Abstract
Plant species exhibiting heavy metal tolerance are instrumental in phytoremediation of metalliferous sites. Most of the time, variations in plant functional traits (PFTs) are overlooked while identifying hyperaccumulators. However, investigating morphological, physiological, and phenological variations can contribute to our knowledge about stress tolerance, and aid in identifying potential hyperaccumulators. In the present study, we investigated variation in morpho-functional traits in Solanum nigrum, a known hyperaccumulator, under lead (Pb) stress. Twenty-one PFTs including 9 above-ground (leaf count, leaf area, specific leaf area, leaf dry matter content, leaf thickness, leaf dry mass, shoot length, stem dry mass, stem diameter), 3 below-ground (root length, root dry mass, and root diameter), 4 reproductive (flower bud count, fruit count, flower count, and fruit dry mass), and 5 photosynthetic traits (total chlorophyll, total carotenoid, chlorophyll a, chlorophyll b, and photosynthetic efficiency) under varying Pb concentrations (500–2000 mg kg−1) were assessed. Pillai’s trace test (MANOVA) depicted significant variations in above-ground, below-ground, and photosynthetic traits, whereas reproductive traits did not vary significantly with progressive metal concentration. However, most of the studied traits except flower count, fruit dry mass, and chlorophyll b varied significantly under Pb stress. The study depicts that enhanced PFT’s plasticity enables S. nigrum to grow in Pb-contaminated soil effectively without impacting plant fitness. Plasticity of morpho-functional traits, therefore, establishes itself as a resourceful approach in successful identification of phytoremediation capacity of a plant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals (HMs) infiltrate the soil environment through diverse sources such as foundries, mines, smelters, combustion of fossil fuel, agriculture, and industrial effluents (Masood et al., 2021; Yan et al., 2020). These are inherently non-essential and are conventionally known for their toxicity, persistence in nature, and bioaccumulative property (Ali et al., 2019). HMs also affect human and animal health through malfunctioning of nerves, kidneys, muscles, etc. (Saher & Kanwal, 2019). In plants, HMs induce inactivation of enzymes, production of cytotoxic compounds (reactive oxygen species, ROS such as hydrogen peroxide, H2O2; hydroxyl radical, OH; superoxide radical, O2•−), DNA fragmentation, denaturation of proteins, and alteration of major metabolic processes such as respiration, photosynthesis, and homeostasis, and disruption of oxidative metabolism (Ghori et al., 2019).
Lead (Pb), the 2nd most toxic metal, is a widely present inorganic pollutant encompassing 0.002% of the Earth’s crust (Zulfiqar et al., 2019). Pb toxicity is a matter of global concern since it impacts all ecological spheres through various anthropogenic activities and pedogenic processes (Pourrut et al., 2011). Although Pb accumulation plays no crucial role in plant physiology, yet the evidence of alteration in various metabolic processes and ultrastructure changes in plants has been reported (Ghori et al., 2019; Kaur et al., 2013, 2014; Zulfiqar et al., 2019). Pb mobilization results in deleterious effect on growth, development and productivity of plants, and composition and structure of soil microbial communities (Pourrut et al., 2011; Zulfiqar et al., 2019). Pb toxicity is species-specific, dependent upon Pb concentration, plant developmental stages, and the period of metal exposure (Gul et al., 2020).
Phenotypic plasticity in plants is predominantly concerned with morphological (leaves, flower, bud, and stem traits), physiological (physiological integration, division of labor), phenological and ecological plasticity of individual species (Xu & Zhou, 2016). As HMs affect the biochemistry and physiology of a plant (Ghori et al., 2019), the resultant alteration can also be witnessed in plant functional traits (PFTs) (Song et al., 2019). Previously, a few studies have demonstrated that HMs induce morpho-functional variations in the plants, which enable the plant to thrive under metal-stressed environments. For example, HMs like Cd, Cu, and Zn induced adaptive plasticity in physiological processes in peanut (Shi & Cai, 2009). Chen et al. (2015) reported that successful colonization of Phytolacca americana in Mn-contaminated soil was related to phenotypic plasticity. Likewise, morpho-functional traits in Leptodictyum riparium changed with metal concentration and provided insights into tolerance mechanism (Maresca et al., 2018).
Plants growing in metalliferous soil either prevent the entry of HMs into the cellular cytoplasm (avoiders) or detoxify metal ions crossing the cell membrane (tolerant) (Millaleo et al., 2010). Accordingly, plants are classified as metal excluders, indicators, and hyperaccumulators. Hyperaccumulators/tolerant plants are instrumental in phytoremediation of metalliferous sites (Saad-Allah & Elhaak, 2017; Suman et al., 2018). In hyperaccumulators, antioxidant machinery has been recognized as instrumental in tolerance, but till date, it has not been dwelled in deep how PFTs vary in hyperaccumulators. Most of the time, variations in PFTs are generally overlooked; if studied, they can help in improving the knowledge about stress tolerance mechanism and identification of potential hyperaccumulators, which can be further used in phytomining. Since detecting morpho-functional plasticity is an effective solar-driven and cost-effective approach, it should serve as an alternative indicator of metal hyperaccumulation. Therefore, we hypothesized that variations in PFTs enable the plant to cope up with metal stress and sustain fitness in HM-enriched environment. To prove our hypothesis, we chose Solanum nigrum L. (family Solanaceae), a well-known hyperaccumulator of Cd, Zn, Pb, Cd, and Ni (Saad-Allah & Elhaak, 2017), as a model plant to study the PFTs’ variation under Pb toxicity. To the best of our knowledge, such type of work has not been undertaken previously. In the present study, we analyzed 21 functional traits: nine above-ground (leaf count, leaf area, specific leaf area, leaf dry matter content, leaf thickness, leaf dry mass, shoot length, stem dry mass, stem diameter), three below-ground (root length, root dry mass, and root diameter), four reproductive (flower bud count, fruit count, flower count, and fruit dry mass), and five photosynthetic traits (total chlorophyll, total carotenoid, chlorophyll a, chlorophyll b, and photosynthetic efficiency) under varying Pb concentrations (0–2000 mg kg−1 soil) for assessing adaptive variations.
Materials and methods
Soil samples and characteristics
The soil samples were collected at a depth of 0–0.2 m from unpolluted sites in Panjab University, Chandigarh, India. The collected soil was air-dried and sieved to remove stones and other debris. After sieving, soil was mixed with manure and sand in the ratio of 3:2:1 (soil:manure:sand) to make the composition uniform. The soil was spiked with lead nitrate [Pb (NO3)2] at different concentrations of Pb (500, 1000, 1500, 2000 mg kg−1). Thereafter, 200 mg kg−1 of ammonium nitrate (NH4NO3) was added to the control soil to normalize the effect of nitrates present in Pb-salt spiked soil. The spiked soil was incubated for 2 weeks for achieving homogenization.
All soil samples were analyzed in triplicates for various soil properties. These were as follows: total nitrogen = 0.69% (Kjeldahl method), total phosphorous = 0.18% (ammonium vanadatemolybdate method], total potassium = 0.52% (ammonium acetate extraction method), and organic carbon = 3.16% (Walkley black method), pH = 6.84 (soil: water = 1:2, w/v), and electrical conductivity = 0.60 mmhos cm−1 (soil: water = 1:2, w/v).
Materials and experiment description
Seeds of S. nigrum were collected locally from plants growing in the wild. Seeds were surface sterilized with 10% sodium hypochlorite for 10 min and then rinsed at least 4 times with sterilized deionized water (Jiang et al., 2016). After sterilization, S. nigrum seeds were sown in seedling trays filled with substrate soil. Seeds were germinated in a growth chamber at 25 °C, 16/8 h day/night photoperiod, and 70% relative humidity (Fagodia et al., 2017).
Experimental design
The experiment was conducted in 10 cm diameter polyethylene plastic pots of 1 kg capacity. The control and spiked soil with different concentrations were poured into the respective pots. Seedlings having 4–6 true leaves and height of 5–6 cm were randomly selected and transferred to plastic pots filled with 1 kg soil, with one seedling per pot. There were six replicates of each treatment arranged in a completely randomized design. Distilled water was used for irrigating pots to maintain the soil moisture at 60% during the experimental duration. Plants were grown under natural light and temperature conditions (average day temperature: 18–25 °C; average night temperature: 8–15 °C) in an experimental dome. After 8 weeks, plants were harvested for the measurement and calculation of different parameters. Then, the plants were rinsed with distilled water before subjecting them for the measurement of PFTs.
Analysis of morphological and reproductive traits
Above- and below-ground traits
Root length (cm) and shoot length (cm) were measured with a centimeter scale, whereas leaves were counted manually. Root, shoot, and leaf were separated and subjected to oven drying at 75 °C for 72 h. Root dry mass (RDM; g), stem dry mass (SDM; g), and leaf dry mass (LDM; g) were measured by weighing balance (Mettler Toledo-ME104, 0.0001 g), while leaf thickness (mm), stem diameter (mm), and root diameter (mm) were measured using a digital Vernier caliper (0.1 mm accuracy). For determining specific leaf area (SLA; mm2 mg−1) and leaf dry matter content (LDMC; mg g−1), the methodology given by Pérez-Harguindeguy et al. (2013) was followed (Table 1). Leaf area (LA; mm2) was determined using a leaf area meter (CI-202, CID Bio-Science, USA).
Reproductive traits
Flower bud count (per plant), flower count (per plant), and fruit count (per plant) were counted manually, and fruit dry mass (g) was measured using an electronic weighing balance.
Determination of photosynthetic traits
From each treatment, leaves of the plant were used for determining photosynthetic traits such as photosynthetic efficiency (Fv/Fm), chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll content, and total carotenoid. Among the studied traits, total chlorophyll content and total carotenoid content were estimated according to the method described by Kaur et al. (2019). Chlorophyll was extracted from 25 mg fresh leaves and was incubated in 5 mL of dimethyl sulphoxide (DMSO) at 60 °C for 1 h as per Hiscox and Israelstam (1979). Subsequently, the absorbance of the solution was read on Shimadzu UV-1800 spectrophotometer at 645 nm, 663 nm, and 470 nm taking DMSO as blank. Amounts of Chl a, Chl b, total chlorophyll and total caotenoid were calculated as per equations provided in Table 1. Total chlorophyll and total carotenoid content were expressed on the dry weight basis as suggested by Rani and Kohli (1991). Photosynthetic efficiency was measured by using OS-30p pulse modulated chlorophyll fluorometer (Opti Sciences, USA).
Statistical analysis
The linear model of ANOVA was used to test the effect of treatments on PFTs of S. nigrum. Treatments were considered as a fixed factor and PFTs were used as a response variable. On the other hand, PFTs were clustered into qualitative functional groups for multivariate analyses (MANOVA), viz., above-ground (leaves count, leaf thickness, LDM, LA, SLA, LDMC, shoot length, stem diameter, and SDM), below-ground (root length, root diameter, and RDM), reproductive (flower bud count, flower count, fruit count, and fruit dry mass), and photosynthetic traits (photosynthetic efficiency, Chl a, Chl b, total chlorophyll content, and total carotenoid content). Significant effects were evaluated with Tukey’s honest significance test for multiple comparisons of group means. All statistical analyses were performed in statistical stats—package and graphical representation in ggplot2 package in R version 3.6.0. Every population sample was used for making graphical visualizations. All the differences were tested at p ≤ 0.05 for determining the statistical significance.
Results
Functional group variation
Multivariate analysis (MANOVA) and Pillai’s test (V) was used to test the significance of quantitative groups (group functional traits treated as factor). Above-ground traits (F = 14.691, p < 0.01), below-ground traits (F = 17.86, p < 0.001), and photosynthetic traits (F = 10.1, p < 0.01) varied significantly with treatments, except for reproductive traits (F = 1.427, p = 0.24) (Table 2).
Above-ground and below-ground trait variations
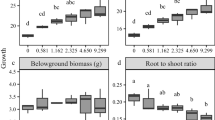
Above-ground traits such as LA (0.101%) and LDMC (14.89%) decreased (Fig. 1b, d), while leaf count (118.46%), SLA (16.43%), leaf thickness (92.50%), and LDM (294.35%) increased linearly (Fig. 1a, c, e, f). The linear model of ANOVA depicted that all above-ground traits like LA (F = 1.2737, p < 0.001), LDMC (F = 2.855, p < 0.1), leaf count (F = 14.8616, p < 0.001), SLA (F = 7.6734, p < 0.001), leaf thickness (F = 47.556, p < 0.001), and LDM (F = 30.0737, p < 0.001) varied significantly (Table 3). Similarly, shoot length, SDM and stem diameter increased by 160.36%, 379.07%, and 11.81%, respectively (Fig. 1g, h, i). ANOVA showed significant interaction with shoot length (F = 8.8848, p < 0.001), SDM (F = 8.8015, p < 0.001), and stem diameter (F = 6.7092, p < 0.01) (Table 3). In addition, below-ground traits such as RDM (127.37%) and root diameter (56.17%) increased, whereas root length (47.98%) decreased significantly in a linear pattern (Fig. 1k, l, j). A significant interaction was also observed with RDM (F = 3.0721, p < 0.05), root diameter (F = 5.8571, p < 0.05), and root length (F = 4.4438, p < 0.05) (Table 3).
Reproductive trait variations
The reproductive traits such as flower bud count, flower count, and fruit count linearly increased with increasing Pb concentration by 191.176%, 800%, and 139.34%, respectively (Fig. 2a, b, c). However, FDM decreased linearly (9.72%) with increasing concentration of Pb (Fig. 2d). ANOVA revealed significant interaction with flower bud count (F = 10.2189, p < 0.001) and fruit count (F = 3.6846, p < 0.05), while flower count (F = 1.6814, p = 0.2015) and FDM (g) (F = 0.1564, p = 0.6942) were non-significant (Table 3).
Photosynthetic traits variation
Photosynthetic traits varied significantly in response to Pb stress. Significant interaction was observed with Chl a (F = 8.8236, p < 0.05), total chlorophyll content (F = 13.103, p < 0.01), total carotenoid content (F = 6.1327, p < 0.05), and photosynthetic efficiency (F = 17.8652, p < 0.001), except for Chl b (F = 2.7722, p = 0.1198) (Table 3). Photosynthetic efficiency (13.42%), Chl a (277.92%), total chlorophyll content (224.58%), and total carotenoid content (75.27%) increased linearly with increasing dose of Pb toxicity (Fig. 3a, b, d, e), except for Chl b (Fig. 3c) as compared with control (Table 3).
Discussion
In our study, most of the PFTs were found to vary significantly with different Pb concentrations, thereby depicting the potential of S. nigrum to exhibit enhanced PFTs in HM-enriched environment. We observed that PFT groups such as above-ground, below-ground, and photosynthetic traits showed highly plastic behavior, while reproductive traits showed the least (Table 3).
Response of above-ground and below-ground traits
Change in growth patterns is a fundamental plant response when encountering biotic and/or abiotic stresses such as heavy metals (Nejat & Mantri, 2017). A slight disturbance in cellular homeostasis triggers an adaptive response in plants. Plant morphology is a parameter utilized for the assessment of plant traits in heterogeneous environments (Abdusalam & Li, 2018). In our results, an increased shoot length and decreased root length were recorded, which might be caused by an uneven distribution of Pb from root to shoot due to an apoplastic and symplastic barrier (Shu et al., 2011). Another reason might be attributed to the phenomenon called hormesis, which is a stimulatory effect in growth reported under the physiologically toxic dose of HMs (Li et al., 2020; Poschenrieder et al., 2013). The observable biomass’s increase or decrease is species-specific, dependent on exposure duration to HM and various experimental conditions (soil properties, microflora, fauna, etc.). The increased biomass could be due to Pb-detoxification (Zulfiqar et al., 2019). This might be correlated with the potential to retain Pb in active non-metabolic regions like cell wall and vacuole that facilitate the growth of the plants without any hindrance (Li et al., 2020). Moreover, in our study, there was an increase in root diameter with increasing Pb concentration. It was corroborated by the findings of Zarinkamar et al. (2013) who reported that in Matricaria chamomilla at the shooting and flowering stages, root diameter increased as Pb concentration caused thickening of cell walls. Pb stimulated parenchyma cells to rapidly begin the secondary growth, which might be the reason for the increase in root diameter and dry weight of roots with increasing concentration of toxicity (Alle et al., 2019).
Leaves are considered as one of the most important plant part due to their role in light capturing and photosynthesis (Ren et al., 2019; Strauss et al., 2020). The studied leaf morphological traits exhibited significant variations with increasing Pb concentration. Leaf area is often characterized with specific adaptations and is known to be sensitive to drought as well as metal stress conditions (Lopez et al., 2008). Decrease in LA can be attributed to inhibition of cell growth and development of plants following Pb toxicity (Shu et al., 2011). LDMC is an important parameter for the evaluation of a species resource utilization strategy; thus, variations in LDMC have direct consequences on plant growth (Li et al., 2005).
Moreover, leaf thickness, SLA, leaf count, and LDM depicted a significant increase with increasing concentration of Pb. An increase in leaf thickness generally indicates structural adaptations for water conservation and herbivore defense (Firn et al., 2019). Increased carbon partitioning in leaf area growth enhances SLA and is a key parameter contributing to the morphological plasticity of species (Weraduwage et al., 2015). Higher SLA optimizes resource allocation, thus has a direct bearing on integrated plant growth, reproductive strategies, and lifespan of species (Lin et al., 2020). High SLA is a determinant of low leaf construction cost and represents a trade-off between surface area (for capturing photons) and resource utilization strategies at biogeographical scale (Firn et al., 2019). The morphological parameters like height of the plant, SLA, and LDMC are effective approaches that efficiently reflect the plants’ response to changing environmental variables (Yue et al., 2019). Furthermore, observed enhanced plant growth might also be attributed to protection from pathogenic attack because of growing in a metal-rich environment (Boyd, 2007).
Response of reproductive traits
Reproduction is the most important stage in the life cycle of plant species, which directly affects species performance (O’Brien, 2019). Heavy metal concentration, atmospheric deposition, and plant growth phase are some of the factors which influence metal absorption and accumulation in vegetables and fruits (Lăcătuşu & Lăcătuşu, 2008). As depicted in our results, FDM decreased with increasing Pb dose. Similar results were reported by Elhindi et al. (2018) in Tagetes erecta upon treatment with Zn, Co, Mn, and Cu. Our results depicted an increase in flower bud count, fruit count, and flower count, which demonstrates the enhanced reproductive fitness of the plant. An increase in number of flowers in Streptanthus polygaloides treated with Ni was recognized as an indication of maintained plant fitness (Jhee et al., 2006). On the contrary, Sankaran and Ebbs (2008) reported that in Brassica juncea heavy metal got translocated to seeds but did not exceed the permissible consumption limits. Similarly, Klatte et al. (2009) reported that seeds experienced the least impact of metal toxicity as compared to other parts of plants. Overall, it can be said that an increase in studied reproductive traits might be resultant of reproductive fitness, which helps to maintain progeny fitness in unfavorable environmental conditions.
Response of photosynthetic traits
Chlorophyll content is a crucial indicator of plant photosynthetic efficiency (Haritha et al., 2017). It is usually measured in plants to assess the impact of environmental stress, as changes in pigment contents are linked to visual symptoms of plant illness and photosynthetic productivity (Chandra & Kang, 2016). Pb is non-essential for cell growth and development of plants; however, it is absorbed through roots and sequestered in vacuoles leading to no significant effect on photosynthetic apparatus (Ahmed & Tajmir-Riahi, 1993; Kaur et al., 2014). The observed changes in photosynthetic pigment content may be attributed to the ability of a plant to maintain its growth and fitness by increasing the level of chlorophyll synthesis under Pb-induced stress (MacFarlane & Burchett, 2001). Improved photosynthetic activity enhances the carbon assimilation that results in biomass accumulation for growth sustenance (Simkin et al., 2019). Our results were contrary to the findings of Peng et al. (2020) who revealed that chlorophyll content decreased in S. nigrum when treated with Cd. Oladele et al. (2019) observed that chlorophyll content significantly decreased on exposure to Pb and Zn treatments in Vigna subterranean and Zea mays. The enhanced carotenoid content under increasing Pb concentration further implies their role in ROS quenching, and thus preventing peroxidation of membrane lipids (Ramel et al., 2012). In our study, photosynthetic efficiency increased with a higher dose of Pb, which resulted in enhanced energy conversion and yield (Simkin et al., 2019), and consequently the ability of S. nigrum to withstand HM stress and maintain growth fitness.
Conclusions
Conventional tools and technology currently employed are either not cost-effective or are labor-intensive, thereby arose the need for an alternative mode of detecting hyperaccumulator capacity of any plant. The study concludes that S. nigrum has the potential to tolerate Pb toxicity through the plasticity of traits as the presence of Pb in contaminated soil enhanced PFTs effectively without impacting plant fitness. Morpho-functional variations, therefore, establishes itself as a resourceful approach in the successful identification of the phytoremediation capacity of a plant. Hence, variations in PFTs provide an additional economical value and help reclamation of HM contaminated soils under field conditions.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abdusalam, A., & Li, Q. (2018). Morphological plasticity and adaptation level of distylous Primula nivalis in a heterogeneous alpine environment. Plant Diversity, 40, 284–291. https://doi.org/10.1016/j.pld.2018.11.003
Ahmed, A., & Tajmir-Riahi, H. A. (1993). Interaction of toxic metal ions Cd2+, Hg2+, and Pb2+ with light-harvesting proteins of chloroplast thylakoid membranes. An FTIR spectroscopic study. Journal of Inorganic Biochemistry, 50(4), 235–243.
Ali, H., Khan, E., & Ilahi, I. (2019). Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. Journal of Chemistry, 2019, 6730305.
Alle, V., Kondratovics, U., Vikmane, M., & Osvalde, A. (2019). Cadmium and lead accumulation and anatomical changes in roots of Vicia faba under heavy metal contamination. International Multidisciplinary Scientific GeoConference: SGEM, 19(3.2), 71–78.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiology 24, 1. https://doi.org/10.1104/pp.24.1.1
Boyd, R. S. (2007). The defense hypothesis of elemental hyperaccumulation: Status, challenges and new directions. Plant and Soil, 293(1–2), 153–176. https://doi.org/10.1007/s11104-007-9240-6
Chandra, R., & Kang, H. (2016). Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. Forest Science and Technology, 12, 55–61.
Chen, C., Zhang, H., Wang, A., Lu, M., Shen, Z., & Lian, C. (2015). Phenotypic plasticity accounts for most of the variation in leaf manganese concentrations in Phytolacca americana growing in manganese-contaminated environments. Plant and Soil, 396(1–2), 215–227.
Elhindi, K. M., Al-Mana, F. A., El-Hendawy, S., Al-Selwey, W. A., & Elgorban, A. M. (2018). Arbuscular mycorrhizal fungi mitigates heavy metal toxicity adverse effects in sewage water contaminated soil on Tagetes erecta L. Soil Science and Plant Nutrititon, 64, 662–668. https://doi.org/10.1080/00380768.2018.1490631
Fagodia, S. K., Batish, D. R., Singh, H. P., Kohli, R. K., Sharma, A., & Sharma, S. (2017). Comparative evaluation of herbicidal activity of Citrus aurantiifolia essential oil and limonene towards some agricultural weeds. International Journal of Tropical Agriculture, 35, 147–155.
Firn, J., McGree, J. M., Harvey, E., Flores-Moreno, H., Schütz, M., Buckley, Y. M., Borer, E. T., Seabloom, E. W., La Pierre, K. J., MacDougall, A. M., Prober, S. M., Stevens, C. J., Sullivan, L. L., Porter, E., Ladouceur, E., Allen, C., Moromizato, K. H., Morgan, J. W., Harpole, W. S., … Risch, A. C. (2019). Leaf nutrients, not specific leaf area, are consistent indicators of elevated nutrient inputs. Nature Ecology & Evolution, 3, 400–406. https://doi.org/10.1038/s41559-018-0790-1
Ghori, N. H., Ghori, T., Hayat, M. Q., Imadi, S. R., Gul, A., Altay, V., & Ozturk, M. (2019). Heavy metal stress and responses in plants. International Journal of Environmental Science and Technology, 16, 1807–1828. https://doi.org/10.1007/s13762-019-02215-8
Gul, I., Manzoor, M., Kallerhoff, J., & Arshad, M. (2020). Enhanced phytoremediation of lead by soil applied organic and inorganic amendments: Pb phytoavailability, accumulation and metal recovery. Chemosphere, 258, 127405.
Haritha, G., Vishnukiran, T., Yugandhar, P., Sarla, N., & Subrahmanyam, D. (2017). Introgressions from Oryza rufipogon increase photosynthetic efficiency of KMR3 rice lines. Rice Science, 24, 85–96. https://doi.org/10.1016/j.rsci.2016.07.006
Hiscox, J. D., & Israelstam, G. F. (1979). A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany, 57, 1332–1334.
Jhee, E. M., Boyd, R. S., Eubanks, M. D., & Davis, M. A. (2006). Nickel hyperaccumulation by Streptanthus polygaloides protects against the folivore Plutella xylostella (Lepidoptera: Plutellidae). Plant Ecology, 183(1), 91. https://doi.org/10.1007/s11258-005-9009-z
Jiang, Q. Y., Tan, S. Y., Zhuo, F., Yang, D. J., Ye, Z. H., & Jing, Y. X. (2016). Effect of Funneliformis mosseae on the growth, cadmium accumulation and antioxidant activities of Solanum nigrum. Applied Soil Ecology, 98, 112–120. https://doi.org/10.1016/j.apsoil.2015.10.003
Kaur, G., Singh, H. P., Batish, D. R., & Kohli, R. K. (2013). Lead (Pb)-induced biochemical and ultrastructural changes in wheat (Triticum aestivum) roots. Protoplasma, 250(1), 53–62. https://doi.org/10.1007/s00709-011-0372-4
Kaur, G., Singh, H. P., Batish, D. R., & Kohli, R. K. (2014). Morphological, anatomical, and ultrastructural changes (visualized through scanning electron microscopy) induced in Triticum aestivum by Pb2+ treatment. Protoplasma, 251, 1407–1416. https://doi.org/10.1007/s00709-014-0642-z
Kaur, A., Kaur, S., Singh, H. P., Batish, D. R., & Kohli, R. K. (2019). Phenotypic variations alter the ecological impact of invasive alien species: Lessons from Parthenium hysterophorus. Journal of Environmental Management, 241, 187–197. https://doi.org/10.1016/j.jenvman.2019.03.129
Klatte, M., Schuler, M., Wirtz, M., Fink-Straube, C., Hell, R., & Bauer, P. (2009). The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses. Plant Physiology, 150, 257–271. https://doi.org/10.1104/pp.109.136374
Lăcătuşu, R., & Lăcătuşu, A. R. (2008). Vegetable and fruits quality within heavy metals polluted areas in Romania. Carpathian Journal of Earth and Environmental Sciences, 3, 115–129.
Li, J., Qiu, Y., Zhao, Q., Chen, D., Wu, Z., Peng, A. A., & Wu, W. (2020). Lead and copper-induced hormetic effect and toxicity mechanisms in lettuce (Lactuca sativa L.) grown in a contaminated soil. Science of The Total Environment, 741, 140440.
Lin, S., Niklas, K. J., Wan, Y., Hölscher, D., Hui, C., Ding, Y., & Shi, P. (2020). Leaf shape influences the scaling of leaf dry mass vs. area: a test case using bamboos. Annals of Forest Science, 77(1), 1-15.
Lopez, F. B., Chauhan, Y. S., & Johansen, C. (2008). Effects of timing of drought stress on leaf area development and canopy light interception of short-duration pigeon pea. Journal of Agronomy and Crop Science, 178, 1–7. https://doi.org/10.1111/j.1439-037X.1997.tb00344.x
MacFarlane, G. R., & Burchett, M. D. (2001). Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the grey mangrove, Avicennia marina (Forsk.) Vierh. Marine Pollution Bulletin, 42, 233–240. https://doi.org/10.1016/S0025-326X(00)00147-8
Maresca, V., Fusaro, L., Sorbo, S., Siciliano, A., Loppi, S., Paoli, L., Monaci, F., Karam, E. A., Piscopo, M., Guida, M., Galdiero, E., Insolvibile, M., & Basile, A. (2018). Functional and structural biomarkers to monitor heavy metal pollution of one of the most contaminated freshwater sites in Southern Europe. Ecotoxicology and Environmental Safety, 163, 665–673. https://doi.org/10.1016/j.ecoenv.2018.07.122
Masood, F., Pandey, R., Singh, H. P., Gupta, A. S., Kaur, S., Batish, D. R., & Kohli, R. K. (2021). Cytotoxic and genotoxic assessment of agricultural soils from an industrial region. Environmental Monitoring and Assessment, 193(8), 526. https://doi.org/10.1007/s10661-021-09289-3
Millaleo, R., Reyes-Díaz, M., Ivanov, A. G., Mora, M. L., & Alberdi, M. (2010). Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. Journal of Soil Science and Plant Nutrition, 10, 470–481.
Nejat, N., & Mantri, N. (2017). Plant immune system: crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defence. Current Issues in Molecular Biology, 23(1), 1-16.
O’Brien, C. (2019). Effects of elevated CO2 on the reproductive performance and early life fitness of long-lived woody plant species. Hawkesbury Institute for the Environment, Western Sydney University, Australia.
Oladele, E. O., Adewumi, O. O., Yahaya, T., & Taiwo, I. A. (2019). Response of Bambara groundnut (Vigna subterranean L.) and maize (Zea mays L.) to heavy metal stress. Beni-Suef University Journal of Basic and Applied Sciences, 8(1), 1–9.
Peng, R., Sun, W., Jin, X., Yu, L., Chen, C., Yue, Z., & Dong, Y. (2020). Analysis of 2,4-epibrassinolide created an enhancement tolerance on Cd toxicity in Solanum nigrum L. Environmental Science and Pollution Research, 27, 16784–16797. https://doi.org/10.1007/s11356-020-08228-y
Pérez-Harguindeguy, N., Díaz, S., Garnier, E., Lavorel, S., Poorter, H., Jaureguiberry, P., et al. (2013). New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany, 61, 167–234. https://doi.org/10.1071/BT12225.
Poschenrieder, C., Cabot, C., Martos, S., Gallego, B., & Barceló, J. (2013). Do toxic ions induce hormesis in plants? Plant Science, 212, 15–25. https://doi.org/10.1016/j.plantsci.2013.07.012
Pourrut, B., Shahid, M., Dumat, C., Winterton, P., & Pinelli, E. (2011). Lead uptake, toxicity, and detoxification in plants. Reviews of Environmental Contamination and Toxicology, 213, 113–136. https://doi.org/10.1007/978-1-4419-9860-6_4
Ramel, F., Birtic, S., Cuiné, S., Triantaphylidès, C., Ravanat, J. L., & Havaux, M. (2012). Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiology, 158, 1267–1278. https://doi.org/10.1104/pp.111.182394
Rani, D., & Kohli, R. K. (1991). Fresh matter is not an appropriate relation unit for chlorophyll content: Experience from experiments on effects of herbicide and allelopathic substance. Photosynthetica, 25, 655–658.
Ren, T., Weraduwage, S. M., & Sharkey, T. D. (2019). Prospects for enhancing leaf photosynthetic capacity by manipulating mesophyll cell morphology. Journal of Experimental Botany, 70, 1153–1165. https://doi.org/10.1093/jxb/ery448
Saad-Allah, K. M., & Elhaak, M. A. (2017). Hyperaccumulation activity and metabolic responses of Solanum nigrum in two differentially polluted growth habitats. Journal of the Saudi Society of Agricultural Sciences, 16, 227–235. https://doi.org/10.1016/j.jssas.2015.08.001
Saher, N. U., & Kanwal, N. (2019). Assessment of some heavy metal accumulation and nutritional quality of shellfish with reference to human health and cancer risk assessment: A seafood safety approach. Environmental Science and Pollution Research, 26, 5189–5201. https://doi.org/10.1007/s11356-018-3764-6
Sankaran, R. P., & Ebbs, S. D. (2008). Transport of Cd and Zn to seeds of Indian mustard (Brassica juncea) during specific stages of plant growth and development. Physiologia Plantarum, 132, 69–78. https://doi.org/10.1111/j.1399-3054.2007.00994.x
Shi, G., & Cai, Q. (2009). Leaf plasticity in peanut (Arachis hypogaea L.) in response to heavy metal stress. Environmental and Experimental Botany, 67, 112–117. https://doi.org/10.1016/j.envexpbot.2009.02.009
Shu, X., Yin, L., Zhang, Q., & Wang, W. (2011). Effect of Pb toxicity on leaf growth, antioxidant enzyme activities, and photosynthesis in cuttings and seedlings of Jatropha curcas L. Environmental Science and Pollution Research, 19, 893–902. https://doi.org/10.1007/s11356-011-0625-y
Simkin, A. J., López-Calcagno, P. E., & Raines, C. A. (2019). Feeding the world: Improving photosynthetic efficiency for sustainable crop production. Journal of Experimental Botany, 70, 1119–1140. https://doi.org/10.1093/jxb/ery445
Song, G., Wang, J., Han, T., Wang, Q., Ren, H., Zhu, H., Wen, X., & Hui, D. (2019). Changes in plant functional traits and their relationships with environmental factors along an urban-rural gradient in Guangzhou, China. Ecological Indicators, 106, 105558. https://doi.org/10.1016/j.ecolind.2019.105558
Strauss, S., Lempe, J., Prusinkiewicz, P., Tsiantis, M., & Smith, R. S. (2020). Phyllotaxis: Is the golden angle optimal for light capture? New Phytologist, 225, 499–510. https://doi.org/10.1111/nph.16040
Suman, J., Uhlik, O., Viktorova, J., & Macek, T. (2018). Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Frontiers in Plant Science, 9, 1476. https://doi.org/10.3389/fpls.2018.01476
Weraduwage, S. M., Chen, J., Anozie, F. C., Morales, A., Weise, S. E., & Sharkey, T. D. (2015). The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Frontiers in Plant Science, 6, 167. https://doi.org/10.3389/fpls.2015.00167
Xu, L., & Zhou, Z. F. (2016). Effects of Cu pollution on the expansion of an amphibious clonal herb in aquatic-terrestrial ecotones. PLoSOne, 11, e0164361. https://doi.org/10.1371/journal.pone.0164361
Yan, A., Wang, Y., Tan, S. N., Mohd Yusof, M. L., Ghosh, S., & Chen, Z. (2020). Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Frontiers in Plant Science, 11, 359. https://doi.org/10.3389/fpls.2020.00359
Yue, X., Zuo, X., Yu, Q., Xu, C., Lv, P., Zhang, J., Knapp, A. K., & Smith, M. D. (2019). Response of plant functional traits of Leymus chinensis to extreme drought in Inner Mongolia grasslands. Plant Ecology, 220(2), 141–149. https://doi.org/10.1007/s11258-018-0887-2
Yulin, L. I., Johnson, D. A., Yongzhong, S. U., Jianyuan, C. U. I., & Zhang, T. (2005). Specific leaf area and leaf dry matter content of plants growing in sand dunes. Botanical Bulletin of Academia Sinica, 46, 127–134
Zarinkamar, F., Saderi, Z., & Soleimanpour, S. (2013). Excluder strategies in response to Pb toxicity in Matricaria chamomilla. Advances in Bioresearch, 4(3), 39–49.
Zulfiqar, U., Farooq, M., Hussain, S., Maqsood, M., Hussain, M., Ishfaq, M., Ahmad, M., & Anjum, M. Z. (2019). Lead toxicity in plants: Impacts and remediation. Journal of Environmental Management, 250, 109557. https://doi.org/10.1016/j.jenvman.2019.109557
Funding
The authors received financial support from the Ministry of Environment, Forest and Climate Change, India. MA received a research fellowship from the Council of Scientific and Industrial Research, India.
Author information
Authors and Affiliations
Contributions
PS, HPS, and DRB conceived the idea for this study. MA, PS, SR, and RKK designed the study. PS and SR conducted the experiments. PS, SR, and MA did the analysis and wrote the first draft. HPS, RKK, and DRB edited the earlier versions of the manuscript. All authors interpreted results and contributed to the final draft of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, P., Ahmad, M., Rathee, S. et al. Bridging the gap: linking morpho-functional traits’ plasticity with hyperaccumulation. Environ Monit Assess 193, 762 (2021). https://doi.org/10.1007/s10661-021-09504-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09504-1