Abstract
The present research describes the successful preparation of melon peel biochar modified with CoFe2O4 (MPBC/CoFe2O4) followed by its usage as a new sorbent to separate, preconcentrate, and determine the toxic heavy metal ions by magnetic solid-phase extraction. The metal ion desorption was performed by 0.1 M HCl solution with a volume of 5.0 mL. Flame atomic absorption spectrometry (FAAS) was utilized for detection of the analyte levels. SEM–EDX, TEM, XRD, and FTIR techniques were carried out to illuminate the structure of MPBC/CoFe2O4. The fundamental variables affecting the adsorption and elution efficiencies of the analyte ions including solution pH, MPBC/CoFe2O4 amount, type and concentration of eluent, adsorption and desorption equilibrium time, and sample volume were optimized. The detection limits were calculated as 0.41, 1.82, and 3.16 µg L−1 for Cu2+, Cd2+, and Pb2+ ions, respectively, with the relative standard deviation of lower than 4.2%. There were no substantial interference effects on the analyte ion recovery due to the presence of foreign ions at high levels. Five minutes of contact time was adequate to attain the adsorption equilibrium. The adsorption capacity of MPBC/CoFe2O4 was obtained as 106.4, 65.4, and 188.7 mg g−1 for Cu2+, Cd2+, and Pb2+ ions, respectively, by utilizing Langmuir isotherm model. The pseudo-second order model is favorable to identify the adsorption kinetics. The method was validated by spike/recovery test, and then, it was successfully implemented to determine the aforementioned analyte levels in sea and stream water, pepper, black cabbage, eggplant, and tomato samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The inevitable increment in the concentration of persistent trace heavy metals in the environment through a variety of industrial, agricultural, and technological activities has attracted more attention in recent years by virtue of their detrimental impacts on living organisms. Lead and cadmium ions are among heavy metals that have extremely toxic effects even at very low concentrations since they induce inhibition or even deprivation of the biological functions of enzymes in living organisms and ultimately cause incredibly negative damages to the nervous, reproductive, and circulatory systems (Huang et al., 2020; Zhao et al., 2016). Moreover, the accumulation of Cd2+ and Pb2+ ions in human body may impair the brain functions and provoke cancer diseases. Although copper is a requisite trace metal for humans, animals, and plants in certain concentrations since it acted as a cofactor in enzymatic reactions, it causes various diseases including cramps, spasms, vomiting, or even death if taken in excess amounts (Dahaghin et al., 2017; Tu et al., 2016). The maximum allowable Cu2+, Cd2+, and Pb2+ ions levels designated by the World Health Organization (WHO)-Food and Agriculture Organization (FAO) in vegetables are 73.0, 0.10, and 0.30 mg kg−1, respectively, while the tolerable levels of these ions in drinking water recommended by WHO are 2.0, 0.01, and 0.003 mg L−1, respectively (Behbahani et al., 2013; Bigdeli & Seilsepour, 2008). Therefore, the precise determination and monitoring of the toxic metal ions contents both in water and food samples are surpassingly important task for scientist.
Numerous analytical techniques including UV–Visible spectrometry, inductively coupled plasma-mass spectrometry, electroanalytical methods, and flame or graphite furnace atomic absorption spectrometry have been employed in the routine detection of trace heavy metal levels. The complicated matrix that consists of a great number of concomitant cations and anions together with heavy metal ions to be analyzed and the lower concentrations of heavy metals than the quantification limit of the aforementioned instrumental techniques restrict the accurate and sensitive analysis (Gouda, 2014). To come through these troubles, a convenient separation and preconcentration method including solid-phase extraction (Yağci et al., 2020), coprecipitation (Tufekci et al., 2013), liquid–liquid extraction (Farajvand et al., 2019), membrane filtration (Soylak et al., 2004), and cloud point extraction (Rahnama & Najafi, 2016) is generally employed prior to analysis.
Solid-phase extraction (SPE) is regarded as a versatile and efficient method with respect to its simplicity and rapidity, usage of less organic solvents in the process, enabling the development of a wide variety of low cost and effective adsorbents, and achieving the high preconcentration factors that allows detecting the extremely low levels of metal ions in the environmental real samples (Tokay & Bagdat, 2019). Besides, the magnetic solid-phase extraction (MSPE) is useful types of SPE method since it enables to separate the utilized adsorbent from high volume of solutions by applying a magnet instead of time consuming filtration or centrifugation. The properties of the selected adsorbents significantly affect the performance of the SPE method (Kanani et al., 2018).
Biochar is a type of carbon-rich substance fabricated by thermal decomposition of organic materials such as apple pomace (Zhang et al., 2019), dew melon peel (Ahmadi et al., 2016), pine bark (Reddy & Lee, 2014), banana and orange peels (Amin et al., 2019), and potato leaves (Zhao et al., 2019) under limited oxygen conditions and at a temperature in the range of 200–900 °C. The utilization of biochar produced from fruit peels has attracted attention due to its abundance and cheapness. Biochar, which is an eco-friendly and cost-effective adsorbent, has a large specific surface area and porous structure, and there are various functional groups on its structure that allow the adsorption of metal ions (Reddy & Lee, 2014). By the reason of low density and small particle size of the biochars, the collection of them from aqueous solutions is considered as a fundamental problem that limits its large-scale application. The magnetic biochar that has an excellent performance in adsorbing the metal ions can be separated easily utilizing an external magnetic field. It is prepared properly by inducing magnetic particles into biochar (Li et al., 2020; Zhang et al., 2019). Magnetic nanoparticles formulated via MFe2O4 have drawn considerable attention as adsorbent due to their magnificent chemical and physical characteristics. In that formula, “M” can be Mn, Zn, Co, Mg, Fe, and Cu atoms (Ramadan, 2019). The cobalt ferrite (CoFe2O4) is commonly preferred magnetic material due to its chemical and thermal stability, high mechanical hardness, easy preparation and separation, and biodegradability. Kanani et al. (2018) have prepared a new magnetic adsorbent by coating MCM-41 with CoFe2O4 and piperazine to separate and preconcentrate the Cu2+, Cd2+, and Pb2+ ions prior to FAAS analysis. Reddy and Lee (2014) have demonstrated an effortless preparation of magnetic biochar with pine bark and CoFe2O4 to be utilized as an adsorbent for retention of Pb2+ and Cd2+ ions from aqueous media. Foroutan et al. (2018) have modified the Phoenix dactylifera stones activated carbon with CoFe2O4 to use as an adsorbent in Cr(VI) uptake.
In the present research, a new and effective magnetic adsorbent, melon peel biochar/CoFe2O4 (MPBC/CoFe2O4) nanoparticles, has been synthesized and characterized. The MPBC/CoFe2O4 has been applied to separate and preconcentrate the Cu2+, Cd2+, and Pb2+ ions in water and vegetable samples by MSPE method. The predominant experimental variables affecting the adsorption and desorption yields of the analyte ions have been evaluated and optimized to obtain the satisfactory recovery values. By this work, a sensitive, effective, economical, and eco-friendly separation and preconcentration technique is considered to be developed for the simultaneous determination of heavy metal ions from food and water samples by FAAS.
Materials and methods
Instrumentation
The morphology and elemental composition of MPBC and MPBC/CoFe2O4 was assessed by the scanning electron microscope with an energy-dispersive X-ray spectrometer (SEM–EDX, ZIESS SIGMA 300). Transmission electron microscope (TEM, Hitachi HT7700) was used to evaluate the microstructure of the adsorbents. The Fourier transform infrared spectrometer (PerkinElmer 1600 FT-IR Spectrophotometer) was utilized to identify the surface functional groups of MPBC and MPBC/CoFe2O4. The structure of the MPBC/CoFe2O4 was scrutinized through X-ray diffraction (XRD) measurements (Rigaku TTR III) with Cu Kα radiation (λ = 1.5406 Å) in the 2Ɵ range of 5–80°. A PerkinElmer AAnalyst 400 model Flame Atomic Absorption Spectrometer was employed to determine the metal ions levels. The device was equipped with deuterium background system and air/acetylene burner. The wavelengths for Cu2+, Cd2+, and Pb2+ ions were selected as 324.75, 228.80, and 283.31 nm, respectively, and other working conditions of the instrument were designated by considering the manufacturer’s recommendations. The pH adjustment was carried out by utilizing a Hanna pH-211 digital pH meter. The batch adsorption and desorption experiments were performed by mechanical shaker (Edmund Bühler GmbH). A closed vessel microwave system (Milestone Ethos D) was operated to digest the vegetable samples. Analytical balance (Sartorius BP 1106) and heating magnetic stirrer (IKA RCT Basic) were utilized in the studies when needed.
Reagents
All the chemical reagents used at different stages of the experiments including nitric acid (HNO3, 65%), sodium hydroxide (NaOH), hydrochloric acid (HCl, 37%), hydrogen peroxide (H2O2, 30%), iron(III) chloride hexahydrate (FeCl3·6H2O), and cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O) and all the salts employed for the interference study are of analytical grade supplied from Sigma-Aldrich (Shanghai, China) and Merck (Darmstadt, Germany) and used without any additional purification process. To obtain the desired concentration of the standard and working solutions of Cu2+, Cd2+, and Pb2+ ions, stock solutions (1000 mg L−1) of these ions prepared in 2% (v/v) HNO3 solution were diluted with distilled water in the appropriate amounts. The glassware utilized in the experiments was kept overnight in 5% (v/v) HNO3 and washed thoroughly with tap water and then with distilled water before use.
Preparation of melon peel biochar/CoFe2O4
The melon was purchased from a greengrocer in Gümüşhane, Turkey, and its peel was collected for the preparation of adsorbent. After washed the melon peels with distilled water, it was cut into small pieces. Then, it was first kept in an open atmosphere for 3 days to remove of moisture and then dried in an oven at 60 °C for 24 h. The sufficient amount of melon peel was put in a porcelain crucible and carbonized in a muffle furnace at 450 °C under oxygen limited conditions. The holding time and heating rate were kept as 60 min and 10 °C/min, respectively. After the resulting melon peel biochar (MPBC) was cooled down to room temperature, it was grinded in an agate mortar and preserved in a plastic container until it was needed (Ahmadi et al., 2016).
The MPBC/CoFe2O4 was prepared briefly as follows: 5.0 g of MPBC was mixed with 3.66 g of Co(NO3)2·6H2O and 6.80 g of FeCl3·6H2O (molar ratio of Fe3+/Co2+ = 2) in 200 mL of aqueous solution. In order to provide an opportunity for the penetration of Fe and Co ions into the MPBC pores, the mixture was stirred vigorously for 20 min and increased the temperature to 90 °C. The NaOH solution (3.0 M) was slowly added until the pH of the solution was 10, and the mixture was stirred for 120 min. Thereafter, the MPBC/CoFe2O4 was collected by filtration, washed thoroughly with distilled water up to neutral pH, and dried in an oven at 105 °C for 24 h (Foroutan et al., 2018; Mehrabi et al., 2017).
Model solutions
The batch technique was carried out to separate and preconcentrate the Cu2+, Cd2+, and Pb2+ ions based on SPE method. The general procedure is as follows: 0.10 g of MPBC/CoFe2O4 (2.0 g L−1) was added to 50 mL of aqueous solution containing 0.4 mg L−1 of Pb2+, 0.1 mg L−1 of Cd2+, and 0.2 mg L−1 of Cu2+ ions. Diluted NaOH or HNO3 solutions were utilized for the adjustment of pH to its optimum value of 5.0. The mixture was shaken on a mechanical shaker at room temperature for 5 min at 400 rpm to achieve the equilibrium. At the end of this period, the metal-loaded MPBC/CoFe2O4 was accumulated at the bottom of the centrifuge tube with an external magnet and then the supernatant was discarded. Afterwards, the adsorbent was treated with 5.0 mL of 0.1 M HCl solution for 5 min to elute the metal ions. Finally, the MPBC/CoFe2O4 was magnetically removed from the solution and the levels of the analyte ions were analyzed in the supernatant by FAAS. The factors affecting the adsorption and desorption conditions of the metal ions including pH, contact time, MPBC/CoFe2O4 quantity, eluent type, concentration and volume, and sample volume were studied in detailed and optimized.
Real sample preparation
The MPBC/CoFe2O4 was implemented successfully for the detection of the analyte ions in water (sea and stream water) and vegetable (pepper, black cabbage, eggplant, and tomato) samples. The seawater (Blacksea, Trabzon, Turkey) and stream water (Değirmendere, Trabzon, Turkey) were filtered by using cellulose nitrate membrane as soon as they were collected. After the samples were acidified, they were kept at 4 °C in the refrigerator in polyethylene bottles until analysis. For implementation of the method, the pH of the 250 mL of water samples (optimum sample volume) was set to 5.0 and the required amount of MPBC/CoFe2O4 was added. Then, the presented SPE method was carried out to determine their Cu2+, Cd2+, and Pb2+ levels. The vegetable samples were purchased from a greengrocer in Gümüşhane, Turkey. Before digestion, the samples were washed thoroughly with distilled water, dried in an oven at 80 °C for 12 h, and then ground in an agate mortar. The vegetable samples were digested by the closed vessel microwave digestion system. For that purpose, 0.750 g of pepper, black cabbage, eggplant, and tomato samples was weighted into the separate Teflon vessels accurately and digested with 6 mL of HNO3 and 2 mL of H2O2 in microwave system (Duran et al., 2009b).
Results and discussions
Adsorbent characterization
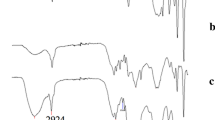
The surface morphology of MPBC and MPBC/CoFe2O4 was assessed by scanning electron microscopy (SEM) technique. The SEM image of MPBC disclosed its rough and slightly porous structure which is suitable for the aggregation of CoFe2O4 nanoparticles (Fig. 1a). The SEM image given in Fig. 1b indicated the attachment of CoFe2O4 on MPBC surface. The EDX spectra of MPBC (Fig. 2a) and MPBC/CoFe2O4 (Fig. 2b) display their elemental composition. MPBC contains 44.36% of C, 54.76% of O, and 0.88% of Fe. When the content of the MPBC/CoFe2O4 was evaluated, it is seen that the amount of Fe (26.04%) increases significantly and a Co peak (7.56%) is also formed in the spectrum. The EDX results indicated the presence of CoFe2O4 on the MPBC surface. Li et al. (2021) observed a similar elemental composition for CoFe2O4-biochar by using SEM–EDX method. The morphology of the adsorbents was further evaluated by utilizing TEM. By comparing the TEM images of MPBC (Fig. 3a) and MPBC/CoFe2O4 (Fig. 3b), it is noticed that CoFe2O4 nanoparticles were distributed on a regular basis dramatically in the MPBC matrix. The XRD pattern of MPBC/CoFe2O4 is shown in Fig. 4. The characteristic peaks (2Ɵ = 30.2°, 35.5°, 43.2°, and 57.2°) correspond to crystal indexes of 220, 311, 400, and 511 indicating the formation of cubic spinel type lattice of MPBC/CoFe2O4 (Rahimi et al., 2018). Similar XRD pattern was obtained by You et al. (2021) for the magnetic corn stalks biochar/ CoFe2O4 complex. FTIR spectroscopy was utilized to acquire information about the surface functional groups of the adsorbents. Both MPBC and MPBC/CoFe2O4 possess several peaks on their structure confirming the presence of polysaccharides and polyphenols. The small peaks at 2926 cm−1 and 1460 cm−1 were related to aliphatic –CH2 stretching while the peaks at 1390, 1112, 1040, and 700–900 cm−1 were attributed to aliphatic –OH, phenolic –OH, C–O, and aromatic C–H species, respectively (Fig. 5a) (Han et al., 2021). By comparing the FTIR spectrum of MPBC and MPBC/CoFe2O4, it is noticed that some peaks have shifted and several new peaks have formed. A stretching vibration at 3320 cm−1 in the FTIR spectra of MPBC/CoFe2O4 was attributed to O–H bonds. The MPBC/CoFe2O4 showed a peak at 570 cm−1 (Fig. 5b), corresponding to the Fe–O stretching mode of the CoFe2O4 (Reddy & Lee, 2014; Zhou et al., 2014). When the characterization results are evaluated in general, it can be concluded that the CoFe2O4 was smoothly loaded onto MPBC.
Effect of pH on the extraction of analyte ions
The pH of aqueous media has a crucial impact with respect to extraction efficiency of the metal ions since the H3O+ ion concentration of solution, the ionization degree of the surface functional groups of an adsorbent, and also speciation of the metal cations are directly affected by the medium pH (Khan et al., 2016). Therefore, the influence of pH on the extraction efficiency of Cu2+, Cd2+, and Pb2+ ions was scrutinized by varying the solution pH in the range of 2.0–8.0 with keeping other experimental parameters constant. Based on the obtained data, the analyte ions recovery increased sharply when the solution pH increased from 2.0 to 5.0, and then, it remained nearly constant in the pH range of 5.0–8.0 (Fig. 6). The recovery percentages were below than 15% at pH 2.0 and higher than 96% in the pH range of 5.0–8.0 for all analyte ions. At lower pH values, the competition between the H3O+ ions and the metal cations to bind to the active adsorption sites on MPBC/CoFe2O4 surface could be resulted in low recovery values. Furthermore, the protonation of MPBC/CoFe2O4 surface at highly acidic pH values induces an electrostatic repulsion between the adsorbent surface and the metal cations. Conversely, when the solution pH was increased, the surface charge of MPBC/CoFe2O4 become more negative because of the presence of excess hydroxide ions, which lead to adsorption of positively charged metal ions substantially (Sun et al., 2015). As a result, the pH 5.0 was specified as optimum pH for the subsequent studies. Suo et al. (2019) also reported that pH 5.0 was the optimum value for simultaneous MSPE of Cr3+, Co2+, Ni2+, Cu2+, Pb2+, Cd2+, and Ag+ ions in environmental water samples by utilizing silica-coated magnetic graphene oxide nanocomposite as an adsorbent.
Evaluation of desorption solution type, concentration, and volume
In SPE procedures, the preference of an appropriate eluent plays a significant role to desorb the extracted analyte ions quantitatively. The complete and rapid desorption of the adsorbed analyte ions from the adsorbent surface is tremendously dependent on the type, concentration, and volume of the eluent used. The HNO3 and HCl solutions are prevalently utilized as desorption solution without causing any interference effects in the analyses step and producing any hazardous wastes (Heidari et al., 2020). That is why, the influences of HNO3 and HCl concentration on the recoveries of Cu2+, Cd2+, and Pb2+ ions were studied in the acid concentration range of 0.1–3.0 M under the optimum conditions. The quantitative recoveries were acquired when used 0.1 M HCl or 0.1 M HNO3 solutions for all analyzed metal ions (Table 1). As the acid concentration increased from 0.5 to 3.0 M, the extraction efficiency of the analyte ions decreased considerably. This decrease may be due to as a result of decomposition of MPBC/CoFe2O4 at high acid concentrations (Khan et al., 2016). So, for further experiments, 0.1 M HCl was chosen as an effective, low cost, and eco-friendly elution solution to extract the analyte ions adsorbed by MPBC/CoFe2O4.
In order to achieve high preconcentration factors, calculated by dividing the optimum sample volume by the eluent volume, it is necessary to choose the lowest volume of the eluent that provides quantitative recovery. Therefore the volume of 0.1 M HCl was assessed within the range of 2.5–10.0 mL. When the volume of HCl was increased from 2.5 to 5.0 mL, the extraction efficiencies increased from 74.4 to 96.8%, from 82.4 to 95.6%, and from 79.3 to 96.9% for Cu2+, Cd2+, and Pb2+ ions, respectively. In the HCl volume ranges of 5.0–10.0 mL, quantitative recovery values were obtained for all analyte ions. Consequently, the volume of 0.1 M HCl was optimized as 5.0 mL for the simultaneous determination of target ions throughout the SPE experiments.
Effect of MPBC/CoFe2O4 quantity
The influence of MPBC/CoFe2O4 dosage on the analyte ions recovery was assessed in the sorbent amount range of 1.0–15.0 g L−1. As the MPBC/CoFe2O4 dosage was increased from 1.0 to 5.0 g L−1, the recoveries of Cu2+, Cd2+, and Pb2+ ions increased from 94.9, 93.5, and 97.3 to 98.6%, 96.7%, and 97.5%, respectively, as a result of increase in the surface area and the binding sites for the adsorption of metal ions (Ghorbani et al., 2020). Although there was no considerable decrease for Cd2+ ions at MPBC/CoFe2O4 amounts higher than 5.0 g L−1, the recovery values for Cu2+ and Pb2+ ions decreased dramatically at higher adsorbent amounts. That is, the extraction efficiencies obtained for Cu2+, Cd2+, and Pb2+ ions at 15.0 g L−1 of MPBC/CoFe2O4 amount were 87.0%, 95.7%, and 75.4%, respectively (Fig. 7). This result may be due to the decrease in the strength of the eluent (5 mL of 0.1 M HCl) at high MPBC/CoFe2O4 amounts. Similar results were obtained by Assi et al. (2019) for the SPE of Cr6+ and Cr3+ ions using magnetic nanoparticles modified by 1,5‑diphenylcarbazide. Accordingly, 2.0 g L−1 of MPBC/CoFe2O4 amount was chosen for further works.
Effect of sample volume
In order to determine the analyte ions accurately and sensitively in real samples, it is required to determine the optimum sample volume to which the method can be applied. The higher optimum sample volume contributes to achieve high preconcentration factors, thus enabling the detection of analyte ions present at very low levels in environmental samples. In this regard, the influence of sample volume was evaluated in the volume range of 50–1000 mL containing 20 µg of Pb2+, 5 µg of Cd2+, and 10 µg of Cu2+ ions and 2.0 g L−1 of MPBC/CoFe2O4 suspension at pH 5.0. The equilibrium time was kept as 5 min for each sample volume. The best extraction efficiencies were achieved by maximum applicable sample volume of 250 mL which are 97.6%, 95.6%, and 96.0% for Cu2+, Cd2+, and Pb2+ ions, respectively. The recovery values decreased distinctly while increasing the sample volume from 250 to 1000 mL. Thus, 250 mL was determined as optimum sample volume for the simultaneous determination of the analyte ions and the preconcentration factor was calculated as 50 when used 5.0 mL of eluent volume.
Effect of contact time for adsorption and desorption
In order to decide the adequate equilibrium time for the retention of Cu2+, Cd2+, and Pb2+ ions onto MPBC/CoFe2O4, the studies were performed in the equilibrium time range of 1–120 min by contacting 20.0 mg L−1 of Pb2+, 2.0 mg L−1 of Cd2+, and 5.0 mg L−1 of Cu2+ ions with 2.0 g L−1 of MPBC/CoFe2O4 suspensions at pH 5.0. When the solutions were shaken for 5 min, the qe (mg g−1) values of all analyte ions achieved their maximum values. Due to the rapid saturation of the active adsorption sites on the MPBC/CoFe2O4 surface, which were available substantially at the initial stages of the process, no significant change in the adsorption efficiency occurred after more than 5 min of agitation. As a consequence, a short contact time of 5 min was selected for complete adsorption of analyte ions on the MPBC/CoFe2O4.
The sufficient time for elution of Cu2+, Cd2+, and Pb2+ ions from MPBC/CoFe2O4 was assessed by changing the time in the range of 1–120 min. In these experiments, 2.0 g L−1 of MPBC/CoFe2O4 was mixed with 0.4 mg L−1 of Pb2+, 0.1 mg L−1 of Cd2+, and 0.2 mg L−1 of Cu2+ ions at pH 5.0. The volume of aqueous solution volume was 50 mL. At the end of the 5 min of adsorption time, the MPBC/CoFe2O4 was separated from the solution and treated with 5.0 mL of 0.1 M HCl solution in the time range of 1–120 min. The metal ions levels in the eluents were measured after being separated the adsorbent from the mixture at the end of each specified time period. The recovery values were 96.4%, 98.1%, and 96.1% for Cu2+, Cd2+, and Pb2+ ions, respectively, at the desorption contact time of 1 min. Hence, 1 min was designated as the optimum desorption time for the analyte ions. However, to make sure whether the adequate elution was obtained, the desorption contact time was specified as 5 min for subsequent studies. The short adsorption and desorption time is considered to be one of the most important advantages of the presented study. Zhu et al. (2016) have optimized the shaking time as 30 min for the SPE of Cr3+, Fe3+, Pb2+, and Mn2+ ions from wastewater using carbon nanotubes functionalized with diethylenetriamine. Sun et al. (2015) have determined both the adsorption and desorption contact time as 7 min for magnetic SPE of different heavy metal ions from biological samples by utilizing magnetic graphene oxide nanocomposite.
Effect of foreign ions
Most of the foreign ions present in the sample matrix may interfere the extraction efficiencies of the analyte ions. Therefore, to assess the performance and practicability of the method, the effects of potentially interfering ions generally found in environmental samples were evaluated by adding these ions at known concentrations to the 50 mL of model solutions containing 0.4 mg L−1 of Pb2+, 0.1 mg L−1 of Cd2+, and 0.2 mg L−1 of Cu2+ ions and 2.0 g L−1 of MPBC/CoFe2O4 suspension at pH 5.0. The solutions were treated according to the optimized SPE procedure. The obtained results revealed that the presence of most of the foreign ions at high levels have no notable effect on the simultaneous extraction and determination of target analyte ions indicating that the proposed method can be applied to the complicated matrices with high selectivity (Table 2).
Adsorption isotherms
In order to estimate the adsorption capacity of MPBC/CoFe2O4 and to interpret the adsorption mechanism of the analyte ions in more detailed, the Langmuir, Freundlich, and Dubinin-Radushkevich (D-R) isotherm models were implemented to the experimental data. For that purpose, the experiments were performed by using different initial metal ion concentrations (50–800 mg L−1) at pH 5.0 by using 2.0 g L−1 of MPBC/CoFe2O4 amount. According to the graph plotted by the qe values versus initial metal ion concentration (figure not shown), it was observed that the adsorption amount (qe) increased from 10.4 to 180.0 mg g−1 for Pb2+, from 17.2 to 65.0 mg g−1 for Cd2+, and from 13.1 to 100.0 mg g−1 for Cu2+ ions as increasing the initial metal ion concentration from 50 to 800 mg L−1.
Langmuir model supports the idea that the adsorption eventuates as a monolayer on the adsorbent surface, which is homogeneous and has active adsorption sites with equal energy (Langmuir, 1918). Conversely, Freundlich model is associated with the multilayer adsorption on heterogeneous adsorbent surface (Freundlich, 1906). The linear forms of Langmuir and Freundlich models are given in Eqs. 1 and 2, respectively.
qe (mg g–1) and Ce (mg L–1) are the adsorption amount per unit mass of MPBC/CoFe2O4 and the equilibrium concentration of metal ions, respectively.
The substantial feature of Langmuir model can be specified by RL given by the following equation:
Co (mg L–1) describes the initial metal ion levels. For the acceptable process, RL should be in the range of 0–1 (McKay et al., 1987).
D-R model is utilized to assess the metal ions adsorption mechanism (Dubinin & Radushkevich, 1947). The linear form of the model can be expressed as follows:
ε is the Polanyi potential and calculated by Eq. 5:
R (8.314 J mol–1 K–1) is the gas constant, T (K) is the temperature, and Ce (mol L–1) is the metal ion levels in aqueous solution at equilibrium. The value of E is observed using Eq. 6:
If the calculated E values are in the range of 8–16 kJ mol−1, the adsorption process occurs through ion exchange. The physical and chemical adsorption mechanism is considered dominant when E values are lesser than 8 kJ mol−1 and higher than 16 kJ mol−1, respectively (Mosai et al., 2020).
The Langmuir, Freundlich, and D-R isotherm constants were obtained from the linear plot of Ce/qe versus Ce, lnqe versus lnCe, and In qe versus ε2, respectively, according to Eqs. 1, 2, and 4. The isotherm constants and correlation coefficients (R2) are given in Table 3. The Langmuir, Freundlich, D-R isotherms, and experimental data are shown in Fig. 8. When the Langmuir and Freundlich models were compared, it can be said that the metal ion adsorption on the homogeneous MPBC/CoFe2O4 surface as a monolayer, since the R2 for all three metal ion adsorption are higher for the Langmuir model. In addition, since the R2 observed from the Freundlich model in the adsorption of Cu2+ ions is relatively high, it can be considered that the Cu2+ adsorption is also multilayered. The adsorption capacity of MPBC/CoFe2O4 was obtained as 188.7, 65.4, and 106.4 mg g–1 for Pb2+, Cd2+, and Cu2+ ions, respectively, by using Langmuir equation. The adsorption capacity of the proposed adsorbent is higher than most of the other reported sorbents given in Table 4 (Duran et al., 2009a; Huang et al., 2020; Khan et al., 2016; Manoochehria et al., 2015; Mashhadizadeh et al., 2014; Soylak et al., 2019; Suleiman et al., 2009; Vellaichamy & Palanivelu, 2011; Xu et al., 2013). Between 50 and 800 mg L−1 of initial metal ion concentrations, the RL values were in the range of 0.35–0.03 for Pb2+, 0.16–0.01 for Cd2+, and 0.53–0.06 for Cu2+ ions which demonstrated the favorability of the process. The n values obtained from the Freundlich model were in the range of 1–10 also supported the suitability of the adsorption of Pb2+, Cd2+, and Cu2+ ions onto MPBC/CoFe2O4 (Le et al., 2019). The calculated E values using D-R model for all three metal ions were in the range of 8–16 kJ mol−1, indicating that the mechanisms of the adsorption of Cu2+, Cd2+, and Pb2+ ions onto MPBC/CoFe2O4 possibly take place via ion exchange mechanisms (Thamilarasi et al., 2018).
Adsorption kinetics
Adsorption kinetics was assessed by considering the pseudo-first-order (Lagergren, 1898) and pseudo-second-order kinetic models (Ho & McKay, 1998) and intraparticle diffusion models (Weber & Morriss, 1963) expressed by Eqs. 7, 8, and 9, respectively.
qe (mg g–1) indicates analyte ion levels adsorbed onto per unit mass of MPBC/CoFe2O4 at equilibrium; qt (mg g–1) denotes the amounts of the analyte ions adsorbed on the MPBC/CoFe2O4 at any time t; k1 (min–1), k2 (g mg–1 min–1), and kid (mg g–1 min–1/2); rate constants of first-order kinetics model, second-order kinetics model, and intraparticle diffusion models, respectively, C (mg g−1); and intraparticle diffusion model constant (Gundogdu et al., 2012).
The pseudo-first order (PFO) and pseudo-second order (PSO) kinetic model parameters were observed from the intercept and slope of the linear plots of ln(qe–qt) versus t and t/qt versus t, respectively, and given in Table 5. The R2 values belonging to the PFO model were obtained as 0.781, 0.931 and 0.903 for Cu2+, Cd2+, and Pb2+ ions, respectively, while for PSO kinetic model, R2 values were 0.999 for all three metal ions. Compared to the PFO, the adsorption mechanism of the analyte ions on MPBC/CoFe2O4 appears to be better assorted with the PSO kinetic model due to the high correlation coefficient and the proximity of qe values obtained from the model to the experimental qe values. This result demonstrated that the chemisorption mechanism is also possible in the process. Similar observations were reported by Buema et al. (2021) for the SPE of Cd2+ ions using hybrid inorganic CoFe2O4/carboxymethyl cellulose polymeric framework nanobeads. On the other hand, the intraparticle diffusion model was examined to explain the adsorption mechanism in more detail. By evaluating the qt versus t1/2 plots, it is seen that the adsorption process consists of three stages: film diffusion, intraparticle diffusion, and equilibrium state. Since the last step occurs very quickly, it is not effective in the adsorption mechanism. Whichever of the film diffusion or intraparticle diffusion steps has the smaller rate constant, that stage is considered to be more effective. As the rate constant values for both stages were compared, it is seen that the rate constants of the second stage have smaller values for all three metal ions; therefore, the intraparticle diffusion mechanism is considered to be effective in the metal ions adsorption onto MPBC/CoFe2O4. However, this assumption is valid when the line passes through its origin so that the C values are zero (Ozdes & Duran, 2014). The C constants were reported as 2.49, 0.88, and 9.76 for Cu2+, Cd2+, and Pb2+ ions, respectively, and hence, both film diffusion and intraparticle diffusion are considered to be impressive in the adsorption of Cu2+, Cd2+, and Pb2+ ions onto MPBC/CoFe2O4 (Ozdes et al., 2014).
Analytical performance of the method
To determine the limit of detection (LOD), the optimized method was applied to ten blank solutions. The LODs were calculated by taking three times the standard deviations of the analysis results of the blank solutions and obtained as 0.41, 1.82, and 3.16 µg L−1 for Cu2+, Cd2+, and Pb2+ ions, respectively. The relative standard deviation (RSD) that gives idea about the precision of the method was calculated by applying the optimized procedure ten times using 50 mL of aqueous solutions containing 0.4 mg L−1 of Pb2+, 0.1 mg L−1 of Cd2+, and 0.2 mg L−1 of Cu2+ ions. Based on the results, the RSDs were 2.34, 4.19 and 3.10% for Cu2+, Cd2+, and Pb2+ ions, respectively. The figures of merits of the proposed method were compared with those of other reported SPE procedures, as summarized in Table 4. It is evident that the MPBC/CoFe2O4-based SPE method has lower RSD and LOD and also higher preconcentration factor when compared to most of the previous methods.
Method accuracy and application to real samples
The aim of optimizing the experimental parameters in detail is to offer a method for simultaneous detection of Cu2+, Cd2+, and Pb2+ ions in environmental liquid and solid samples. On the other hand, the accuracy and applicability of the optimized method were estimated by spiking different amounts of analyte ions to sea and stream waters (Table 6) and to pepper, black cabbage, eggplant, and tomato samples (Table 7). Sufficient conformity was observed between the added and found analyte levels, demonstrating the validity of the suggested method. As a conclusion, the proposed method was applied successfully to determine the heavy metal levels of water and vegetable samples (Table 8).
Conclusions
In this paper, a simple, effective, and environmentally friendly magnetic solid-phase extraction technique using melon peel biochar modified with CoFe2O4 as a new adsorbent was developed. The experimental parameters were optimized, and the method validation was performed by the spike/recovery tests. Thereafter, the MPBC/CoFe2O4 was successfully utilized for separation, preconcentration, and simultaneous determination of Cu2+, Cd2+, and Pb2+ ions in waters and some vegetable samples without significant matrix interferences. There is no need to use any special laboratory equipment in the process. This demonstrated the cheapness of the method. The quantitative recovery values were obtained in a wide pH range (4.0–8.0). The low adsorption and desorption contact time, low LOD and RSD values, and the obtained high metal adsorption capacity of MPBC/CoFe2O4 are considered as crucial advantages of the suggested method. As a result, it is considered that this study provide a new and beneficial approach for the assessment of trace heavy metal ions in waters and vegetable samples.
Availability of data and material
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
References
Ahmadi, M., Kouhgardi, E., & Ramavandi, B. (2016). Physico-chemical study of dew melon peel biochar for chromium attenuation from simulated and actual wastewaters. Korean Journal of Chemical Engineering, 33(9), 2589–2601.
Amin, M. T., Alazba, A. A., & Shafiq, M. (2019). Comparative study for adsorption of methylene blue dye on biochar derived from orange peel and banana biomass in aqueous solutions. Environmental Monitoring and Assessment, 191, 735.
Assi, N., Azar, P. A., Tehrani, M. S., Husain, S. W., Darwish, M., & Pourmand, S. (2019). Selective solid phase extraction using 1,5-diphenylcarbazide-modified magnetic nanoparticles for speciation of Cr(VI) and Cr(III) in aqueous solutions. International Journal of Environmental Science and Technology, 16, 4739–4748.
Behbahani, M., Salarian, M., Amini, M. M., Sadeghi, O., Bagheri, A., & Bagheri, S. (2013). Application of a new functionalized nanoporous silica for simultaneous trace separation and determination of Cd(II), Cu(II), Ni(II), and Pb(II) in food and agricultural products. Food Analytical Methods, 6, 1320–1329.
Bigdeli, M., & Seilsepour, M. (2008). Investigation of metals accumulation in some vegetables irrigated with waste water in Shahre Rey-Iran and toxicological implications. American-Eurasian Journal of Agricultural & Environmental Sciences, 4, 86–92.
Buema, G., Borhan, A. I., Herea, D. D., Stoian, G., Chiriac, H., Lupu, N., Roman, T., Pui, A., Harja, M., & Gherca, D. (2021). Magnetic solid-phase extraction of cadmium ions by hybrid self-assembled multicore type nanobeads. Polymers, 13, 229.
Dahaghin, Z., Mousavi, H. Z., & Sajjadi, S. M. (2017). Trace amounts of Cd(II), Cu(II) and Pb(II) ions monitoring using Fe3O4@graphene oxide nanocomposite modified via 2-mercaptobenzothiazole as a novel and efficient nanosorbent. Journal of Molecular Liquids, 231, 386–395.
Dubinin, M. M., & Radushkevich, L. V. (1947). Equation of the characteristic curve of activated charcoal. Proceedings of the Academy of Sciences of the USSR, Physical Chemistry Section, 55, 331–333.
Duran, A., Tuzen, M., & Soylak, M. (2009a). Preconcentration of some trace elements via using multiwalled carbon nanotubes as solid phase extraction adsorbent. Journal of Hazardous Materials, 169, 466–471.
Duran, C., Bulut, V. N., Gundogdu, A., Ozdes, D., Yildirim, N., Soylak, M., Senturk, H. B., & Elci, L. (2009b). Carrier element-free coprecipitation with 3-phenly-4-o-hydroxybenzylidenamino-4,5-dihydro-1,2,4-triazole-5-one for separation/preconcentration of Cr(III), Fe(III), Pb(II) and Zn(II) from aqueous solutions. Journal of Hazardous Materials, 167(1–3), 294–299.
Farajvand, M., Kiarostami, V., Davallo, M., & Ghaedi, A. (2019). Simultaneous extraction of Cu2+ and Cd2+ ions in water, wastewater, and food samples using solvent-terminated dispersive liquid–liquid microextraction: Optimization by multiobjective evolutionary algorithm based on decomposition. Environmental Monitoring and Assessment, 191, 287.
Foroutan, R., Mohammadi, R., Ramavandi, B., & Bastanian, M. (2018). Removal characteristics of chromium by activated carbon/ CoFe2O4 magnetic composite and Phoenix dactylifera stone carbon. Korean Journal of Chemical Engineering, 35(11), 2207–2219.
Freundlich, H. M. F. (1906). Uber die adsorption in lösungen. Zeitschrift Für Physikalische Chemie, 57, 385–470.
Ghorbani, Y. A., Ghoreishi, S. M., & Ghani, M. (2020). Derived N-doped carbon through core-shell structured metal-organic frameworks as a novel sorbent for dispersive solid phase extraction of Cr(III) and Pb(II) from water samples followed by quantitation through flame atomic absorption spectrometry, Microchemical Journal, 155, 104786.
Gouda, A. A. (2014). Solid-phase extraction using multiwalled carbon nanotubes and quinalizarin for preconcentration and determination of trace amounts of some heavy metals in food, water and environmental samples. International Journal of Environmental Analytical Chemistry, 94, 1210–1222.
Gundogdu, A., Duran, C., Senturk, H. B., Soylak, M., Ozdes, D., Serencam, H., & Imamoglu, M. (2012). Adsorption of phenol from aqueous solution on a low-cost activated carbon produced from tea ındustry waste: Equilibrium, kinetic, and thermodynamic study. Journal of Chemical Engineering Data, 57, 2733–2743.
Han, L., Nie, X., Wei, J., Gu, M., Wu, W., & Chen, M. (2021). Effects of feedstock biopolymer compositions on the physiochemical characteristics of dissolved black carbon from lignocellulose-based biochar. Science of the Total Environment, 751, 141491.
Heidari, M., Ghanemi, K., & Nikpour, Y. (2020). Applying Al2O3@Ag@trithiocyanuric acid as an efficient metal ion scavenger for the selective extraction of iron (III) and lead (II) from environmental waters. Ecotoxicology and Environmental Safety, 203, 110995.
Ho, Y. S., & McKay, G. (1998). Kinetic models for the sorption of dye from aqueous solution by wood. Journal of Environment Science Health. Part B: Process Safety and Environmental Protection, 76, 183–191.
Huang, L., Huang, W., Shen, R., & Shuai, Q. (2020). Chitosan/thiol functionalized metal–organic framework composite for the simultaneous determination of lead and cadmium ions in food samples. Food Chemistry, 330, 127212.
Kanani, N., Bayat, M., Shemirani, F., Ghasemi, J. B., Bahrami, Z., & Badiei, A. (2018). Synthesis of magnetically modified mesoporous nanoparticles and their application in simultaneous determination of Pb(II), Cd(II) and Cu(II). Research on Chemical Intermediates, 44, 1689–1709.
Khan, M., Yilmaz, E., Sevinc, B., Sahmetlioglu, E., Shah, J., Jan, M. R., & Soylak, M. (2016). Preparation and characterization of magnetic allylamine modified graphene oxide-poly(vinylacetate-co-divinylbenzene) nanocomposite for vortex assisted magnetic solid phase extraction of some metal ions. Talanta, 146, 130–137.
Lagergren, S. (1898). About the theory of so-called adsorption of soluble substance. Kung Sven. Veten. Hand., 24, 1–39.
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40, 1361–1403.
Le, V. T., Tran, T. K. N., Tran, D. L., Le, H. S., Doan, V. D., Bui, Q. D., & Nguyen, H. T. (2019). One-pot synthesis of a novel magnetic activated carbon/clay composite for removal of heavy metals from aqueous solution. Journal of Dispersion Science and Technology, 40(12), 1761–1776.
Li, X., Wang, C., Zhang, J., Liu, J., Liu, B., & Chen, G. (2020). Preparation and application of magnetic biochar in water treatment: A critical review. Science of the Total Environment, 711, 134847.
Li, S., Wu, Y., Zheng, Y., Jing, T., Tian, J., Zheng, H., Wang, N., Nan, J., & Ma, J. (2021). Free-radical and surface electron transfer dominated bisphenol A degradation in system of ozone and peroxydisulfate co-activated by CoFe2O4-biochar. Applied Surface Science, 541, 147887.
Manoochehria, M., Asgharinezhad, A. A., & Shekari, N. (2015). Synthesis, characterisation and analytical application of Fe3O4@SiO2@polyaminoquinoline magnetic nanocomposite for the extraction and preconcentration of Cd(II) and Pb(II) in food samples. Food Additives & Contaminants: Part A, 32, 737–747.
Mashhadizadeh, M. H., Amoli-Diva, M., Shapouri, M. R., & Afruzi, H. (2014). Solid phase extraction of trace amounts of silver, cadmium, copper, mercury, and lead in various food samples based on ethylene glycol bis-mercaptoacetate modified 3-(trimethoxysilyl)-1-propanethiol coated Fe3O4 nanoparticles. Food Chemistry, 151, 300–305.
McKay, G., El-Guendi, M., & Nassar, M. (1987). Equilibrium studies during the removal of dyestuffs from aqueous solutions using bagasse pith. Water Research, 21, 1513–1520.
Mehrabi, F., Vafaei, A., Ghaedi, M., Ghaedi, A. M., Dil, E., & Asfaram, A. A. (2017). Ultrasound assisted extraction of Maxilon Red GRL dye from water samples using cobalt ferrite nanoparticles loaded on activated carbon as sorbent: Optimization and modeling. Ultrasonics Sonochemistry, 38, 672–680.
Mosai, A. K., Chimuka, L., Cukrowska, E. M., Kotze, I. A., & Tutu, H. (2020). Removal of platinum (IV) from aqueous solutions with yeast functionalized bentonite. Chemosphere, 239, 124768.
Ozdes, D., & Duran, C. (2014). Preparation of a new sorbent, cetyltrimethylammonium bromide (CTAB)- modified obsidian, for the separation and preconcentration of Pb(II) and Cd(II) ions in food and water samples. Atomic Spectroscopy, 35, 118–126.
Ozdes, D., Duran, C., Senturk, H. B., Avan, H., & Bicer, B. (2014). Kinetics, thermodynamics and equilibrium evaluation of adsorptive removal of methylene blue onto natural ıllitic clay mineral. Desalination and Water Treatment, 52, 208–218.
Rahimi, Z., Sarafraz, H., Alahyarizadeh, Gh., & Shirani, A. S. (2018). Hydrothermal synthesis of magnetic CoFe2O4 nanoparticles and CoFe2O4/MWCNTs nanocomposites for U and Pb removal from aqueous solutions. Journal of Radioanalytical and Nuclear Chemistry, 317, 431–442.
Rahnama, R., & Najafi, M. (2016). The use of rapidly synergistic cloud point extraction for the separation and preconcentration of trace amounts of Ni (II) ions from food and water samples coupling with flame atomic absorption spectrometry determination. Environmental Monitoring and Assessment, 188, 150.
Ramadan, R. (2019). Physical study of cobalt ferrite and its application in purification of water. Applied Physics A, 125, 825.
Reddy, D. H. K., & Lee, S.-M. (2014). Magnetic biochar composite: Facile synthesis, characterization, and application for heavy metal removal. Colloids and Surfaces a: Physicochemical and Engineering Aspects, 454, 96–103.
Soylak, M., Acar, D., Yilmaz, E., El-Khodary, S. A., Morsy, M., & Ibrahim, M. (2019). Magnetic graphene oxide as an efficient adsorbent for the separation and preconcentration of Cu(II), Pb(II), and Cd(II) from environmental samples. Journal of AOAC International, 100, 1544–1550.
Soylak, M., Narin, I., Divrikli, U., Saracoglu, S., Elci, L., & Dogan, M. (2004). Preconcentration-separation of heavy metal ions in environmental samples by membrane filtration-atomic absorption spectrometry combination. Analytical Letters, 37, 767–780.
Suleiman, J. S., Hu, B., Peng, H., & Huang, C. (2009). Separation/preconcentration of trace amounts of Cr, Cu and Pb in environmental samples by magnetic solid-phase extraction with Bismuthiol-II-immobilized magnetic nanoparticles and their determination by ICP-OES. Talanta, 77, 1579–1583.
Sun, J., Liang, Q., Han, Q., Zhang, X., & Ding, M. (2015). One-step synthesis of magnetic graphene oxide nanocomposite and its application in magnetic solid phase extraction of heavy metal ions from biological samples. Talanta, 132, 557–563.
Suo, L., Dong, X., Gao, X., Xu, J., Huang, Z., Ye, J., Lu, X., & Zhao, L. (2019). Silica-coated magnetic graphene oxide nanocomposite based magnetic solid phase extraction of trace amounts of heavy metals in water samples prior to determination by inductively coupled plasma mass spectrometry. Microchemical Journal, 149, 104039.
Thamilarasi, M. J. V., Anilkumar, P., Theivarasu, C., & Sureshkumar, M. V. (2018). Removal of vanadium from wastewater using surface-modified lignocellulosic material. Environmental Science and Pollution Research, 25, 26182–26191.
Tokay, F., & Bagdat, S. (2019). Solid phase extraction and preconcentration of some metal ions using Schiff base immobilised silica gel followed by ICP-OES. International Journal of Environmental Analytical Chemistry, 99, 1528–1539.
Tu, Y., Ju, S., & Wang, P. (2016). Flame atomic absorption spectrometric determination of copper, lead, and cadmium in Gastrodiae rhizoma samples after preconcentration using magnetic solid-phase extraction. Spectroscopy Letters, 49, 249–256.
Tufekci, M., Bulut, V. N., Elvan, H., Ozdes, D., Soylak, M., & Duran, C. (2013). Determination of Pb(II), Zn(II), Cd(II) and Co(II) ions by flame atomic absorption spectrometry in food and water samples after preconcentration by coprecipitation with Mo(VI)-diethyldithiocarbamate. Environmental Monitoring and Assessment, 185, 1107–1115.
Vellaichamy, S., & Palanivelu, K. (2011). Preconcentration and separation of copper, nickel and zinc in aqueous samples by flame atomic absorption spectrometry after column solid-phase extraction onto MWCNTs impregnated with D2EHPA-TOPO mixture. Journal of Hazardous Materials, 185, 1131–1139.
Weber, W. J., Jr., & Morriss, J. C. (1963). Kinetics of adsorption on carbon from solution. Journal of the Sanitary Engineering Division, American Society of Civil Engineers, 89, 31–60.
Xu, H., Wu, Y., Wang, J., Shang, X., & Jiang, X. (2013). Simultaneous preconcentration of cadmium and lead in water samples with silica gel and determination by flame atomic absorption spectrometry. Journal of Environmental Sciences, 25, S45–S49.
Yağci, Ö., Akkaya, E., & Bakirdere, S. (2020). Nano-sized magnetic Ni particles based dispersive solid-phase extraction of trace Cd before the determination by flame atomic absorption spectrometry with slotted quartz tube: A new, accurate, and sensitive quantification method. Environmental Monitoring and Assessment, 192, 583.
You, Y., Shi, Z., Li, Y., Zhao, Z., He, B., & Cheng, X. (2021). Magnetic cobalt ferrite biochar composite as peroxymonosulfate activator for removal of lomefloxacin hydrochloride. Separation and Purification Technology, 272, 118889.
Zhang, S., Ji, Y., Dang, J., Zhao, J., & Chen, S. (2019). Magnetic apple pomace biochar: Simple preparation, characterization, and application for enriching Ag(I) in effluents. Science of the Total Environment, 668, 115–123.
Zhao, D., Li, J., Li, C., Juhasz, A. L., Scheckel, K. G., Luo, J., Li, H.-B., & Ma, L. Q. (2016). Lead relative bioavailability in lip products and their potential health risk to women. Environmental Science and Technology, 50(11), 6036–6043.
Zhao, J., Liang, G., Zhang, X., Cai, X., Li, R., Xie, X., & Wang, Z. (2019). Coating magnetic biochar with humic acid for high efficient removal of fluoroquinolone antibiotics in water. Science of the Total Environment, 688, 1205–1215.
Zhou, L., Ji, L., Ma, P.-C., Shao, Y., Zhang, H., Gao, W., & Li, Y. (2014). Development of carbon nanotubes/CoFe2O4 magnetic hybrid material for removal of tetrabromobisphenol A and Pb(II). Journal of Hazardous Materials, 265, 104–114.
Zhu, X., Cui, Y., Chang, X., & Wang, H. (2016). Selective solid-phase extraction and analysis of trace-level Cr(III), Fe(III), Pb(II), and Mn(II) ions in waste water using diethylenetriamine-functionalized carbon nanotubes dispersed in graphene oxide colloids. Talanta, 146, 358–363.
Funding
The financial support was received from the Unit of the Scientific Research Projects of Karadeniz Technical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozdes, D., Duran, C. Preparation of melon peel biochar/CoFe2O4 as a new adsorbent for the separation and preconcentration of Cu(II), Cd(II), and Pb(II) ions by solid-phase extraction in water and vegetable samples. Environ Monit Assess 193, 642 (2021). https://doi.org/10.1007/s10661-021-09389-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09389-0