Abstract
A study of the horizontal and vertical distribution of phytoplankton in Lake Sevan was conducted in mid-July 2018 during the maximum Cyanoprokaryota blooms. The summer biomass of algae in the pelagic zone of the lake corresponded to values of mesatrophic waters. The Cyanoprokaryota biomass in Lake Sevan has horizontal and vertical heterogeneity and is related to the depth, morphometry of the lake parts, distribution of currents, transparency, temperature, and biomass of Cladocera. The specific number of phytoplankton species and water transparency decreases in areas where water blooms occur. The following factors contribute to the increase in the average phytoplankton biomass when compared to 2013–2017: (1) increased water temperature, (2) weakening of zooplankton grazing pressure due to the disappearance of Daphnia magna, and (3) increased phosphorus load due to increased contribution of D. longispina and Diaphanosoma lacustris to the zooplankton biomass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Lake Sevan (Armenia) is the largest water body in the Caucasus; it is situated at an altitude of ~1900 m above sea level. The area of the lake is ~1262 km2, the average depth is 44.3 m, and the maximum depth is 98.7 m (Chilingaryan and Mnatsakanyan, 2010; Integral’naya otsenka…, 2011). The lake is subdivided into two parts, Bolshoi and Malyi Sevan, differing in the time of formation, origin, and morphometry. The Sevan National Park was founded to preserve water quality and biological resources in Lake Sevan and the surrounding areas. According to the Ramsar International Convention, the water body is included into the list of specially protected objects. Extensive economic exploitation of the lake started in the 1930s. Since that time, there was a period of a decrease in the water level in Lake Sevan (~20 m); however, the water level began rising significantly in 2002 (Chilingaryan and Mnatsakanyan, 2010). Such fluctuations of the water level affected the dynamics of qualitative and quantitative composition of hydrobionts and the trophic state of the lake. During the initial period of the water level rise, biomass and species richness of zooplankton increased and fish stocks decreased, which resulted in considerable changes in the quantitative parameters of phyto- and bacterioplankton (Krylov et al., 2013; Gerasimov et al., 2010). A decline in poaching levels promotes an increase in the ichthyomass, which causes structural reorganizations of zooplankton and a decrease in its biomass (Krylov et al., 2016, 2018). Taking into account that weakening of the top-down control and an increase in nutrient loading due to flooding periodically lead to the increase in intensity of Cyanoprokaryota bloom in Lake Sevan (Ovsepyan et al., 2010; Ovsepyan and Khachikyan, 2016), a study of the autotrophic chain under conditions of the rise in water level is of special importance.
The aim of this work is to study the phytoplankton distribution in Lake Sevan during the period of mass Cyanoprokaryota bloom.
MATERIALS AND METHODS
Integral phytoplankton samples were collected with a 4-L Molchanov bathometer from the surface to the bottom every 1–5 m in the middle of July 2018 at two transects in Malyi (MS) and Bolshoi (BS) Sevan and one semitransect in BS and in the part overgrown with macrophytes (1) and open (2) part of the shallow bay in MS formed after the water-level rise (Fig. 1). To study the vertical phytoplankton distribution, samples were collected at different horizons of the water column every 5–10 m at three deepwater stations (I-5, II-13 and 20). Samples were concentrated in turn by direct filtration under weak pressure through membrane filters with a pore diameter of 5 and 1.2 µm to determine the qualitative and quantitative composition of phytoplankton. Phytoplankton was fixed with Lugol’s solution with the addition of formaldehyde and glacial acetic acid (Metodika…, 1975). The cells were counted and identified using a Carl Zeiss Primo Star light microscope; the biomass was determined by the counting-volume technique. Phytoplankton was estimated according to the total and specific number of species (number of species in a sample), biomass, ratio of taxonomic groups, and dominant species. Taxa with a biomass of ≥10% of the total were classified as dominant. At the same time, zooplankton samples were collected with a bucket in shallow parts (to 2 m) and 25–50 L of water was filtered through gauze with a mesh size of 64 µm; at other depths samples were collected using a Juday net with a mesh size of 64 µm, pulling it from the bottom to the surface, and with a 4-L Molchanov bathometer every 1–5 m. The cameral treatment of zooplankton samples was carried out according to the standard procedure (Metodika…, 1975); the biomass was calculated taking into account the size of the organisms (Balushkina and Vinberg, 1979).
The relationship between the biomass of phytoplankton, green algae, and cyanoprokaryotes and some abiotic and biotic parameters of the lake was determined using the Spearman’s rank correlation coefficient (p < 0.05). The Kholmogorov–Smirnov test was used to check for normal distribution of the data. The hierarchical cluster analysis was performed based on the complete list of algae at each station. Grouping of habitats according to the presence or absence of the species was made by Ward’s method.
Hydrophisical characteristics of water (temperature, oxygen content, and conductivity) were measured with a YSI ProPlus handheld multiparameter meter with 1–2 m increments. Water transparency was measured using a Secchi disk.
Sentinel-2 satellite images were obtained from USGS Earth Explorer website (https://earthexplorer. usgs.gov/). Atmospheric correction was made for all selected images using SCP module of Quantum GIS software. Further, channels 3, 5, 7 (B3: 542.5–577.5 nm, resolution of 10 m, B5: 697.5–712.5 nm, resolution of 20 m, B7: 773.0–7930 nm, resolution of 20 m) were used in the combination of 7–5–3. This combination provides images similar to natural colors, but makes it possible to clearly distinguish shallow zones from the coastal area and the color of water appears to vary from light blue to dark blue. Cyanoprokaryotes are colored from emerald green to ultramarine depending on the stage of development.
RESULTS
Abiotic parameters. Water transparency varied from 0.2 to 4 m; the lowest values were recorded at stations I-7–I-9 in MS and at depth of 9 and 15 m of the semi-transect III at a deepwater station 20 in BS (Table 1). Water temperature varied from 20.2 to 23.6°С on the surface and from 7.5 to 22.4°С near the bottom; the difference between surface and near-bottom layers increased with increasing depth. Water conductivity varied from 623 to 766 mS/cm in the surface horizon and from 522 to 784 mS/cm in the near-bottom layer. The maximum concentrations of dissolved oxygen were recorded in the surface layer in BS. The highest difference in the oxygen concentration was observed between surface and near-bottom horizons at semi-transect III in BS (III-18).
Floristic composition of phytoplankton in the lake. In July 2018, 80 taxa of the rank below genus belonging to 17 orders, 55 genera, and 8 divisions were identified in the phytoplankton composition in Lake Sevan. Green algae (41% of the total number of species), diatoms (21%), and cyanoprokaryotes (14%) made the greatest contribution to the floristic diversity of algae.
The species richness of phytoplankton varied from 11 to 28 species. The largest number of species in a sample was recorded in the deepwater part of the lake with depths of 15–30 m, I-4 and II-12 (28 and 27 species, respectively (Fig. 2). The relative richness of species reduced with decreasing depth (r = 0.50, p = 0.04, n = 17). The exception was semitransect III, where the reverse trend was seen. It is found that the number of species in a sample decreased with increasing biomass of cyanoprokaryotes (r = –0.52, p = 0.03, n = 17).
In a shallow bay of MS, 67 taxa of the rank below genus belonging to 40 genera, 13 orders, and 7 divisions were detected. The greatest number of taxa belonged to the division of green algae (43% of their total number). Fifty-five species of phytoplankton were recorded in the open part of the shallow bay and 48 species in the part overgrown with macrophytes in MS, which is considerably higher than in the main area of MS and BS.
When clustering the floristic composition of phytoplankton, three groups of stations were distinguished: (1) a shallow bay of MS, (2) BS above the depths of >2 m, and (3) MS and parts above depths of ≤2 m in BS (Fig. 3). The largest number of taxa was recorded at the first group of stations (67 taxa of the rank below genus), among which there were 29 taxa of green algae and 10 taxa of diatoms and cyanoprokaryotes (each of them). In contrast to other groups of stations, this group of stations was characterized by a high diversity of the genus Scenedesmus and the presence of species of the genus Cosmarium. In addition, the diversity of cyanoprokaryotes was higher at these stations. Among them, species were detected which did not occur in the main area of Lake Sevan: Aphanocapsa delicatissima West & G.S. West, Merismopedia tenuissima Lemmermann, Merismopedia punctata Meyen, Anabaena sp., Planktothrix agardhii (Gomont) Anagnostidis & Komárek, and Oscillatoria tenuis C. Agardh ex Gomont. The second group of stations was characterized by the lowest number of taxa (37 taxa of the rank below genus); the division of green algae was the most diverse (18), but the number of diatoms was the lowest and dinoflagellates were absent. The third group of stations (61 taxa of the rank below genus) was also characterized by the highest diversity of green algae (24) but, at the same time, the maximum diversity of diatoms from the genera Epithemia, Gomphonema, Navicula, Nitzschia, and Rhoicosphenia was found.
Biomass of phytoplankton. The maximum biomass of phytoplankton was observed at shallow station I-9 with a depth of 2 m in MS (110.46 mg/L). As the depth increased at transect I, the biomass of phytoplankton decreased (Fig. 4a) and it decreased more than 70 times at the deepest station I-5 (62 m).
The high biomass of phytoplankton cells was also observed in a shallow part I-1 (3 m), but it was only 4.5 times higher than at station I-5. In BS, the trend toward declining the algae biomass recurred (Fig. 4b): its lowest values were recorded at station II-13 with a depth of 32 m (1.87 mg/L) and were 3 and 9 times higher, respectively, at a depth of 2 m (stations II-10 and II-16). On the contrary, the biomass increased with increasing depth at semitransect III (Fig. 4c), but the mass of phytoplankton cells was insignificantly higher in respect to the data obtained at transects I and II (2 times). In general, a negative significant correlation was found between the phytoplankton biomass and depth in the lake (r = –0.48, p = 0.04, n = 19).
The biomass of phytoplankton in the lake was largely composed of cyanoprokaryotes (Figs. 4а–4c). Their biomass decreased with increasing depth, which is indicated by a significant correlation (r = –0.45, p = 0.05, n = 19, Fig. 2). The proportion of cyanoprokaryotes in the total biomass varied from 30 to 99%, Anabaena lemmermannii P.G. Richter was an absolutely dominant species, and the proportion of other species was less than 1% of the biomass. The proportion of green algae was considerable in the total phytoplankton biomass (0–72%). Large-sized algae Oonephris obesa (West & G.S. West) Fott (0–72%) were among the dominant species; other representatives of this division constituted no more than 1% in the total biomass. The contribution of other divisions of phytoplankton (diatoms, dinophytes, cryptophytes, golden algae, and euglenids) did not exceed 2% of the total biomass of algae.
The biomass of algae ((7.21 mg/L) in the open part of the shallow bay in BS was higher than in the part overgrown with higher aquatic vegetation (4.97 mg/L) (Fig. 5), which is comparable with values of phytoplankton biomass at similar depths in the main area. Dinoflagellates prevailed in the biomass (73–74% of the total biomass); species of the genus Glenodinium dominated. Green algae constituted from 17 to 19% of the total biomass due to the dominance of Tetraëdron minimum (A. Braun) Hansgirg (12–13% of the total biomass). The proportion of cyanoprokaryotes was 4‒5% of the total biomass of algae. Anabaena lemmermannii, dominant in the main area of Lake Sevan, was absent in the considered part.
The maximum biomass of phytoplankton at deepwater stations was recorded in the surface water layer; it was two times higher in BS than in MS (Figs. 6а–6c). The biomass of algae decreased 3–33 times toward the near-bottom horizon. The maximum difference in the phytoplankton biomass was observed in BS between surface and near-bottom horizons (a decrease of 10–33 times). The phytoplankton biomass was basically formed by cyanoprokaryotes and green algae in all horizons of deepwater parts. Cyanoprokaryotes (50–94% of the total biomass) dominated in the surface layer due to Anabaena lemmermannii. Their biomass decreased and that of green algae increased due to the dominance of Oonephris obesa as the depth increased. The proportion of other divisions (diatoms, dinophytes, cryptophytes, golden algae, and euglenids) was in total no more than 6% of the total phytoplankton biomass.
Dependence of phytoplankton distribution in the lake on abiotic and biotic factors. Water transparency in MS positively correlated with the biomass of green algae and negatively correlated with the total biomass of phytoplankton and cyanoprokaryotes (Table 2).
A significant positive correlation was found between water temperature and the biomass of cyanoprokaryotes in MS. The control by plankton invertebrates was a key factor determining phytoplankton development. During the study period, the biomass of zooplankton was high; it was 18.2 ± 6.1 g/m3 in MS and 16.4 ± 5.6 g/m3 in BS. It was mainly composed of Cladocera, 80.7 and 78.6%, respectively. Cyanoprokaryotes correlated with the biomass of Cladocera differently in two parts of the lake: positively in MS and negatively in BS. In addition, the biomass of green algae negatively correlated with the biomass of Cladocera in MS and the total biomass of phytoplankton positively correlated with the biomass of green algae in BS.
DISCUSSION
The development of phytoplankton in the lake in July 2018 was determined by water bloom caused by cyanoprokaryotes Anabaena lemmermannii. The mass development of the species in the lake was first recorded in 1964–1965, when the water level decreased by 17 m (Legovich, 1979). Since then, the species was constantly recorded in the composition of summer phytoplankton (Ovsepyan et al., 2010). The distribution of A. lemmermannii in the lake area was uneven in July 2018: the maximum bloom was observed in shallow parts (Figs. 4, 7).
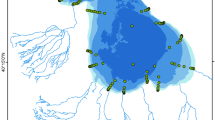
It is known that the distribution and quantitative parameters of phytoplankton depend on the topology of the water body and its temperature regime (Abakumov and Izrailsky, 2012). BS is characterized by more vast littoral zone (Chilingaryan and Mnatsakanyan, 2010; Integral’naya otsenka…, 2011) and higher water temperatures in the summer season, which is indicated by the data: it was on average 22.2 ± 1.2°С in the surface layer in the littoral zone and 21.7 ± 1.2°С in the pelagic zone of BS, whereas the water temperature in MS was 21.2 ± 0.1°С and 21.4 ± 0.5°С, respectively. Cyanoprokaryotes prefer well-warmed waters, as confirmed by significantly positive correlations between the biomass of cyanoprokaryotes and water temperature obtained in some water bodies (Kozitskaya, 1991; Korneva et al., 2014). However, our studies demonstrated that MS was characterized by higher trophic status in July 2018, although comparatively high parameters of phytoplankton were recorded earlier in BS (Legovich, 1979; Ovsepyan and Gambaryan, 2013; Ovsepyan and Khachikyan, 2016). This is apparently due to certain features, first to the uneven distribution of cyanoprokaryotes in the lake area determined by currents. Thus, the maximum biomass of A. lemmermannii was recorded in the southwestern part of the littoral zone of MS, which is characterized by the highest surface velocity of water current inflowing through the strait. The main water exchange between two parts of the lake occurs along the shores of the strait, and the current velocity here is 1.5–2 times higher than in its central part (Filatov, 1991). This apparently determined the similarity of the algae composition in MS and shallow zone of BS (Fig. 3), the communities of which, unlike the deepwater zone of BS, were characterized by higher species richness, including green algae and diatoms and mixotrophs, dinophytes, and euglenids. In addition, the peak of bloom in the most rapidly warming waters of BS occurred during the period before sampling and the active development of cyanoprokaryotes continued in a deeper MS. During our studies in July 2018, the average biomass of algae was 18.23 mg/L in MS and 4.05 mg/L in BS; the average biomass of cyanobacteria was 17.04 mg/L in MS and 2.93 mg/L in BS. This is evidenced by the data of satellite image analysis (Fig. 7). Thus, in the image obtained on July 1, 2018, we observe the beginning of Cyanoprokaryota bloom in a shallow southeastern part of BS (the area of the Masrik River). Later on, it developed, reaching the maximum by July 11, 2018, when the satellite once again passed over the area under study. The processes of the beginning of Cyanoprokaryota dying off are clearly visible in the southeastern part and in the littoral zone of the southwestern part of BS; the peak of their bloom shifts toward the middle and northwestern part of BS. In MS, the active bloom is notable in the area of Chayachiy Island and along the boundary of cyclonic and anticyclonic currents. By the end of the month (July 26, 2018), we can observe the practically complete absence of bloom in BS and vast areas of cyanoprokaryote in MS.
The active development of cyanoprokaryotes negatively affects the species richness of plankton algae (Korneva, 1993). As a rule, littoral parts are distinguished by the largest number of algae species (Sakharova and Korneva, 2018; Pokrovskaya et al., 1983), which is facilitated by the high changeability of environmental factors and the nutrient enrichment of littoral zones (Sakharova and Korneva, 2018). However, cyanobacteria have a competitive advantage in the consumption of nutrients and cause light limitation for other phytoplankton species (Paerl and Huisman, 2009). There was such a situation in Lake Sevan in July 2018, when the mass development of cyanoprokaryotes led to the reduction in the relative richness of algae, which is evidenced by the negative correlation between the biomass of cyanoprokaryotes and the number of species in a sample. In addition, the active development of cyanoprokaryotes decreases water transparency (Paerl and Huisman, 2008), which was observed in Lake Sevan and confirmed by a significant negative correlation between the biomass of cyanoprokaryotes and water transparency in MS (Table 2).
Changes in the water level play an important role in the summer phytoplankton development. Its rise leads to the appearance of well-warmed vast areas overgrown with higher aquatic vegetation and an increase in the concentration of nutrients (Sakharova and Korneva, 2018). A high variability of environmental factors and active nutrient enrichment of the littoral zone leads to the high biodiversity of these parts; they are a source of species richness in the water body as a whole (Pollock et al., 1998; Hanley and Barnard, 1999; Naiman et al., 2005). In this regard, the study of flooded coastal areas in Lake Sevan is of particular importance. The increase in the water level in Lake Sevan caused the formation of a vast shallow bay overgrown with higher aquatic plants, which is characterized by slow water exchange with the lake (Airapetyan et al., 2014). The specific community of plankton algae was formed in the bay, which is evidenced by phytoplankton clustering according to the species composition (Fig. 3) and the peculiarities of the ratio of the main taxonomic groups in the phytoplankton biomass. The species and specific richness of phytoplankton reached comparatively larger values in a shallow bay in MS. In addition, dinophyte algae were recorded in the composition of dominant species which are capable of mixotrophic feeding and develop en masse in water bodies with high concentrations of organic substances (Sládečeková and Sládeček, 1993; Sakharova and Korneva 2017). The difference in the biomass between the open area and a part overgrown by macrophytes may be due to the activity of higher plants, which have an advantage in consumption of nutrients (Pokrovskaya et al., 1983). This explains the high buffer function of overgrown shallow zones, which reduce the high inputs of nutrients to the water body (Schlosser and Karr, 1981; Decamps et al., 2004).
Despite the fact that the lake corresponded to mesotrophic water bodies in respect to values of the algae biomass averaged for a vegetation period (Ovsepyan et al., 2010; Ovsepyan and Khachikyan, 2016), the vertical distribution of phytoplankton in July differed from that in other deep stratified mesotrophic lakes (Ekosistema ozera Pleshcheevo, 1989; Trifonova, 1990). In view of active water bloom and low transparency, the maximum biomass of algae was recorded in the surface layer, which is typical of eutrophic water bodies (Trifonova, 1990). The role of large-celled green algae Oonephris obesa increased in the meta- and hypolimnion.
As was mentioned above, the phytoplankton biomass in the lake corresponded to values typical of mesotrophic waters (Kitaev, 2007); however, in July 2018, the phytoplankton biomass substantially increased in the lake compared to 2013–2017 (Ovsepyan et al., 2010; Ovsepyan and Khachikyan, 2016) (Table 3). This is apparently due to the increase in water temperature in the lake, which is indirectly evidenced by the data on average daily air temperature in July (Table 3).
In addition to the above factors, the top-down control plays a major role in the phytoplankton development, which is indicated by significant negative correlations between Cladocera and the biomass of dominant phytoplankton divisions (green algae and cyanoprokaryotes). The positive correlation between Cladocera and cyanoprokaryotes in MS is probably due to the higher concentration of the latter in this part of the lake. When forming large aggregations, they are not available for consumption by plankton invertebrates.
An increase in the density of fish populations reported in the lake during the past years led to a reduction in the abundance and biomass of zooplankton (Gerasimov et al., 2016; Krylov et al., 2016). However, the total abundance and biomass of plankton invertebrates increased in 2018 due to Cladocera, which could also affect the state of phytoplankton. This is due, first and foremost, to the disappearance of Daphnia magna Straus, 1820 from the composition of plankton invertebrates which formed the basis of zooplankton in 2011–2014 and played a key role in the structural organization of plankton in Lake Sevan (Krylov et al., 2016, 2018).
In this regard, the zooplankton grazing pressure on phytoplankton decreased in 2018, which may be one of reasons for a sufficient increase in the algal biomass. The abundance and biomass of smaller species, D. longispina O.F. Mueller, 1776 and Diaphanosoma lacustris Kořinek, 1981, simultaneously increased with the disappearance of D. Magna: compared to the period before the appearance of Daphnia magna, the abundance of the first species increased on average 1.4 times; the biomass increased 1.8 times; and the abundance and biomass of the second species increased 36.6 and 38.6 times, respectively. Compared to the period of the mass development of D. magna they increased 3.3 and 2.5 times and 6.8 and 9.1 times, respectively. It is known that species of Cladocera characterized by smaller sizes are distinguished by the highest rates of phosphorous excretion (Ferrante, 1976). An increase in the phosphorus excretion in 2018 (0.77 ± 0.18 gP/(m3 day)) to values exceeding the values during the period before Daphnia magna introduction to the lake (0.33 ± 0.09 gP/(m3 day)), and during its mass development (0.13 ± 0.03 gP/(m3 day)) due to D. longispina and Diaphanosoma lacustris also could promote an increase in the phytoplankton biomass in July 2018. Cyanoprokaryota bloom in water bodies under the increasing phosphorus load, including excretion by zooplankton, has been described in some works (Vollenweider, 1971; Sirenko and Gavrilenko, 1978).
CONCLUSIONS
The phytoplankton biomass in Lake Sevan in July 2018 corresponded to values typical of mesotrophic waters. The development of cyanoprokaryotes in Lake Sevan had horizontal and vertical heterogeneity and depended on depth, distribution of currents, temperature, and biomass of Cladocera. The relative richness of phytoplankton and water transparency decreased in parts affected by water bloom.
The following factors contributed to an increase in the average phytoplankton biomass when compared to 2013–2017: (1) increase in the water temperature, (2) weakening of zooplankton grazing pressure due to the disappearance of Daphnia magna, and (3) an increase in the phosphorus load due to the increasing contribution of D. longispina and Diaphanosoma lacustris to the zooplankton biomass.
REFERENCES
Abakumov, A.I. and Izrailsky, Y.G., Environment influence on the phytoplankton distribution in a basin, Math. Biol. Bioinf., 2012, vol. 7, no. 1, pp. 274–283. https://doi.org/10.17537/2012.7.274
Airapetyan, A.O., Krylov, A.V., and Gabrielyan, B.K., Zooplankton of two types of shallow water sites of the Sevan Lake formed during water level rise, Biol. Zh. Arm., 2014, vol. 66, no. 1, pp. 12–17.
Balushkina, E.B. and Vinberg, G.G., Dependence between weight and length of body in planktonic animals, in Obshchie osnovy izucheniya vodnykh ekosistem (General Principles of the Study of Aquatic Ecosystems), Leningrad: Nauka, 1979, pp. 169–172.
Chilingaryan, L.A. and Mnatsakanyan, B.A., Hydrology of the Sevan Lake basin, in Ekologiya ozera Sevan v period povysheniya ego urovnya (Ecology of the Sevan Lake during Water Level Rise), Makhachkala: Nauka, 2010, pp. 28–40.
Decamps, H., Pinay, G., Naiman, R., Petts, G.E., McClain, M. E., Hillbricht-Ilkowska, A., Hanley, T.A., Holmes, R.M., Quinn, J., Gibert, J., Planty Tabacchi, A., Schiemer, F., Tabacchi, E., and Zalewski, M., Riparian zones: where biogeochemistry meets biodiversity in management practice, Pol. J. Ecol., 2004, vol. 52, no. 1, pp. 3–18.
Ekosistema ozera Pleshcheevo (Ecosystem of the Pleshcheevo Lake), Leningrad: Nauka, 1989.
Ferrante, J.G., The role of zooplankton in the intrabiocenotic phosphorus cycle and factors affecting phosphorus excretion in a lake, Hydrobiologia, 1976, vol. 49, no. 3, pp. 203–214.
Filatov, N.N., Gidrodinamika ozer (Lake Hydrodynamics), St. Petersburg: Nauka, 1991.
Gerasimov, Yu.V., Gabrielyan, B.K., Malin, N.I., and Rubenyan, A.R., Long-term dynamics and modern state of fish reserves in the Sevan Lake, in Ekologiya ozera Sevan v period povysheniya ego urovnya. Rezul’taty issledovanii Rossiisko-Armyanskoi biologicheskoi ekspeditsii po gidroekologicheskomu obsledovaniyu ozera Sevan (Armeniya) (2005–2009 gg.) (Ecology of the Sevan Lake during Water Level Increase. The Study Results of Russian-Armenian Biological Expedition on Hydroecological Observation of the Sevan Lake (Armenia) in 2005–2009), Makhachkala: Nauka, 2010, pp. 249–278.
Gerasimov, Yu.V., Borisenko, E.S., Malin, N.I., Rubenyan, A.R., Vardanyan, T.V., Barsegyan, N.E., Zhgareva, N.N., Bazarov, M.I., and Gabrielyan, B.K., Fishes, in Ozero Sevan. Ekologicheskoe sostoyanie v period izmeneniya urovnya vody (Lake Sevan: Ecological State during Water Level Dynamics), Yaroslavl: Filigran’, 2016, pp. 207–252.
Hanley, T.A. and Barnard, J.C., Spatial variation in population dynamics of Sitkamice in floodplain forests, J. Mammal., 1999, vol. 80, no. 3, pp. 866–879.
Integral’naya otsenka ekologicheskogo sostoyaniya ozera Sevan (GEO–Lake Sevan). Doklad Association For SHD”/UNEPCom (Integral Assessment of Ecological State of the Sevan Lake (GEO–Lake Sevan): A Report of For SHD”/UNEPCom Association), Yerevan, 2011.
Kitaev, S.P., Osnovy limnologii dlya gidrobiontov i ikhtiologov (Fundamentals of Limnology for Hydrobiologists and Ichthyologists), Petrozavodsk: Karel. Nauchn. Tsentr, Ross. Akad. Nauk, 2007.
Korneva, L.G., Phytoplankton of Rybinsk Reservoir: composition, Specific distribution, and eutrophication consequences, in Sovremennoe sostoyanie ekosistemy Rybinskogo vodokhranilishcha (Modern State of Ecosystem of Rybinsk Reservoir), St. Petersburg: Gidrometeoizdat, 1993, pp. 50–113.
Korneva, L.G., Solov’eva, V.V., Zhakovskaya, Z.A., Ruskikh, Ya.V., and Chenova, E.N., Phytoplankton and content of cyanotoxins in Rybinsk, Gorky, and Cheboksary reservoirs during abnormally hot summer 2010, Voda: Khim. Ekol., 2014, no. 8, pp. 24–29.
Kozitskaya, V.N., Influence of temperature on growth and reproduction of algae with various types of pigment systems, Gidrobiol. Zh., 1991, vol. 27, no. 5, pp. 62–71.
Krylov, A.V., Gerasimov, Yu.V., Gabrielyan, B.K., Borisenko, E.S., Hakobyan, S.A., Nikogosyan, A.A., Malin, M.I., and Ovsepyan, A.A., Zooplankton in Lake Sevan during the period of high water level and low fish density, Inland Water Biol., 2013, vol. 6, no. 3, pp. 203–210. https://doi.org/10.1134/S1995082913030085
Krylov, A.V., Hayrapetyan, A.O., Gerasimov, Yu.V., and Malin, M.I., Changes in the structural parameters of the summer zooplankton in the pelagic zone of Lake Sevan (Armenia) during an increase in fish abundance and biomass, Inland Water Biol., 2016, vol. 9, no. 1, pp. 73–78. https://doi.org/10.1134/S1995082916010090
Krylov, A.V., Kosolapov, D.B., Kosolapova, N.G., Hovsepyan, A.A., and Gerasimov, Yu.V., The plankton community of Sevan Lake (Armenia) after invasion of Daphnia (Ctenodaphnia) magna Straus, 1820, Biol. Bull. (Moscow), 2018, vol. 45, no. 5, pp. 505–511. https://doi.org/10.1134/S0002332918050089
Legovich, N.A., About water blooming in the Sevan Lake, in Ekologiya gidrobiontov ozera Sevan (Ecology of Hydrobionts of the Sevan Lake), Yerevan: Akad. Nauk Arm. SSR, 1979, no. 17, pp. 51–74.
Metodika izucheniya biogeotsenozov vnutrennikh vodoemov (A Method of Analysis of Biogeocenosises of Inland Reservoirs), Moscow: Nauka, 1975.
Naiman, R.J., Bechtold, J.S., Drake, D., Latterell, J.J., O’Keefe, T.C., and Balian, E.A., Origins, patterns, and importance of heterogeneity in riparian systems, in Ecosystem Function in Heterogeneous Landscapes, New York: Springer-Verlag, 2005, pp. 279–309.
Ovsepyan, A.A. and Gambaryan, L.R., The data on development of phytoplanktonic community of the Sevan Lake, Tr. Bryansk.Gos. Univ., 2013, vol. 2, pp. 193–195.
Ovsepyan, A.A. and Khachikyan, T.G., Pelagial phytoplankton of the Sevan Lake, in Ozero Sevan. Ekologicheskoe sostoyanie v period izmeneniya urovnya vody (Lake Sevan: Ecological State during Water Level Dynamics), Yaroslavl: Filigran’, 2016, pp. 39–60.
Ovsepyan, A.A., Gambaryan, L.R., Oganesyan, R.O., and gusev, E.S., Planktonic algae of the Sevan Lake, in Ekologiya ozera Sevan v period povysheniya ego urovnya (Ecology of the Sevan Lake during Water Level Rise), Makhachkala: Nauka, 2010, pp. 90–104.
Paerl, H.W. and Huisman, J., Blooms like it hot, Science, 2008, vol. 320, pp. 57–58. https://doi.org/10.1126/science.1155398
Paerl, H.W. and Huisman, J., Climate change: a catalyst for global expansion of harmful cyanobacterial blooms, Environ. Microbiol. Rep., 2009, vol. 1, no. 1, pp. 27–37. https://doi.org/10.1111/j.1758-2229.2008.00004.x
Pokrovskaya, T.N., Mironova, N.Ya., and Shil’krot, G.S., Makrofitnye ozera i ikh evtrofirovanie (Eutrophication of Macrophytic Lakes), Moscow: Nauka, 1983.
Pollock, M.M., Naiman, R.J., and Hanley, T.A., Plant species richness in riparian wetlands—a test of biodiversity theory, Ecology, 1998, vol. 79, no. 1, pp. 94–105.
Sakharova, E.G. and Korneva, L.G., Phytoplankton communities of Volga Reach open shallows of Rybinsk Reservoir at the area affected by the vital activity products of grey heron (Ardea cinerea L.), Contemp. Probl. Ecol., 2017, vol. 10, no. 2, pp. 140–146. https://doi.org/10.1134/S199542551702010X
Sakharova, E.G. and Korneva, L.G., Phytoplankton in the littoral and pelagial zones of the Rybinsk Reservoir in years with different temperature and water level regimes, Inland Water Biol., 2018, vol. 11, no. 1, pp. 6–12. https://doi.org/10.1134/S1995082918010157
Schlosser, I.J. and Karr, J.R., Water quality in agricultural watersheds: impact of riparian vegetation during baseflow, Water Resour. Bull., 1981, vol. 17, pp. 233–240.
Sirenko, L.A. and Gavrilenko, M.Ya., Tsvetenie vody i evtrofirovanie (Water Blooming and Eutrophication), Kiev: Naukova Dumka, 1978.
Sládečeková, A. and Sládeček, V., Bioindication within the aquatic environment, Acta Environ. Univ. Comenianae, 1993, vol. 7, nos. 1–2, pp. 3–69.
Trifonova, I.S., Ekologiya i suktsessiya ozernogo fitoplanktona (Ecology and Succession of Lake Phytoplankton), Leningrad: Nauka, 1990.
Vollenweider, R.A., Scientific Fundamentals of the Eutrophication of Lakes and Flowing Waters with Particular Reference to Nitrogen and Phosphorus as Factors in Eutrophication, Paris: Org. Econ. Co-op. Dev., 1971.
FUNDING
We are grateful to the crew of the Gidrolog expeditionary vessel for assistance in collecting the material. This study was supported by the Russian Foundation for Basic Research (project no. 18-54-05003 Arm_а) and was performed within the framework of a state assignment (project nos. АААА-А18-118012690096-1, АААА-А18-118012690106-7, and АААА-А18-118012690104-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests.
The authors declare that they have no conflicts of interest.
Statement on the welfare of animals.
This article does not contain any studies involving animals performed by any of the authors.
Additional information
Translated by N. Ruban
Rights and permissions
About this article
Cite this article
Sakharova, E.G., Krylov, A.V., Sabitova, R.Z. et al. Horizontal and Vertical Distribution of Phytoplankton in the Alpine Lake Sevan (Armenia) during the Summer Cyanoprokaryota Bloom. Contemp. Probl. Ecol. 13, 60–70 (2020). https://doi.org/10.1134/S1995425520010072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995425520010072