Abstract
The validated hydride generation atomic absorption spectrometry (HG-AAS) method has been used to investigate total arsenic in groundwater. Under optimal experimental conditions, the concentration of arsenic in groundwater can be analysed in the range of 0.5 to 50 µg/L, with a method detection limit of 0.15 µg/L. Its recovery in the field is from 96.3 to 99.8%, with high repeatability. The method was used to observe the total arsenic pollution in groundwater collected in Phu Tho Province, Vietnam. A total of 364 groundwater samples were analysed. The results showed that arsenic pollution was significant, with 15.93% of the samples higher than the maximum permissible level of arsenic. About 20.69% of the contaminated samples had a total arsenic ten times higher (100 µg/L) than the maximum permissible level of arsenic. The pollution source was also considered by comparing the arsenic level in the groundwater with arsenic in the surface water in the same areas. Thus, the use of the high-accuracy and sensitive method, HG-AAS, supplies valuable data on groundwater pollution for water resources management and environmental protection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is one of the representative hazardous elements that cause significant health complications, such as liver disease (Hsu et al., 2016), cardiovascular disease (Navas-Acien et al., 2019), respiratory disease (Sancheza et al., 2016), type 2 diabetes mellitus (Beck et al., 2017) and cancers (Martinez et al., 2011; Wei et al., 2019). As exposure occurs primarily through ingestion of contaminated food and drinking water, besides inhalation and absorption through the skin. Arsenic in drinking water is a direct cause of human health problems and thus, is a severe worldwide human health concern. The amount of arsenic in drinking water accumulates over time, and if the dose is large enough, it will cause disease (Hong et al., 2014).
Arsenic pollution in groundwater has both natural and anthropogenic causes. The natural causes are geological and sedimentary processes under the influence of oxidation, reduction or biochemical processes (Bissen & Frimmel, 2003). The primary natural sources include geological formations (e.g. sedimentary deposits/rocks, volcanic rocks and soils), geothermal fluids, coal and volcanic activities (Missimer et al., 2018; Smedley & Kinniburgh, 2002). Anthropogenic sources include anthropogenic activities such as coal burning, emissions, As-containing wastewater, pesticides, irrigation water and industrial waste (Nriagu et al., 2007; Shankar et al., 2014).
There have been many reports of arsenic poisoning from drinking water, nearly 50% of the raw water of which is supplied by groundwater. According to Smith et al. (2000) and Flanagan et al. (2012), estimated tens of millions of people in Bangladesh have been exposed to arsenic-contaminated water, resulting in approximately 24,000 deaths each year. Kwanyuen et al. (2017) reported that in Ron Phibun town, Thailand, about 1000 people had been diagnosed with As-related skin disorders. Alarcón-Herrera et al. (2013) claimed that 8.81 million people were exposed to a high level of arsenic in groundwater. Ravenscroft et al. (2009) revealed that South American countries such as Guatemala and El Salvador have high As content in their water resources, detected from volcanogenic.
Vietnam has one of the highest levels of natural contamination of arsenic in groundwater (Shaji et al., 2020). Nguyen et al. (2018, 2020) reported that in Thai Nguyen Province, 75% of groundwater samples had more than the permissible limit of arsenic in groundwater set by the World Health Organization. Winkel et al. (2011) and Stopelli et al. (2020) also found arsenic-contaminated groundwater in deep aquifer exploitation areas in Ha Noi and in the Red River delta. Although several studies have observed the level of arsenic contamination in groundwater, the fulfillment data in many areas in Vietnam, especially in mineral-rich regions, mountainous areas and highlands, have not been thoroughly studied yet.
In this study, a validated method based on HG-AAS was used to investigate total arsenic contamination in groundwater in Phu Tho Province, Vietnam. This province has many pegmatite mines of original hydrothermal and sulphide, such as kaolin, fenspat, mica, quartzite, pyrite and iron, which can be the natural sources of arsenic in groundwater. About 40 wards in most communes in Phu Tho province, especially in mineral areas with over 360 groundwater samples, will be collected for the observation. Surface water samples will also be collected at the arsenic-contaminated location to find out if the cause of the pollution is natural or artificial. The research results can provide reliable data for managing and using groundwater resources most safely.
Materials and methods
Materials and reagents
All the reagents used in this study were of analytical grade. Arsenic standard solution (1000 mg/L AsV) traceable to SRM from NIST (H3AsO4 in 0.5 mol/L HNO3) was purchased from Merck (Merck, Darmstadt, Germany). Potassium iodide (purity ≥ 99.5%), L-ascorbic acid (99% purity) and hydrochloric acid (36.5–38%) were purchased from Sigma-Aldrich (Singapore). All the solutions were prepared and diluted using ultra-pure water with an electrical resistivity > 18.3 MΩ⋅cm produced by the Barnstead Water Purification Systems.
Groundwater sampling
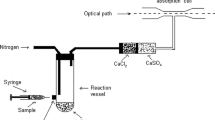
Groundwater was sampled according to ISO 5667–11:2009 (ISO, 2020). The samples were collected from water wells that were used for drinking and living. The water wells were either dug wells (depth: 15–20 m) or drilled wells (depth > 25 m). Groundwater was pumped out for 10–15 min from the drilled wells and drained for 5–10 min from the dug wells before the sampling. The groundwater samples were collected in 1-L polypropylene cans and acidified with 1 mL of concentrated nitric acid before they were brought to the laboratory for storage, sample preparation and analysis. The sampling position is presented in Fig. 1.
Sample preparation and analysis
The samples were prepared in the following three steps according to ISO 17378–2:2014 (ISO, 2019). First, 20 mL of hydrochloric acid (ρ = 1.15 g/mL) was added to 4 mL of a mixed solution of potassium iodide and ascorbic acid [3 g potassium iodide KI and 5 g L ( +) − ascorbic acid in 100 mL of water] in a round-bottom flask that contained 50 mL of groundwater sample. Second, this mixture was gently heated for 15 min at 50 °C to convert all the arsenic into the AsIII form. Finally, the solution was cooled to room temperature, transferred to a volumetric flask and diluted to a final volume of 100 mL. A total of 0.5 mL of this solution was injected into the flow injection hydride generator (Perkin Elmer FIAS 100 Flow Injection for AAS) and analysed using atomic absorption spectrometry (Perkin Elmer AAnalyst 800, USA) at the operating conditions, as shown in Table 1. The calibration curve was prepared in the same way as the sample, except that the sample was replaced with a blank matrix (arsenic-free) spiking various amounts of AsV.
Results and discussion
HG-AAS method verification
To confirm the accuracy of the HG-AAS method that was used in this study, its merits such as linearity, method detection limit (MDL), accuracy and precision were sequentially evaluated, according to the standards of the Association of Official Analytical Chemists (AOAC, 2016) and ISO/IEC 17,025–2017 (ISO, 2017).
The linearity was observed with various concentrations of AsIII, from 0.5 to 50 µg/L. The calibration curves obtained with the coefficient correlation (R2) were higher than 0.997. R2 was stable on 3 different days. The MDLs in the determination of arsenic were calculated from the three-time standard deviation (SD) that was calculated from 11 repetitive analysis spiked samples. As shown in Table 2, the average MDL for As determination ranged from 0.10 to 0.15 µg/L, whereas the published MDL of this method was 0.15 µg/L. According to the maximum permissible As limit of 10 µg/L, the obtained MDLs were highly sensitive to the determination of arsenic in groundwater. The average concentration to the MDL value, from 5.7 to 6.5, confirmed that the spike level in the experiments had high concordance.
The accuracy of the method was evaluated based on the recovery value, repeatability and intermediate reproducibility. By repeatedly analysing the sample with different spiking concentrations of arsenic, the recovery value and the relative standard deviation (RSD) percentage were calculated and are shown in Table 2. The recoveries varied from 96.3 to 99.8%, much better than the required value (60 to 115% at the corresponding level). This demonstrated the high trueness of the quantification. The maximum RSD in the arsenic determination was 3.69%. According to the RSD Horwitch function or the AOAC, the obtained RSD was much lower than the maximum acceptable value for a concentration of 100 µg/L (15%). Therefore, the method exhibited excellent repeatability precision for determining arsenic in groundwater.
The uncertainty was measured based on a combination of “top-down” and “bottom-up” approaches (ISO/IEC, 2008; EURACHEM/CITAC, 2012). From the primary sources of uncertainty of the method, which were precision, bias and reference standard purity, the uncertainty measurement was 10.52%. The merits are summarised in Table 2.
Observation of arsenic pollution in groundwater
Arsenic pollution in groundwater was observed in the 364 samples collected in 36 wards in Phu Tho Province. It was found that 84.61% of the samples were positive for As (> 0.5 µg/L). The arsenic contamination of the groundwater was 15.93%, which exceeded the maximum permissible level of 10 µg/L. The samples with As concentrations 10 times higher than the allowable threshold accounted for 20.69% of the contaminated water samples (Fig. 2; Table S1 in Supporting Information), with the highest concentration reaching 267 µg/L. In 10 of the 36 areas (27.78%), the groundwater had an arsenic concentration above the maximum permissible level (Fig. 3).
Source of arsenic contamination of groundwater
To assess the source of the arsenic in the groundwater, 56 surface water samples (from ponds, lakes and rivers) near the 14 groundwater sampling areas, where the groundwater had arsenic concentrations higher than 5 µg/L, were also observed. Anthropogenic sources usually cause arsenic pollution in the surface water as agricultural, industrial and other human activity wastes. Although the surface water samples positive for arsenic (> 0.5 µg/L) were 76.78% of the total of 56 surface water samples, the samples that had arsenic concentrations higher than 10 µg/L accounted for only 3.57%.
The difference between the arsenic contamination in groundwater samples (total arsenic higher than 5 µg/L), and the surface water samples at the same sampling position was significant (Fig. 4). The paired t-test results for the mean between the surface water and the groundwater samples yielded a p-value (two-tail) of 0.00065, which is much smaller than 0.05, confirming that the arsenic contaminations in the two studied aquatic environments were entirely different (Table 3).
Six areas with concentrations much higher than 10 µg/L (red column in Fig. 3) are low-lying areas stretching along the Red River, where porous aquifers have loose, soft formations. These results are consistent with those of previous research on groundwater pollution in the downstream area of the Red River (Stopelli et al., 2020; Winkel et al., 2011). The high groundwater abstraction from peat-rich aquifers may enhance the dissolution of arsenic-rich iron oxyhydroxides and, thus, increase arsenic concentrations (Winkel et al., 2011). All these results prove that the source of arsenic pollution cannot be from human activity and industrial waste. Thus, we can conclude that the arsenic in the groundwater is from natural sources.
Conclusion
This study is the first complete study on arsenic pollution in groundwater in Phu Tho, the northern midland mountainous province of Vietnam. There are many mineral deposits and peat-rich aquifers in the province that may cause groundwater pollution. Based on the validated HG-AAS method, this work assessed the arsenic-contaminated level in groundwater in 36 areas. The results showed that the level of pollution was considerable. There were 27.78% sampling areas that had average arsenic concentrations higher than the maximum permissible level. It is recommended that groundwater in these areas be arsenic-treated before it is used for drinking, for health safety. The sources of arsenic pollution were also examined in this study by comparing the concentrations of arsenic in the surface water with groundwater in arsenic-contaminated areas. Almost of the surface water samples were not arsenic-contaminated, which demonstrated that the sources of the pollution were natural and underground. All the results prove valuable data for environmental management and technology.
Data availability
All the data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Alarcón-Herrera, M. T., Bundschuh, J., Nath, B., Nicolli, H. B., Gutierrez, M., Reyes-Gomez, V. M., & Sracek, O. (2013). Co-occurrence of arsenic and fluoride in groundwater of semi-arid regions in Latin America: Genesis, mobility and remediation. Journal of Hazardous Materials, 262, 960–969. https://doi.org/10.1016/j.jhazmat.2012.08.005
Association of Official Analytical Chemists. (2016). Appendix F: Guidelines for standard method performance requirements, Journal of AOAC International. http://www.eoma.aoac.org/app_f.pdf
Beck, R., Styblo, M., & Sethupathy, P. (2017). Arsenic exposure and Type 2 diabetes: MicroRNAs as mechanistic links? Epidemiology of Diabetes, 17, 18. https://doi.org/10.1007/s11892-017-0845-8
Bissen, M., & Frimmel, F. H. (2003). Arsenic – A review. Part I: Occurrence, toxicity, speciation, mobility. Acta hydrochimica et hydrobiologica, 31(1), 9–18. https://doi.org/10.1002/aheh.200390025
EURACHEM/CITAC. (2012). Guide quantifying uncertainty in analytical measurement, (3th ed.).
Flanagan, S. V., Johnston, R. B., & Zhen, Y. (2012). Arsenic in tube well water in Bangladesh: Health and economic impacts and implications for arsenic mitigation. Bulletin of the World Health Organization, 90(11), 839–846. https://doi.org/10.2471/BLT.11.101253
Hong, Y. S., Song, K. H., & Chung, J. Y. (2014). Health effects of chronic arsenic exposure. Journal of Preventive Medicine and Public Health, 47(5), 245–252. https://doi.org/10.3961/jpmph.14.035
Hsu, L. I., Wang, Y. H., Hsieh, F. I., Yang, T. Y., Jeng, R. W. J., Liu, C. T., Chen, C. L., Hsu, K. H., Chiou, H. Y., Wu, M. M., & Chen, C. J. (2016). Effects of arsenic in drinking water on risk of hepatitis or cirrhosis in persons with and without chronic viral hepatitis. Clinical Gastroenterology and Hepatology, 14, 1347–1355. https://doi.org/10.1016/j.cgh.2016.03.043
International Standard, ISO 5667–11:2009. Water quality – Sampling – Part 11: Guidance on sampling of groundwaters, Reviewed and confirmed in 2020. https://www.iso.org/standard/42990.html
International Standard, ISO 17378–2:2014. Water quality – Determination of arsenic and antimony – Part 2: Method using hydride generation atomic absorption spectrometry (HG-AAS), Reviewed and confirmed in 2019. https://www.iso.org/standard/55220.html
International Standard, ISO/IEC 17025–2017. General requirements for the competence of testing and calibration laboratories. https://www.iso.org/publication/PUB100424.html
International Standard, ISO/IEC GUIDE 98–3:2008. Uncertainty of measurement – Part 3: Guide to the expression of uncertainty in measurement (GUM:1995). https://www.iso.org/standard/50461.html
Kwanyuen, S., Wattasit, S., Sumol, P., & Robert, S. (2017). Environmental arsenic exposure and risk of diabetes type 2 in Ron Phibun subdistrict, Nakhon Si Thammarat Province, Thailand: Unmatched and matched case–control studies. Risk Management and Healthcare Policy, 10, 41–48. https://doi.org/10.2147/RMHP.S128277
Martinez, V. D., Vucic, E. A., Becker-Santos, D. D., Gil, L., & Lam, W. L. (2011). Arsenic exposure and the induction of human cancers. Journal of Toxicology, 431287, 1–13. https://doi.org/10.1155/2011/431287
Missimer, T., Teaf, C., Beeson, W., Maliva, R., Woolschlager, J., & Covert, D. (2018). Natural background and anthropogenic arsenic enrichment in Florida soils, surface water, and groundwater: A review with a discussion on public health risk. International Journal of Environmental Research and Public Health, 15(10), 2278. https://doi.org/10.3390/ijerph15102278
Navas-Acien, A., Sanchez, T. R., Mann, K., & Jones, M. R. (2019). Arsenic exposure and cardiovascular disease: Evidence needed to inform the dose-response at low levels. Environmental Epidemiology, 6, 81–92.
Nguyen, T. H., Hoang, H. N. T., Bien, N. Q., Tuyen, L. H., & Kim, K. W. (2020). Contamination of heavy metals in paddy soil in the vicinity of Nui Phao multi-metal mine, North Vietnam. Environmental Geochemistry and Health, 42, 4141–4158. https://doi.org/10.1007/s10653-020-00611-5
Nguyen, T. P. M., Nguyen, T. P. T., Bui, T. H., & Nguyen, T. H. (2018). Concentration of arsenic in groundwater, vegetables, human hair and nails in mining site in the Northern Thai Nguyen province, Vietnam: Human exposure and risks assessment. Human and Ecological Risk Assessment: An International Journal, 1–12. https://doi.org/10.1080/10807039.2018.1483189
Nriagu, O. J., Bhattacharya, P., Mukherjee, A. B., Bundschuh, J., Zevenhoven, R., & Loeppert, R. H. (2007). Arsenic in soil and groundwater: An overview. Trace Metals and Other Contaminants in the Environment, 9, 3–60. https://doi.org/10.1016/S1875-1121(06)09001-8
Ravenscroft, P., Brammer, H., & Richards, K. S. (2009). Arsenic pollution: A global synthesis. Wiley-Blackwell.
Sancheza, T. R., Perzanowskia, M., & Grazianoa, J. H. (2016). Inorganic arsenic and respiratory health, from early life exposure to sex-specific effects: A systematic review. Environmental Research, 147, 537–555. https://doi.org/10.1016/j.envres.2016.02.009
Shankar, S., Shanker, U., & Shikha,. (2014). Arsenic contamination of groundwater: A review of sources, prevalence, health risks, and strategies for mitigation. The Scientific World Journal, 2014, 1–18. https://doi.org/10.1155/2014/304524
Shaji, E., Santosh, M., Sarath, K. V., Prakash, P., Deepchand, V., & Divya, B. V. (2020). Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geoscience Frontiers. https://doi.org/10.1016/j.gsf.2020.08.015
Smedley, P. L., & Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry, 17(5), 517–568. https://doi.org/10.1016/S0883-2927(02)00018-5
Smith, A. H., Lingas, E. O., & Rahman, M. (2000). Contamination of drinking-water by arsenic in Bangladesh: A public health emergency. Bulletin of the World Health Organization, 78(9), 1093–1103. PMC2560840
Stopelli, E., Duyen, V. T., Mai, T. T., Trang, P. T. K., Viet, P. H., Lightfoot, A., Rolf, K., Magnus, S., Elisabeth, E., Agnes, K., Thomas, N., Martyna, G., Monique, P., Andreas, K., Sara, K., Bhasker, R., Olaf, C., Benjamin, B., Henning, P., Lenny, H. E. W., & Berg, M. (2020). Spatial and temporal evolution of groundwater arsenic contamination in the Red River delta, Vietnam: Interplay of mobilisation and retardation processes. Science of the Total Environment, 717, 137–143. https://doi.org/10.1016/j.scitotenv.2020.137143
Wei, S., Zhang, H., & Tao, S. (2019). A review of arsenic exposure and lung cancer. Toxicology Research. https://doi.org/10.1039/c8tx00298c
Winkel, L. H., Trang, P. T. K., Lan, V. M., Stengel, C., Amini, M., Ha, N. T., Viet, P. H., & Berg, M. (2011). Arsenic pollution of groundwater in Vietnam exacerbated by deep aquifer exploitation for more than a century. Proceedings of the National Academy of Sciences, 108, 1246–1251. https://doi.org/10.1073/pnas.1011915108
Acknowledgements
The authors would like to thank the Industrial University of Ho Chi Minh City and the VNU University of Education for financial supports.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Dinh Vu Le designed the study, analysed the data, wrote and submitted the manuscript. Pham Thi Kim Giang evaluated the experimental procedures and results. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le, D.V., Giang, P.T.K. & Nguyen, V.T. Investigation of arsenic contamination in groundwater using hydride generation atomic absorption spectrometry. Environ Monit Assess 195, 84 (2023). https://doi.org/10.1007/s10661-022-10707-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10707-3