Abstract
Fragmentation creates heterogeneous landscapes with different interactions and trajectories. In this scenario, the understanding in how forest fragment sizes influence forest species patterns can lead the way for biodiversity management and conservation in agricultural landscapes. The aim of this study was to compare the density and the diameter distribution of Bertholletia excelsa, Carapa guianensis and Cedrelinga cateniformis among fragments of different sizes, located at the Agro-Extractivist Settlement Project Praialta Piranheira, Pará State, Brazil. Data were collected from adult and sapling trees in six forest fragments. Adult and sapling density was compared among fragment sizes by the Mann–Whitney test, while diameter distributions were compared by a summary statistic calculated using the scores of a PCA produced with the results of the binomial logit model between tree DBH and the cumulative DBH proportions. The density of adult trees of three species is similar among different-sized fragments. Only the saplings of C. cateniformis showed a significant difference in density among fragment sizes. The DBH distribution of adults, for all three species, seems to also not respond to fragmentation, since the differences in the DBH distribution curves among fragments were large. The results of this study suggest that in areas established for agroextractivism, all fragments (large and small) no longer have sustainable populations (except only one C. guianensis population), probably due to anthropic pressure exerted by agroextractivism. So, it is important to ensure the structural and environmental quality of the forest fragments for the conservation of the species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Amazonian terrestrial ecosystem that has been occupied by man for thousands of years presents the greatest biodiversity on the planet (Levis et al., 2017). Only in trees, it contains between 6,000 and 16,000 species (Ter Steege et al., 2013; Silk et al. 2015) and represents a third of the productivity and evapotranspiration of the terrestrial surface (Malhi et al., 2014). However, forest losses are already considered irreparable (Barlow, 2016; Lovejoy & Nobre, 2018) with deforestation reaching 784,666 km2 (Fearnside, 2017; Newbold et al., 2015) and the number of rare tree species at risk of extinction from habitat loss may be from hundreds to thousands (Hubbell et al., 2008). Based on simulations, it is predicted that by 2050, habitats may be reduced, and Amazonian plant species will be extinguished at rates from 5 to 9% (Feeley & Silman, 2009).
The original forest matrix became fragments surrounded by secondary forests, pastures and agricultural areas (Sousa et al., 2017), producing a heterogeneity of environments (Ewers & Didham, 2006). After forest fragmentation, changes occur in ecological processes, e.g., studies have shown that small fragments tend to have fewer species richness and abundance than in large fragments, both for plant and animal species (Gibson, 2013; Haddad et al., 2015). However, other studies have shown that the size of the fragment is not significant for litter thickness indices (Portela & Santos, 2007), species richness (Fahrig, 2013), seedling mortality due to litterfall (Scariot, 2000) and for the structure of tolerant and intermediary species population (Jurinitz, 2010). Based on a review on landscape-scale investigations, Fahrig (2017) reports that biodiversity responses to habitat fragmentation ‘per se’ are more often positive rather than negative; namely, a group of small habitat patches generally has lower ecological value than large patches of the same total area. However, Fletcher et al. (2018) based on extrapolation from patch-scale patterns and mechanisms (effects of patch size and isolation and edge effects) to landscape-scale effects of habitat fragmentation showed that it is necessary to understand why and when these habitat fragmentation effects occur, how they interact with other human-induced changes, and under what situations fragmentation effects will be positive or negative. Finally, Fahrig et al. (2019) argue that such extrapolation is unreliable because: (1) it ignores other mechanisms, especially those acting at landscape scales (e.g., increased habitat diversity, spreading of risk, landscape complementation) that can counteract effects of the documented patch-scale mechanisms; and (2) extrapolation of a small-scale mechanism to a large-scale pattern is not evidence of that pattern but, rather a prediction that must be tested at the larger scale.
These contradictory results open a gap on the importance of forest fragment sizes, especially those located in areas around anthropic activities, such as in agricultural mosaics. In the Brazilian Amazon, agricultural mosaics are commonly found in areas of small family farmers, composed of multiple types of land use, such as agricultural crops, pastures and secondary forests, interconnected to remaining forest fragments of different sizes (Do Vale et al., 2018). These forest fragments still keep species that have been explored for centuries, such as Bertholletia excelsa Bonpl. (castanha-do-brasil), Carapa guianensis Aubl. (andiroba) and Cedrelinga cateniformis (Ducke) Ducke (cedro-arana). These species are historically eminent by its economic importance in wood and seeds exploitation, which are mainly conducted by traditional communities, such as indigenous, riparian, afrodescendants (quilombolas) and agro-extractivists (Henriques & Penido, 2014; Levis et al., 2017; Santos et al., 2019). The population structure of these species is influenced by several environmental factors, such as dispersal, predation, rainfall seasonality and nutrient availability (Klimas et al., 2012c; Tomimatsu & Ohara, 2010). However, anthropic influence plays a major role, such as the extraction of the seeds (Guariguata et al., 2017; Oliveira et al., 2014; Peres et al., 2003) and wood (Fernandes et al., 2018; Salomão, 2014) by the communities.
In areas of agricultural mosaics, due to the complexity and heterogeneity of these environments (Ewers & Didham, 2006), there is a need for more detailed information on the effect of fragment size on forest species, especially those of greater economic interest. In this context, the present study aims to compare the population structure of B. excelsa, C. guianensis and C. cateniformis in different-sized forest fragments. In the studied agro-extractivist community, these three species have the highest economic and social values, with the first two standing out mainly for being of multiple use. Emphasis has been laid in adult and sapling density and the diameter distribution for the evaluation of the potential regeneration and stability of the adult trees. The outcomes can help other Amazonian traditional communities in the management of these regionally important species, reflecting on the importance of individuals intensity and spatialization for wood and seed extraction.
2 Material and methods
2.1 Study area
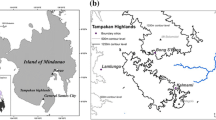
The study was conducted in the Agro-Extractivist Settlement Project Praialta Piranheira (PAE), located in the municipality of Nova Ipixuna, Southeast Pará State, Brazil. The settlement was created covering an area of 22,000 hectares with 600 families in the year of 1997; however, the number of families decreased over time, and in 2017, there were 330 families settled (INCRA, 2017) (Fig. 1). The first known extractivist families arrived in the region in 1933; until the 1980s, the region was sparsely inhabited, where the extraction of fish in Tocantins River, wood and mainly Brazil nut (B. excelsa) was predominant, as well as mineral extraction, such as diamonds. On August 22, 1997, the PAE was created (Oliveira et al., 2014).
The original vegetation of the PAE is a tropical forest, classified as a Submontane Dense Ombrophylous Forest (IBGE, 2012); the climate, according to the Köppen classification system, is Am, a very humid equatorial climate, characterized by two well-defined periods, the rainy season (November to April) and the dry period (May to October), with mean annual rainfall of 2,000 mm and mean annual temperature of 26 °C (INMET, 2017). The area is located on the Bacajá geological domain, composed of rehydrated granulites of tonalitic and granulitic composition, containing pyroxenes that give rise to soils rich in quartz, and small amounts of potassic feldspars affected by metamorphism with fragmented crystals, resulting in soils with fine textures (Vasquez & Rosa-Costa, 2008). Soils are poor and predominantly kaolinitic and morphologically argisols (Zenero et al., 2016).
Over the years, several agricultural activities have been developed, such as cultivation of annual crops; extractivism of wood from several species, Brazil nuts (B. excelsa), cupuaçu (Theobroma grandiflorum) and andiroba (C. guianensis), and, to a lesser extent, the raising of small animals. Nevertheless, the most important activity in the settlement is beef and dairy farming, which is antagonistic to the initial proposal (Oliveira et al., 2014).
The advances in livestock farming have contributed to forest fragmentation (Oszwald et al., 2010) and civil organizations began to express concerns about the fate of the PAE, which led, since 2005, to a collective creation of a community forest management plan. The plan had the participation of agro-extractivists, researchers from the Support Group for Family Agriculture in the Frontier Region (GRAAL) and the Federal University of Pará (UFPA). This plan covered both timber and non-timber forest products; among the non-timber forest products, the extraction of C. guianensis oil was the most important activity due to the large existing market (Oliveira et al., 2014).
In spite of these initiatives, the settlement endured a change in the landscape, creating an agricultural mosaic composed of different types of land use, such as agricultural crops, pastures, secondary forests, originated from fallow areas and mature forests that were mainly riparian forest or conserved forests located in areas far from the roads (Oszwald et al., 2011).
2.2 Sampling of forest fragments
To define which forest fragments would be studied, a Landsat-8 image (scene 223/64) from the year 2016, acquired at Earth Explorer (www.earthexplorer.usgs.gov/), was used; this image had less than 10% of clouds. First, vegetation cover was classified into two classes, forest and non-forest. For this purpose, a composition in RGB (Red-Green-Blue) was made from the bands 4, 5 and 6, followed by a supervised classification in Spring Software version 5.3, using the Bhattacharya classifier. This classifier analyzes each pixel and has an acceptance threshold of 95%. During the classification, a segmentation (similarity 6 and pixel area 20) was performed, with a mean performance of the validated sampling in 93.28% and mean confusion of 7.70%.
The areas of all forest fragments were measured using Quantum GIS software version 2.18. A total of 336 forest fragments were quantified, with size areas between 0.09 and 484.04 ha (median = 6.658 ha, standard error = 3.457 ha, coefficient of variation = 2.398 ha). The frequency of fragment size did not show a normal distribution (Shapiro–Wilk test: S–W = 0.421 and p = 0.00001), with most fragments sizes up to 50 ha (Fig. 2). The frequency distribution of the PAE fragments area was used to aid in the selection of the size of the studied fragments (Fig. 2). The forest fragments can be classified into small, those of up to 50 ha, and large, those with an area greater than 50 ha, according to Ribeiro et al. (2009) and Jurinitz et al. (2013). Considering this classification, six fragments were selected: three large fragments (L1 = 208.65 ha; L2 = 205.78 ha; L3 = 172.22 ha) and three small fragments (S1 = 22.61 ha; S2 = 19.18 ha; S3 = 15.13 ha). The six selected forest fragments are located in the central part of the PAE. The PAE border area was avoided because other factors, besides the activities of the PAE, can also act on the populations. On one side, they could be influenced by Tucuruí Lake, where the soils are wetter, and on the other sides, they can be influenced by large adjoining farms with a high level of landscape disturbance.
2.3 Species
The three species studied are widely distributed across the Amazon basin and have high economic value in the region. B. excelsa has been exploited by its production of wood, which nowadays it is illegal, and its nuts that are commercialized in the international market as Brazil nut, consisting in an important subsistence activity for many traditional communities in Amazonia (Guariguata et al., 2017). Brazil nut germination occurs in the shade and the seedlings can survive long time in the understory, but the growth occurs mainly in the presence of light that is why the species is sometimes classified as shade-tolerant and sometimes as light-dependent (Scoles et al., 2016). Pollination is made by medium and large bees (Cavalcante et al., 2018), and their seeds have a dormancy period that can exceed one year, with an extremely slow germination; the dispersal of the seeds occurs mainly by Dasyprocta sp. (a small rodent called agouti) (Tonini et al., 2014) and humans (Levis et al., 2017).
C. guianensis has also its wood and seeds exploited (Klimas et al., 2012a, 2012b, 2012c). Its wood is of high quality, comparable to cedar (Cedrela odorata L.) and mahogany (Swietenia macrophylla King), but heavier and denser (Shanley & Medina, 2005). From the seeds are extracted an oil with medicinal, repellents and emollients properties used in traditional medicine and by cosmetic and phytotherapeutic industries (Santos et al., 2014). C. guianensis is plastic, which confers morphological differences and ability to occupy various habitats, living from sea level, in flooded areas (várzeas and igapós), to the high grounds in the forests (terra-firme) at 1400 m of altitude (Klimas et al., 2012c). It is classified as partially shade-tolerant (Schwartz et al., 2012, 2014). The dispersal of the seeds is mainly barocoric, with secondary dispersal by medium and large mammals that are also important predators of the seeds, especially in the food shortage period. For the trees close to water, the dispersal can be hydrocoric (Santos et al., 2014).
C. cateniformis is culturally important because of its high-valued wood (Lombard et al., 2008), much used in the furniture sector due to its coloration that varies from reddish brown to rosy-gray (Fernandes et al., 2018). C. cateniformis is a species of low wood density (Schulze et al., 2008), with adults present in the canopy or above the canopy, as emergent trees (Salomão, 2009). Classified as shade-tolerant presents irregular flowering and fruiting (Alencar et al., 1979) with anemochoric dispersal (Luna, 2004).
2.4 Population sampling
The population structure of each species was analyzed using three variables: adult and sapling density and adult diameter distribution. In each forest fragment, all adult individuals, with diameter at breast height (DBH) ≥ 10 cm of B. excelsa, C. guianensis and C. cateniformis, were identified and measured in a census. For each species, three trees were selected as matrices in the central part of each fragment for sapling count; matrix-trees had good crown formation, the absence of broken branches, the absence of vine and termite. Saplings (individuals with DBH < 10 cm and height > 10 cm) were counted in three 25 × 25 m plots installed near each matrix-tree. In total, there were 54 plots (6 fragments × 3 matrix-trees × 3 plots per matrix-tree) for each species; with 27 plots in small fragments and 27 plots in large fragments.
The plots were arranged one meter from the matrix-trees of B. excelsa and C. guianensis, because these species have barocoric dispersal as primary, consequently many seeds fall near the matrix; this methodology was also used by Zuidema and Boot (2002), Cotta et al. (2008) and Guedes et al. (2014). For C. cateniformis, the plots were allocated 5 m away from the matrix-tree, since seed dispersal is anemocoric and regeneration was occurring farther from the adult tree (Mariana Oliveira, field observation). A parataxonomist and a resident agro-extractivist of the settlement performed the identification of the individuals. The data collection period occurred between November 2016 and April 2017, i.e., during the rainy season.
2.5 Data analysis
The density of adult trees was measured by the ratio of the total number of individuals to the area of the fragments in hectare. To avoid pseudoreplication, the sapling density (in m2) was calculated from the average of the individuals found in each matrix-tree, later the average of the fragment. Adult and sapling density was compared between large and small fragments by the non-parametric Mann–Whitney test. This test was standardized for all species in the two strata studied, because in some cases, the data did not normalized.
Diameter (DBH) size distribution of adult trees was analyzed as in Peres et al. (2003). First, tree DBH was ordered from smallest to largest and expressed as cumulative proportions varying from 0 to 1; then, a binomial logit model between tree DBH and the cumulative proportions was adjusted for each species per fragment. The intercept and slope of the model were analyzed representing, respectively, the DBH size and variation did found in population. To represent DBH size and variation, the median and variance of DBH were used, respectively. Pearson's correlation was performed to confirm the relationships between intercept and DBH median, slope and DBH variance and also between intercept and slope. To facilitate data analysis, DBH frequency histograms were established using the rule of Sturges (1926) to define the number of DBH size classes. Finally, to compare DBH size distribution between large and small fragments, a single summary statistic (s*) was calculated using the scores of the first axis of a PCA produced with the intercept and slope of the models for each species considering all six fragments. This s* measures the shape of the cumulative size distribution (Peres et al., 2003).
All statistical analyzes were considered at the 5% probability level and were performed in R Platform version 3.4.2 (R Foundation for Statistical Computing, Vienna, AT, 2017) using the Stats, Mass and Lattice package.
3 Results
3.1 Adult and sapling density
Although the density of adult trees varied greatly among the fragments (Table 1), there was no significant difference between large and small fragments for any of the studied species: B. excelsa (U = 2; p = 0.4); C. cateniformis (U = 3; p = 0.7); and C. guianensis (U = 3, p = 0.79). The sapling density also showed a very high variation among fragments; however, the variation was much higher in the small fragments (Table 1). The sapling density did not show significant difference between large and small fragments for B. excelsa (U = 59; p = 0.095), nor for C. guianensis (U = 60; p = 0.092). However, the sapling density of C. cateniformis was significantly higher in large fragments (U = 73, p = 0.004, Table 1).
3.2 Diameter distribution
Frequency histograms and the cumulative DBH distributions of the species showed different patterns among fragments (Figs. 3, 4 and 5). For B. excelsa (Fig. 3), the adjustments of models applied to each population resulted in a high negative correlation between intercepts and DBH median (-0.94), while slopes had a negative correlation with DBH variance (-0.89). Intercepts and slopes also showed a negative correlation between them (-0.68) (Fig. 6a), i.e., the higher the intercept (or lower the median), the lower the slope (or higher the variance). The L1 and L3 fragments were the only ones with positive s* values (Fig. 7).
Relationship between intercepts and slopes originated from the fit of the binomial logit model between tree diameters and cumulative proportions of the populations of Bertholletia excelsa, Cedrelinga cateniformis and Carapa guianensis studied in large (blue: L1, L2, L3) and small (red: S1, S2, S3) fragments. For C. cateniformis, a plot amplification was performed to verify differences between points very close
The populations of C. cateniformis were all very small, but the adjustments of models applied to each population produced intercepts representing the DBH median in a positive correlation (0.58), while the slopes have a negative correlation with DBH variance (− 0.50). There was also a negative correlation between intercepts and slopes (-0.99) (Fig. 6b), i.e., the greater intercept (greater the median), the lower the slope (greater the variance). S2 was the only fragment that had a positive s* value (3.13), remaining quite distant from the others (Fig. 7). All other fragments had very similar values for intercept, slope and s*, however, it is apparent that among S2, S1, L2, L1, L3 and S3 (in that order) DBH median and variance increased. The populations at the ends of the settlement, S2 and S3, had the lowest number of individuals (2 and 3, respectively); however, DBH variation in S3 was much greater, covering 4 size classes, while in S2, individuals were in two adjacent size classes (Fig. 4).
C. guianensis did not present individuals in one small fragment (S1), and in the other fragments, it presented very small populations, but unlike the small populations of C. cateniformis, the DBH median had a very low correlation with the intercept (− 0.22); correlation with DBH mean was even lower (0.017). However, DBH mode showed a positive correlation (0.660) with the intercept. The slopes had a negative correlation with DBH variance (− 0.99), and there was also a negative correlation between intercepts and slopes (− 0.68) (Fig. 6c), i.e., the greater the intercept (greater the mode), the lower the slope (greater the variance). S3 was the only fragment that showed a positive s* value (Fig. 7), which implies in a smaller population (Fig. 5; only three size classes with a maximum DBH of 57.3 cm) and a lower DBH variance. In other fragments, the values of s* were all negative, but close to zero, with L2 presenting the lowest value (s* = − 1.13), implying in a greater mode (63.66) and a greater (and very high) variance (1115.48).
4 Discussion
4.1 Sapling density
In the PAE, the size of the fragments does not seem to influence the sapling density of B. excelsa and C. guianensis, while the sapling density of C. cateniformis was significantly higher in large fragments. For B. excelsa and C. guianensis, the results suggest that sapling density did not change in the face of fragmentation. This occurred because time since fragmentation was still too short to reveal differences or, perhaps, fragments are large enough to maintain population dynamics (Laurance et al., 2002). Considering that saplings include individuals with height ≥ 10 cm, the hypothesis of a too short time period was discarded, as these individuals germinated in the last reproductive events, i.e., after fragmentation. According to Oszwald et al. (2010), the PAE had a notably marked dynamic cover between 1997 and 2004, when 60% of the changes in land-use types occurred. Consequently, the PAE fragmentation intensified only 20 years before data collection.
When there is a terrestrial matrix in a heterogeneous landscape, with a good amount of original habitat, populations preserve their structures, regardless of the size of the fragments, as the habitat quantity hypothesis suggests (Fahrig, 2013). This cushion effect on the fragmentation allows the movement of the species and the stability of the predator–prey dynamics. Accordingly, it is possible to obtain seed flow and seed dispersal due to functional connectivity within the landscape (Fahrig, 2017). These same mechanisms may be occurring in the PAE fragments.
The attenuated effects of fragmentation have been found for various groups of organisms and in many parts of the world, such as for butterfly assemblages in Tokyo, Japan (Soga & Koike, 2012), spider diversity in southern Judea, Israel (Fahrig, 2013) and for richness of shade-tolerant and intermediary plants in Brazil's Atlantic Forest (Jurinitz, 2010). However, these mild effects of fragmentation should be analyzed with caution, especially considering: (1) the effect of fragment isolation that is determinant in the process of matrix permeability; (2) the use of different methodologies, as they can lead to different and biased results; and (3) approach levels, usually measures of richness ignore species identity (Bueno et al., 2018; Haddad et al., 2017).
Despite the use of the same methodology for the three species considering the specificities of each population structure, as suggested by the aforementioned precautions, the results were different for the sapling density of C. cateniformis, which was lower in small fragments. This pattern difference among species also suggests that the effects of fragmentation cannot be generalized. Each species has a certain reaction bound to its tolerance habitat amplitude (Lees & Peres, 2008) and to ecological processes (e.g., dispersal and predation) that are influenced by the landscape structure and occur at different spatial scales (Smith et al., 2011). In addition, there are environmental changes that promote variations in physical gradients, such as light intensity, humidity reduction and microclimate changes (Leimu et al., 2010).
Such environmental changes should be monitored as they may be sufficient to extinguish some populations locally, especially rare species (like as C. cateniformis), that are more susceptible to the negative effects of fragmentation present in small fragments (Bueno et al., 2018; Laurance & Vasconcelos, 2009). These species require a large area to survive and to contain the random demographic effect, which has influence on birth rates and mortality (Shafer 1981). Unfortunately, little is known about C. cateniformis dynamic in the eastern Amazon.
Sapling density, as well as adult density, also varied greatly among the fragments. This variation is also common across the Amazon and can be caused by different factors, such as the period of data collection, since the dry season can increase the mortality rate of these individuals by up to 65%, as observed for B. excelsa (Zuidema & Boot, 2002), and therefore, sapling density might be lower. Other factors such as the absence of pollinators, pests, diseases, competition and abundance of the dispersers and predators may also be responsible for variation in sapling density (Klimas et al., 2007; Porcher et al., 2018; Tonini et al., 2014; Wadt et al., 2005, 2008). For C. guianensis, two different mechanisms seem to act in this variation: First, the spacing pattern among matrix-trees can be farther in drier areas, decreasing pollination (Cloutier et al., 2007); second, climatic factors (Klimas et al., 2012c). However, in the medium or long term, the average sapling density of C. guianensis remains stable, as was observed in the PAE, where Oliveira et al. (2014) found density similar to the current study (0.43 ind m−2).
4.2 Adult density
The size of the fragments does not seem to influence the adult density of the studied species. Considering that these species have slow growth (Brienen & Zuidema, 2006a; Schöngart et al., 2015, 2017), they need a long time to reach DBH ≥ 10 cm; therefore, the adult density probably represents the populations structure before PAE fragmentation. The large variation found in adult density stands out among the results. This variation is commonly found among tree species in tropical forests, since different factors affect individuals along both their range of distribution in area and time (Tomimatsu & Ohara, 2010). Among the factors, the diversity of the Amazonian biophysical environments is well known (Hoorn et al., 2010). In PAE forests, Zenero et al. (2016) showed that even in soils of similar classification, soil properties can vary greatly. The inherent soil properties (variation in composition and depth) are important to the provision of soil ecosystem services, such as soil storage capacity of plant-available water, as shown by Grimaldi et al. (2014), who studied sample points from PAE in addition to other locations.
Although the adult density varied widely among the studied fragments, the values are as expected for undisturbed forests, which reinforces the idea that this density represents the populations structure before PAE fragmentation. The average density of B. excelsa adult trees in the PAE, for example, was similar to that found in the Tapajós National Forest (0.68 ind ha−1 considering DBH > 15 cm) (Carvalho, 1981). The density of C. guianensis found in this study is also within the range of variation shown by Oliveira et al. (2014), however, well below to those found in other regions of the Amazon (Plowden, 2004; Schwartz et al., 2012). C. guianensis is a geographically widespread species (Pereira & Tonini, 2012) with a competitive and adaptive advantage to different environments; however, it presents lower density in dry land than in flooded areas (Klimas et al., 2007, 2012c). Explaining the variation found in the adult density of C. cateniformis is even more difficult, since it is a species normally considered as rare (Condé et al., 2016; Schulze et al., 2008); therefore, the species is not present in most density studies. However, we can see that in the PAE, density was also close to those found by Schulze et al. (2008) in the Brazilian Amazon, with densities between 0.75 and < 0.01 ind ha−1 considering DBH > 20 cm. In Bolivia, it seems that C. cateniformis has a higher density, as Brienen and Zuidema (2006b) found 0.80 ind ha−1 considering DBH > 50 cm.
4.3 Diameter distribuition
The DBH distribution of adults seems to also not respond to fragmentation, since the values of s* were mixed between small and large fragments for all three species (Fig. 7). For B. excelsa, large fragments as L1 and L3, the only ones with positive s* values, presented populations with a higher median DBH (few juveniles), a wide variety of DBH classes and a more accentuated sigmoidal curve (Fig. 3). The values of s * decrease between L1, L3, S2, S1, L2 and S3 (in that order) and the smaller the s* there is a tendency for populations to change from a more sigmoidal shape, with less juveniles, to a more exponential curve, with a greater predominance of juveniles. The populations in L1 and L2 are closer to a more senescent population, as described by Peres et al. (2003). The population structure of exploited long-lived species, such as B. excelsa, is more sensitive to small changes in certain size classes than to reproduction itself (Zuidema & Boot, 2002).
For C. cateniformis and C. guianensis, correlation between intercept and DBH median or mode was positive, different from that found for B. excelsa, so there was greater caution when analyzing the results, since they were inverted. For these two species, the only positive s* values were found in populations of small fragments (S2 for C. cateniformis and S3 for C. guianensis) (Fig. 7). For C. cateniformis, decrease in s* values between S2, S1, L2, L1, L3 and S3 (in that order) can also be noticed by the change from a more geometric shape, where the populations were very small, with the absence of juveniles in the first class (as S2 and S1) to a more sinusoids shape, in larger populations, with the presence of juveniles, but without their predominance (as L1, L2 and L3) (Fig. 4). S3 presented the smallest s* due to the great variation in DBH; however, the shape of its curve is more similar to the shapes of S1 and S2, with very small populations. Finally, none C. cateniformis populations had a stable structure (Fig. 4).
For C. guianensis, the differences in the shape of the DBH distribution curve among fragments were more profound, so the median did not serve to represent the population size, and the use of mode reflects the large number of individuals present in the same DBH class. The decrease in s* values between S3, L1, S2, L3 and L2 (Fig. 7) can also be noticed by the change in the curves from a more sigmoidal shape, where there were no juveniles (as S3 and L1), to a more logistical curve, with a predominance of juveniles, such as L2, the only one population in C. guianensis that showed a DBH distribution curve typical of sustainable populations (Fig. 5).
A greater quantity of juveniles does not make a population more stable that is why a combined analysis in diameter size and variation helps to better analyze the logistical pattern that should be presented by stable populations. Over time, there may also be a temporal variation in population demographic rates, decreasing the number of juveniles (Virillo et al., 2011), who are commonly affected by different light intensities (Tonini et al., 2008), reproductive events, seedling mortality, climate factors and those linked to pollinators, dispersers and predators (Klimas et al., 2007, 2012c; Porcher et al., 2018; Tonini et al., 2014; Wadt et al., 2005, 2008). These factors can act in non-fragmented areas, but there may be a worsening in fragmented areas (Aguilar et al., 2006; Dirzo et al., 2014; Morellato et al., 2016). Further study of these factors will allow a better understanding of the effect of fragmentation on PAE. In non-fragmented areas, the low density in the first classes of DBH was also found in other B. excelsa populations (Salomão, 2009; Tonini et al., 2014; Zuidema & Boot, 2002).
Another factor that can influence these results are the effects of logging and seed collection, as they affect the recruitment of individuals in the lower classes. In PAE, these factors cannot be disregarded, since besides the seed collection of C. guianensis and B. excelsa, there is also the logging of C. cateniformis and B. excelsa; although prohibited by law, B. excelsa wood extraction is still a common practice in the region (Salomão, 2009). These factors also help explain the differences in the DBH distributions among fragments.
For B. excelsa, studies have shown stability in the DBH distribution even under high seed collection pressure, such as those in the Kayapó indigenous area in the Pará State (Ribeiro et al., 2014a, 2014b); in the community of Rio Trombetas Valley, also in Pará (Scoles & Gribel, 2012); and in Bolivia, where Zuidema and Boot (2002) showed that although 93% of the seeds were collected, this did not limit the finding of individuals in the lower classes. However, seed collection affected the stability of the population in Acre State (Peres et al., 2003). These contradictory results may be related to the history and the intensity of seed collection over time.
4.4 Implications for conservation
The three studied species presented density similar, but their DBH size distributions showed different patterns among the six fragments, with no clear relationship with the size of the fragments. The vast majority of populations were small and with great variation in DBH size, which makes them very vulnerable to seed collection and or logging. Of all species and fragments, only one population of C. guianensis in fragments L2 showed greater stability. Previous study on C. guianensis in the PAE showed density high and diameter distribution similar as stable population (Schwartz et al., 2008); however, the authors did not consider, as in this study, the effect of fragmentation.
For these three species, population viability analysis has been recommended, including important demographic rates such as germination, mortality, recruitment and the effect of seed collection to evaluate changes in population structure, both for adults and for seedlings (Wadt et al., 2005). An adequate management plan is necessary for the sustainability of the exploitation of these species, considering silvicultural treatments adapted to each fragment, since the population structure differs.
The results of this study suggest that when comparing different populations (1) the use of the adults and young densities does not reveal good results, mainly due to the great spatial variation presented by the species; and this great spatial variation is also found in undisturbed forests; (2) in this sense, the use of the analysis of diameter distribution is better, through this analysis it is also possible to compare the population size; (3) in the PAE, all fragments no longer have sustainable populations (except rare situations), probably due to anthropic pressure exerted by agroextractivism. So, it is important to ensure the structural and environmental quality of the fragments for the conservation of the species. In this sense, establishing a simple plan for spatializing logging and seed collection would be recommended, to avoid greater pressure on some fragments and to track demographic fluctuations over time, because some larger populations may be large enough to sustain the population of a given species over a certain period of time, but eventually may become locally extinct due to environment changes, easier exploitation, altered pollination patterns, connectivity loss and so on.
Finally, it is important to expand the studies to other forest fragment sizes and in other rural settlements in the region, since they are a common reality in the Amazon. Likewise, it is necessary to advance in population studies to understand how the use of forest species, even with management plans, influences their structure and spatialization over time.
References
Aguilar, R., Ashworth, L., Galetto, L., & Aizen, M. A. (2006). Plant reproductive susceptibility to habitat fragmentation: Review and synthesis through a meta-analysis. Ecology Letters, 9(8), 968–980.
Alencar, J. D. C., Almeida, R. A. D., & Fernandes, N. P. (1979). Fenologia de espécies florestais em floresta tropical úmida de terra firme na Amazônia Central. Acta Amazonica, 9(1), 163–199.
Barlow, J., Lennox, G. D., Ferreira, J., Berenguer, E., Lees, A. C., Nally, R. M., et al. (2016). Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature, 535, 144–147.
Brienen, R. J., & Zuidema, P. A. (2006a). Lifetime growth patterns and ages of Bolivian rain forest trees obtained by tree ring analysis. Journal of Ecology, 94, 481–493.
Brienen, R. J., & Zuidema, P. A. (2006b). The use of tree rings in tropical forest management: Projecting timber yields of four Bolivian tree species. Forest Ecology and Management, 226(1–3), 256–267.
Bueno, A. S., Dantas, S. M., Henriques, L. M. P., & Peres, C. A. (2018). Ecological traits modulate bird species responses to forest fragmentation in an Amazonian anthropogenic archipelago. Diversity and Distributions, 24(3), 387–402.
Carvalho, J. O. P. (1981). Distribuição diamétrica de espécies comerciais e potenciais em floresta tropical úmida natural na Amazônia. Belém: Embrapa Amazônia Oriental, Séries anteriores (Infoteca-E), 23. https://www.infoteca.cnptia.embrapa.br/bitstream/doc/378780/1/CPATUBP23.pdf.
Cavalcante, M. C., Galetto, L., Maués, M. M., Pacheco Filho, A. J. S., Bomfim, I. G. A., & Freitas, B. M. (2018). Nectar production dynamics and daily pattern of pollinator visits in Brazil nut (Bertholletia excelsa Bonpl.) plantations in Central Amazon: Implications for fruit production. Apidologie, 49, 505–516.
Cloutier, D., Kanashiro, M., Ciampi, A. Y., & Schoen, D. J. (2007). Impact of selective logging on inbreeding and gene dispersal in an Amazonian tree population of Carapa guianensis Aubl. Molecular Ecology, 16(4), 797–809.
Condé, T. M., Tonini, H., da Silva, F., Barni, P. E., Celes, C. H. S., de Araujo, R. F., Campos, M. A. A., & de Miranda, D. L. C. (2016). Padrão espacial de espécies madeireiras da Amazônia pelo método de coordenadas cartesianas e espaciais. Pesquisa Florestal Brasileira, 36(86), 115–125. https://doi.org/10.4336/2016.pfb.36.86.1111
Cotta, J. N., Kainer, K. A., Wadt, L. H., & Staudhammer, C. L. (2008). Shifting cultivation effects on Brazil nut (Bertholletia excelsa) regeneration. Forest Ecology and Management, 256(1–2), 28–35.
Dirzo, R., Young, H. S., Galetti, M., Ceballos, G., Isaac, N. J. B., & Collen, B. (2014). Defaunation in the Anthropocene. Science, 345, 401–406.
Do Vale, I., Miranda, I. S., Mitja, D., Santos, A. M., Lima, T. T. S., & Costa, L. G. S. (2018). Successional processes in agricultural mosaics in the eastern Amazon. Agriculture, Ecosystems and Environment, 256, 51–60.
Ewers, R. M., & Didham, R. K. (2006). Confounding factors in the detection of species responses to habitat fragmentation. Biological Reviews, 81(1), 117–142.
Fahrig, L. (2013). Rethinking patch size and isolation effects: The habitat amount hypothesis. Journal of Biogeography, 40(9), 1649–1663.
Fahrig, L. (2017). Ecological responses to habitat fragmentation per se. Annual Review of Ecology, Evolution, and Systematics, 48, 1–23.
Fahrig, L., Arroyo-Rodríguez, V., Bennett, J. R., Boucher-Lalonde, V., Cazetta, E., Currie, D. J., et al. (2019). Is habitat fragmentation bad for biodiversity? Biological Conservation, 230, 179–186.
Fearnside, P. (2017). Deforestation of the Brazilian Amazon. In Oxford Research Encyclopedia, environmental science. USA: Oxford University Press. https://doi.org/10.1093/acrefore/9780199389414.013.102.
Feeley, K. J., & Silman, M. R. (2009). Extinction risks of Amazonian plant species. Proceedings of the National Academy of Sciences, 106(30), 12382–12387.
Fernandes, N. C. L., Valle, M. L. A., & Calderon, C. M. A. (2018). Physical and Anatomical Characteristics of Cedrela odorata L and Cedrelinga cateniformis Ducke. Floresta e Ambiente. https://doi.org/10.1590/2179-8087.100814
Fletcher, R. J., Jr., Didham, R. K., Banks-Leite, C., Barlow, J., Ewers, R. M., Rosindell, J., et al. (2018). Is habitat fragmentation good for biodiversity? Biological Conservation, 226, 9–15.
Gibson, L., Lynam, A. J., Bradshaw, C. J., He, F., Bickford, D. P., Woodruff, D. S., et al. (2013). Near-complete extinction of native small mammal fauna 25 years after forest fragmentation. Science, 341(6153), 1508–1510.
Grimaldi, M., Oszwald, J., Dolédec, S., Hurtado, M. P., Miranda, I. S., Sartre, X. A., et al. (2014). Ecosystem services of regulation and support in Amazonian pioneer fronts: Searching for landscape drivers. Landscape Ecology, 29, 311–328.
Guariguata, M. R., Cronkleton, P., Duchelle, A. E., & Zuidema, P. A. (2017). Revisiting the ‘cornerstone of Amazonian conservation’: A socioecological assessment of Brazil nut exploitation. Biodiversity and Conservation, 26(9), 2007–2027.
Guedes, M. C., Neves, E. D. S., Rodrigues, E. G., Paiva, P., Costa, J. B. P., Freitas, M. F., et al. (2014). Castanha na roça: Expansão da produção e renovação dos castanhais em áreas de agricultura itinerante no Amapá, Brasil. Boletim Do Museu Paraense Emilio Goeldi Ciências Naturais, 9(2), 381–398.
Haddad, N. M., Brudvig, L. A., Clobert, J., Davies, K. F., Gonzalez, A., Holt, R. D., et al. (2015). Habitat fragmentation and its lasting impact on Earth’s ecosystems. Science Advances, 1(2), 1500052. https://doi.org/10.1126/sciadv.1500052
Haddad, N. M., Gonzalez, A., Brudvig, L. A., Burt, M. A., Levey, D. J., & Damschen, E. I. (2017). Experimental evidence does not support the Habitat Amount Hypothesis. Ecography, 40(1), 48–55. https://doi.org/10.1111/ecog.02535
Henriques, M. G., & Penido, C. (2014). The therapeutic properties of Carapa guianensis. Current Pharmaceutical Design, 20(6), 850–856.
Hoorn, C., Wesselingh, F. P., ter Steege, H., Bermudez, M. A., Mora, A., Sevink, J., et al. (2010). Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science, 330(6006), 927–931.
Hubbell, S. P., He, F., Condit, R., Borda-de-Água, L., Kellner, J., & ter Steege, H. (2008). How many tree species are there in the Amazon and how many of them will go extinct? Proceedings of the National Academy of Sciences, 105(Supplement 1), 11498–11504. https://doi.org/10.1073/pnas.0801915105
IBGE. (2012). Manuais técnicos em geociências: Manual técnico da vegetação brasileira - Sistema fitogeográfico; Inventário das formações florestais e campestres; Técnicas e manejo de coleções botânicas; Procedimentos para mapeamentos. 2ed. Rio de Janeiro: IBGE.
INCRA. (2017). informações gerais sobre os assentamentos da Reforma Agrária; SR 27 – Pará/Marabá. Instituto Nacional de Colonização e Reforma Agrária. http://painel.incra.gov.br/sistemas/Painel/ImprimirPainelAssentamentos.php?cod_sr=27&Parameters%5BPlanilha%5D=Nao&Parameters%5BBox%5D=GERAL&Parameters%5BLinha%5D=4 Accessed 20 June 2019.
INMET. (2017). Estação Meteorológica de Observação de Superfície Automática. Instituto Nacional de Meteorologia - Ministério da Agricultura, Pecuária e Abastecimento, Brasília. http://www.inmet.gov.br/portal/index.php?r=bdmep/bdmep Accessed 24 Feb 2017.
Jurinitz, C.F. (2010). Ecologia das populações de duas espécies arbóreas em fragmentos florestais no Planalto Atlântico Paulista. Universidade de São Paulo, São Paulo. Phd Thesis. http://www.teses.usp.br/teses/disponiveis/41/41134/tde-08112010-101716/en.php Accessed 25 June 2019.
Jurinitz, C. F., Oliveira, A. A., & Bruna, E. M. (2013). Abiotic and biotic influence son early-stage survival in two shadetolerant tree species in brazil’s atlantic forest. Biotropica, 45(6), 728–736.
Klimas, C. A., Cropper, W. P., Jr., Kainer, K. A., & Wadt, L. H. (2012a). Viability of combined timber and non-timber harvests for one species: A Carapa guianensis case study. Ecological Modelling, 246, 147–156.
Klimas, C. A., Kainer, K. A., & Wadt, L. H. (2007). Population structure of Carapa guianensis in two forest types in the southwestern Brazilian Amazon. Forest Ecology and Management, 250(3), 256–265.
Klimas, C. A., Kainer, K. A., & Wadt, L. H. (2012b). The economic value of sustainable seed and timber harvests of multi-use species: An example using Carapa guianensis. Forest Ecology and Management, 268, 81–91.
Klimas, C. A., Kainer, K. A., Wadt, L. H., Staudhammer, C. L., Rigamonte-Azevedo, V., Correia, M. F., et al. (2012c). Control of Carapa guianensis phenology and seed production at multiple scales: A five-year study exploring the influences of tree attributes, habitat heterogeneity and climate cues. Journal of Tropical Ecology, 28(1), 105–118.
Laurance, W. F., Lovejoy, T. E., Vasconcelos, H. L., Bruna, E. M., Didham, R. K., Stouffer, P. C., et al. (2002). Ecosystem decay of Amazonian forest fragments: A 22-year investigation. Conservation Biology, 16(3), 605–618.
Laurance, W. F., & Vasconcelos, H. L. (2009). Conseqüências ecológicas da fragmentação florestal na Amazônia. Oecologia Brasiliensis, 13(3), 434–451.
Lees, A. C., & Peres, C. A. (2008). Avian life-history determinants of local extinction risk in a hyper-fragmented neotropical forest landscape. Animal Conservation, 11(2), 128–137.
Leimu, R., Vergeer, P., Angeloni, F., & Ouborg, N. (2010). Habitat fragmentation, climate change, and inbreeding in plants. Annals of the New York Academy of Sciences, 1195(1), 84–98.
Levis, C., Costa, F., Bongers, F., Peña-Claros, M., Clement, C. R., Junqueira, A. B., et al. (2017). Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science, 355, 925–931.
Lombard, L., Bogale, M., Montenegro, F., Wingfield, B. D., & Wingfield, M. J. (2008). A new bark canker disease of the tropical hardwood tree Cedrelinga cateniformis in Ecuador. Fungal Diversity, 31, 73–81.
Lovejoy, T. E., & Nobre, C. (2018). Amazon Tipping Point. Sci. Adv., 4, eaat2340.
Luna, F. M. S. (2004). Ethnobotany of the communities of the upper Rio Nangaritza. Lyonia, 7(2), 105–122.
Malhi, Y., Gardner, T. A., Goldsmith, G. R., Silman, M. R., & Zelazowski, P. (2014). Tropical forests in the Anthropocene. Annual Review of Environment and Resources, 39, 125–159.
Morellato, L. P. C., Alberton, B., Alvarado, S. T., Borges, B., Buisson, E., Camargo, M. G. G., et al. (2016). Linking plant phenology to conservation biology. Biological Conservation, 195, 60–72.
Newbold, T., Hudson, L. N., Hill, S. L. L., Contu, S., Lysenko, I., Senior, R. A., et al. (2015). Global effects of land use on local terrestrial biodiversity. Nature, 520(7545), 45. https://doi.org/10.1038/nature14324
Oliveira, M. O., Jardim, F. C. S., & Araujo, C. S. (2014). Fruto da floresta: Impacto da coleta de sementes de andiroba (Carapa guianensis) na sustentabilidade das espécies no sudeste do Pará (1st ed.). Novas edições acadêmicas.
Oszwald, J., Lefebvre, A., Arnauld De Sartre, X., Gond, V., Thales, M., & Fretas, R. L. (2010). Analyse des directions de changement des états de surface végétaux pour renseigner la dynamique du front pionnier de Maçaranduba (Brésil) entre 1997 et 2006. Télédétection, 9(2), 97–111. https://hal.archives-ouvertes.fr/hal-00623686
Oszwald, J., Gond, V., Doledec, S., & Lavelle, P. (2011). Identification d’indicateurs de changement d’occupation du sol pour le suivi des mosaïques paysagères. Bois Et Forêts Des Tropiques, 307, 7–21.
Pereira, M. R. N., & Tonini, H. (2012). Fenologia da andiroba (Carapa guianensis Aubl., Meliaceae) no sul do estado de Roraima. Ciência Florestal, 22(1), 47–58.
Peres, C. A., Baider, C., Zuidema, P. A., Wadt, L. H., Kainer, K. A., Gomes-Silva, D. A., et al. (2003). Demographic threats to the sustainability of Brazil nut exploitation. Science, 302(5653), 2112–2114. https://doi.org/10.1126/science.1091698
Plowden, C. (2004). The ecology and harvest of andiroba seeds for oil production in the Brazilian Amazon. Conservation and Society, 2, 251–272. http://www.conservationandsociety.org/text.asp?2004/2/2/251/49329
Porcher, V., Thomas, E., Gomringer, R. C., & Lozano, R. B. (2018). Fire-and distance-dependent recruitment of the Brazil nut in the Peruvian Amazon. Forest Ecology and Management, 427, 52–59.
Portela, R. D. C. Q., & Santos, F. A. M. D. (2007). Produção e espessura da serapilheira na borda e interior de fragmentos florestais de Mata Atlântica de diferentes tamanhos. Brazilian Journal of Botany, 30(2), 271–280.
Ribeiro, M. B. N., Jerozolimski, A., Robert, P., & Magnusson, W. E. (2014a). Brazil nut stock and harvesting at different spatial scales in southeastern Amazonia. Forest Ecology and Management, 319, 67–74.
Ribeiro, M. B. N., Jerozolimski, A., Robert, P., Salles, N. V., Kayapó, B., Pimentel, T. P., et al. (2014b). Anthropogenic landscape in southeastern Amazonia: Contemporary impacts of low-intensity harvesting and dispersal of Brazil nuts by the Kayapó Indigenous people. PloSone, 9(7), 102187. https://doi.org/10.1371/journal.pone.0102187
Ribeiro, M. C., Metzger, J. P., Martensen, A. C., Ponzoni, F. J., & Hirota, M. M. (2009). The Brazilian Atlantic Forest: How much is left, and how is the remaining forest distributed? Implications for Conservation. Biological Conservation, 142(6), 1141–1153.
Salomão, R. P. (2009). Densidade, estrutura e distribuição espacial de castanheira-do-brasil (Bertholletia excelsa H. & B.) em dois platôs de floresta ombrófila densa na Amazônia setentrional brasileira. Boletim do Museu Paraense Emilio Goeldi, Ciências Naturais, 4(1), 11–25.
Salomão, R. P. (2014). A castanheira: História natural e importância socioeconômica. Boletim Do Museu Paraense Emilio Goeldi, Ciências Naturais, 9(2), 259–266.
Santos, M. N., Cunha, H. F. A., Lira-Guedes, A. C., Gomes, S. C. P., & Guedes, M. C. (2014). Saberes tradicionais em uma unidade de conservação localizada em ambiente periurbano de várzea: Etnobiologia da andirobeira (Carapa guianensis Aublet). Boletim Do Museu Paraense Emílio Goeldi, Ciências Humanas, 1(1), 93–108.
Santos, R. S., Coelho-Ferreira, M., Lima, P. G., & Magalhaes, M. P. (2019). Useful plants and their relation to archaeological sites in the Serra de Carajás, Brazil. Anais Da Academia Brasileira De Ciências, 91(1), e20170909. https://doi.org/10.1590/0001-3765201920170909
Scariot, A. (2000). Seedling mortality by litterfall in Amazonian forest fragments. Biotropica, 32(4), 662–669.
Schöngart J., Bräuning A., Barbosa A. C. M. C., Lisi C. S., & de Oliveira J. M. (2017). Dendroecological studies in the neotropics: History, status and future challenges. In M. Amoroso, L. Daniels, P. Baker, & J. Camarero (Eds.), Dendroecology. Ecological Studies (Analysis and Synthesis) (Vol. 231). Cham: Springer. https://doi.org/10.1007/978-3-319-61669-8_3.
Schöngart, J., Gribel, R., Ferreira da Fonseca-Junior, S., & Haugaasen, T. (2015). Age and growth patterns of Brazil nut trees (Bertholletia excelsa Bonpl.) in Amazonia, Brazil. Biotropica, 47(5), 550–558.
Schulze, M., Grogan, J., Landis, R. M., & Vidal, E. (2008). How rare is too rare to harvest? management challenges posed by timber species occurring at low densities in the Brazilian Amazon. Forest Ecology and Management, 256(7), 1443–1457.
Schwartz, G., Lopes, J. C., Kanashiro, M., Mohren, G. M., & Peña-Claros, M. (2014). Disturbance level determines the regeneration of commercial tree species in the eastern Amazon. Biotropica, 46(2), 148–156.
Schwartz, G., Nascimento, N. A., & Menezes, A. J. E. A. (2008). Estrutura populacional de espécies de interesse florestal não-madeireiro no Sudeste do Pará. Brasil. Amazônia: Ciência & Desenvolvimento, 4(7), 117–128.
Schwartz, G., Peña-Claros, M., Lopes, J. C., Mohren, G. M., & Kanashiro, M. (2012). Mid-term effects of reduced-impact logging on the regeneration of seven tree commercial species in the Eastern Amazon. Forest Ecology and Management, 274, 116–125.
Scoles, R., Canto, M. S., Almeida, R. G., & Vieira, D. P. (2016). Sobrevivência e frutificação de Bertholletia excelsa Bonpl. em áreas desmatadas em Oriximiná. Pará. Floresta e Ambiente, 23, 555–564.
Scoles, R., & Gribel, R. (2012). The regeneration of Brazil nut trees in relation to nut harvest intensity in the Trombetas River valley of Northern Amazonia, Brazil. Forest Ecology and Management, 265, 71–81.
Shanley, P., & Medina, G. (2005). Frutíferas e plantas úteis na vida Amazônica. Belém: Imazon.
Slik, J. W. F., Arroyo-Rodríguez, V., Aiba, S., Alvarez-Loayza, P., Alves, L. F., Ashton, P., et al. (2015). An estimate of the number of tropical tree species. Proceedings of the National Academy of Sciences, 112(24), 7472–7477.
Smith, A. C., Fahrig, L., & Francis, C. M. (2011). Landscape size affects the relative importance of habitat amount, habitat fragmentation, and matrix quality on forest birds. Ecography, 34(1), 103–113.
Soga, M., & Koike, S. (2012). Relative importance of quantity, quality and isolation of patches for butterfly diversity in fragmented urban forests. Ecological Research, 27(2), 265–271.
Sousa, T. R., Costa, F. R. C., Bentos, T. V., Leal Filho, N., Mesquita, R. C. G., & Ribeiro, I. O. (2017). The effect of forest fragmentation on the soil seed bank of Central Amazonia. Forest Ecology and Management, 393, 105–112.
Sturges, N. A. (1926). The choice of class interval. J. Am. Statistical Assoc., 21, 65–66.
Ter Steege, H., Pitman, N., Sabatier, D., Baraloto, C., Salomão, R., Guevara, J. E., et al. (2013). Hyperdominance in the Amazonian tree flora. Science, 342(6156), 1243092. https://doi.org/10.1126/science.1243092
Tomimatsu, H., & Ohara, M. (2010). Demographic response of plant populations to habitat fragmentation and temporal environmental variability. Oecologia, 162(4), 903–911.
Tonini, H., Costa, P., & Kaminski, P. E. (2008). Estrutura e produção de duas populações nativas de castanheira-do-Brasil (Bertholletia excelsa O Berg) em Roraima. Floresta, 38(3), 445–457. https://doi.org/10.5380/rf.v38i3.12410
Tonini, H., Lopes, C. E. V., Borges, R. A., Kaminski, P. E., Alves, M. D. S., & Fagundes, P. D. O. (2014). Fenologia, estrutura e produção de sementes em castanhais nativos de Roraima e características socioeconômicas dos extrativistas. Boletim Do Museu Paraense Emilio Goeldi, Ciências Naturais, 9(2), 399–414.
Vasquez, M.L., Rosa-Costa, L.T. (2008). Geologia e recursos minerais do Estado do Pará: Sistema de Informações Geográficas - SIG: texto explicativo dos mapas geológico e tectônico e de recursos minerais do Estado do Pará. Belém: CPRM.
Virillo, C. B., Martins, F. R., Tamashiro, J. Y., & Santos, F. A. M. D. (2011). Is size structure a good measure of future trends of plant populations? An empirical approach using five woody species from the Cerrado (Brazilian savanna). Acta Botanica Brasilica, 25(3), 593–600.
Wadt, L. H., Kainer, K. A., & Gomes-Silva, D. A. (2005). Population structure and nut yield of a Bertholletia excelsa stand in Southwestern Amazonia. Forest Ecology and Management, 211(3), 371–384.
Wadt, L. D. O., Kainer, K. A., Staudhammer, C. L., & Serrano, R. O. P. (2008). Sustainable forest use in Brazilian extractive reserves: Natural regeneration of Brazil nut in exploited populations. Biological Conservation, 141(1), 332–346.
Zenero, M. D. O., Silva, L. F. S. D., Castilho, S. C. D. P., Vidal, A., Grimaldi, M., & Cooper, M. (2016). Characterization and classification of soils under forest and pasture in an agroextractivist project in eastern Amazonia. Revista Brasileira De Ciência Do Solo, 40, e0160165. https://doi.org/10.1590/18069657rbcs20160165
Zuidema, P. A., & Boot, R. G. (2002). Demography of the Brazil nut tree (Bertholletia excelsa) in the Bolivian Amazon: Impact of seed extraction on recruitment and population dynamics. Journal of Tropical Ecology, 18(1), 1–31.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oliveira, M.G., Araujo, C.S., Do Vale, I. et al. Tree population structure in fragments of different sizes in the Eastern Amazon. Environ Dev Sustain 24, 5743–5763 (2022). https://doi.org/10.1007/s10668-021-01681-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10668-021-01681-w