Abstract
Different fractions of organic matter in surface sediments from three transects along the eastern margin of the Arabian Sea (AS) were quantified to determine the sources of organic matter, and also to study its impact on microbial community structure. From the extensive analyses of different biochemical parameters, it was evident that the distribution of total carbohydrate (TCHO), total neutral carbohydrate (TNCHO), proteins, lipids, and uronic acids (URA) concentrations and yield (% TCHO-C/TOC) are affected by organic matter (OM) sources and microbial degradation of sedimentary OM. Monosaccharide compositions from surface sediment was quantified to assess the sources and diagenetic fate of carbohydrates, suggesting that the deoxysugars (rhamnose plus fucose) had significant inverse relationship (r = 0.928, n = 13, p < 0.001) with hexoses (mannose plus galactose plus glucose) and positive relationship (r = 0.828, n = 13, p < 0.001) with pentoses (ribose plus arabinose plus xylose). This shows that marine microorganisms are the source of carbohydrates and there is no influence of terrestrial OM along the eastern margin of AS. During the degradation of algal material, the hexoses seem to be preferentially used by heterotrophic organisms in this region. Arabinose plus galactose (glucose free wt %) values between 28 and 64 wt% indicate that OM was derived from phytoplankton, zooplankton, and non-woody tissues. In the principal component analysis, rhamnose, fucose, and ribose form one cluster of positive loadings while glucose, galactose, and mannose form another cluster of negative loadings which suggest that during OM sinking process, hexoses were removed resulting in increase in bacterial biomass and microbial sugars. Results indicate sediment OM to be derived from marine microbial source along the eastern margin of AS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arabian Sea is among the most productive marine regions due to intense seasonal upwelling, as well as open water upwelling, deeper mixed layer, and seasonal reversal of surface circulation (Wyrtki, 1971; Madhupratap et al., 1996; Shetye & Gouveia, 1998; Prasanna Kumar et al., 2002; Naqvi et al., 2006). It is also one of the largest water body of oxygen-deficient waters (Wyrtki, 1971; Naqvi et al., 2006). Furthermore, it experiences quite a large precipitation, as high as ~ 400 cm during four summer months between June and September (Agnihotri & Kurian, 2008). The freshwater inputs result in very strong but shallow thermohaline stratification. Yet, the high nutrient concentrations in the runoff and freshly upwelled water induce high surface biological productivity (Madhupratap et al., 1996; Naqvi et al., 2006). The low oxygen and high productivity in the Arabian Sea favor accumulation of organic matter (OM) in the continental margin (Hedges et al., 1999; Naqvi et al., 2006; Sánchez et al., 2013). Such high biological productivity (Madhupratap et al., 1996, 2003) and sedimentation rates (Cowie & Hedges, 1994) influence the carbon burial/flux and composition of many biochemical compounds (Fernandes et al., 2014). For instance, organic content and humic acids (Sardessai, 1994) in the Arabian Sea sediment show distinct regional variations along the western margin of India.
The OM in the continental margin sediments can be supplied from autochthonous sources such as phytoplankton and allochthonous sources such as terrestrial inputs by rivers (Hedges et al., 1997; Zhang et al., 2014). Bulk organic carbon, nitrogen, stable carbon, nitrogen isotopes, and C:N ratios or contents of humic material are used widely to elucidate the source and fate of OM in the terrestrial, estuarine, coastal regions, and continental margins (Bhosle & Dhople, 1988; Goni et al., 2003; Guo et al., 2020; Khodse et al., 2008; Krishna et al., 2013; Pan et al., 2019). The major concern with these methods are the changes in C/N ratio need not be because of preferential loss of nitrogen but it can be also due to source change and inorganic nitrogen immobilization. Humic materials are operationally defined and are chemically uncertain, thus humic materials need not inevitably reflect bioavailability. Organic carbon used for comparing diagenetic maturity can complicate the interpretation due to physical processes (Cowie & Hedges, 1984).
Biopolymers derived from decayed organic matter may be more advantageous to study the stages of degradation and investigate diagenetic fate of organic matter as they differ in solubility, composition, and resistance to microbial attack. Compared to other biopolymers such as lipids, chlorophyll, lignin which are mostly process specific, carbohydrates and proteins offer the potential benefit as they are important organic components of marine as well as terrestrial organisms.
Monosaccharide distribution on sedimentary organic matter (SOM), particulate organic matter (POM), dissolve organic matter (DOM), and humic substances in coastal and oceanic environments helps in understanding their diagenetic fate and nature of organic matter (Amon & Benner, 2003; Benner & Opsahl, 2001; Khodse et al., 2008; Ogier et al., 2001; Quijada et al., 2015; Smith et al., 2021; Tareq & Ohta, 2011; Ware et al., 2022; Zhu et al., 2020). Individual sugars and sugar ratios are used to differentiate marine, terrestrial, silicious, and carbonaceous inputs to the particulate matter and sediments (Cowie & Hedges, 1984; Ogier et al., 2001; de Cunha et al., 2002; Duan et al., 2017; Nouara et al., 2019). Characterization of OM such as carbohydrate concentration and monosaccharide composition can provide useful information on the origin of OM in marine sediments.
In the present study, spatial variability of biomolecules such as carbohydrates, proteins, and lipids have been analyzed; quantitative determinations of the monosaccharides is done with the aim to discern sources and fate of these molecules in the sediments from the eastern Arabian Sea continental margin.
Materials and methods
Sample collection
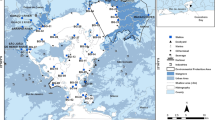
Using a box corer, 13 box cores were collected during the ocean research vessel (ORV) Sindhu Sankalp cruise (SSK-046, February, 2013) from different locations in the eastern Arabian Sea (Fig. 1). Immediately after collection, the cores were sectioned at 5 cm intervals. The top 5 cm sections were lyophilized and ground to a fine powder using agate pestle and mortar, and stored at – 20 °C until analysis.
The sampling locations are depicted graphically. The sampling stations of Goa coast were G5, GS1, GS4, GS5, and GS6, and the sediments were collected at depths of 26 m, 198 m, 780 m, 1045 m, and 1208 m, respectively. The sampling stations on the Mangalore coast were MS1, MS2, MS8, and MS9, and the sediments were collected at 202 m, 418 m, 1798 m, and 1986 m, respectively. COS1, COS2, COS8, and COS9 from the Cochin coast served as sampling stations, and sediments were collected at depths of 200 m, 400 m, 1780 m, and 2000 m, respectively
Analysis of various bulk parameters
Total organic carbon (TOC), total nitrogen (TN), total carbohydrates (TCHO), total neutral carbohydrates (TNCHO), total proteins, total lipids, and total uronic acids (URA) were quantified by following the standard methods described in Khodse et al. (2008). Briefly, sediment samples were treated with 1 N HCl to remove the inorganic carbon and traces. HCl was removed by washing the sediments several times with UV-Milli-Q water. The sediments were dried at 60 °C and then used for TOC and TN analysis. A known quantity of the sediment was packed into a tin foil and analyzed for TOC and TN using a NCS analyzer (CE Instruments, Model-2500) and 2,5,bis-(5-tertbutyl-benzoxazol-2-yl)-thiophen (C26H26N2O2S) was used as a standard.
TCHO was analyzed using the phenol–sulfuric acid method of Dubois et al. (1956) as described in Khodse et al. (2008). TNCHO concentration and composition were determined using a capillary gas chromatographic (GC) method described in Khodse et al. (2008). The sample was treated with 12 M H2SO4 at room temperature for 2 h and diluted to 1.2 M H2SO4 using cold UV-Milli-Q water, flushed with N2, sealed and hydrolyzed for 3 h at 100 °C. After cooling, an internal standard (inositol) was added. The sample was neutralized, treated with NaBH4, acetylated, and analyzed using a Shimadzu GC Model-GC-2010 equipped with a flame ionization detector (FID), a programmable on-column injector and a fused silica column coated with CPSil-88 (25 m, i.d. 0.32 mm). Both the detector and injector were maintained at 300 °C. The oven temperature was programmed as follows: 70 to 150 °C at 30 °C/min and then at 3 °C/min to 230 °C, at which it was maintained for 10 min. Quantification of TNCHO was achieved by peak area integration using the data handling system installed in the instrument. The TNCHO concentration is defined as the sum of all the identified aldoses. The contribution of individual aldoses to TNCHO is expressed as wt%. Analytical reproducibility in four replicate samples of the GC method was ± 8.9%. Blank sample was treated and analyzed using same procedure. The precision of the analytical method based on 6 replicates was better than ± 6%. The detection limits were 0.021–0.143 mg/ml for sugars analyzed.
Total proteins were extracted from the sediments using 1 N NaOH, and were analyzed by the bicinchoninic acid method (Smith et al., 1985). Total lipids from sediment samples were extracted using a modified method of Bligh and Dyer (1959) described in Harji et al. (2008). Briefly, using dichloromethane (DCM):methanol (MeOH) (2:1), freeze-dried sediment samples were ultrasonically extracted (15 × 3 min). This step was repeated two more times. To remove any traces of water, the extracts were pooled, filtered using Whatman No. 1 filter paper, and treated with anhydrous sodium sulfate. The extract was prepared to a known volume (1 ml) by using rotary evaporator at 40 °C under reduced pressure. The pre-weighed piece of Whatman filter paper was then applied with a known aliquot. The paper was weighed after drying in a vacuum desiccator. The weight of the total lipids was calculated by subtracting the weight of the empty Whatman paper from the weight of paper with solvent extract (Zaghden et al., 2005). Acidic carbohydrate such as TURA was analyzed according to the method of Filisctti-Cozzi and Carpita (1991). Using this method, more than 90% of the added uronic acid standard (glucuronic acid) could be recovered. Analytical variability of the method based on three replicates was less than 10%.
Statistical analysis
Correlation coefficients between the parameters were calculated using Excel software program. To evaluate spatial variations, paired t test was carried out using STATISTICA. Principal component analysis (PCA) was done on concentrations of TCHO, protein, lipid, TNCHO, URA, and individual monosaccharides to decipher the OM source and its diagenetic processes. PCA was performed using the statistical software package version 5. The data matrix used for PCA consisted of individual degradation indicators. The raw data matrix was normalized to nullify the influence of the components with higher values. Required normalization was done using the log transformation of the parameters and factors were extracted when the eigenvalues were more than 1.
Results
Variability in TOC and TN
The TOC and TN varied from 30.1 to 95.7 mg g−1 dry wt and 0.5 to 7.1 mg g−1 dry wt respectively (Fig. 2a, b). TOC values were generally higher in the slope sediments. TOC concentrations showed large variation within transect. Their highest observed concentrations were in the sediment, GS6 off Goa. Large variations in TOC/TN ratios from 9.5 to 47.2 were evident (Fig. 2c) with the lowest ratio of 9.5 in the sample from COS9 (2000 m) and the highest of 47.2 in the sediment (200 m) from GS1 (Fig. 2c). TOC/TN ratio significantly decreased from 47.2 (200 m) to 13.4 (1200 m) for off Goa sediments. TOC/TN for Mangalore and Kochi had small variation with increasing depth (Fig. 2c).
Total carbohydrates
TCHO concentration varied with respect to the sampling location without exhibiting any particular trend TCHO concentrations varied from station to station with the maximum of 13.44 mg g−1 dry wt at COS2 (400 m) and the minimum 2.88 mg g−1 at COS1 (200 m) (Fig. 3). TCHO concentrations generally decreased with increasing water depth (Fig. 3) except at two (MS1 and COS1) locations. Higher concentrations of TCHO (mean = 9.8 ± 4.8 mg g−1 dry wt) were observed off Kochi than off Mangalore (mean = 6.6 ± 3.8 mg g−1 dry wt) and Goa (mean = 8.3 ± 1.9 mg g−1 dry wt) (Fig. 3). The yield accounted for 1.5 to 12.49% in these sediments (Fig. 5a). TCHO yields decreased with increasing water column depth in the Goa sediments as well as in the Kochi sediments (Fig. 5a). There was no particular trend in TCHO yields in sediments off Mangalore.
Total proteins
Analogous to TCHO distribution pattern of total protein concentrations varied from 0.44 to 9.73 mg g−1 dry wt sediment (Fig. 3) and accounted for 0.3 to 10% of TOC (Fig. 5a). At MS2, protein content was high (9.23 mg g−1 dry wt) compared to other stations. The ratios of protein-carbon to TOC showed significant spatial variations and followed a trend similar to those of TCHO (Fig. 5a). Highest protein/TCHO ratio was at MS2 and the lowest at COS1 (Fig. 4). Protein/TCHO ratio decreased with increasing depth off Goa and no particular trend was seen in protein/TCHO ratios in the samples off Mangalore or Kochi (Fig. 4).
Total lipids
With quite a lot of variability between stations, total lipids varied from 5.0 to 13.0 mg g−1 dry wt sediment (Fig. 3). The highest concentration was recorded at MS2 and the lowest at MS9. Lipid-carbon accounted for 3.1% to 10.0% of TOC (Fig. 5a). Lipid/TCHO ratio, an indicator of metabolic differences of microorganisms as well as preservation of lipids in the sediment organic matter, increased with increasing water depth for sediments off Goa and showed a decreasing trend in sediments off Mangalore and Kochi (Fig. 4). Corroborating with low TCHO and total proteins, the lipid/TCHO ratio was high at MS1.
Spatial variation in carbon contribution of total carbohydrate-carbon (TCHO-C); protein-C, total lipid-C (a), total neutral sugars-carbon (TNCHO-C), uronic acid-carbon (URA-C), and total labile carbon (sum of all biochemical-carbon) (b) in the surface sediments along the eastern margin of the Arabian Sea
Total neutral carbohydrates
Large variability in total neutral carbohydrate (TNCHO) concentrations was evident in all the sediments analyzed during this study (Fig. 3). In sediments off Goa, their concentrations decreased with increasing water depth (Fig. 3). No such particular trend was seen in samples from off Mangalore and Kochi (Fig. 3). Higher concentrations were recorded at COS9 7.4 mg g−1 (Fig. 3). TNCHO-C yield ranged from 0.2 to 3.7% of TOC (Fig. 5b). TNCHO yield decreased with increasing depth for only off Goa sediments. TNCHO yield increased with sediment depth for off Mangalore. Kochi sediments not followed any particular trend for TNCHO yield.
Total uronic acids
Uronic acids are acidic carbohydrates that play an important role in marine sediments. URA concentration ranged from 0.18 to 1.92 mg g−1 dry wt sediment (Fig. 3). Generally, higher concentrations of URA were observed at MS2, MS8, COS2, and COS8 (Fig. 3). URA-C yield varied from 0.11 to 1.67% (Fig. 5b). URA/TCHO ratio decreased in sediments in sediments only off Kochi (Fig. 4).
Concentrations of monosaccharides
Capillary gas chromatographic analysis revealed the presences of arabinose, ribose, galactose, glucose, xylose, rhamnose, fucose, and mannose in all the surface sediments (Fig. 6). The overall composition of different sugars was quite different in the sediments off Goa, Mangalore, and Kochi (Figs. 6 and 8). Galactose was the most abundant monosaccharide (19 to 40 wt %), followed by glucose (10 to 37 wt %) and mannose (9 to 22 wt %) (Fig. 6). Among other sugars rhamnose (1 to 12 wt %), fucose (1 to 9 wt %), arabinose (3 to 13 wt %), xylose (0 to 16 wt %), and ribose (1 to 18 wt %) were contributed to TNCHO (Fig. 6). Lower concentration of glucose and high deoxysugars (rhamnose plus fucose) and ribose were recorded at COS8 (Fig. 6). Carbohydrate digenetic parameters were used to investigate the source of OM (Table 2). Monosaccharide composition was quite variable in the sediments off Goa, Mangalore, and Kochi (Fig. 7). The trend of average concentration of rhamnose, fucose, ribose, and xylose were increased, whereas arabinose, galactose, and glucose were decreased in off Goa, Mangalore, and Kochi sediments (Fig. 7).
Variation in the average monosaccharide composition (wt%) in the sediments collected along the eastern margin of the Arabian Sea. Abbreviations used are given in the legend of Fig. 6
Carbohydrate digenetic parameters were used to discern the source of OM (Table 3). Monosaccharide concentration expressed in wt% to investigate the OM sources in surface sediments of eastern Arabian Sea (Table 3). The ratio of rhamnose plus fucose to arabinose plus xylose varied 0.8 to 1.6 in surface sediment. The contribution of ribose plus fucose and arabinose plus galactose were ranged from 11.3 to 21.5 and 38.3 to 54.0 in all three transect sediments (Table 3). Deoxysugars had significant positive correlation (arabinose plus xylose = 1.333 (deoxy sugars) − 28.16, r = 0.962, n = 13, p < 0.001) with arabinose and xylose in all three transect. Sedimentary sugars and sugars ratios were compared with diagenetic indicators used for the identification of sedimentary OM (Table 2). Deoxysugars showed significant negative correlation with hexoses, galactose. Average monosaccharide composition of each transect was quite variable in the sediments off Goa, Mangalore, and Kochi (Fig. 7). The trend of average concentration of rhamnose, fucose, ribose, and xylose increased, whereas arabinose, galactose, and glucose decreased in off Goa, Mangalore, and Kochi sediments (Fig. 7).
In PCA analysis, two principal components were recognized that accounted for 62% of the total variance. The first principal component (PC1) accounted for 45% and the second principal component (PC2) accounted for 17% of total variance. Plot of PCA loadings of sediment component clearly separates the variables into three clusters and thus providing better insight into the relationships that exist among the variables (Fig. 8). Rhamnose, fucose, and ribose clustered together and projected positively on PC1. These results indicate high load of microbial OM in the samples. On the other hand, negative loadings of glucose, galactose, and mannose on PC1 suggest their utilization during degradation processes. TCHO, proteins, and URA are positively correlated and clustered together (Fig. 8).
Principal componant analysis (PCA) loading factors of TCHO, protein, URA, TNCHO, lipid, rham, fuc, rib, gal, man, glu, ara, and xyl in the surface sediment collected along the eastern margin of the Arabian Sea. The variance of each factor is shown on X and Y axis. Abbreviations used are given in the legends of Figs. 2 and 5
Discussion
Diagenetic pattern of organic matter in the sediments
Spatial and regional differences of TOC in the sediments are probably due to variable accumulation rates and preservation of OM in the sediments. The main reason for it could be the low-oxygen waters on the sea floor between 200 and 1500 m water depth and also the high sedimentation rates (Naqvi et al., 2006; Paropkari et al., 1992) in this area. Variability of TOC and TN in the sediments off Goa, Mangalore, and Kochi is also attributable to the difference in organic matter source and primary productivity along the eastern margin of the Arabian Sea. TOC content in sediment depends on sediment texture (Prakash et al., 1999; Sakhare, 2007) and inputs from terrestrial OM (Cowie & Hedges, 1984; Krishna et al., 2013). Soil texture is the relative ratio between percentage of sand, silt, and clay in a soil mass; fine-grained sediments like sandy loam and sandy clay loam have higher OC content than sand; soil organic matter is reported to increases with the increase of soil clay contents (Hartati & Sudarmadji, 2016). Overall, TN in sediment samples did not follow similar trend of TOC which implies that TOC and TN have different origins. As Cowie and Hedges (1996) suggests, observed high TN at GS6 may favor higher bacterial biomass during the degradation of OM or/ immobilization of nitrogen.
The TOC/TN ratio is a useful indicator and helpful for recognizing the digenetic state of OM. Usually in the terrestrial OM, the C/N ratio ranges from 20 to 200 (Hedges et al., 1986; Kim et al., 2006) unlike the low ratio of < 4 to 6 that is autochthonously produced in marine ecosystem (Elser et al., 2000). The OM rich in nitrogenous material such as microalgae with low TOC/TN ratio is known to favor net bacterial mineralization, whereas those poor in nitrogen such as of terrestrial origin with high TOC/TN ratio favors net bacterial immobilization (Kristensen et al., 1995). Lobbes et al. (2000) proposes TOC/TN ratio of 9.5 to fresh OM derived from phytoplankton and bacteria., while any ratio above 9.5 must be implicated due to degraded OM derived from marine detrital material (Lobbes et al., 2000). In our study, COS9 showed TOC/TN ration of 9.5 while other stations were in the range of 10 to 20. TOC/TN ratio at GS1 and COS8 showed interesting results, the ratio increased to 47. This contrasting behavior may be due to the abundance of glycine reach plankton in the sediment sample. This behavior is explained in surface sediments of BOB-6 reported by Fernandes et al. (2014), where high proportion of glycine was reported in the sediment samples showing more degraded OM at surface sample compared to deeper sediments. High proportion of glycine due to glycine-rich plankton was also responsible for the low Degradation index values in the sediments of Southern Ocean (Ingalls et al., 2003).
Distribution of carbohydrates in surface sediments
Carbohydrates are constituents that act as storage and structural polymers in marine and terrestrial sources. Storage carbohydrates are labile and preferentially utilized by heterotrophic organisms (Hernes et al., 1996; Khodse & Bhosle, 2011) and therefore accumulation of relatively inert structural carbohydrates in marine sediments (Burdige et al., 2000; Khodse et al., 2008; Lazareva & Romankevich, 2012) are observed. Sedimentary carbohydrates are important as energy source for benthic and many heterotrophic organisms (Hernes et al., 1996; Lazareva & Romankevich, 2012). TCHO and TNCHO differences are recorded in the sediments off Goa, Mangalore, and Kochi, suggesting differences in OM quality and degradation state. These TCHO variability can be linked with the source of carbohydrate such as 40% of bacteria and 75 wt% of vascular plants, 20–40 wt% of phytoplankton (Parsons et al., 1984), 3 to 53% in benthic organisms (Lazareva & Romankevich, 2012), and 3–26% of sedimentary organic carbon (Burdige et al., 2000; Khodse et al., 2008). TNCHO are more labile and preferentially removed by heterotrophic organisms, resulting in the fewer structural carbohydrates in sediments (Hernes et al., 1996). TCHO concentrations in Arabian Sea sediments are higher than those recorded earlier for the Bay of Bengal and lower than Cretan Sea (Table 1). This suggests higher TCHO preservation measured in the Arabian Sea sediments probably because of higher phytoplankton productivity in the Arabian Sea (Prasanna Kumar et al., 2002) and lower oxygen concentration in the water sediment interface (Alagarsamy, 2003; Naqvi et al., 2006). Low TOC/TN ratio associated with higher carbohydrate concentration is to be taken as indicative of marine OM as the source of carbohydrates.
As Cowie and Hedges (1984) reported, the high TOC/TN ratio associated with low TCHO implies terrestrial/degraded OM as source of carbohydrate in these sediments. Youssef et al. (2014) reported that carbohydrate distribution was also affected by inorganic mineral deposits such as carbonate, fluoride, magnesium, and calcium content in the surface sediments. Lazareva and Romankevich (2012) suggested that high TCHO in benthic organisms is attributed to high abundance of microbenthic polychaete species in Kochi sediments which might be responsible for variability of biochemical parameters in the sediments (Musale & Desai, 2011).
Distribution of total proteins in surface sediments
In sediments, proteins are indicators of microbial degradation processes (Ragusa et al., 2004; Romankevich, 1984). At the water–sediment interface, the proteins are important energy source for the benthic organisms. Sedimentary protein concentrations are influenced by several factors like phytoplankton abundance, species compositions, and degradation state of OM. Protein concentrations and protein/TCHO ratios were higher at MS2, COS2, and COS8 perhaps due to higher protein in the OM produced during early growth phase (Myklestad, 1977). Diatoms produce more protein during early growth phase and more TCHO in stationary growth phase due to depleted nutrient concentration (Myklestad, 1977; D’souza & Bhosle, 2001). Proteins are mineralized faster than carbohydrate, leading to higher amount of fresh OM (Isla et al., 2006). Earlier studies suggest that protein/TCHO ratio is high in the productive areas such as estuaries and coastal regions (Pusceddu et al., 1999; Isla et al., 2006). Protein/TCHO ratios in sediments off Goa, Mangalore, and Kochi were lower than those reported earlier from other marine sites (Fabiano & Danovaro, 1999; Isla et al., 2006; Neira et al., 2001). Large variations in protein concentrations and protein/TCHO ratios in sediments are due to the variability in phytoplankton productivity, terrestrial inputs, and microbial degradation state of OM (Hernes et al., 1996; Ittekkot et al., 1984). Low protein/TCHO ratio at MS1 and COS1 indicates that the OM was derived during early growth stage or aged OM compared to other stations (D’Souza et al., 2005).
Distribution of total lipids in surface sediments
Lipids are important components of phytoplankton and bacterial cells. Total lipids and lipid/TCHO ratio in sediments have been used to describe the energetic quality of OM (Grémare et al., 2002). Higher lipid concentration of 13 mg g−1dry wt observed at MS2 coupled with low C/N ratio and high concentration of TCHO and protein may imply fresh derivation of OM from marine detritus (Henderson et al., 1991). Muhlebach and Weber (1998) reported that zooplankton fecal pellets concentrate lipids (e.g., Sterols) and reduce nitrogen during the transport of OM into the sediments. Sedimentary lipid concentration reported in this study is relatively higher compared to other locations (Table 1). For instance, total lipid concentration can be influenced by several factors viz. abundance of phytoplankton, bacteria and terrestrial plant material (Harji et al., 2010).
Uronic acids
Many marine organisms including bacteria, fungi, phytoplankton, microalgae, as well as plants and animals produce uronic acid (Abad et al., 2011; Bergamaschi et al., 1999; Decho, 1990). Available information on the distribution and cycling of uronic acids in the marine environment is quite sparse. URA showed significant positive correlation (R = 0.914, p < 0.001) with TCHO and (R = 0.847, p < 0.001) proteins suggesting that these compounds originated from common origin. URA concentrations and URA/TCHO ratios observed from the eastern Arabian Sea are relatively lower than earlier reported from surface sediments (Khodse et al., 2008). Variations of URA/TCHO ratios from sediments off Goa, Mangalore, and Kochi are not consistent with any particular trend with increasing water depth suggesting that URA are not accumulated owing to their utilization by heterotrophic organisms. Due to the negative charge, URA forms a complex with sedimentary particles, thus microbial utilization and degradation and ionic binding are the factors that may influence the concentration of URA in the marine sediments.
Normalized carbon concentration of TCHO, TNCHO, proteins, lipids, and URA and diagenetic state of organic matter
Normalized carbon compound (TCHO-C/TOC %) concentrations allow estimating their reactivity with respect to total organic material and to understand the digenetic state of organic matter (Kerhervé et al., 2002; Kaiser & Benner, 2009). Biological polymeric carbon (BPC) is considered as sum of TCHO, TNCHO, proteins, lipids, and URA-carbon and accounted 7.1 to 37.2% (mean = 18.3 ± 9.1) of TOC accumulated in the bottom sediments. BPC supports the feeder communities which get benefited from the highly nutritious food source. The TCHO, TNCHO, proteins, lipids, and URA carbon contribution is higher off Kochi (except# GS) sediments probably due to higher phytoplankton abundance (Sardessai, 1994). Higher TCHO, proteins, lipids, and URA yield associated with low TOC/TN (12.1) at GS implies the presence of higher quantities of microbially derived material at this station. The BPC carbon yield is 37% of TOC; this value is lower than those reported (70% of TOC) for sediments in other environments (Grémare et al., 2002; Isla et al., 2006).
Surface sediment carbohydrate sources
Individual sugar and sugar ratios allow distinguishing marine and terrestrial sources because neutral sugar pattern for metabolically active organisms are more variable than vascular plant tissue (Cowie & Hedges, 1984; Schulz and Boyle, 2005). Ribose plus fucose are diagnostic components to separate marine and terrestrial carbohydrate sources. Ribose plus fucose accounted > 10 wt% of TNCHO off Goa, Mangalore, and Kochi sediments which indicates that marine OM is the source of TNCHO (Table 2). Fucose is a major component of phytoplankton and bacteria and rarely present in terrestrial plants (Cowie & Hedges, 1984; Kappelmann et al., 2019). Ribose is a vital component of nucleotides which is commonly found in small organisms than terrestrial plants. Glucose content in surface sediments varied from 10 to 36 wt% of TNCHO, and this wide variation is reported due to the heterotrophic removal of glucose during the transport of surface OM to deep sediments (Ittekkot et al., 1984). Low glucose and high abundance of rhamnose, fucose, and ribose recorded at COS8 implies loss of glucose, also supported by Opsahl and Benner (1999). Low glucose in water column is explained by Ogier et al. (2001), where the author mentioned that glucose being a storage polysaccharide for cyanobacteria, during its decay glucose can be easily removed by aquatic biota during sinking. Also, lower abundance of glucose indicates preferential or selective removal of glucose by heterotrophic microorganisms (Khodse & Bhosle, 2012).
Xylose and arabinose are major components of terrestrial plants than marine organisms (D’Souza et al., 2005). The ratio of rhamnose plus fucose to arabinose plus xylose is > 0.5 off Goa, Mangalore, and Kochi suggesting marine microbial biomass as source of carbohydrates (He et al., 2010). Galactose was most abundant in TNCHO pool. Bacteria and diatoms cell wall polysaccharides are major source of galactose (Bernaerts et al., 2018; Decho, 1990). Both arabinose plus galactose contribution in sediments were used to investigate woody and non-woody source of carbohydrates. Pectin rich non-woody tissue (leaves and grasses) have more arabinose and galactose monomers than woody (Aspinall, 1970; Kögel-Knabner, 2002). The contributions of both monomers in Table 2 reflect non-wood (phytoplankton, zooplankton, angiosperm leaves, and grasses) carbohydrate source (Guggenberger et al., 1994; Tareq & Ohta, 2011) Table 3.
Monosaccharides in sediments off Goa, Mangalore, and Kochi (Fig. 7) imply that monosaccharide composition is controlled by microbial processes in the water sediment interface (Guggenberger et al., 1994; Opsahl & Benner, 1999; Panagiotopoulos & Sempere, 2005a, b). Rhamnose, fucose, and ribose increased from Goa to Kochi (Fig. 7) and may be due to the higher phytoplankton productivity (Jyothibabu et al., 2010; Madhupratap et al., 1996) and bacterial abundance () in southern than northern locations. The Arabian Sea is known for oxygen minimum zone (200 m to 1500 m) along the eastern margin. Perhaps rhamnose, fucose, and ribose are mostly derived by heterotrophic bacteria growing in anoxic water during the organic matter degradation (Johnson & Cummins, 1972; Ogier et al., 2001). The preservation of microbial synthesize sugars might have supported preferential consumption of protein rather than TCHO during anoxic degradation of OM (Harvey et al, 1995).
PCA is useful to suggest high positive factors loading for ribose, fucose, rhamnose, URA, protein, and TCHO, which indicate that their quantities are affected by fresh OM derived from marine microbial source. Marine phytoplankton and bacteria contain large amount of carbohydrates viz. rhamnose, fucose, ribose (Hicks et al., 1994; Bergamaschi et al., 1999; D’Souza et al., 2005; Khodse & Bhosle, 2010), URA, and proteins (Khodse & Bhosle, 2010). The negative loadings for mannose, galactose glucose, and TNCHO are useful to interpret simultaneous decrease of these storage sugars that are readily removed by in situ organisms during sinking (Handa, 1969; Panagiotopoulos & Sempere, 2005a, b). More studies are needed for understanding the biochemical preservation, fate, and cycling in the Arabian Sea sediments.
Conclusions
Preservation processes of biochemical compositions in the surface sediments allow us to investigate the sources and variability of OM concentration. In situ environmental and microbial processes in the eastern margin of the Arabian Sea appear to be the governing factors responsible for the observed variability in all of the bulk OM parameters. Terrestrial sources seem to be important as far as their contribution to preserved OM in this part of the eastern margin of the Arabian Sea. Concentrations and carbon-normalized yields of TCHO, TNCHO, proteins, URA, and lipids showed wide spatial variations. This can be attributed to differences in biological production, sources, terrestrial inputs, and microbial degradation. Higher concentration of TCHO, proteins, URA, rhamnose, fucose, and ribose could be linked to higher phytoplankton productivity and bacterial abundance in the southern location. Carbohydrate digenetic parameters suggest that sedimentary OM is derived from marine origin and non-woody source material. Further, persistently low oxygen concentration in the surface layers of the water column might be responsible for distribution and preservation of biochemical compounds in the water–sediment interface in the eastern Arabian Sea.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Abad, M. J., Bedoya, L. M., & Bermejo, A. P. (2011). Marine compounds and their antimicrobial activities. Science against Microbial Pathogens: Communicating Current Research and Technological Advances, 51, 1293–1306.
Agnihotri, R., & Kurian, S. (2008). Recently sedimentary records from eastern Arabian Sea: Implications to Holocene monsoonal variability. Earth Science India, 1, 258–287.
Alagarsamy, R. (2003). Organic matter cycling in sediments of the Oman Margin. Chemistry and Ecology, 19, 419–429.
Amon, R. M., & Benner, R. (2003). Combined neutral sugars as indicators of the diagenetic state of dissolved organic matter in the Arctic Ocean. Deep Sea Research Part I: Oceanographic Research Papers, 50(1), 151–169.
Aspinall, G. O. (1970). Polysaccharides. Pergamon Press.
Benner, R., & Opsahl, S. (2001). Molecular indicators of the sources and transformations of dissolved organic matter in the Mississippi river plume. Organic Geochemistry, 32(4), 597–611.
Bernaerts, T. M., Gheysen, L., Kyomugasho, C., Kermani, Z. J., Vandionant, S., Foubert, I., & Van Loey, A. M. (2018). Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides. Algal Research, 32, 150–161.
Bergamaschi, B. A., Walters, J. S., & Hedges, J. (1999). Distributions of uronic acids and 0-methyl sugars in sinking and sedimentary particles in two coastal marine environments. Geochimica Cosmochimica Acta, 63, 413–425.
Bhosle, N. B., & Dhople, V. M. (1988). Distribution of some biochemical compounds in the sediments of the Bay of Bengal. Chemical Geology, 67, 341–352.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Burdige, D. J., Skoog, A., & Gardner, K. (2000). Dissolved and particulate carbohydrates in contrasting marine sediments. Geochimica Cosmochimica Acta, 64, 1029–1041.
Corinaldesi, C., Dell’Anno, A., & Danovaro, R. (2007). Early diagenesis and trophic role of extracellular DNA in different benthic ecosystems. Limnology and Oceanography, 52(4), 1710–1717.
Cowie, G. L., & Hedges, J. I. (1984). Carbohydrate sources in a coastal marine environment. Geochimica Cosmochimica Acta, 48, 2075–2087.
Cowie, G. L., & Hedges, J. I. (1994). Biochemical indicators of diagenetic alteration in natural organic-matter mixtures. Nature, 369, 304–307.
Cowie, G. L., & Hedges, J. I. (1996). Digestion and alteration of the biochemical constituents of a diatom (Thalassiosira weisflogii) ingested by an herbivorous zooplankton (Calanus pacificus). Limnology and Oceanography, 41(4), 581–594.
da Cunha, L. C., Serve, L., & Blazi, J. L. (2002). Neutral sugars as biomarkers in the particulate organic matter of a French Mediterranean river. Organic Geochemistry, 33, 953–964.
Danovaro, R., Dell, A., Martorano, D., Parodi, P., Marrale, N. D., & Fabiano, M. (1999). Seasonal variation in the biochemical composition of deep-sea nematodes: bioenergetic and methodological considerations. Marine Ecology Progress Series, 179, 273–283.
Danovaro, R., Dell’Anno, A., & Fabiano, M. (2001). Bioavailability of organic matter in the sediments of the Porcupine Abyssal Plain, northeastern Atlantic. Marine Ecology Progress Series, 220, 25–32.
Decho, A. W. (1990). Microbial exopolymer secretion in ocean environments: Their roles in food webs and Marine processes. Oceanography and Marine Biology Annual Review, 28, 73–153.
D’souza, F., & Bhosle, N. B. (2001). Variation in the composition of carbohydrates in the Dona Paula Bay (west of India) during May/June 1998. Oceanologica Acta, 24, 221–237.
D’Souza, F., Garg, A., & Bhosle, N. B. (2005). Seasonal variation in the chemical composition and carbohydrates signature compounds of biofilm. Aquatic Microbial Ecology, 41, 199–207.
Duan, D., Zhang, D., Yang, Y., Wang, J., Chen, J. A., & Ran, Y. (2017). Source, composition, and environmental implication of neutral carbohydrates in sediment cores of subtropical reservoirs. South China. Biogeosciences, 14(18), 4009–4022.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric methods for determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
Elser, J. J., Fagan, W., Denno, F., Dobberfuhl, R. F., Folarin, D. R., Huberty, A., Interlandi, S., Kilham, S. S., McCauley, E., Schulz, K. L., Siemann, E. H., & Sterner, R. W. (2000). Nutritional constraints in terrestrial and freshwater food web. Nature, 408, 578–580.
Fabiano, M., & Danovaro, R. (1999). Meiofauna distribution and mesoscale variability in two sites of the Ross Sea (Antarctica) with contrasting food supply. Polar Biology, 22(2), 115–123.
Fernandes, L., Garg, A., & Borole, D. V. (2014). Amino acid biogeochemistry and Bacterial contribution to sediment organic matter along the western margin of the Bay of Bengal. Deep Sea Research I, 83, 81–92.
Filisctti-Cozzi, T. M. C. C., & Carpita, N. C. (1991). Measurement of uronic acids without interference from neutral sugars. Analytical Biochemistry, 197, 157–162.
Fontana, C., Grenz, C., & Pinazo, C. (2010). Sequential assimilation of a year-long time-series of SeaWiFS chlorophyll data into a 3D biogeochemical model on the French Mediterranean coast. Continental Shelf Research, 30(16), 1761–1771.
Garcia-Rodriguez, F., Puerto, L. D., Venturini, N., Pita, A. L., Brugnoli, E., Burone, L., & Muniz, P. (2011). Diatoms, protein and carbohydrate sediment content as proxies for coastal eutrophication in Montevideo, Rio de la Plata Estuary, Uruguay. Brazilian Journal of Oceanography, 59, 293–310.
Grémare, A., Medernach, L., DeBovée, F., Amouroux, J., Vétion, G., & Albert, P. (2002). Relationships between sedimentary organics and benthic meiofauna on the continental shelf and the upper slope of the Gulf of Lions (NW Mediterranean). Marine Ecology Progress Series, 234, 85–94.
Goni, M. A., Teixeira, M. J., & Perkey, D. W. (2003). Sources and distribution of organic matter in a river-dominated estuary (Winyah Bay, SC, USA). Estuarine, Coastal and Shelf Science, 57, 1023–1048.
Guggenberger, G., Christensen, B. T., & Zech, W. (1994). Land-use effects on the composition of organic matter in particle-size separate of soil: I. Lignin and carbohydrate signature. European Journal of Soil Science, 45, 449–458.
Guo, Q., Wang, C., Wei, R., Zhu, G., Cui, M., & Okolic, C. P. (2020). Qualitative and quantitative analysis of source for organic carbon and nitrogen in sediments of rivers and lakes based on stable isotopes. Ecotoxicology and Environmental Safety, 195, 110436
Handa, N. (1969). Carbohydrate metabolism in the marine diatom Skeletonema costatum. Marine Biology, 4, 208–214.
Harji, R. R., Bhosle, N. B., Garg, A., Sawant, S. S., & Venkat, K. (2010). Sources of OM and microbial community structure in the sediments of Visakhapatnam harbour, east coast of India. Chemical Geology, 276, 309–317.
Harji, R. R., Yvenat, A., & Bhosle, N. B. (2008). Sources of hydrocarbons in sediments of the Mandovi estuary and the Marmugoa harbour, west coast of India. Environment International, 34, 959–965.
Hartati, W., & Sudarmadji, T. (2016). Relationship between soil texture and soil organic matter content on mined-out lands in Berau, East Kalimantan, Indonesia. Nusantara Bioscience, 8(1).
Harvey, H. R., Tuttle, J. H., & Bell, J. T. (1995). Kinetics of phytoplankton decay during simulated sedimentation: Changes in biochemical composition and microbial activity under oxic and anoxic conditions. Geochimica Et Cosmochimica Acta, 59, 3367–3377.
He, B., Dai, M., Huang, W., Liu, Q., Chen, H., & Xu, L. (2010). Sources and accumulation of organic carbon in the Pearl river estuary sediment as indicated by elemental, stable carbon isotopic, and carbohydrate compositions. Biogeosciences Discuss, 7, 2889–2926.
Hedges, J. I., Keil, R., & Benner, R. (1997). What happens to terrestrial organic matter in the ocean? Organic Geochemistry, 27, 195–2012.
Hedges, J. I., Clark, W. A., Quay, P. D., Rickey, J. E., Devol, A. H., & Satos, U. M. (1986). Compositions and fluxes of particulate organic material in the Amazon River. Limnology and Oceanography, 31, 717–738.
Hedges, J. I., Hu, F. S., Devol, A. H., Hartnett, H. E., Tsamakis, E., & Keil, R. G. (1999). Sedimentary organic matter preservation: A test for selective degradation under oxic conditions. American Journal of Science, 299, 529–555.
Henderson. R. J., Olsen, R. E. & Eilertsen, H. C. (1991). Lipid composition of phytoplankton from the Barents Sea and environmental influences on the distribution pattern of fixed carbon among photosynthetic end products. pp. 229–237 in Sakshaug, E., Hopkins, C. C. E. and (dritsland. N. A. (eds.): Proceedings of the Pro Mare Symposium on Polar Marine Ecology, Trondheim. 12–16 May 1990.
Hernes, P. J., Hedges, J. I., Peterson, M. L., Wakeham, S. G., & Lee, C. (1996). Neutral carbohydrates of particulate material in the central equatorial Pacific. Deep Sea Research I, 43, 1181–1204.
Hicks, R. E., Owen, C. J., & Aas, P. (1994). Deposition, resuspension and decomposition of particulate organic matter in the sediment of Lake Itasca, Minnesota, USA. Hydrobiologia, 284, 79–91.
Isla, E., Rossi, S., Palanques, A., Gili, J.-M., Gerdes, D., & Arntz, W. (2006). Biochemical composition of marine sediment from the eastern Weddell Sea (Antarctica): High nutritive value in a high benthic-biomass environment. Journal of Marine System, 60, 255–267.
Ittekkot, V., & Arain, R. (1986). Nature of particulate matter in the river Indus, Pakistan. Geochimica et Cosmochimica Acta, 50, 1643–1653.
Ingalls, A. E., Lee, C., Wakeham, S. G., & Hedges, J. I. (2003). The role of biominerals in the sinking flux and preservation of amino acids in the Southern Ocean along 170 oW. Deep-Sea Research II, 50, 713–738.
Ittekkot, V., Deuser, W. G., & Degens, E. T. (1984). Seasonality in the fluxes of sugars, amino acids, and amino sugars to the deep ocean: Panama Basin. Deep-Sea Research, 31, 1071–1083.
Johnson, J. L., & Cummins, C. S. (1972). Cell wall composition and deoxyribonucleic acid similarities among the anaerobic coryneforms, classical propionibacteria, and strains of Arachnia propionica. Journal of Bacteriology, 1047–1066.
Jyothibabu, R., Madhu, N. V., Habeebrehman, H., Jayalakshmy, K. V., Nair, K. K. C., & Achuthankutty, C. T. (2010). Re-evaluation of ‘paradox of mesozooplankton’ in the eastern Arabian Sea based on ship and satellite observations. Journal of Marine Systems, 81, 235–251.
Kaiser, K., & Benner, R. (2009). Biochemical composition and size distribution of organic matter at the Pacific and Atlantic time-series stations. Marine Chemistry, 113, 63–77.
Kappelmann, L., Krüger, K., Hehemann, J. H., Harder, J., Markert, S., Unfried, F., & Teeling, H. (2019). Polysaccharide utilization loci of North Sea Flavobacteriia as basis for using SusC/D-protein expression for predicting major phytoplankton glycans. The ISME Journal, 13(1), 76–91.
Kerhervé, P., Buscail, R., Gadel, F., & Serve, L. (2002). Neutral monosaccharides in surface sediments of the northwestern Mediterranean Sea. Organic Geochemistry, 33(4), 421–435.
Khodse, V. B., & Bhosle, N. B. (2012). Nature and sources of suspended particulate organic matter in a tropical estuary during the monsoon and pre-monsoon: Insights from stable isotopes (δ13CPOC, δ15NTPN) and carbohydrate signature compounds. Marine Chemistry, 145–147, 16–28.
Khodse, V. B., & Bhosle, N. B. (2010). Differences in carbohydrate profiles in batch culture grown planktonic and biofilm cells of Amphora rostrata Wm. Sm. Biofouling, 26, 527–537.
Khodse, V. B., & Bhosle, N. B. (2011). Bacterial utilization of size fractionated dissolved organic matter. Aquatic Microbial Ecology, 64, 299–309.
Khodse, V. B., Fernandes, L., Bhosle, N. B., & Sardessai, S. (2008). Carbohydrates, uronic acids and alkali extractable carbohydrates in contrasting marine sediments: Distribution, size fractionation and partial chemical characterization. Organic Geochemistry, 39, 265–283.
Kim, J. H., Schouten, S., Buscail, R., Ludwig, W., Bonnin, J., Sinninghe Damsté, J. S., & Bourrin, F. (2006). Origin and distribution of terrestrial organic matter in the NW Mediterranean (Gulf of Lions): Exploring the newly developed BIT index. Geochemistry, Geophysics, Geosystems, 7(11).
Kögel-Knabner, I. (2002). The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biology and Biochemistry, 34(2), 139–162.
Krishna, M. S., Naidu, S. A., Subbaiah, Ch. V., Sarma, V. V. S. S., & Reddy, N. P. C. (2013). Distribution and sources of organic matter in surface sediments of the eastern continental margin of India. Journal of Geophysical Research. Biogeosciences, 118, 1484–1494. https://doi.org/10.1002/2013JG002424
Kristensen, E., Ahmed, S. I., & Devol, A. H. (1995). Aerobic versus anaerobic decomposition of organic matter in marine sediments: Which is faster? Limnology and Oceanography, 40, 1430–1437.
Kumar, M. D., Rajendran, A., Somasundar, K., Haake, B., Jenisch, A., Shuo, Z., Ittekkot, V., & Desai, B. N. (1990). Dynamics of dissolved organic carbon in the northwestern Indian Ocean. Marine Chemistry, 31, 299–316.
Lazareva, E. V., & Romankevich, E. A. (2012). Carbohydrates as indicators of biogeochemical processes. Okeanologiya, 2012(52), 362–371.
Lobbes, J. M., Fitznar, H. P., & Kattner, G. (2000). Biogeochemical characteristics of dissolved and particulate organic matter in Russian rivers entering the Arctic Ocean. Geochimica Et Cosmochimica Acta, 64(17), 2973–2983.
Madhupratap, M., Prasanna Kumar, S., Bhattathiri, P. M. A., Kumar, M. D., Raghukumar, S., Nair, K. K. C., & Ramaiah, N. (1996). Mechanism of the biological response of winter cooling in the NE Arabian Sea. Nature, 384, 549–552.
Madhupratap, M., Gauns, M., Ramaiah, N., Kumar, S. P., Muraleedharan, P. M., De Sousa, S. N., & Muraleedharan, U. (2003). Biogeochemistry of the Bay of Bengal: Physical, chemical and primary productivity characteristics of the central and western Bay of Bengal during summer monsoon 2001. Deep Sea Research Part II: Topical Studies in Oceanography, 50(5), 881–896.
Meyer-Reil, L. A. (1983). Benthic response to sedimentation events during autumn to spring at a shallow water station in the Western Kiel Bight: II. Analysis of benthic bacterial populations. Marine Biology, 77, 247–256.
Muhlebach, A., & Weber, K. (1998). Origin and fate of dissolved sterols in the Weddell Sea, Antarctica. Organic Geochemistry, 29, 1595–1607.
Musale, A. S., & Desai, D. V. (2011). Distribution and abundance of macrobenthic polychaetes along the South Indian coast. Environmental Monitoring and Assessment, 178, 423–436.
Myklestad, S. (1977). Production of carbohydrates by marine planktonic diatoms. II. Influence of N/P ratio in the growth medium on the assimilation ratio, growth rate, and production of cellular and extracellular carbohydrates by Chaetoceros affinis and Skeletonema costatum. Journal of Experimental Marine Biology and Ecology, 29, 161–179.
Nair Manju, P., Akhil, P. S., & Sujatha, C. H. (2013). Geochemistry of core sediment from Antarctic region.
Naqvi, S. W. A, Naik, H., Jayakumar, D. A., Shailaja, M. S. and Narvekar, P. V. (2006) Seasonal oxygen deficiency over the western continental shelf of India. In: Neretin, l., (ed.), Past and Present Water Column Anoxia, NATO Science Series, IV. Earth and Environmental Sciences, 64, Springer, 195–224.
Neira, C., Sellanes, J., Levin, L. A., & Arntz, W. E. (2001). Meiofaunal distributions on the Peru margin: Relationship to oxygen and organic matter availability. Deep Sea Research Part I: Oceanographic Research Papers, 48(11), 2453–2472.
Nouara, A., Panagiotopoulos, C., & Sempéré, R. (2019). Simultaneous determination of neutral sugars, alditols and anhydrosugars using anion-exchange chromatography with pulsed amperometric detection: Application for marine and atmospheric samples. Marine Chemistry, 213, 24–32.
Ogier, S., Dissnar, J. R., Alberic, P., & Bourdier, G. (2001). Neutral carbohydrate geochemistry of particulate material (trap and core sediments) in an eutrophic lake (Aydat, France). Organic Geochemistry, 32, 151–162.
Opsahl, S., & Benner, R. (1999). Characterization of carbohydrates during early diagenesis of five vascular plant tissues. Organic Geochemistry, 30, 83–94.
Pan, Z., Gao, Q. F., Dong, S. L., Wang, F., Li, H. D., Zhao, K., & Jiang, X. Y. (2019). Effects of abalone (Haliotis discus hannai Ino) and kelp (Saccharina japonica) mariculture on sources, distribution, and preservation of sedimentary organic carbon in Ailian Bay, China: Identified by coupling stable isotopes (δ13C and δ15N) with C/N ratio analyses. Marine Pollution Bulletin, 141, 387–397.
Panagiotopoulos, C., & Sempere, R. (2005a). Molecular distribution of carbohydrates in large marine particles. Marine Chemistry, 95, 31–49.
Panagiotopoulos, C., & Sempere, R. (2005b). The molecular distribution of combined aldoses in sinking particles in various oceanic conditions. Marine Chemistry, 95, 31–49.
Paropkari, A. L., Babu, C. P., & Mascarenhas, A. (1992). A critical evaluation of depositional parameters controlling the variability of organic carbon in Arabian Sea sediments. Marine Geology, 107, 213–226.
Parsons, T. R., Takahashi, M., & Hargrave, B. (1984). Biological Oceanographic Processes (3rd ed.). Pergamon Press.
Prakash, B. C., Brumsack, H.-J., & Schnetger, B. (1999). Distribution of organic carbon in surface sediments along the eastern Arabian Sea:A revisit. Marine Geology, 162, 91–103.
Prasanna Kumar, S., Muraleedharan, P. M., Prasad, T. G., Gauns, M., Ramaiah, N., de Souza, S. N., Sardessai, S., & Madhupratap, M. (2002). Why is the Bay of Bengal less productive during summer monsoon compared to the Arabian Sea. Geophysical Research Letters, 29, 2235. https://doi.org/10.1029/2002GL016013
Pusceddu, A., Cattaneo-Vietti, R., Albertelli, G., & Fabiano, M. (1999). Origin, biochemical composition and vertical flux of particulate organic matter under the pack ice in Terra Nova Bay (Ross Sea, Antarctica) during late summer 1995. Polar Biology, 2, 124–132.
Quijada, M., Riboulleau, A., Guerardel, Y., Monnet, C., & Tribovillard, N. (2015). Neutral aldoses derived from sequential acid hydrolysis of sediments as indicators of diagenesis over 120,000 years. Organic Geochemistry, 81, 53–63.
Ragusa, S. R., McNevin, D., Qasem, S., & Mitchell, C. (2004). Indicators of biofilm development and activity in constructed wetlands microcosms. Water Research, 38(12), 2865–2873.
Romankevich, E. A. (1984) Geochemistry of organic matter in the ocean. Springer Verlag, 334p.
Rossi, S., Grémare, A., Gili, J.-M., Amouroux, J. M., Jordana, E., & Vétion, G. (2003). Biochemical characteristics of settling particulate organic matter at two north-western Mediterranean sites: A seasonal comparison. Estuarine, Coastal and Shelf Science, 58, 423–434.
Sakhare, V. B. (2007). Advances in aquatic ecology (Vol. 1). Daya Publishing house.
Sánchez, A., López-Ortiz, B. E., Aguíñiga-García, S., & Balart, E. (2013). Distribution and composition of organic matter in sediments of the oxygen minimum zone of the Northeastern Mexican Pacific: Paleoceanographic implications. Journal of Iberian Geology, 39(1), 111–120.
Sardessai, S. (1994). Organic carbon and humic acids in sediments of the Arabian Sea and factors governing their distribution. Oceanologica Acta, 17, 263–270.
Schulz, B., & Boyle, C. (2005). The endophytic continuum. Mycological Research, 109(6), 661–686.
Shetye, S. R., & Gouveia, A. D. (1998) Coastal circulation in the North Indian Ocean. Coastal segment (14, SW). In: Robinson, A.R. and Brink, K.H.(eds.) The Sea, v.11, John Wiley & Sons, New York, pp.523- 556.
Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, C. K., Gocke, N. M., Olson, B. J., & Klenk, D. C. (1985). Measurement of protein using bicinchoninic acid. Analytical Biochemistry, 150, 76–85.
Smith, M. A., Kominoski, J. S., Gaiser, E. E., Price, R. M., & Troxler, T. G. (2021). Stormwater runoff and tidal flooding transform dissolved organic matter composition and increase bioavailability in urban coastal ecosystems. Journal of Geophysical Research: Biogeosciences, 126(7), e2020JG006146.
Tareq, S. M., & Ohta, K. (2011). Distribution of combined monosaccharides in sediments from the lake Rawa Danau, West Java, Indonesia: Sources and diagenetic fate of carbohydrates in a tropical wetland. Geochemical Journal, 45, 1–13.
Tselepides, A., Polychronaki, T., Marrale, D., Akoumianaki, I., Dell'Anno, A., Pusceddu, A., & Danovaro, R. (2000). Organic matter composition of the continental shelf and bathyal sediments of the Cretan Sea (NE Mediterranean).
Ware, S. A., Hartman, B. E., Waggoner, D. C., Vaughn, D. R., Bianchi, T. S., & Hatcher, P. G. (2022). Molecular evidence for the export of terrigenous organic matter to the north Gulf of Mexico by solid-state 13C NMR and Fourier transform ion cyclotron resonance mass spectrometry of humic acids. Geochimica Et Cosmochimica Acta, 317, 39–52.
Wyrtki, K. (1971). Oceanographic Atlas of the international Indian Ocean Expedition (p. 531). National Science Foundation.
Youssef, D. H., El-Said, G. F., & Shobier, A. H. (2014). Distribution of total carbohydrates in surface sediments of the Egyptian Mediterranean coast, in relation to some inorganic factors. Arabian Journal of Chemistry, 7(5), 823–832.
Zaghden, H., Kallel, M., Louati, A., Elleuch, B., Oudot, J., & Saliot, A. (2005). Hydrocarbons in surface sediments from the Sfax coastal zone, (Tunisia) Mediterranean Sea. Marine Pollution Bulletin, 50(11), 1287–1294.
Zhang, Y., Kaiser, K., Li, L., Zhang, D., Ran, Y., & Benner, R. (2014). Sources, distributions, and early diagenesis of sedimentary organic matter in the Pearl River region of the South China Sea. Marine Chemistry, 158, 39–48.
Zhu, R., Tolu, J., Deng, L., Fiskal, A., Winkel, L. H., & Lever, M. A. (2020). Improving the extraction efficiency of sedimentary carbohydrates by sequential hydrolysis. Organic Geochemistry, 141, 103963.
Acknowledgements
We thank the Director of the institute for his help and encouragements. All the scientific and crew members of the cruise SSK-046 of ORV Sindhu Sankalp are thanked for their cooperation during sampling. Financial supports from the CSIR funds under PSC0206 and OLP2005 are gratefully acknowledged. This is NIO contribution number is 7022.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khodse, V.B., Amberkar, U., Khandeparker, R. et al. Variability of biochemical compounds in surface sediments along the eastern margin of the Arabian Sea. Environ Monit Assess 195, 414 (2023). https://doi.org/10.1007/s10661-023-10991-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-10991-7