Abstract
High-altitude freshwater lakes are experiencing ever-increasing risk of harmful algal blooms (HABs) on the face of climate change and a growing demand on agricultural production. The biggest alpine lake of the Caucasus, Lake Sevan, has “blooming” recently almost every year. Thus, the study of phytoplankton community’ development patterns in Lake Sevan is gaining urgency. The aim of the work has been the study of the seasonal dynamics of quantitative and qualitative characteristics of phytoplankton in Lake Sevan. Also, we have tried to determine the features of horizontal distribution of phytoplankton within different seasons with the focus on Cyanobacteria distribution to identify current spatial–temporal features of HABs in Lake Sevan. Seasonal ground data collected from the photic zone of 178 stations in 2016–2018 was analysed and spatially interpolated. The results of analysis of seasonal dynamics of phytoplankton groups abundance have revealed the increased role of Cyanobacteria in the algal “blooms”. Particularly, HABs were led by the dominant species of the genus Dolichospermum/Anabaena that are potentially toxic. Univariate analysis of variance with the post hoc Tukey test has proved the significance of changes in the quantitative parameters of phytoplankton development within years with the peak in 2018. Some antagonistic relations between the groups of phytoplankton under the HAB events were also shown through factorial and correlation analysis. Spatial interpolations revealed very limited extents of HABs compared with “blooms” led by Bacillariophyta species. HABs were mainly occurring in the littoral zone in a close proximity to the estuaries of Lake Sevan major tributaries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the World Resources Institute (2019), Armenia has been experiencing high baseline water stress. Thus, the preservation of water resources is gaining urgency in the face of the climate and anthropogenic challenges. Lake Sevan is the largest freshwater reservoir in the Caucasus region and one of the largest high-altitude (1900 m a.s.l.) lakes in the world (Winston et al., 2015). The geologic history of the lake extends back to the late Miocene, but the recent morphology was established only in Pleistocene and Holocene (Wilkinson, 2020), which makes it one of the youngest major freshwater ecosystems in Eurasia.
It is common to think of the alpine lakes as pristine ecosystems with lack of anthropogenic pressures. However, some evidences are neglecting this stereotype and making to conclude about the necessity to perform specific studies on this issue. Particularly, climate change brings shifts in biological hydrological, chemical and temperature cycles of lakes (Rinke et al., 2010). The results of long-term study of lakes in the central Austrian part of the Alps allow to conclude that the epilimnion’s water temperature in summer is very sensitive to a change in air temperature (Thompson et al., 2005). In some deeper alpine lakes like Geneva in Switzerland, temperature increase has also noticed due to climate change (Perroud & Goyette, 2010). It’s been also shown (Salmaso et al., 2003) that in deep alpine lakes, the internal recycling of nutrients and consequently development of phytoplankton are strongly dependent from water temperature and intensity of vertical mixing in winter and early spring. Prolonged thermal stratification, occurred in particular in summer, can influence hypolimnetic oxygen conditions, dissolved nutrient concentration and phytoplankton composition (Wilhem & Adrian, 2008). Oxygen depletion and higher temperatures increase nutrient release processes at the sediment water interface (Søndergaard et al., 2003). In many lakes investigated, the analysed data indicate a rising temperatures favour Cyanobacteria in several ways. However, Cyanobacteria generally grow better at higher temperatures (often above 25 °C) than do other phytoplankton species such as diatoms and green algae. This gives Cyanobacteria a competitive advantage at elevated temperatures (Paerl & Huisman, 2008). However, there is a lack of studies related to alpine lakes worldwide.
Despite unique environmental conditions, Lake Sevan is experiencing several anthropogenic pressures due to its significant economic role for Armenia (Babayan et al., 2006). As a result, the pure oligotrophic status of the lake has been critically modified within the twentieth century (Hovhannisyan & Ghazaryan, 1986) due to over-exploitation of its water (Chilingaryan & Mnatsakanyan, 2010) for irrigation and production of electricity and fish stocks (Gabrielyan, 2010). Since then, phytoplankton has become the main producer in the trophic chain. Also, phytoplankton is intensely used for the monitoring of the ecosystem health, as a widely accepted indicator of the ecological status of lakes (EU WFD 2000/60/EC, 2000).
The first noticeable changes in the phytoplankton community of Lake Sevan were recorded in the mid-1960s of the last century, due in part to a drastic artificial drop in water level by 18 m. In that period, the average annual biomass of phytoplankton increased tenfold compared with the oligotrophic period (from 0.32 to 3.5 g/m3). Along with the increase, Cyanobacteria (Dolichospermum/Anabaena, Microcystis, Aphanizomenon) and their “blooming” were recorded (Legovich, 1979) for the first time. Since then, Cyanobacteria have been causing harmful algal blooms (HABs) periodically (Hambaryan, 2001; Parparov, 1990). Although the drainage basin of Lake Sevan has been declared a national park since 1978, pressure mitigation strategies are not working properly yet, as the results of the recent studies clearly indicate the activation of eutrophication processes in the lake (Gevorgyan et al., 2020; Hambaryan et al., 2020b).
To combat the negative tendencies, a decision was made to increase the water level by more than 6 m (the Law on Lake Sevan, 2001). The process has led to the emergence of new wetlands, which hydrological features, nutrient and temperature regime are affecting the whole ecosystem (Asatryan et al., 2016). Thus, the study of the spatial patterns of phytoplankton in the lake is highly relevant nowadays. Considering an active growth of phytoplankton in Lake Sevan (Hambaryan et al., 2020a) within different seasons, the study of its seasonal succession has gained a special interest recently, because some groups of algae are potentially toxic and the frequency of HABs is increasing (Gevorgyan et al., 2020).
The aim of the work has been the study of the seasonal dynamics of the quantitative and qualitative characteristics of phytoplankton in Lake Sevan using the ground data. Another objective is to determine the features of horizontal distribution of phytoplankton within different seasons with the focus on Cyanobacteria distribution. This will allow identifying spatial–temporal features of HABs in Lake Sevan and thus to improve management strategies in the drainage basin.
Materials and methods
Study area and sampling stations
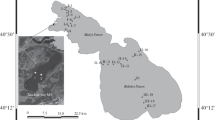
Due to the geologic formation features, Lake Sevan consists of two unequal parts: (1) the northern part, Small Sevan (SS), with a surface of 338.6 m2, a volume of 14.09 km3 and a maximum depth of 80 m, and (2) the southern part, Big Sevan (BS) with a surface of 940 m2, a volume of 24.1 km3 and a maximum depth of 36 m (Hydrological regime of lake Sevan, 2017). Thereby, it seems meaningful to check the hypothesis of significance in spatial–temporal dynamics of phytoplankton development within the two lake parts. Considering that algal “blooms” usually start from a littoral zone (up to 15 m depths) and then spread throughout the whole lake (Hambaryan et al., 2020a; Sakharova et al., 2020), the sampling site network covered most intensely the littoral zone of the lake. To estimate the seasonal dynamics of phytoplankton in Lake Sevan, sampling was conducted during every May, July and October of the 2016–2018 period from the total of 178 stations distributed along 22 semitransects, as well as three additional monitoring stations distributed in the deepwater parts of Lake Sevan (Fig. 1). Considering the morphology of Lake Sevan, the transects were constructed perpendicular to the lakeshore. Sampling stations were distributed along the transects at the following water column depths, where possible 2 m, 4 m, 7 m, 10 m, 15 m, 20 m, 25 m and 30 m. However, all the material was strictly collected from the upper layer of the photic zone (0.5 m deep), which allows discussing the patterns of phytoplankton horizontal dynamics. As the depths at BS are significantly lower, the last samples have been taken usually from the 25 m depth zone. The deepwater station in SS was chosen at the 60 m depth zone, and two deepwater stations in BS were chosen at the 33 m depth zone. In some seasons, the number of sampling stations was reduced due to artificial obstacles for sampling in some directions of Lake Sevan.
Sampling procedure and processing
A 1-l water sample taken from each site was preserved with 40% formaldehyde solution and stored in a dark place for 10–12 days. Then, the samples were concentrated by sedimentation method by gradually decreasing the volume from 1000 mL to 100 and then to 10 mL (Guide, 1983; Guide, 1992). The identification of phytoplankton was performed by using the key determinants (Tsarenko, 1990; Streble & Krauter, 2001; Berg et al., 2012; Hambaryan & Shahazizyan, 2014; Guiry & Guiry, 2021). The qualitative and quantitative parameters of different groups of phytoplankton were derived under a microscope in the Nageotte counting chamber (0.1 mL) (Devlin et al., 2014; Kitaev, 2007).
Based on the number of phytoplankton cells/litre, the inverse distance weighted (IDW) tool was used in ArcMap10.5 software to interpolate data and gain the model of spatial distribution of phytoplankton in Lake Sevan for all studied seasons. For the visualisation of spatial model, four groups of phytoplankton development were distinguished from weak development to bloom based on expert judgement (Table 1).
To avoid the bias that the data is not representative enough for the assessment of horizontal dynamics patterns in Lake Sevan because of seasonal sampling, we also checked the freely available true colour satellite images from Sentinel-2 (from 2016–11-01 to 2018–10-19) and Landsat 8 (from 2016–5-01 to 2016–11-01) in Sentinel Hub EO Browser (https://apps.sentinel-hub.com/eo-browser/). The main search criterion was that the cloud cover above Lake Sevan should be less than 15% and we mainly looked at L2A or L2 images (atmospherically corrected).
Statistical analyses
To justify the results of IDW interpolation and check the hypothesis of antagonistic relations between the groups of phytoplankton, we run univariate general linear models in IBM SPSS statistics 17. In particular, the year, season and parts of Lake Sevan (BS and SS) as well as phytoplankton groups were considered fixed factors in factorial analyses, and the number of cells/litre was considered dependent parameter. Homogeneity factor was checked through Levene’s statistics, and the equity of means was checked through Welch and Brown–Forsythe tests. Tukey post hoc test was used for all factorial analyses except analysing the effect of parts of Lake Sevan. The calculation of F value through one-way ANOVA test allowed understanding whether the quantitative development of phytoplankton groups is significantly different between SS and BS. For that we assumed that when the variance between the groups is higher than the variance within the groups, the factors influencing phytoplankton development in SS and BS should be significantly different as well.
As the temperature was considered one of the main abiotic factors influencing seasonal dynamics of phytoplankton, the mean monthly temperatures for the photic zones of northwest and southeast of SS, as well as southeast and southwest parts of BS for the period of 2016–2018, were derived from “Hydrometeorology and Monitoring Center” (HMC) SNCO. To analyse the spatial–temporal variance features of temperature and the concordance between the abundance of three main groups of phytoplankton and temperature, the plot of dependence was constructed. For all these analyses, we initially eliminated data for the Xanthophyta and Euglenophyta groups, as they were recorded rarely and could significantly lower the accuracy of assessments. Additionally, to check the hypothesis of antagonism between various groups of phytoplankton, the intercorrelation analysis was conducted for all seasons.
Results
Satellite images
Satellite image search showed 25 appropriate results for Sentinel-2 (supplement 1) and 4 results for Landsat 8. True colour images distinctively showed that there were no obvious algal “blooms” between the periods of sampling that we may have missed. So, the carried sampling was considered representative enough to reveal the common dynamics of phytoplankton for the measured period.
Quantitative parameters of phytoplankton community
The main groups of phytoplankton indicated were Bacillariophyta (diatoms), Cyanophyta (blue-green), Chlorophyta (green), Xanthophyta (yellow-green) and Euglenophyta (euglena).
The results of seasonal dynamics of abundance of phytoplankton (Fig. 2) show that some succession of the main groups and their functional significance in the formation of the community occurs as well along with the quantitative changes. Particularly, the role of Cyanobacteria in the community regularly increases in summer, and the role of diatoms increases in autumn. During the whole period of studies, HABs were led by the dominant species of the genus Dolichospermum/Anabaena, Dolichospermum/Anabaena flos-aquae, Dolichospermum/Anabaena cylindrica Lemmerm and Dolichospermum/Anabaena circinalis Rabenhorst ex Bornet & Flahault, 1886, which its toxicity has been already proven for some years. In 2018, when the strong HAB occurred in July, the increase in the number of green and yellow-green algae cells was followed in autumn. Moreover, the yellow-green algae were encountered strictly in the littoral zone and were completely absent during HAB in July 2018. Also, the number of Cyanobacteria in BS was almost always higher than in the SS during the same seasons.
During autumn peaks, the dominant species of diatoms were Aulacoseira granulata (Ehrenberg) Simonsen, 1979, which caused some local algae “blooms”. Two other species of Melosira, M. varians and M. italica, were also recorded during such “blooms”. Some other species grew significantly in that period like Ceratoneis arcus, Cocconeis pediculus (2016), Stephanodiscus astraea, Fragilaria construens (Ehrenberg) Grun. (2017) and Diatoma mesodon, Stephanodiscus hantzschii Grunow in Cleve & Grunow and Cyclotella kutzingiana (2018).
From more than 20 species of Cyanobacteria, Aphanothece clathrata W. et. G.S. West f. Clathrata and Microcystis aeruginosa (Kütz) Elenk. were present at all seasons and everywhere but with low quantities (Fig. 3). Other species like Aphanothece stagnina (Sprengel) B.-Peters et Geitl, M. rosenbergii (Komárek) Komárek, Phormidium breve (Kütz.) Anagn. et Kom., Phormidium foveolarum Gomont, Dactylococcopsis acicularis Lemmerm., Chroococcus turgidus (Kützing) Nägeli, Oscillatoria limnetica Lemmerm., Oscillatoria limosa C.Agardh ex Gomont 1892, Oscillatoria lauterbornii Schmidle, Oscillatoria geminata var. sulphurea Strzeszewski, Spirulina sp., Merismopedia elegans A.Braun ex Kützing, Aphanizomenon flos-aquae L.Ralfs flos-aquae, Gloeocapsa sanguinea (Agardh) Kützing and Gomphosphaeria lacustris Chodat have no obvious patterns of distribution. In overall, species diversity gets lower in 2018, especially during summer bloom of Cyanobacteria of Dolichospermum/Anabaena genus. Dolichospermum/Anabaena flos-aquae (Lingb.) Breb. has dominated during summer blooms, while the abundance of species Dolichospermum/Anabaena lemmermannii P.G.Richt. and Dolichospermum/Anabaena spiroides var. crassa Lemm. has been lesser.
Statistical analyses
Factorial analysis of annual differences
To check the hypothesis of significant differences in the phytoplankton development among the years, one-way ANOVA test was performed. The Levene’s test proves the significance of homogeneity of variance (value 16.072, significance 0.000) for the number of cells/litre dependent variable. Thus, the derived F value (6.354, significance 0.02) claimed the null hypothesis that the changes among years between the groups of phytoplankton were bigger than within the groups. Both Welch (significance 0.036) and Brown–Forsythe (significance 0.035) tests prove the null hypothesis of equality of means for the dependent variable.
However, the results of Tukey’s test show that confidently significant differences exist only between the data for 2018 and the remaining 2 years of studies (Table 2).
Univariate analysis of variance
The results of univariate analysis of variance for the effects of factors like years, seasons, parts of Lake Sevan and phytoplankton groups as well as their paired interceptions on phytoplankton quantitative parameters show no significant effect only for the parts of Lake Sevan and relevant interceptions (Table 3).
The results of Tukey’s test for season parameter (Table 4) show that statistically confident significant changes in the quantitative parameters of phytoplankton occurred between autumn and the remaining two seasons but not among spring and early summer seasons, which is probably due to thermal regime of the lake.
Also, the results of Tukey’s test for phytoplankton groups parameters claim the confident significant difference between Bacillariophyta and the other groups (Table 5) but not among Cyanophyta and Chlorophyta.
In addition to factorial analysis, we conducted correlation analysis for the groups of phytoplankton in different seasons of 2016–2018 (Table 6). The results claim the existence of some ties between the development of different groups of algae in Lake Sevan. Particularly, confident correlations among all groups were found only in May 2016, when no HABs were recorded. Such results show that HABs are really disintegrating the normal development patterns of phytoplankton groups in Lake Sevan. Stronger positive correlations were found between Cyanophyta and Chlorophyta groups, especially during HABs.
Temperature factor
Temperature variance in different stations has shown that the most stable conditions were in the Shorja part of SS (southeast of SS) and the highest variance is in the Martuni station of BS (southwest of BS) (Fig. 4). Moreover, the highest mean monthly temperature in the period of 2016–2018 was also recorded for Martuni station. In general, the maximum range of mean monthly temperatures reached 23 °C there in 2017.
Usually, the temperatures at Martuni station start getting lower a bit earlier during the autumn (Fig. 4) which is also influencing the development of phytoplankton, as different groups have various optimum ranges.
The analysis of the dependency of development of different groups of algae from temperature shows a little concordance in general (Fig. 5). Intense growth of Bacillariophyta usually starts at lower temperatures and has been more intense during 2016–2018. Chlorophyta group shows less dependence from temperature and in general grows well when water heats up to around 16 °C. Cyanophyta prefers higher temperatures and usually blooms when water heats more than 21 °C. Such observations could help to build some probability models for algal “blooms” in Lake Sevan.
Spatial analysis of phytoplankton development
The results of IDW interpolation for the number of cells/litre parameter show some spatial–temporal patterns of phytoplankton development (Fig. 6). Particularly, blooms were not typical for May period but were intense since July. Local “blooms” in spring and early summer typically start from the littoral zones at parts where some major rivers flow into the lake. Autumn “blooms” are covering wider area in both parts of Lake Sevan owing to strong development of diatom group. The concentrations of phytoplankton are higher near the river mouth parts of Gavaraget, Argichi, Masrik and Dzknaget rivers that ensure flux of the organic matter and nutrients from the settlements and intense agricultural lands. According to IDW model, the strong “blooms” more commonly extend to the whole central zone in SS but rarely in BS. However, this still should be properly checked with the use of wider satellite data and additional ground sampling.
The dynamics of phytoplankton in both SS and BS indicate a growing risk of eutrophication processes in the lake during the period of studies. Also, an increase in the proportion of the blue-green algae indicates a higher risk of HABs. Although the point sampling showed higher quantitative development of blue-green algae in SS, the interpolation model (Fig. 7) has shown that the risk of HABs was equally high for both parts of Lake Sevan.
The observation of a stronger “bloom” in the territory of SS in 2018 is just a matter of sampling time, because the satellite imagery shows the dynamics of spreading of algal bloom from BS to SS in late July of 2018.
Discussion
The patterns of algal “bloom” in Lake Sevan
Algal “blooms” depend on many abiotic and biotic factors like the temperature, nutrient load, trophic relations, antagonistic relations and the state of fish and crayfish stocks. Upon the artificial decrease in the water level of Lake Sevan, the fluctuations in temperatures are getting higher during different seasons, which strongly influences the phytoplankton community. According to the Fourth National Report on Climate Change (2020), the average annual temperature in the territory of Armenia has increased by 1.23 °C, and the precipitations have decreased by 9% in the period of 1929–2016, which creates an artificial need for irrigation water and thus increases the pressure on Lake Sevan water resources. Along with the tendency of accelerated warming, this leads to the extension of favourable period for the intense development of phytoplankton. As the frequency of heat waves and the average temperatures have been increasing also during the last 20 years, the risk of HABs will gradually increase as well. The role of the temperature factor in the development of algal “blooms” grows significantly considering the relative stability in overall economic activity in the drainage basin, as well as the fact that the population number remains stable or at least is not growing (RA, 2021). However, some artificial interventions in the hydroecosystem, like regular water abstraction from the lake in the warm period or more water abstraction from the main tributaries for the irrigation, could play a major role too.
The temperature optimum of different groups of algae varies significantly, which determines the change in the species composition over the seasons. In the summer of 2018, an intense development of blue-green and green algae was recorded both in the SS and BS, due in part to the more intense heating of the aquatic environment (the average values were 20–21 °C). It should be noticed that the summer “blooms” of Cyanobacteria and green algae are also the result of competition for similar conditions for growth and development. A suppression of the development of the diatom group was observed along with their development in the summer. In particular, the most common group for the lake during the oligotrophic period (Vladimirova, 1947) — diatoms, is being substituted more frequently by green and blue-green algae in different seasons. However, in autumn, with a decrease in water temperature to + 10 + 12 °C, an increase in the proportion of cold-water diatom species is still being observed. Moreover, in the winter of 2017, the lake was covered with ice, which, probably, was the reason for the weak development of all groups of algae but diatoms. In general, such a model in the development of phytoplankton functional groups has been described for various lakes (Senerpont Domis et al., 2007). The cyanobacterial species of the genus Dolichospermum/Anabaena usually dominate in summer, and the analysis of the water samples for the presence of anatoxin-a and microcystin carried out in 2019 has showed a positive result (Hambaryan et al., 2020b). The amount of anatoxin-a in 0.5 mL of water was 0.147 μg/L (test protocol N1827, product code 1/1720; dated 08.06.2019). During the recent years, the concentrations of some nutrients (e.g. ammonium and phosphate ions) have increased as well (Gevorgyan et al., 2020), which can be directly connected with the growth of blue-green algae (Carmichael, 2001). On the other hand, Cyanobacteria have a competitive advantage in the consumption of nitrogen (Paerl & Huisman, 2009). Freshwater Cyanobacteria can dominate both in pelagic and benthic habitats. They can produce effective allelochemical compounds that interfere with the growth of other groups of algae (Gross et al., 2003). Xanthophyceae also shows their highest diversity in acidic waters enriched with dissolved organic matter (Ott & Oldham-Ott, 2003).
In general, phytoplankton quantitative fluctuations are stronger in BS, which allows making a conclusion on a higher instability and vulnerability of phytoplankton community towards changes in abiotic factors there. As the southeast of Lake Sevan is the shallowest part and its thermal regime is quite different from the remaining parts of the lake, the algal “blooms” led by the blue-green algae will more probably start earlier in this part of the lake. Spatial extents of such “blooms” cover wider zones in SS; however, more detailed studies are necessary to get the directions of “bloom” expansion in both parts. It is not less important to get the patterns of underwater currents too.
Ecosystem management perspectives
In recent years, there are many literary references for the clarification of the issues of eutrophication of lakes, which show the importance of studying phytoplankton dynamics for models of aquatic ecosystem management (e.g. Elliott & Thackeray, 2004; Frisk et al., 1999; Hillmer et al., 2008; Reynolds & Irish, 1997; Rukhovets et al., 2003; Seip, 1991; Vinçon-Leite & Casenave, 2019). In many models, predictions are based on the quantitative parameters and the functional characteristics describing the community (e.g. Hallstan et al., 2012; Jones & Elliott, 2007; Kerimoglu et al., 2017; Mieleitner & Reichert, 2006, 2008). These models can be used to predict both short-term (Huang et al., 2012, 2014) and long-term (e.g. Elliott et al., 2005; Markensten et al., 2010) changes in the composition, as well as spatial heterogeneities in the development of the phytoplankton community (e.g. Hillmer et al., 2008; Leon et al., 2011, 2012), which are individual for each ecosystem. As the main producer in Lake Sevan, phytoplankton plays a crucial role in the trophic chain of the lake. With HABs, a number of negative impacts on the ecosystem functioning are observed, including loss of aquatic biodiversity, low transparency, surface scums and deterioration of oxygen regime (Merel et al., 2013). The disintegration of cyanobacterial cells leads to an increase in the concentration of nutrients in deep stratified lakes and mixing of water masses, which has manifested by a shift in phytoplankton towards the development of greens and diatoms (Visser et al., 2016). Thus, any improvements in the ecosystem management plans should be based on the comprehensive assessment of the spatial and temporal features of phytoplankton development. Although remote sensing (RS) technologies provide new perspectives in environmental monitoring and algal “bloom” studies (e.g. Medvedev et al., 2020; Shi et al., 2017), without an extensive ground data, they are not readily available yet for such big freshwater lakes like Sevan. Moreover, with the launch of Sentinel-2 and delivery of 10-m resolution images having “red-edge band”, many tools have been developed for a more accurate chlorophyll-a calculation using RS data (Beck et al., 2016; Bramich et al., 2021). But they need some accuracy testing using bulky ground data for Lake Sevan too. Thus, the current study provides some scientific bases that should contribute to the further development of RS methods for Lake Sevan aiming at algal bloom assessment and monitoring in the face of the current transformations.
Considering that planned increase in the water level of the lake continues but the coasts are not properly cleared from vegetation yet, the actions should be activated in this direction. Also, the revealed patterns show that more attention should be paid to the tributaries of Lake Sevan.
Conclusion
The study concludes on some dangerous transformations in the phytoplankton community of the biggest freshwater reservoir of the Caucasus. Particularly, both the intensity of algal “blooms” and the role of Cyanobacteria in that have increased. Considering temperature preferences of different groups of algae, such transformation indicates the future perspectives of Lake Sevan ecosystem under the increasing threat of global warming. However, the most intense “blooms” were registered in autumn seasons mainly due to Bacillariophyta species growth. Spatial analysis of algal “blooms” allows to conclude that HABs in Lake Sevan are still more local and probably connected with the load from tributaries as spatially they are close to estuaries. Thus, we strive to think that proper water resource management in the basin could decrease the frequency of HABs very efficiently. The made spatial analysis and derived data also could serve for the development of remote sensing tools for Lake Sevan and similar high-altitude lakes under the threat of algal “blooms”.
References
Asatryan, V. L., Barseghyan, N. E., Vardanyan, T. V., Yepremyan, H. V., Hayrapetyan, A. H., Dallakyan, M. R., & Gabrielyan, B. K. (2016). Analysis of the state of biocenoses that formed in shallow areas of Small Sevan (Armenia) during the period of lake’s water level rise. Inland Water Biology, 9, 1–7. https://doi.org/10.1134/S199508291601003X
Babayan, A., Hakobyan, S., Jenderedjian, K., Muradyan, S., & Voskanov, M. (2006). Lake Sevan: Experience and lessons learned brief (Lake basin management initiative). International Lake Environment Committee Foundation, Kusatsu, Japan. Retrieved November 7, 2021, from http://worldlakes.org/uploads/sevan_01oct2004.pdf
Beck, R., Zhan, S., Liu, H., Tong, S., Yang, B., Xu, M., Ye, Zh., Huang, Y., Shu, S., Wu, Q., Wang, S., Berling, K., Murray, A., Emery, E., Reif, M., Harwood, J., Young, J., Nietch, Ch., Macke, D., … Su, H. (2016). Comparison of satellite reflectance algorithms for estimating chlorophyll-a in a temperate reservoir using coincident hyperspectral aircraft imagery and dense coincident surface observations. Remote Sensing of Environment, 178, 15–30. https://doi.org/10.1016/j.rse.2016.03.002
Berg, L. K. -H., Hoef-Emden, K., & Melkonian, M. (2012). Der Kosmos-Algenfuhrer: Die Wichtigsten Subwasseralgen im Mikroskop. Kosmos, 368 p.
Bramich, J., Bolch, C. J. S., & Fischer, A. (2021). Improved red-edge chlorophyll-a detection for Sentinel 2. Ecological Indicators, 120, 106876. https://doi.org/10.1016/j.ecolind.2020.106876
Carmichael, W. W. (2001). Health effects of toxin-producing cyanobacteria: “The CyanoHABs.” Human and Ecological Risk Assessment, 7, 1393–1407.
Chilingaryan, L.A. & Mnatsakanyan, B.A. (2010). Hydrology of Lake Sevan basin. In Krylov A. (eds.) Ecology of Lake Sevan during the period of water level rise. Makhachkala: Nauka DNC, pp. 28–40.
Devlin, M., Best, M., Bresnan, E., Scanlan, C., & Baptie, M. (2014). Water Framework Directive: The development and status of phytoplankton tools for ecological assessment of coastal and transitional waters. United Kingdom. Technical Report WFD-UKTAG 93.
Elliott, J., & Thackeray, S. (2004). The simulation of phytoplankton in shallow and deep lakes using PROTECH. Ecological Modelling, 178, 357–369. https://doi.org/10.1016/j.ecolmodel.2004.02.012
Elliott, J., Thackeray, S., Huntingford, C., & Jones, R. (2005). Combining a regional climate model with a phytoplankton community model to predict future changes in phytoplankton in lakes. Freshwater Biology, 50, 1404–1411. https://doi.org/10.1111/j.1365-2427.2005.01409.x
European Commission Directive 2000/60/EC of the European Parliament and of the Council of 23. (2000). October 2000 establishing a framework for community action in the field of water policy. Official Journal of the European Communities.
Fourth National Report on Climate Change. (2020). Retrieved August 18, 2021, from http://nature-ic.am/Content/announcements/11676/FNC_Arm.pdf
Frisk, T., Bilaletdin, A., Kaipainen, H., Malve, O., & Mols, M. (1999). Modelling phytoplankton dynamics of the eutrophic Lake Vortsjarv, Estonia. Hydrobiologia, 414, 59–69. https://doi.org/10.1023/A:1003802912687
Gabrielyan, B. K. (2010). Fish of Lake Sevan. Yerevan, Gitutyun. 252p. (in Russian).
Gevorgyan, G., Rinke, K., Schultze, M., Mamyan, A., Kuzmin, A., Belykh, O., Sorokovikova, E., Hayrapetyan, A., Hovsepyan, A., Khachikyan, T., Aghayan, S., Fedorova, G., Krasnopeev, A., Potapov, S., & Tikhonova, I. (2020). First report about toxic cyanobacterial bloom occurrence in Lake Sevan Armenia. International Review of Hydrobiology, 105(5–6), 131–142. https://doi.org/10.1002/iroh.202002060
Gross, E. M. (2003). Allelopathy of aquatic autotrophs. First publ. in: Critical Reviews in Plant Science 22, pp. 313–339. Retrieved August 5, 2021, from http://www.ub.uni-konstanz.de/kops/volltexte/2008/4868/
Guide on methods for the hydrobiological analysis of surface water and bottom sediments. (1983). Abakumov, V. A. (Ed.). M.: Gidrometeoizdat, 196 p. (in Russian).
Guide on the hydrobiological monitoring of freshwater ecosystems. (1992). Abakumov, V. A. (Ed.). St. Petersburg, Russia: Gidrometeoizdat. (in Russian).
Guiry, M. D., & Guiry, G. M. (2021). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Retrieved May 01, 2021, from https://www.algaebase.org.
Hallstan, S., Johnson, R. K., Willén, E., & Grandin, U. (2012). Comparison of classification-then modelling and species-by-species modelling for predicting lake phytoplankton assemblages. Ecological Modelling, 231, 11–19. https://doi.org/10.1016/j.ecolmodel.2012.01.018
Hambaryan, L. R. (2001). Phytoplankton succession during the period of the re-lowering of Lake Sevan level. PhD dissertation abstract, Yerevan, 21 pp. (in Russian).
Hambaryan, L., Shahazizyan, I. (2014). Determinant and educational manual for genera of freshwater algae. Yer.: YSU Press, 61 p. (in Armenian).
Hambaryan, L., Khachikyan, T., & Ghukasyan, E. (2020a). Changes in the horizontal development of phytoplankton of the littoral of Lake Sevan (Armenia) in conditions of water level fluctuations. Limnology and Freshwater Biology, 4, 662–664 https://doi.org/10.31951/2658-3518-2020a-A-4-662.
Hambaryan, L. R., Stepanyan, L. G., Mikaelyan, M. V., & Gyurjyan, Q. G. (2020b). The bloom and toxicity of cyanobacteria in Lake Sevan. Proceedings of the Yerevan State University, Chemistry and Biology, 54(2), p. 168–176. https://doi.org/10.46991/PYSU:B/2020b.54.2.168
Hillmer, I., van Reenen, P., Imberger, J., & Zohary, T. (2008). Phytoplankton patchiness and their role in the modelled productivity of a large, seasonally stratified lake. Ecological Modelling, 218, 49–59. https://doi.org/10.1016/j.ecolmodel.2008.06.017
Hovhannisyan, R. H., & Ghazaryan, I. A. (1986). Succession of phytoplankton community during different periods of Lake Sevan water level lowering. Abstracts of the 1st congress of hydrobiologists of Moldova, Kishinev, pp. 105–106. (in Russian)
Huang, J., Gao, J., & Hoermann, G. (2012). Hydrodynamic-phytoplankton model for short term forecasts of phytoplankton in Lake Taihu, China. Limnologica, 42, 7–18. https://doi.org/10.1016/j.limno.2011.06.003
Huang, J., Gao, J., Hoermann, G., & Fohrer, N. (2014). Modeling the effects of environmental variables on short-term spatial changes in phytoplankton biomass in a large shallow lake Lake Taihu. Environment and Earth Science, 72, 3609–3621. https://doi.org/10.1007/s12665-014-3272-z
Hydrological regime of Lake Sevan. (2017). Report of service of the hydrometeorology and active influence on atmospheric phenomena of MES RA (in Armenian). Retrieved March 25, 2021, from http://mes.am/u_files/file/vova/havelvac/Sevan%20grquyk%2023_03_2017.pdf
Jones, I. D., & Elliott, J. A. (2007). Modelling the effects of changing retention time on abundance and composition of phytoplankton species in a small lake. Freshwater Biology, 52, 988–997. https://doi.org/10.1111/j.1365-2427.2007.01746.x
Kerimoglu, O., Hofmeister, R., Maerz, J., Riethmüller, R., & Wirtz, K. W. (2017). The acclimative biogeochemical model of the southern North Sea. Biogeosciences, 14, 4499–4531. https://doi.org/10.5194/bg-14-4499-2017
Kitaev, S. P. (2007). Osnovy limnologii dlya gidrobiontov i ikhtiologov (Fundamentals of limnology for hydrobiologists and ichthyologists), Petrozavodsk: Karel. Nauchn. Center, Russ. Akad. Sciences, 394p.
Legovich, N. O. (1979). Blooms of Lake Sevan (observations during 1964–1972). Proceedings of Sevan Hydrobiological Station, 17, 51–74. (in Russian).
Leon, L. F., Smith, R. E. H., Hipsey, M. R., Bocaniov, S. A., Higgins, S. N., Hecky, R. E., Antenucci, J. P., Imberger, J. A., & Guildford, S. J. (2011). Application of a 3D hydrodynamic-biological model for seasonal and spatial dynamics of water quality and phytoplankton in Lake Erie. Journal of Great Lakes Research, 37, 41–53. https://doi.org/10.1016/j.jglr.2010.12.007
Leon, L. F., Smith, R. E. H., Malkin, S. Y., Depew, D., Hipsey, M. R., Antenucci, J. P., Higgins, S. N., Hecky, R. E., & Rao, R. Y. (2012). Nested 3D modeling of the spatial dynamics of nutrients and phytoplankton in a Lake Ontario nearshore zone. Journal of Great Lakes Research, 38, 171–183. https://doi.org/10.1016/j.jglr.2012.02.006
Markensten, H., Moore, K., & Persson, I. (2010). Simulated lake phytoplankton composition shifts toward cyanobacteria dominance in a future warmer climate. Ecological Applications, 20, 752–767. https://doi.org/10.1890/08-2109.1
Medvedev, A., Telnova, N., Alekseenko, N., Koshkarev, A., Kuznetchenko, P., & Asmaryan, Sh. (2020). UAV-derived data application for environmental monitoring of the coastal area of Lake Sevan, Armenia with a Changing Water Level. Remote Sensing, 12(22), 3821. https://doi.org/10.3390/rs12223821
Merel, S., Walker, D., Chicana, R., Snyder, S., Baurès, E., & Thomas, O. (2013). State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environment International, 59, 303–327. https://doi.org/10.1016/j.envint.2013.06.013
Mieleitner, J., & Reichert, P. (2006). Analysis of the transferability of a biogeochemical lake model to lakes of different trophic state. Ecological Modelling, 194, 49–61. https://doi.org/10.1016/j.ecolmodel.2005.10.039
Mieleitner, J., & Reichert, P. (2008). Modelling functional groups of phytoplankton in three lakes of different trophic state. Ecological Modelling, 211, 279–291. https://doi.org/10.1016/j.ecolmodel.2007.09.010
Ott D. W. & Oldham-Ott C. K. (2003). Eustigmatophyte, raphidophyte, and tribophyte algae. In J. D. Wehr & R. G. Sheath (Eds):. Aquatic Ecology, Freshwater Algae of North America, (pp. 423–469). Academic Press. https://doi.org/10.1016/B978-012741550-5/50012-X
Paerl, H. W., & Huisman, J. (2008). Blooms like it hot. Science, 320, 57–58. https://doi.org/10.1126/science.1155398
Paerl, H. W., & Huisman, J. (2009). Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environmental Microbiology Reports, 1(1), 27–37. https://doi.org/10.1111/j.1758-2229.2008.00004.x
Parparov, A. S. (1990). Some characteristics of the community of autotrophs of Lake Sevan in connection with its eutrophication. Hydrobiologia, 191(1), 15–21. https://doi.org/10.1007/978-94-009-0467-5_3
Perroud, M., & Goyette, S. (2010). Impact of warmer climate on Lake Geneva water-temperature profiles. Boreal Environment Research, 15, 255–178.
RA, Gegharkunik marz, in figures (2021). Regional statistics from Statistics committee of the Republic of Armenia. Retrieved December 01, 2021, from https://armstat.am/file/doc/99527793.pdf
Reynolds, C. S., & Irish, A. E. (1997). Modelling phytoplankton dynamics in lakes and reservoirs: The problem of in-situ growth rates. Hydrobiologia, 349, 5–17. https://doi.org/10.1023/A:1003020823129
Rinke, K., Yeates, P., & Rothhaupt, K.-O. (2010). A simulation study of the feedback of phytoplankton on thermal structure via light extinction. Freshwater Biology, 55, 1674–1693. https://doi.org/10.1111/j.1365-2427.2010.02401.x
Rukhovets, L., Astrakhantsev, G., Menshutkin, V., Minina, T., Petrova, N., & Poloskov, V. (2003). Development of Lake Ladoga ecosystem models: Modeling of the phytoplankton succession in the eutrophication process I. Ecological Modelling, 165, 49–77. https://doi.org/10.1016/S0304-3800(03)00061-9
Sakharova, E. G., Krylov, A. V., Sabitova, R. Z., Tsvetkov, A. I., Hambaryan, L. R., Mamyan, A. S., Gabrielyan, B. K., Hayrapetyan, A. O., & Khachikyan T. G. (2020). Horizontal and vertical distribution of phytoplankton in alpine Lake Sevan (Armenia) during the summer water blooms of Cyanoprokaryota. Contemporary Problems of Ecology, 13(1), 60–70. Pleiades Publishing, Ltd.
Salmaso, N., Mosello, R., Garibaldi, L., Decet, F., Brizzio, M. C., & Cordella, P. (2003). Vertical mixing as a determinant of trophic status in deep lakes: A case study from two lakes south of the Alps (Lake Garda and Lake Iseo). Journal of Limnology, 62, 33–41. https://doi.org/10.4081/jlimnol.2003.s1.33
Seip, K. L. (1991). The ecosystem of a mesotrophic lake-I. Simulating plankton biomass and the timing of phytoplankton blooms. Aquatic Sciences, 53, 239–262. https://doi.org/10.1007/BF00877061
Senerpont Domis, L. N., Wolf, M. M., & Huisman, J. (2007). Climate-induced shifts in an experimental phytoplankton community: A mechanistic approach. Hydrobiologia, 584, 403–413. https://doi.org/10.1007/s10750-007-0609-6
Shi, K., Zhang, Y., Zhou, Y., Liu, X., Zhu, G., Qin, B., & Gao, G. (2017). Long-term MODIS observations of cyanobacterial dynamics in Lake Taihu: Responses to nutrient enrichment and meteorological factors. Scientific Reports, 7, 40326. https://doi.org/10.1038/srep40326
Søndergaard, M., Jensen, J. P., & Jeppesen, E. (2003). Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia, 506–509, 135–145. https://doi.org/10.1023/B:HYDR.0000008611.12704.dd
Streble, H., & Krauter, D. (2001). Das Leben im Wassertropfen (p. 415). Kosmos.
The law on Lake Sevan. (2001). Retrieved May 01, 2021, from https://www.arlis.am/documentview.aspx?docid=891
Thompson, R., Kamenik, Ch., & Schmidt, R. (2005). Ultra-sensitive alpine lakes and climate change. Journal of Limnology, 64, 139–152. https://doi.org/10.4081/jlimnol.2005.139
Tsarenko, P. M. (1990). Short determinant chlorococcus algae of the Ukraine. Kiev: Naukova Dumka, 106 p. (in Russian)
Vinçon-Leite, B., & Casenave, C. (2019). Modelling eutrophication in lake ecosystems: A review. Science of the Total Environment, 651, 2985–3001. https://doi.org/10.1016/j.scitotenv.2018.09.320
Visser, P. M., Ibelings, B. W., Bormans, M., & Huisman, J. (2016). Artificial mixing to control cyanobacterial blooms: A review. Aquatic Ecology, 50, 423–441; https://doi.org/10.1007/s10452-015-9537-0
Vladimirova, K. S. (1947). Phytoplankton of Lake Sevan. Proceedings of Sevan Hydrobiological Station, 9, 69–144. (in Russian).
Wilhelm, S., & Adrian, R. (2008). Impact of summer warming on the thermal characteristics of a polymictic lake and consequences for oxygen, nutrients and phytoplankton. Freshwater Biology, 53, 226–237. https://doi.org/10.1111/j.1365-2427.2007.01887.x
Wilkinson, I. P. (2020). Lake Sevan: Evolution, biotic variability and ecological degradation. In: Large Asian lakes in the changing world (eds. Steffen Mischke). Springer international publishing. https://doi.org/10.1007/978-3-030-42254-7_2.
Winston, Yu., Cestti, R. E., & Lee, J. Y. (2015). Toward integrated water resources management in Armenia. The World Bank. https://doi.org/10.1596/978-1-4648-0335-2
World Resources Institute. (2019). 17 Countries, home to one-quarter of the world’s population, face extremely high water stress. Retrieved August 18, 2021, from https://www.wri.org/insights/17-countries-home-one-quarter-worlds-population-face-extremely-high-water-stress
Acknowledgements
The authors want to thank the “Hydrometeorology and Monitoring Center” (HMC) SNCO of the Ministry of Environment for the provision of long-term temperature observation database for Lake Sevan.
Funding
Sampling and processing of phytoplankton was financially supported by the Foundation for Restoration of Sevan Trout Stocks and Development for Aquaculture.
Author information
Authors and Affiliations
Contributions
All the material of phytoplankton was sampled and processed by Lusine Hambaryan, Lilit Stepanyan, Anahit Hovsepyan, Termine Khachikyan and Armine Mamyan. Satellite imagery processing was realised by Asatryan Vardan. The first draft of the manuscript was written by Asatryan Vardan and Lusine Hambaryan. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asatryan, V., Stepanyan, L., Hovsepyan, A. et al. The dynamics of phytoplankton seasonal development and its horizontal distribution in Lake Sevan (Armenia). Environ Monit Assess 194, 757 (2022). https://doi.org/10.1007/s10661-022-10446-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-022-10446-5