also affecting the organisms living there.
Numerous studies have been conducted on metal pollution in various marine organisms in Turkish waters (Topçuoğlu et al. 2004, 2010; Kayhan et al. 2007, 2016; Türkmen 2011; Balkıs et al. 2012, 2013; Erdem et al. 2015; Mülayim and Balkıs 2015; Özden and Tunçer 2015; Güner et al. 2016; Korkmaz et al. 2017; Durmus et al. 2018; Bat et al. 2019, 2023; Duyar and Bilgin 2019; Türksönmez and Diler 2019; Yağcı 2019; Bayrakli 2021; Erguden et al. 2021; Kılıç et al. 2021; Köker et al. 2021; Yildirim et al. 2022; Acarlı et al. 2023; Koçbaş et al. 2023). In marine pollution monitoring studies, certain indicator organisms are used to search long-term pollution. These organisms are selected for their low mobility and their ability to accumulate pollutants. These organisms act as passive samplers and provide information on the pollution trend in the region.
This study aims to determine the levels of toxic elements such as Se, As, Hg, Cr, Cd, Pb, Cu, Zn, non-toxic major elements such as Ni, Mn, Al, Fe, and rare earth elements such as Co and V in different marine organisms (mussels, crabs, fish, and macroalgae) collected from the northern part of the Sea of Marmara. The study also investigates the possible sources of this metal pollution and aims to reveal the current state of metal pollution in the Sea of Marmara based on the results obtained.
Material and Method
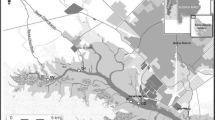
The samples used in this study (mussels, crabs, fish, and macroalgae) were collected from stations in the northern part of the Sea of Marmara, including Büyükçekmece (Station 1), Silivri (Station 2), Tekirdağ (Station 3) and Şarköy (Station 4) between May and September 2019 (Fig. 1). Measurements of Se, As, Hg, Cr, Cd, Pb, Cu, Zn, Ni, Mn, Al, Fe, Co, and V were conducted on the obtained samples. Ten individuals of crab samples (Eriphia verrucosa) used in the study were collected by trammel net and diving, while five individuals of sole fish (Solea solea) samples were collected by local fishermen. Ten individuals of mussels (Mytilus galloprovincialis) and about 500 gr of the fresh weight macroalgae samples (Ulva lactuca) were collected by hand. The collected samples were placed in nylon bags, transported to the laboratory, and stored in a deep freezer. The crab samples were divided into two categories based on size, less than 7 cm and greater than 7 cm. Both sizes were found at the Tekirdağ and Şarköy stations. Soft tissues were extracted from mussels, crabs, and fish, and all samples to be examined were dried using the freeze-drying technique with a Freeze Dryer device. Subsequently, 1 g of each sample was weighed and placed in teflon tubes, and a wet digestion process was performed. For this purpose, 5 ml of concentrated HNO3 and 2 ml of concentrated H2O2 were added to the tubes, and the tube openings were tightly sealed (UNEP 1982, 1984 and 1985). The teflon tubes were then placed in a microwave digestion device (CEMX5) and left at 120 °C for 2 h to break down the samples with acid. Following this process, the samples were filtered through a disk filter, adjusted to 25 ml with 1 M HNO3, and made ready for analysis using a Perkin Elmer Nexion 1000 ICP-MS device.
The Minimum Limit of Detection (MLOD) values for this analytical method are as follows: Fe 0.025 ppm, As: 0.25 ppb, Se: 0.25 ppb, V: 0.0025 ppm, Co: 0.025 ppm, Pb: 0.01 ppm, Cd: 0.0025 ppm, Cu: 0.01 ppm, Cr: 0.025 ppm, Mn: 0.0025 ppm, Al: 0.125 ppm, Ni: 0.0025 ppm, Hg: 0.025 ppb, Zn: 0.025 ppm. The Minimum Limit of Quantification (MLOQ) values are: Fe: 0.075 ppm, As: 0.75 ppb, Se: 0.75 ppb, V: 0.0075 ppm, Co: 0.075 ppm, Pb: 0.03 ppm, Cd: 0.0075 ppm, Cu: 0.03 ppm, Cr: 0.075 ppm, Mn: 0.0075 ppm, Al: 0.375 ppm, Ni: 0.0075 ppm, Hg: 0.075 ppb, Zn: 0.075 ppm. Spearman's rank correlation was used to examine the relationships using SPSS v.25 software between species based on the element accumulations in the organisms obtained in the study (Siegel 1956).
Results and Discussion
In this study, measurements of toxic elements such as Se, As, Hg, Cr, Cd, Pb, Cu, Zn, non-toxic major elements such as Ni, Mn, Al, Fe, and rare earth elements such as Co and V were carried out in samples of mussels, crabs, fish, and macroalgae. The values within each sample group were evaluated independently.
Mussels
The elemental concentrations recorded in the mussel samples in the study were Fe 0.00–6058.36 µg g−1 dry weight (dw), Pb 0.00–30.01 µg g−1 dw, Cd 0.00–9.93 µg g−1 dw, Cu 4.89–302.08 µg g−1 dw, Cr 0.65–21.12 µg g−1 dw, Mn 2.75–331.47 µg g−1 dw, Al 0.00–8134.95 µg g−1 dw, Ni 0.00–56.26 µg g−1 dw, Hg 0.45–2.12 µg g−1 dw, Zn 18.23–2802.00 µg g−1 dw, As 0.04–0.66 µg g−1 dw, Se 0.00–0.03 µg g−1 dw, V 0.53–25.89 µg g−1 dw and Co 0.00–10.98 µg g−1 dw (Table 1). Based on the results, the order of toxic elements in mussel samples from the inside Büyükçekmece port, from the lowest to the highest concentration, is Se < As < Hg < Cr < Cd < Pb < Cu < Zn; non-toxic major elements are Ni < Mn < Al < Fe, and rare earth elements are Co < V; outside the Büyükçekmece port, Pb < Se < As < Cd < Cr < Hg < Cu < Zn for toxic elements; Fe = Al < Ni < Mn for non-toxic major elements, and Co < V for rare earth elements; outside the Silivri port, toxic elements is Se < As < Hg < Cd < Cr < Pb < Cu < Zn, non-toxic major elements are Ni < Mn < Al < Fe, and rare earth elements are Co < V; inside the Tekirdağ port, toxic elements are Pb = Cd = Se < As < Cr < Hg < Cu < Zn, non-toxic major elements are Al = Ni < Mn < Fe, and rare earth elements are Co < V; outside the Tekirdağ port, Se < As < Hg < Pb < Cd < Cr < Cu < Zn for toxic elements, non-toxic major elements are Ni < Mn < Al < Fe, and rare earth elements are Co < V; outside the Şarköy port, toxic elements is Se < As < Hg < Cd < Pb < Cr < Cu < Zn, non-toxic major elements are Ni < Mn < Fe < Al, and rare earth elements are Co < V.
Crabs
The elemental concentrations in the crab samples were Fe 92.68–379.19 µg g−1 dw, Pb 0.25–0.85 µg g−1 dw, Cd 125 0.01–0.78 µg g−1 dw, Cu 3.65–39.50 µg g−1 dw, Cr 1.03–1.70 µg g−1 dw, Mn 1.53–9.83 µg g−1 dw, Al 7.68–366.05 126 µg g−1 dw, Ni 0.75–3.23 µg g−1 dw, Hg 0.63–1.28 µg g−1 dw, Zn 92.87–226.55 µg g−1 dw, As 0.02–1.84 µg g−1 dw, Se 0.02–0.04 µg g−1 dw, V 1.25–9.06 µg g−1 dw and Co 0.00–4.65 µg g−1 dw (Table 1). As for the values for crabs, the order of toxic elements in Büyükçekmece port is Se < As < Cd < Pb < Hg < Cr < Cu < Zn, while the non-toxic major elements are Ni < Mn < Al < Fe, and the rare earth elements are Co < V; in Silivri port, the order of toxic elements is Se < Cd < As < Pb < Hg < Cr < Cu < Zn, non-toxic major elements are Ni < Mn < Al < Fe, and rare earth elements are Co < V; in Tekirdağ crab (> 7 cm), the order for toxic elements is Cd < Se < As < Pb < Hg < Cr < Cu < Zn, the non-toxic major elements are Ni < Mn < Al < Fe, and the rare earth elements are Co < V; in Tekirdağ crab (< 7 cm), the order of toxic elements is As = Cd < Se < Pb < Hg < Cr < Cu < Zn, non-toxic major elements are Ni < Mn < Al < Fe, and rare earth elements are Co < V; in Şarköy crab (> 7 cm), the order of toxic elements is Cd < Se < As < Pb < Hg < Cr < Cu < Zn, non-toxic major elements are Ni < Mn < Al < Fe, and rare earth elements are Co < V; in Şarköy crab (< 7 cm), the order for toxic elements is Se < Cd < Pb < Hg < Cr < As < Cu < Zn, non-toxic major elements are Ni < Mn < Al < Fe, and rare earth elements are Co < V.
Fishes
The elemental concentrations in the fish samples were Fe 0.00–282.15 µg g−1 dw, Pb 0.00–0.28 µg g−1 dw, Cd 0.00–0.05 µg g−1 dw, Cu 2.98–40.91 µg g−1 dw, Cr 0.24–4.13 µg g−1 dw, Mn 3.64–24.55 µg g−1 dw, Al 0.00– 4638.65 µg g−1 dw, Ni 0.18–0.80 µg g−1 dw, Hg 0.03–3.37 µg g−1 dw, Zn 0.00–132.86 µg g−1 dw, As 0.00–0.07 µg g−1 dw, Se 0.00–0.00 µg g−1 dw, V 0.13–1.78 µg g−1 dw and Co 0.00–0.00 µg g−1 dw (Table 1). Regarding the values for fish samples, the order of toxic elements in Silivri is Cd = Se < As < Pb < Cr < Hg < Cu < Zn, while the non-toxic major elements are Ni < Mn, and the rare earth elements are Co < V; in Tekirdağ, the order for the toxic elements is Pb < Se < As < Cd < Cr < Hg < Cu < Zn, the non-toxic major elements are Al < Ni < Mn < Fe, and the rare earth elements are Co < V; in Şarköy, the order for the toxic elements is Pb = Cd = Zn = Se = As < Hg < Cu < Cr, the non-toxic major elements are Ni < Mn < Fe < Al, and the rare earth elements are Co < V.
Macroalgaes
The elemental concentrations in the macroalgae samples were Fe 1412.25–7499.78 µg g−1 dw, Pb 0.96–37.20 µg g−1 dw, Cd 0.68–5.00 µg g−1 dw, Cu 24.75–775.63 µg g−1 dw, Cr 6.23–26.19 µg g−1 dw, Mn 45.93–427.59 µg g−1 151 dw, Al 600.55–13,788.43 µg g−1 dw, Ni 6.55–45.79 µg g−1 dw, Hg 0.04–1.25 µg g−1 dw, Zn 10.18–447.47 µg g−1 152 dw, As 0.01–0.05 µg g−1 dw, Se 0.00–0.02 µg g−1 dw, V 7.55–72.54 µg g−1 dw and Co 0.00–3.41 µg g−1 dw (Table 1). As for the values for macroalgae samples, the order of toxic elements inside the Büyükçekmece port is Se < As < Hg = Cd < Cr < Pb < Zn < Cu, while the non-toxic major elements are Ni < Mn < Fe < Al, and the rare earth elements are Co < V; outside the Büyükçekmece port, the order for toxic elements are Se < As < Hg < Cd < Pb < Cr < Cu < Zn, the non-toxic major elements are Ni < Mn < Fe < Al, and the rare earth elements are Co < V; inside the Silivri port, the order of toxic elements are Se < As < Hg < Cd < Pb < Zn < Cr < Cu, non-toxic major elements are Ni < Mn < Fe < Al, and the rare earth elements are Co < V; inside the Tekirdağ port, the order for toxic elements are Se < As < Hg < Cd < Cr < Pb < Zn < Cu, the non-toxic major elements are Ni < Mn < Fe < Al, and the rare earth elements are Co < V; outside the Tekirdağ port, the order of toxic elements are Se < As < Hg < Cd < Pb < Cr < Zn < Cu, non-toxic major elements are Ni < Mn < Al < Fe, and the rare earth elements are Co < V; inside the Şarköy port, the order of toxic elements are As = Se < Hg < Cd < Cr < Pb < Zn < Cu, non-toxic major elements are Ni < Mn < Al < Fe, and the rare earth elements are Co < V; outside the Şarköy port, the order for toxic elements is Se < As < Hg < Pb < Cd < Cr < Cu < Zn, non-toxic major elements are Ni < Mn < Al < Fe, and the rare earth elements are Co < V.
When the correlation was applied to the values of other toxic elements except Fe and Al outside the ports of Tekirdağ and Şarköy, it was found that macroalgae, mussels, crabs and fish showed a positive correlation (p < 0.01) with each other in terms of elements (Table 2). This finding indicates that elements are transferred through the food chain.
When examining the correlation of elements in mussels (Table 3), the following relationships were observed: Fe had a strong positive correlation (p < 0.01) with Al and V, and a positive correlation (p < 0.05) with Pb, Cr, and Co. Cd had a strong positive correlation (p < 0.01) with Ni, and a positive correlation (p < 0.05) with Co. It had a negative correlation (p < 0.05) with Hg. Cr had a strong positive correlation (p < 0.01) with Mn and Al, and a positive correlation (p < 0.05) with Zn and Co. Mn had a strong positive correlation (p < 0.01) with Zn, and a positive correlation (p < 0.05) with Al, Ni and V. Al had a positive correlation (p < 0.05) with V. Ni had a strong positive correlation (p < 0.01) with Co and a positive correlation (p < 0.05) with V. Hg had a negative correlation (p < 0.05) with Zn and Se. Zn had a positive correlation (p < 0.05) with Se.
Regarding the correlation of elements in crabs (Table 4), the following relationships were observed: Fe had a strong positive correlation (p < 0.01) with Al, and a positive correlation (p < 0.05) with Cd, Mn, Ni, and Co. It had a negative correlation (p < 0.05) with Hg and Zn. Pb had a positive correlation (p < 0.05) with Ni. Cd had a strong positive correlation (p < 0.01) with Ni and Co, and a positive correlation (p < 0.05) with Mn. Cu had a negative correlation (p < 0.05) with Mn. Mn had a strong positive correlation (p < 0.01) with Co. Ni had a positive correlation (p < 0.05) with Co. Zn had a negative correlation (p < 0.05) with V and Co. Finally, V had a positive correlation (p < 0.05) with Co.
Regarding the correlation of toxic elements in fish (Table 5), the following relationships were observed: Cu had a strong positive correlation with Zn, and a strong negative correlation with Cr, Mn, and V. Cr had a strong positive correlation with Mn and V, and a strong negative correlation with Zn (p < 0.01). Mn had a strong positive correlation with V, and a strong negative correlation with Zn. Ni had a strong positive correlation with Co (p < 0.01). Hg had a strong positive correlation with As and Se. Zn had a strong negative correlation with V. As had a strong positive correlation with Se (p < 0.01).
When examining the correlation of toxic elements in macroalgae (Table 6), the following relationships were observed: Fe had a strong positive correlation with Mn and Co (p < 0.01), and a positive correlation with Cr (p < 0.05). Pb had a positive correlation with Cu and Se (p < 0.05). Cd had a strong positive correlation with V (p < 0.01), and a positive correlation with Ni (p < 0.05). Cu had a strong positive correlation with Se (p < 0.01). Cr had a positive correlation with Mn, Ni, As, and Co (p < 0.05). Mn had a strong positive correlation with Co (p < 0.01). Ni had a strong positive correlation with As and V (p < 0.01).Hg had a strong positive correlation with Zn (p < 0.01). As had a strong positive correlation with V (p < 0.01).
Looking at the correlation tables, the strong correlation of Fe and Al with all the elements, especially in mussels and crabs, is related to the fact that the structures of the elements are bound to iron aluminosilicate structures when they enter the sea from land and these structures accumulate in the organisms. The positive correlation of Pb, Cr, and Co in mussels and crabs can be explained by similarities in the mechanisms of element uptake by these organisms. It can also be explained by similarities in their feeding mechanisms and biochemical compositions. The negative correlation between Se and Hg, due to their antagonistic effects, is quite natural. Similarly, the positive correlation between Se and Zn is significant due to the affinity of selenium for sulfur, which has similar in chemical structure.
Se < As < Hg < Cr < Cd < Pb < Cu < Zn, which is the sequence of elements from smallest to largest, can be observed based on their natural abundance. Se and As are naturally present in relatively low levels. In addition, Hg is present in aquatic environments in a very low soluble form due to the salinity of the marine environment. It is therefore natural for Se, As, and Hg to be detected at lower levels than other trace element. In general, Cd disturbs this order. Cd has a high solubility in water, but enters the natural environment only through anthropogenic activities, and its presence in living tissues depends on local, periodic or continuous inputs. Therefore, changes in the ranking in living tissues can be explained by these reasons.
Three main mechanisms determine the solubility of elements in seawater. These mechanisms are the precipitation of elements with their insoluble salts, the precipitation of elemental sulfides in the presence of reducing sulfides, and the adsorption of elements onto inorganic compounds such as inorganic iron sulfide, hydrous ferric oxide, hydrous manganese dioxide, apatite, clay and organic matter (Krauskopf 1956).
As a result of precipitation and dissolution mechanisms, the concentration of elements in seawater is controlled, and more soluble forms of elements, as well as elements bound to organic structures or food, enter the food chain and pose a threat to human health. In this respect, organisms that are permanently present in the aquatic environment are indicator species to show the level of elemental pollution in that environment. Upon examination, it is observed that zinc and copper, both of which readily chelate with proteins, are present in high concentrations in all organisms, except for iron, which is relatively major (West and Crouch 2022). The order of their presence is related to factors such as the pH of the environment, the amount of input to the aquatic environment, the abundance of chelating structures in organic matter, such as carboxyl, hydroxyl, and amine groups, and the ability of elements to form ligands with each other.
In a study conducted by Erdem et al. (2015) in the Black Sea, the metal values in Eriphia verrucosa were measured as follows: Zn > Cu > Fe > Cr > Pb > Ni, and it was found that except for Pb, the values were within the limits values set by FAO/WHO and the Turkish Food Codex. Mülayim and Balkis (2015), in their research on E. verrucosa in the Black Sea, reported the following metal levels: Cr levels ranged from 0.4 to 1.2 mg kg−1, Cd levels ranged from 0.9 to 25.2 mg kg−1, Pb levels ranged from 0.3 to 4 mg kg−1, and Hg levels were at 0.1 mg kg−1. They found that Cd and Pb values were above or at the limit of the Turkish Food Codex values. Bat and Öztekin (2016) found high levels of Cu, Zn, and Fe in their study on E. verrucosa. In another study by Durmus et al. (2018) on E. verrucosa, the trace element values were found as: Zn > Fe > Cu > Mn > Se > Ni > Co, and the measured Cd (0.17–0.32 µg g−1 w/w) and Pb (0.13–0.36 µg g−1 w/w) values were found lower than the codex values. Bayrakli (2021), metal contents (Zn, Cu, Fe, As, Al, Mn, Hg, Pb, Cd) of E. verrucosa in the Black Sea were found to be below the acceptable levels of the Turkish Food Codex and the EU Commission. In this study, the values of certain elements in E. verrucosa were determined at all stations as follows: The lowest Cr value was 1.03 µg g−1 dw at the Silivri station, and the highest was 1.70 µg g−1 dw at the Büyükçekmece station. The lowest Cd value was 0.01 µg g−1 dw at the Şarköy and Tekirdağ stations, and the highest value was 0.78 µg g−1 dw at the Büyükçekmece station. The lowest Cu value was 3.65 µg g−1 dw at the Büyükçekmece station, and the highest was 39.5 µg g−1 dw at the Silivri station. The lowest Pb value was 0.25 µg g−1 dw at the Şarköy station, and the highest was 0.85 µg g−1 dw at the Büyükçekmece station. The Hg value was the lowest at 0.63 µg g−1 dw in crabs (< 7 cm) at the Şarköy station, and the highest was 1.28 µg g−1 dw in crabs (> 7 cm) at the same station. The lowest Zn value was 92.87 µg g−1 dw in crabs (< 7 cm) at the Şarköy station, and the highest was 226.55 µg g−1 dw at the Tekirdağ station in crabs (> 7 cm). The lowest Fe value was 92.68 µg g−1 dw in crabs (> 7 cm) at the Şarköy station, and the highest was 379.19 µg g−1 dw in crabs (< 7 cm) at the same station. The lowest Ni value was 0.75 µg g−1 dw in crabs (> 7 cm) at the Şarköy station, and the highest was 3.23 µg g−1 dw at the Büyükçekmece station. The lowest Mn value was 1.53 µg g−1 dw in crabs (> 7 cm) at the Tekirdağ station, and the highest was 9.83 µg g−1 dw at the Büyükçekmece station. The high levels of these elements in the edible parts of crabs may be due to their habitat and feeding habits.
When examining the correlations in fish, it is observed that Cr, Mn, and V elements have similar applications, and they are found in fuels or in petroleum. The presence of these elements in this region is related to the source of pollution. Copper and zinc have the same affinity, which explains their positive correlation. The antagonistic relationship between As and Se is also evident in the correlations. In the study by Türkmen (2011), on Solea solea, the metal levels were determined as follows: Cd: 0.02–0.38 mg kg−1, Co: < 0.01–0.43 mg kg−1, Cr: 0.11–1.78 mg kg−1, Cu: 0.30–1.82 mg kg−1, Fe: 7.19–59.7 mg kg−1, Mn: 0.08–1.11 mg kg−1, Ni: 0.01–3.27 mg kg−1, Pb: 0.17–1.13 mg kg−1, and Zn: 4.05–6.80 mg kg−1. These values were reported to be below the recommended tolerable daily and weekly intakes levels for human consumption. In the study by Korkmaz et al. (2017) on S. solea in the Mersin region, metal concentrations were measured as follows: Cr (< 0.001 mg kg −1), Mn (0.33–3.88 mg kg −1), Fe (3.86–25.76 mg kg −1), Ni (0.06–0.63 mg kg −1), Cu (0.01–1.61 mg kg −1), Zn (5.16–45.95 mg kg −1), Cd (< 0.0004 mg kg −1), Pb (0.03–0.99 mg kg −1). In the study by Kılıç et al. (2021) conducted in the İskenderun, Mersin, and Antalya Gulfs on S. solea, the wet weight metal results were found as follows: Cd values ranged from 0.004 to 0.01 µg g, Co values ranged from 0.01 to 0.03 µg g, Ni values ranged from 0.04 to 0.05 µg g, and Pb values ranged from 0.08 to 0.10 µg g. In the present study on S. solea, the metal values were detected as follows: The lowest Cu value was 2.98 µg g−1 dw at the Şarköy station, and the highest was 40.91 µg g−1 dw at the Tekirdağ station. The lowest Cr value was 0.24 µg g−1 dw at the Tekirdağ station, and the highest was 4.13 µg g−1 dw at the Şarköy station. The lowest Mn value was 3.64 µg g−1 dw at the Tekirdağ station, and the highest was 24.55 µg g−1 dw at the Şarköy station. The lowest Ni value was 0.18 µg g−1 dw at the Tekirdağ station, and the highest was 0.80 µg g−1 dw at the Silivri station. The lowest Zn value was 0.00 µg g−1 dw at the Şarköy station, and the highest was 132.86 µg g−1 dw at the Tekirdağ station. The lowest Cd value was 0.00 µg g−1 dw at the Şarköy and Silivri stations, and the highest was 0.05 µg g−1 dw at the Tekirdağ station. The lowest Pb value was 0.00 µg g−1 dw at the Tekirdağ and Şarköy stations and the highest was 0.28 µg g−1 dw at the Silivri station.
In a study conducted by Topçuoğlu et al. (2004) in the Sea of Marmara, the following metal values were found in M. galloprovincialis: Cd as 1.26–2.88 µg g−1; Co as < 0.05–1.89 µg g−1; Cr as < 0.06–3.54 µg g−1; Ni as < 0.1–13.96 µg g−1, Zn as 208.3–319.9 µg g−1, Fe as 120–415 µg g−1, Mn as 4.5–11.7 µg g−1, Pb as < 0.1–5.2 µg g−1, Cu as 6.7–9.5 µg g−1. In the study by Balkıs et al. (2013), conducted between 2005 and 2009 in the Black Sea, they reported the following metal levels in M. galloprovincialis: Pb as < 0.01–25 µg g−1, Cd as < 0.01–6.0 µg g−1, Hg as < 0.01–3.6 µg g−1, Cu as 0.8–80 µg g−1, Zn as 13–801 µg g−1, Fe as 21–2363 µg g−1 and Mn as 1.1–86 µg g−1. Kayhan et al. (2016) conducted a study on M. galloprovincialis on the coast of Tuzla and reported that the Cd levels were low, but the Pb levels exceeded the acceptable values according to the Turkish Food Codex. Yildirim et al. (2022), in their study in the south of the Sea of Marmara, found that all trace elements measured were below the legal limits. Acarlı et al. (2023), in their study on the Yalova coast (Sea of Marmara), showed that the levels of copper, zinc, mercury and lead in mussels were suitable for human consumption, while the levels of arsenic and cadmium exceeded the legal limits in some stations. Bat et al. (2023), determined that the heavy metal levels of M. galloprovincialis that collected at the Samsun coast of the Black Sea are safe for human health. In the present study, the metal concentrations in M. galloprovincialis were found as follows: The lowest value of Cd was 0.00 µg g−1 dw inside the Tekirdağ Port, and the highest value was 9.93 µg g−1 dw inside the Büyükçekmece Port. The lowest value of Co was 0.00 µg g−1 dw outside the Büyükçekmece Port and inside the Tekirdağ Port, and the highest value was 10.98 µg g−1 dw outside the Şarköy Port. The lowest value of Cr was 0.65 µg g−1 dw outside the Büyükçekmece Port, and the highest value was 21.12 µg g−1 dw outside the Şarköy Port. The lowest value of Ni was 0.00 µg g−1 dw inside the Tekirdağ Port, and the highest value was 56.26 µg g−1 dw outside the Şarköy Port. The lowest value of Zn was 18.23 µg g−1 dw inside the Tekirdağ Port, and the highest value was 2802.00 µg g−1 dw outside the Tekirdağ Port. The lowest value of Fe was 0.00 µg g−1 dw outside the Büyükçekmece Port, and the highest value was 6058.36 µg g−1 dw outside the Şarköy Port. The lowest value of Mn was 2.75 µg g−1 dw inside the Tekirdağ Port, and the highest value was 331.47 µg g−1 dw outside the Şarköy Port. The lowest value of Pb was 0.00 µg g−1 dw outside the Büyükçekmece Port and inside the Tekirdağ Port, and the highest value was 30.01 µg g−1 dw outside the Silivri Port. The lowest value of Cu was 4.89 µg g−1 dw inside the Tekirdağ Port, and the highest value was 302.08 µg g−1 dw outside the Tekirdağ Port. The lowest value of Hg was 0.45 µg g−1 dw outside the Tekirdağ Port, and the highest value was 2.12 µg g−1 dw inside the Tekirdağ Port.
The relationship between Cu and Se in macroalgae is similar to that in mussels, whereas the relationship between Cr and Mn is similar to that in fish. In their study conducted in the Sea of Marmara, Topçuoğlu et al. (2004) determined the following metal values in U. lactuca: Cd < 0.02–1.10 µg g−1, Co < 0.05–1.01 µg g−1, Cr < 0.06–4.90 µg g−1, Ni < 0.1–16.91 µg g−1, Zn < 0.2–286.4 µg g−1, Fe 0.1–2197 µg g−1, Mn 0.1–105.2 µg g−1, Pb < 0.1–15.4 µg g−1, Cu 0.1–29.1 µg g−1. Ozyigit et al. (2017) reported the following metal values in U. lactuca in their study on the Istanbul coasts: Cd (0.45–3.22 mg/kg), Cu (6.67–18.32 mg/kg), Fe (553.32–989.33 mg/kg), Mn (8.27–25.32 mg/kg), Pb (4.93–19.32 mg/kg), and Zn (15.16–41.30 mg/kg). Duyar and Bilgin (2019), in their study with U. lactuca collected from the Sinop coast in the Black Sea determined the metal values in the following order: Fe > Mn > Zn > Ni > Cu > Pb > Cd. Erguden et al. (2021), in their study in the Mediterranean Sea (Iskenderun and Mersin Bay), found Fe values that were high in U. lactuca. Koçbaş et al. (2023), in their study in the northern Aegean, they found the Fe concentration to be in the range of 304.76–1140.54 mg kg−1 and the cadmium to be < 0.01 in the U. lactuca. In the present study, the metal values were determined as follows: The lowest value Cd was inside the Silivri Port as 0.68 µg g−1 dw, highest value outside the Şarköy Port was 5.00 µg g−1 dw. The lowest value of Cr inside the Tekirdağ Port was 6.23 µg g−1 dw, highest value inside the Büyükçekmece Port was 26.19 µg g−1 dw. The lowest value of Pb inside the Silivri Port was 0.96 µg g−1 dw, highest value inside the Tekirdağ Port was 37.2 µg g−1 dw. The lowest value of Zn inside the Silivri Port was 10.18 µg g−1 dw, highest value inside the Büyükçekmece Port was 447.47 µg g−1 dw. The lowest value of Cu inside the Silivri Port was 24.75 µg g−1 dw, highest value inside the Şarköy Port was 775.63 µg g−1 dw. The lowest value of Mn outside the Tekirdağ Port was 45.93 µg g−1 dw, highest value inside the Büyükçekmece Port was 427.59 µg g−1 dw. The lowest value of Fe outside the Tekirdağ Port was 1412.25 µg g−1 dw, highest value inside the Büyükçekmece Port was 7499.78 µg g−1 dw. The lowest value of Co outside the Tekirdağ Port and inside the Silivri Port were 0.00 µg g−1 dw, highest value inside the Büyükçekmece Port was 3.41 µg g−1 dw.
According to the Turkish Food Codex (2011), it was found that Pb and Cd values in mussel samples exceeded the limits at all stations except station 1 (out of port) and station 3 (in the port) also, Hg values exceeded the limits at all stations except station 3 (out of port). In crab samples, while Pb values below the limits at three stations [Station 3 (> 7 cm), Station 4 (> 7 and < 7 cm)], Cd values were found below the limits except for station 1. On the other hand, Hg values exceeded limits at all stations (Table 1). The high metal levels in mussels, which are filter feeding, are expected due to their feeding behaviour. Similarly, metals such as, Cd, Pb, and Hg tend to accumulate in shellfish because they compete with elements such as Ca and Mg, which form the shell structure. Because of this competition for accumulation, the elements tend to be higher in such organisms.
Conclusion
This study analysed the accumulation of toxic elements in macroalgae, mussels, crabs, and fish obtained from the northern part of the Sea of Marmara. It can be said that the edible marine organisms used in the study could pose a potential risk to human health. Metals in the marine environments can present a potential risk to both to marine life in the region and to humans who consume these organisms. The Sea of Marmara, which is heavily polluted, is therefore an important environment to monitor. Proper wastewater treatment in the Sea of Marmara and the planning of environmentally friendly industrial activities are crucial for reducing the risk of metal pollution in the marine ecosystem. It can be stated that the metals entering the Sea of Marmara are attributed to various anthropogenic activities in the coastal zone. In order to ensure proper management of marine resource, it is necessary to have comprehensive knowledge of the status of the region's biodiversity and the threats it faces. Regular marine monitoring studies should be carried out in this region, which includes numerous industrial and tourism structures. This will help to monitor the effects of changes in the marine ecosystem.
Data Availability
The datasets generated by this study are included in the paper and can be obtained from the corresponding author upon approval of a request.
References
Acar U, Yılmaz OS, Çelen M, Ateş AM, Gülgen F, Şanlı FB (2021) Determination of Mucilage in The Sea of Marmara Using Remote Sensing Techniques with Google Earth Engine. International Journal of Environment and Geoinformatics (IJEGEO) 8(4):423–434. https://doi.org/10.30897/ijegeo.957284
Acarlı S, Vural P, Yildiz H (2023) An assessment of the cultivation potential and suitability for human consumption of mediterranean mussels (Mytilus galloprovincialis Lamarck, 1819) from the Yalova coast of the Marmara Sea. Menba J Fish Faculty 9(1):12–24. https://doi.org/10.58626/menba.1282775
Aksu A, Balkis N, Taskin OS, Ersan MS (2011) Toxic metal (Pb, Cd, As and Hg) and organochlorine residue levels in hake (Merluccius merluccius) from the Marmara Sea, Turkey. Environ Monit Assess 182:509–521. https://doi.org/10.1007/s10661-011-1893-1
Aktan Y, Dede A, Çiftçi PS (2008) Mucilage event associated with diatoms and dinoflagellates in Sea of Marmara, Turkey. An IOC Newsletter on toxic algae and algal blooms. The Intergovernmental Oceanographic Commission of UNESCO 36:1–3
Algan O, Balkis N, Çağatay MN, Sarı E (2004) The sources of metal contents in the shelf sediments from the Marmara Sea, Turkey. Environ Geol 46:932–950
Balkis N, Atabay H, Turetgen I, Albayrak S, Balkis H, Tüfekçi V (2011) Role of single-celled organisms in mucilage formation on the shores of Buyukada Island (the Marmara Sea). J Mar Biol Assoc UK 91:771–781
Balkıs N, Aksu A, Hiçsönmez H (2012) Metal levels in biota from the Southern Black Sea, Turkey. J Black Sea/mediterranean Environ 18(2):134–143
Balkıs N, Aksu A, Hiçsönmez H (2013) Pollution Monitoring using Mytilus galloprovincialis and fishes: A case study on the Southern Black Sea Shelf. Asian J Chem 25(1):450–454
Balkis-Ozdelice N, Durmus T, Balci M (2021) A preliminary study on the ıntense pelagic and benthic mucilage phenomenon observed in the Sea of Marmara. Intl J Environ Geoinform (IJEGEO) 8(4):414–422
Bat L, Öztekin HC (2016) Heavy metals in Mytilus galloprovincialis, Rapana venosa and Eriphia verrucosa from the Black Sea coasts of Turkey as bioindicators of pollution. Walailak J Sci and Tech (WJST) 13(9):715–728
Bat L, Şahin F, Öztekin A (2019) Assestment of Heavy Metals Pollution in Water and Sediments and Polychaetes in Sinop Shores of the Black Sea. KSÜ Tarım Ve Doğa Derg 22(5):806–816
Bat L, Yardım Ö, Arıcı E, Hasançavuşoğlu Z, Öztekin A (2023) Heavy Metals Risk Assessment for Consumption of Wild Mediterranean Mussels Mytilus galloprovıncialis Lamarck, 1819 Along Samsun Coasts of The Black Sea. Pakistan J Marine Sci 32(2):129–144
Bayrakli B (2021) Concentration and potential health risks of trace metals in warty crab (Eriphia verrucosa Forskal, 1775) from Southern Coasts of the Black Sea Turkey. Environ Sci Pollut Res 28(12):14739–14749
Beşiktepe S, Özsoy E, Ünlüata Ü (1993) Filling of the Marmara Sea by the Daradanelles lower layer inflow. Deep-Sea Res 49(9):1815–1838
Beşiktepe Ş, Sur Hİ, Özsoy E, Latif MA, Oğuz T, Ünlüata Ü (1994) The Circulation and Hydrography of the Marmara Sea. Prog Oceanogr 34(4):285–334
Bettini S, Ciani F, Franceschini V (2006) Recovery of the Olfactory Receptor Neurons in the African Tilapia mariae Following Exposure to Low Copper Level. Aquat Toxicol 76(3):321–328. https://doi.org/10.1016/j.aquatox.2005.10.009
Durmus M, Ayas D, Aydın M, Kosker AR, Ucar Y, Ozogul Y (2018) The Effect of Sex and Seasonality on the Metal Levels of Warty Crab (Eriphia verrucosa) in the Black Sea. J Aquat Food Prod Technol 27(6):749–758. https://doi.org/10.1080/10498850.2018.1485196
Duyar HA, Bilgin S (2019) Heavy Metal Concentrations in Different Marine Organism Obtained from Black Sea Turkey. Fresenius Environ Bull 28(7):5281–5286
Erdem ME, Turan H, Kaya Y (2015) Mineral and trace element contents of Warty Crab (Eriphia verrucosa) and Brown Shrimp (Crangon crangon). İstanbul Üniversitesi Su Ürünleri Dergisi 30(2):26–31
Erguden SA, Erguden D, Ciftci N, Akbora HD, Ayas D (2021) Metal levels of macroalgae from Iskenderun and Mersin Bay Turkey Eastern Mediterranean. Fresenius Environ Bull 30(07):8365–8371
Ergul HA, Balkis- Ozdelice N, Koral M, Aksan S, Durmus T, Kaya M, Kayal M, Ekmekci F, Canlı O (2021) The early stage of mucilage formation in the Marmara Sea during spring 2021. J Black Sea / Mediterranean Environ 27(2):232–257
Güner Y, Kayhan FE, Balkıs N, Aksu A, Kaymak G (2016) Cd and Pb Levels in the Soft Tissue of Black Mussel (Mytilus galloprovincialis) Collected from Marmara Sea, Pendik District Coastal Area, Istanbul Turkey. Pakistan J Zool 48(2):605–607
Kayhan FE, Balkıs N, Aksu A (2006) İstanbul Balık Halinden Alınan Akdeniz Midyelerinde (Mytilus galloprovincialis) Arsenik Düzeyleri. Ekoloji 15(61):1–5
Kayhan FE, Gulsoy N, Balkis N, Yüce R (2007) Cadmium (Cd) and lead (Pb) levels of Mediterranean mussel (Mytilus galloprovincialis Lamarck, 1819) from Bosphorus, Istanbul Turkey. Pak J Biol Sci 10(6):915–919. https://doi.org/10.3923/pjbs.2007.915.919
Kayhan FE, Çağlar N, Aksu A, Ertug NDY, Sesal C (2016) Trace Metal Concentration and Sea Water Quality of Tuzla Shipyard Area, Istanbul, Turkey Using Mediterranean Mussels (Mytilus galloprovincialis) for Monitoring Aquatic Pollution. Pak J Zool 48:597–600
Kılıç E, Can MF, Yanar A (2021) Assestment of some heavy metal accumulation and potential health rsik for three fish species from three consecutive bay in North-Eastern Mediterranean Sea. Marine Life Sci 3(1):24–38
Koçbaş F, Çulha ST, Gündoğdu A, Türkçü N (2023) Comparison of heavy metal concentrations in marine macroalgae of the Northern Aegean Sea, Türkiye. Eurasian J Med Biol Sci 3(1):23–33
Korkmaz C, Ay Ö, Çolakfakioğlu C, Cicik B, Erdem C (2017) Heavy Metal Levels in Muscle Tissues of Solea solea, Mullus barbatus, and Sardina pilchardus Marketed for Consumption in Mersin Turkey. Water Air Soil Pollut 228:315. https://doi.org/10.1007/s11270-017-3503-5
Köker L, Aydın F, Gaygusuz Ö, Akçaalan R, Çamur D, İlter H, Ayoğlu FN, Altın A, Topbaş M, Albay M (2021) Heavy Metal Concentrations in Trachurus mediterraneus and Merlangius merlangus captured from Marmara Sea, Turkey and Associated Health Risks. Environ Manage 67:522–531. https://doi.org/10.1007/s00267-020-01352-y
Krauskopf KB (1956) Factors controlling the concentration of thirteen trace metals in sea-water. Geochim Cosmochim Acta 9(1):1–32. https://doi.org/10.1016/0016-7037(56)90055-2
Kurun A, Balkıs H, Balkıs N (2006) Accumulations of total metal in shrimp species (Palaemon adspersus, Palaemon serratus, Parapenaeus longirostris) and bottom surface sediments in the Northern inner shelf of the Sea of Marmara. Environ Monit Assess 135:353–367. https://doi.org/10.1007/s10661-007-9655-9
Kurun A, Balkıs N, Erkan M, Balkıs H, Aksu A, Erşan MS (2010) Total metal levels in crayfish Astacus leptodactylus (Eschscholtz, 1823) and surface sediments in Lake Terkos, Turkey. Environ Monit Asses 169:385–395. https://doi.org/10.1007/s10661-009-1181-5
Mason RP (2013) Thrace Metals in Aquatic Sytem. Wiley, Blackwell, pp.431. ISBN 978–1–4051–6048–3
Mülayim A, Balkıs H (2015) Toxic metal (Pb, Cd, Cr and Hg) levels in Rapana venosa (Valenciennes, 1846), Eriphia verrucosa (Forskal, 1775) and sediment samples from the Black Sea littoral (Thrace, Turkey). Mar Pollut Bull 95:215–222
Odzak N, Zvonaric T, Kljakovic Z, Horvat M (2000) Biomonitoring of mercury in the Kastela Bay using transplanted mussels. Sci Total Environ 261:61–68
Okuş E, Balkıs N, Müftüoğlu E, Aksu A (2007) Metal (Pb, Cd and Hg) inputs via the rivers to the Southern Marmara Sea Shelf, Turkey. J Black Sea/mediterrenean Environ 13(1):35–38
Ozyigit I I, Uyanik OL, Sahin NR, Yalcin IE, Demir G (2017) Monitoring the Pollution Level in Istanbul Coast of the Sea of Marmara Using Algal Species Ulva lactuca L. Polish J Environ Stud 26(2):773–778. https://doi.org/10.15244/pjoes/66177
Özden S, Tunçer S (2015) Ulva rigida’daki Ağır Metal (Pb, Cu, Zn ve Fe) Düzeyleri (Dardanel, Çanakkale). BEU Fen Bilimleri Dergisi 4(1):35–42
Raposo JC, Bartomome EL, Cortazar EE, Arana EJ, Zabaljauregui EM, de Diego EM, Zuloaga EO, Madariaga EJM, Etxebrria EM (2009) Trace Metals in Oysters, Crassotrea sps., from UNESCO Protected Natural Reserve of Urdaibai: Space-Time Observations and Source Identification. Bull Environ Contam Toxicol 83:223–229
Siegel S (1956) Nonparametric statistics for behavioral sciences. McGraw-Hill, New York
Topçuoğlu S, Kırbaşoğlu Ç, Yılmaz YZ (2004) Heavy Metal Levels in Biota and Sediments in the northern coast ofMarmara Sea. Environ Monit Assess 96:183–189. https://doi.org/10.1023/B:EMAS.0000031726.01364.47
Topçuoğlu S, Kılıç Ö, Belivermiş M, Ergül HA, Kalaycı G (2010) Use of algae as biological indicator of heavy metal pollution in Turkish marine environment. J Black Sea/mediterranean Environ 16(1):43–52
Tüfekçi V, Balkis N, Beken ÇP, Ediger D, Mantıkçı M (2010) Phytoplankton composition and environmental conditions of a mucilage event in the Sea of Marmara. Turk J Biol 34:199–210
Turkish Food Codex (2011) Official Gazette of Republic of Turkey. Notifications changes to the maximum levels for certain contaminants in foodstuffs (in Turkish) . Notification No: 28157
Türkmen A (2011) Türkiye Denizleri’nden Yakalanan Dil Balığı (Solea solea L., 1758) Türünün Kas ve Karaciğer Dokularında Ağır Metal Düzeylerinin Belirlenmesi. Karadeniz Fen Bilimleri Dergisi 2(1):139–151
Türksönmez Ç, Diler A (2019) Seasonal determination of heavy metal levels of anchovy (Engraulis encrasicolus L., 1758) obtained from the Marmara Sea. Acta Biologica Turcica 32(4):242–247
Ünlüata Ü, Oğuz T, Latif MA, Özsoy E (1990) On the physical oceanography of the Turkish Straits. In: Pratt, L.J. (eds) The Physical Oceanography of Sea Straits. NATO ASI Series, 318, 25–60. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-0677-8_2
Ullyot P, Pektaş H (1952) Çanakkale Boğazı’ndaki yıllık temperatür ve tuzluluk değişmeleri hakkında ilk araştırmalar. İstanbul Üniversitesi Fen Fakültesi Hidrobiyoloji Araştırma Enstitüsü Yayınları Hidrobiyoloji Mecmuası Seri A 1(1):19–33
UNEP (1982) Reference methods for marine pollution studies. 14
UNEP (1984) Determination of total Cd, Zn, Pb and Cu in selected marine organisms by flameless AAS. Reference Methods for Marine Pollution Studies, 11
UNEP GESAMP (1985). Cadmium, lead and tin in the marine environment. UNEP Regional Seas Reports and Studies, 56
West S, Crouch H (2022) Analitik Kimya Temel İlkeler Cilt 1. Bilim Yayınları. Çeviri Edit: Esma Kılıç, Hamza Yılmaz, Pınar Esra Erden . ISBN :9789755560250. Sayfa Sayısı :774
Yağcı T (2019) Antalya, Rize ve Yalavo Kıyı Sularından Örneklenen Karides Türlerinin (Aristeus antennatus (Risso, 1816), Palaemon adspersus (Rathke, 1837), Parapenaus longirostris (Lucas,1846) Metal İçeriklerinin Değerlendirilmesi. Acta Aquatica Turcica 15(3):298–306. https://doi.org/10.22392/actaquatr.514050
Yildirim P, Cardak M, Colakoglu S, Türker G, Ormanci HB (2022) Seasonal variations and human health risk assessment of trace elements in the bivalve ecosystem in the Sea of Marmara. Food Addit Contam Part B 15(3):221–234
Yüce H, Türker A (1991) Marmara Denizi’nin fiziksel oşinografik özellikleri ve Akdeniz suyunun Karadeniz’e girişi, Uluslararası Çevre Sorunları Sempozyumu Tebliğleri, İstanbul Marmara Rotary Kulübü, İstanbul, 284–303
Acknowledgements
The present study is a M.Sc. thesis entitled ‘‘Determination of heavy metal accumulation in Eriphia verrucosa (Forskal, 1775) which is obtained from North of Sea of Marmara’’ by Mertcan Tartıcı. The authors are grateful to Dr. Husamettin Balkis, Dr. Abdullah Aksu and Dr. Turgay Durmuş from Istanbul University for their valuable assistance in field sampling and in elements analyses.
Funding
This work was supported by the Research Fund of Istanbul University. Grant number: FYL-2019–33514.
Author information
Authors and Affiliations
Contributions
Mertcan Tartıcı, Benin Toklu Alıçlı, Nuray Çağlar Balkıs conceived the project, heavy metals and statistical analyses, wrote the article. Mertcan Tartcı, Benin Toklu Alıçlı sampling materials.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tartıcı, M., Alıçlı, B.T. & Balkıs, N.Ç. Analysis of Metal Accumulation in Some Marine Organisms Obtained from the Northern Sea of Marmara, Türkiye. Thalassas 40, 995–1005 (2024). https://doi.org/10.1007/s41208-024-00709-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-024-00709-y