Abstract
Industrialization and extraction of natural resources have resulted in large-scale environmental contamination and pollution. We have collected the soil samples from five different industrial areas of Mettur (Chemplast Sanmar Limited, SIDCO-1, SIDCO-2, SIDCO-3, thermal power plant), Salem district, Tamil Nadu, India, and estimated the physical properties (pH, EC, and alkalinity), chemical properties (major and minor elements), and heavy metal analysis. Thermal power plant soil sample showed higher pH 5.01, EC 29.33 μmhos/cm compared with rest of the samples. Acidic nature of the soil samples near thermal power plant was due to the effect of ash disposal. The high electrical conductivity is due to the disposal of soluble electrolytes and deposition of dust particles released from Thermal Power Plant. Alkalinity of the SIDCO-2 soil (410 ppm) was higher than that of rest of the soil samples. Soil samples show higher concentrations of chloride (10,400 ppm) from thermal power plant when compared with soil sample collected from all 15 sample areas. It was found that heavy metal concentrations lie in the following ranges: Cu (3.780–86.360 ppm) > Pb (0.018–1.710 ppm) > As (0.053–0.342 ppm) in Mettur area. The maximum concentration of copper (Cu) found in SIDCO-1 (86.360 ppm) was due to electroplating industry, smelting and refining, mining, and biosolids. Maximum concentrations of arsenic (As) recorded (0.342 ppm) in thermal Power plant was due to ash disposal from the coal-fired thermal power plant. And maximum concentrations of lead (Pb) (1.710 ppm) in Chemplast area are due to the effluent discharge of manufacturing units like PVC resins, chlorochemicals, and piping systems in Chemplast which are main source of heavy metal pollutants. Therefore, major mining and smelting of metalliferous ores, burning of leaded gasoline, municipal sewage, industrial wastes enriched with Pb, and paints, which exceeded WHO (2011) and BIS (2003) recommended standard for lead (0.090 ppm) and arsenic (0.010 ppm). The geo-accumulation index (Igeo) study indicates that there is no significant contamination with lead and arsenic but there is a moderate contamination with copper (86.360 ppm). According to the calculated values of PLI, area 1 (0.061) has been contaminated high compared with other areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollution is the most important crisis causing excessive level of dangerous pollutants. For the growth and sustainability of life, environment plays an important role. The environmental problems in India are growing rapidly. Large amounts of toxic waste have been dispersed in thousands of contaminated sites spread across our nation. Thus, every one of us is being exposed to contamination from past and present industrial practices and emissions in natural resources (air, water, and soil) even in the most remote regions. The increasing economic development and a rapidly growing population that has taken the country from 300 million people in 1947 to more than one billion people today is putting a strain on the environment, infrastructure, and the country’s natural resources. Industrial pollution, soil erosion, deforestation, rapid industrialization, urbanization, and land degradation are all worsening problems.
Overexploitation of the country’s resources either land or water and the industrialization process have resulted in environmental degradation of resources (Meagher and Heaton 2005). Environmental pollution is one of the most severe issues facing humanity and other life forms on our planet these days (Ahmad et al. 2012).
Industrial pollution is being a major cause of environmental degradation (Lee et al. 2006). Numerous studies have already demonstrated that areas in close proximity to industrial activities are marked by noticeable contamination of air, soil, and water (Guo et al. 2012). Hence, such activities can affect the air we breathe, the water we use, and the soil we stand on and can ultimately lead to illness and/or harm to the residents in the affected area (Kabir et al. 2012). Among the range of contaminants that may be found in soil, potentially toxic elements or heavy metals are of particular interest for a number of reasons (Soriano et al. 2012). First, they show a tendency, under normal circumstances, to accumulate in soil and have a long persistence time because of the interactions with particular soil components (Nazzal et al. 2013). The most important sources of heavy metals in the environment are the anthropogenic activities such as mining (Feng et al. 2008), smelting procedures, steel and iron industry, chemical industry, traffic, and agriculture as well as domestic activities (Jantschi et al. 2008; Stihi et al. 2006). Chemical and metallurgical industries are the most important sources of heavy metals in soil (Pantelica et al. 2008; Jantschi et al. 2008; Schutze et al. 2007).
Heavy metal pollution has become one of the important environmental problems worldwide (Guven and Akinci 2011; Wu et al. 2009). Increasing industrialization and urbanization has given great problem of heavy metal pollution which is listed as priority pollutants by the US Environmental Protection Agency (2000). It is reported that As, Cd, Pb, Cr, Cu, Hg, and Ni are the most common heavy metals detected in polluted areas (Adelekan and Abegunde 2011). Between 1850 and 1990, production of heavy metals increased nearly 10-fold (Nriagu et al. 1996). The main threats to human health from heavy metals are associated with exposure to lead, cadmium, mercury, and arsenic (Suciu et al. 2008; Holmes et al. 2009). Metal pollutants are particularly difficult to remediate from the soil, water, and air because, unlike organic pollutants that can be degraded to harmless small molecules, toxic element such as lead, mercury, cadmium, copper, and zinc are immutable by biochemical reactions (de Vries et al. 2007). Among heavy metals, lead and copper are one of the most hazardous pollutants of the environment (Verma et al. 2016).
Assessing the problems caused by contaminated soil typically involves soil chemistry as well as laboratory and field studies to fully assess the extent and significance of any adverse environmental effects (Mathiyazhagan et al. 2012). Therefore, assessment of these metals from industrially contaminated sites is important for safety assessment of physicochemical and metal properties of contaminated soil, as well as it is essential to know about the possibilities to evacuate human major diseases (Bahemuka and Mubofu 1999). The deadlier diseases like edema of eyelids, tumor, congestion of nasal mucous membranes and pharynx, stuffiness of the head, and gastrointestinal, muscular, reproductive, neurological, and genetic malfunctions caused by some of these heavy metals have been documented (Tsuji and Karagatzides 2001: He et al. 2005; Zhao et al. 2012). The proposed work aims to assess the heavy metal concentration in soil and alarming the risk of heavy metals through contaminated sites having long-term exposure in and around Salem district. This will also help in evaluating environmental approach for better utilization of soil from industrially contaminated areas in the near future.
Materials and method
Study area
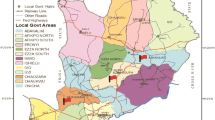
Mettur is an industrial area located in the north east corner of Salem district of Tamil Nadu in India. It is elevated 238 m above the sea level and 10.7 km between the geographical coordinates 8° 52′ 0″ North and 77° 22′ 0″ East, 16.4 km WxNW of Nangavalli, 14.4 km NW of Jalakandapuram, and 19.3 km NxNE of Ammapettai. It coordinates 2.1 N 11.80000 E 77.80000.
Mettur literally means “town on the hills.” Once an agricultural area, it now hosts a number of chemical industries due to the existence of dam. The River Kaveri, the life line of north and central Tamilnadu, enters the south Indian state through Mettur.
The Mettur Dam which is named as Stanley Reservoir was built in 1934, across a gorge where the River Kaveri enters the plains. It is a major source of drinking water. According to Professor Janakarajan, Madras Institute of Development Studies, the Kaveri irrigates 24 lakh acres of land across central and eastern Tamilnadu.
Stretches of the Kaveri are deemed to be among the “most polluted zones of the state,” according to a State of Environment of Tamilnadu report released in 2005. At least 1100 industries operate in the Kaveri basin, according to Tamil Nadu Pollution Control Board (TNPCB) survey mentioned in the State of Environment report. The estimated waste water discharge is 16.2 crore liters per day (lpd), of that, 870 lakh lpd is discharged directly into the Kaveri. Salem district, with 640 lakh lpd, followed by Trichy 57.64 lakh lpd are the largest contributors of effluent to the Kaveri, according to the report Premkumar (2007).
Among the first things that greet the Kaveri as it enters Tamil Nadu at Mettur is the toxic effluents from more than half a dozen major industrial operations including five units owned by Chemplast Sanmar, one owned by MALCO, and two thermal power plants owned by MALCO and Tamil Nadu Electricity Board. Industrialization in Mettur began as early as 1936, when Mettur Chemicals and Industries Corporation (MCIC), now Chemplast Sanmar, set up India’s first caustic chlorine factory. Over the years, Chemplast Sanmar added a range of facilities including a chloromethane plant, and a PVC plant including units to produce ethylene dichloride (EDC) and vinyl chloride monomer (VCM). Madras Aluminum Company (MALCO) set up a refinery-cum-smelter near the reservoir in 1965. A number of chemical industries were set up on the banks of the River Kaveri in Mettur as part of Small Industries Development Corporation (SIDCO) industrial estate. Most of the industries are clustered around three Panchayats (units of local self-government)—P.N. Patti, Veerakalpudur, and Gonur. Chemplast’s Plant 2 and Plant 3 are the key units dealing with chlorine and chlorinated organic chemicals. Plant 2 manufactures ethylene dichloride, vinyl chloride monomer and polyvinyl chloride. Plant 3 manufactures chlorinated solvents such as carbon tetrachloride, methylene chloride, chloroform, chlorine, and caustic soda through the mercury cell route.
Sampling area
For the present study, soil samples were taken from the following industrial areas of Mettur (Fig. 1):
-
Area 1. Chemplast Sanmar Limited
-
Area 2. SIDCO-1
-
Area 3. SIDCO-2
-
Area 4. SIDCO-3
-
Area 5. Thermal power plant
Chemplast Sanmar Limited is a Chennai-based chemical company in Mettur, Tamil Nadu. It is part of Sanmar Group which has business in chemicals, shipping, engineering, and metals. It is a major manufacturer of PVC resins, chlorochemicals, and piping systems. SIDCO (Small Industries Development Corporation Limited) was established to promote small-scale industries in Tamil Nadu, supplying raw materials—metals for processing. The Mettur Thermal Power Station is a coal-fired electric power station located in Salem district of Tamil Nadu. It is operated by Tamil Nadu Generation and Distribution Corporation Limited. In all the five areas, samples were collected from 2-m distance intervals from the sewage outlet of the industries. Three different samples from each of the five different areas (3 ×5 = 15) in Mettur industrial estate were taken for analysis.
Sample collection
At each sampling site, soil samples were collected separately by a random selection, from surface (0–15-cm soil layer) with a small core sampler. The soil samples collected from the factory outlet were found to be wet and in slurry condition. The soil samples collected from different areas of Mettur were kept in clean polyethylene bags, labeled, and brought to the laboratory for analyses. Each soil sample of about 1 kg in powder form was collected after drying them in room temperature.
Soil samples were air-dried under a sterile and moisture-free condition for further processing. The samples were sieved through a 2-mm mesh screen and each as soil sample was divided into two parts. One part held for analyzing their physical parameters such as pH, nature, and color. The other part was used for the determination of metal analysis. Soil pH was determined by using a digital pH meter (Baltensweiler and Zimmermann 2010). The nature of the soil was found to be colloid form appearance and greenish black in color. Electrical conductivity, alkalinity, chloride, phosphate, sulphate, total organic carbon, available nitrogen, lead, arsenic, and copper were analyzed for all the soil samples and the results were tabulated.
Sample analysis
Determination of physicochemical properties
The soil samples (10.0 g) were sieved through a 100-mm mesh and put into a 50-mL beaker containing 25 mL of distilled water. The solution was sharply agitated for 2 min and kept undisturbed for 30 min. Then, the pH and electrical conductivity (EC) values were determined by the respective meters. Chloride was determined argentometrically by using AgNO3. Sulphate was analyzed turbidometrically using BaCl2. Sodium and potassium were estimated using a flame photometer after extracting with neutral ammonium acetate. For alkalinity analysis, titration method was used with phenolphthalein and methyl orange as indicators. Calcium, magnesium, phosphate, and total available nitrogen were estimated by method previously reported by Mathew et al. (2003). Total organic carbon (TOC) concentration was determined by titration method using potassium dichromate as titrate using a method previously reported by Gaudette et al. (1974).
pH of the soil
Soil pH was determined by using a digital pH meter.
Electrical conductivity
Electrical conductivity was determined using a conductivity meter.
Determination of heavy metal analysis
The soil samples homogenized by coning and quartering were dried at 75 °C for 48 h and then ground to fine powder. The dried and sieved soil samples were digested by HCl and concentrated HNO3 in 3:1 ratio (Mathiyazhagan and Natarajan 2011). The solution was cooled, filtered, and diluted with 25 ml of distilled water. The digested liquid was filtered through a Whatman No. 0.5 filter paper, and the total heavy metal content of the filtrate was analyzed by using atomic absorption spectrometry (AAS) (Baisberg-Pahlsson 1989).
Result and discussion
Physicochemical parameters of soil samples
The present study shows that the metal concentrations of top soil can be used as a powerful geochemical tool for monitoring the impact of anthropogenic activity. The physiological properties like pH, electro conductivity, and alkalinity of soil sample were done and the results are tabulated in Table 1.
pH
Thermal power plant soil sample shows the lower pH (5.01) when compared with rest of the samples (Fig. 2). Acidic nature of the pH of the soil samples near the thermal power plant was due to the effect of ash disposal. The solid wastes produced from the coal-fired thermal power plants are mainly of two types, i.e., fly ash and bottom ash. Bottom ash is the coarse-grained fraction that is collected from the bottom of the boiler and is disposed of by the wet disposal method in a slurry form to nearby waste disposal sites (ash ponds or water resources) (Mandal and Sengupta 2006).
Electrical conductivity
Electrical conductivity nature of the thermal plant soil sample was found to be high (29.33 μmhos/cm) when compared with Chemplast and SIDCO-1, SIDCO-2, and SIDCO-3 areas (Fig. 3). The electrical conductivity is the measure of soluble electrolytes present in the soil samples. The measurement of conductivity is for measuring the current that gives a clear idea of soluble salt present in the soil. Higher EC indicated the low quality of ground water and soil, because EC is an indirect indicator of plant nutrient uptake and salinity concerns and may lead to the formation of insoluble heavy metal salts. Consequently, EC was also significantly low in the Chemplast (18.45, 18.40, and 18.35 μmhos/cm) and SIDCO-1, SIDCO-2, and SIDCO-3 (20.36 to 23.34 μmhos/cm) areas. The relatively low EC in the area confirmed the deposition of dust particulates in the surrounding area, which saturated the soil, reduced the exchange sites on soil particles and led to lower EC (Ojha and Chaudhary 2017).
Alkalinity
The alkalinity of the samples ranged between 295 and 410 ppm (Fig. 4). The higher (410 ppm) value of alkalinity was recorded in SIDCO-2 than the rest of the soil samples. It is reported that the higher amount of alkalinity is harmful for the proper growth of cultivated species. The alkalinity of sample SIDCO 2 was found to be higher (410 ppm) than the rest of the soil samples (Ojha and Chaudhary 2017) as shown in Table 1.
Determination of elements
Determination of major and minor trace elements in and around Mettur industrial areas (15 areas) for chloride, phosphate, sulphate, total organic carbon, and total available nitrogen were analyzed as shown in Figs. 5, 6, 7, 8, and 9. Soil samples shows higher concentrations of chloride (10,400 ppm) from thermal power plant (sample 13) when compared with soil sample collected from all the other sample areas. Excess of phosphate (4600 ppm) in the thermal power plant soil samples was recorded and is given in Table 2. Mutual correlation of the major and minor trace elements in the soil samples suggests a common origin for sulphate, carbon, and nitrogen. Concentration of sulphate and carbon was found to be 26,900 ppm (thermal plant 13) and 194,400 ppm (thermal plant 13) respectively compared with other compounds in this area. Nitrogen was found to be 20,000 ppm in thermal plant area when compared with soil sample areas of Mettur. Total organic carbon (TOC) concentrations in soil sample surface of thermal power plant sediments were relatively high (194,100 ppm). The high organic matter flux to sediments was due to direct discharge of untreated domestic wastes and insufficiently treated industrial wastes.
Determination of heavy metal content in soil samples
The heavy metal concentrations (Cd, Cr, Cu, Fe, Ni, and Pb) were determined using atomic absorption flame emission spectrophotometer (AAFES—6200 Shimadzu). The total heavy metal content in the sediments decreases in the order of Cu> Pb > As. Statistical summary and other comparisons of the metal contents are presented in Table 4. Srinivas et al. (2000) listed the Indian standard and WHO permissible limit for the range of values of the physicochemical parameters of ground water (Table 3). The units are converted into ppm according to Harter (2003).
Assessment of heavy metal using atomic adsorption spectroscopy
Table 4 shows that the measured heavy metal contents varied greatly as follows: Cu, 86.360–3.780 ppm; Pb, 1.710–0.018 ppm; and As, 0.342–0.053 ppm.
Assessment of copper
The highest value for copper (Cu) 86.32 ppm was from SIDCO-1, while the lowest value 3.78 ppm was found in thermal power plant areas. The reason is due to untreated discharge of effluents from electroplating, smelting, refining, and mining units (Luo et al. 2011).
Assessment of lead
The highest value 1.710 ppm for lead (Pb) was found in sample collected from Chemplast area and the lowest value 0.018 ppm was recorded in SIDCO-1. The presence of Pb was confirmed by the manufacturing units like PVC resins, chlorochemicals, and piping systems in Chemplast which are main source of heavy metal pollutants. Therefore, major mining and smelting of metalliferous ores, burning of leaded gasoline, municipal sewage, paints, and industrial wastes enriched in Pb (Gisbert et al. 2003; Seaward and Richardson 1990).
Assessment of arsenic
The highest value for arsenic (As) 0.342 ppm was from thermal power plant area, while the lowest value 0.053 ppm was found in SIDCO-2 area. The major reason for augmented value of arsenic present in soil was due to semiconductors, petroleum refining, wood preservatives, animal feed additives, coal power plants, herbicides, volcanoes, mining, and smelting (Nriagu 1994; Walsh et al. 1977). Since the power plant in Mettur is coal-fired thermal power plant, value of As in the plant area recorded far above the ground.
The study shows that the Mettur site area is highly polluted, which might be due to the discharge of effluent from various industries and municipal wastes. The present values of 15 sites reported heavy metals in all the studied stations were higher than the previous results reported by Jayakumar et al. (2015) for copper during the study of Cauvery River, Mettur, Tamil Nadu (Figs. 10, 11, and 12). Similar observations were reported by deleterious effect of lead (Ogeleka et al. 2016), and accumulation of Cu and lead in river bed dam soil has been reported by Nazir et al. (2015). The present study highlights the importance of periodical monitor of surface soil quality to sort the inhibitory chemicals, affecting the groundwater resources in Mettur industrial region.
Assessment of pollution load index and geo-accumulation index (Igeo)
To analyze the level of pollution in more detail, the pollution load index (PLI) is used. The PLI of elements is calculated using following formula (Salomons and Forstner 1984):
where PLI is the pollution load index and CFPb, CFAs, and CFCu, are the contamination factor (CF) for different metals.
The contamination factor, which is the ratio between the concentration (X) of a metal and its corresponding background value (BG), is arrived as
where X is the metal concentration and BG is the metal background value.
Background values used here are the standards reported by Alloway (1990) and Turekian and Wedepohl (1961).
To define the degree of pollution, it is necessary to establish the accepted background value for the soil samples. Enrichment may then be defined as the difference between the measured value and the background value. A quantitative measure of the extent of metal pollution in the investigated area was assessed using the geo-accumulation index (Igeo) proposed by Muller (1979) and expressed as follows:
where Cn denotes the measured concentration, Bn is the background value (average shale) of element n and the value 1.5 is the background matrix correction factor. The geo-accumulation index consists of seven grades (0 to 6), indicating different degrees of enrichment above the background values and ranging from unpolluted to very highly polluted sediment quality.
Geo-accumulation index
The mean values of Igeo for the elements lead, arsenic, and copper are shown in Table 6. Based on the Igeo data and Muller’s geo-accumulation index listed in Table 5, the Igeo value for Pb was − 11.330 to 0 and As was − 8.201 to − 5.511 and Cu was − 3.625 to 0.888. According to Olubunmi and Olorunsola (2010), the negative Igeo values show that the soil sediment is not significantly polluted and values less than 1 show that soil is moderately polluted with the respective metals. These Igeo values signify that the soil in these areas is moderately contaminated copper heavy metal and not polluted with lead and arsenic (Table 6).
Contamination factor
Contamination factor values have been calculated using the formula and shown in Table 7. The maximum contamination factor values for the heavy metals arsenic, copper, and lead are 0.055, 0.032, and 2.776 and the average values are 0.017, 0.017, and 0.743. These values of sample were utilized further for calculating pollution load index (PLI).
Pollution load index
Table 7 shows the minimum, maximum, and average values of PLI for the different locations sampled for the selected area in Metttur. The average pollution load index throughout the sampling area of Mettur is 0.074 whereas the highest PLI value is 0.355. Table 8 and Fig. 13 compare the average of PLI in various sampling areas of Mettur. PLI values are 0 in the samples collected from areas 3 and 4 and it is high in samples collected from areas 1 (0.067), 2 (0.021), and 5 (0.056). Area 1 shows the maximum PLI value which indicates this area is contaminated more than the other area.
Conclusion
This study provides the comprehensive analysis of physicochemical properties and heavy metal status in surface soil of industrial area of Mettur in Salem district. Top soil samples collected from the five different areas were taken for analysis. Soil pH, EC, alkalinity, chloride, phosphate, sulphate, total organic carbon, available nitrogen, lead, arsenic and copper were analyzed. Thermal power plant soil sample shows the lower pH (5.01) when compared with rest of the samples. Acidic nature of the soil samples near the thermal power plant is due to the effect of ash disposal. Electrical conductivity of the thermal plant soil sample was found high (29.33 μmhos/cm) which indicates the presence of soluble salt in it. The higher (410 ppm) value of alkalinity recorded in SIDCO-2 will be harmful to the growth of cultivated species. Major elements like chloride (10,400 ppm), phosphate (4600 ppm), sulphate (26,900 ppm), carbon (194,400 ppm), nitrogen (20,000 ppm), and total organic carbon (194,100 ppm) were found high in thermal power plant area, which is due to direct discharge of untreated superfluous and insufficiently treated industrial wastes. The heavy metal concentrations (Cu, Pb, and As) were determined using AAS and recorded in the order of Cu > Pb > As. The maximum concentration of copper (Cu) found in SIDCO-1 (86.360 ppm) is due to disposal from electroplating, mining, smelting, and refining industries. Maximum concentration of arsenic (0.342 ppm) is due to ash disposal from the coal-fired thermal power plant. And the higher concentration of lead (1.710 ppm) in Chemplast area is due to the effluent discharge from the manufacturing units like PVC resins, chlorochemicals, and piping systems in it. The geo-accumulation index (Igeo) study indicates that there is no contamination with lead and arsenic but there was a moderate contamination with copper (86.360 ppm). The calculated PLI value reveals that area 1 (0.067) is polluted more. From this study, it is understood that the soil quality in industrial area of Mettur is deteriorating and becoming potentially hazardous to public health. Hence, an immediate attention is needed to control the discharge of waste and effluents by adopting proper effluent treatment techniques and continuous monitoring by the authorities.
References
Adelekan, B. A., & Abegunde, K. D. (2011). Heavy metals contamination of soil and groundwater at automobile mechanic villages in Ibadan, Nigeria. International Journal of the Physical Sciences, 6(5), 1045–1058.
Ahmad, M. A., Guar, R., & Gupta, M. (2012). Comparative biochemical and RAPD analysis in two verities of rice (Oryza sativa) under arsenic stress by using various biomarkers. Journal of Hazardous Materials, 217–218, 141–148.
Alloway, B. J. (1990). Heavy metals in soils (pp. 100–124). Glasgow: Blackie and Son Ltd..
Bahemuka, T. E., & Mubofu, E. B. (1999). Heavy metals in edible green vegetables grown along the sites of the Sinza and Msimbazi Rivers in Dares Salaam, Tanzania. Food Chemistry, 66, 63–66.
Baisberg-Pahlsson, A. M. (1989). Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants. Water, Air, and Soil Pollution, 47(3–4), 287–319.
Baltensweiler, A, & Zimmermann S. (2010). Modeling soil acidity in Switzerland using spatial statistics tools. Proceedings of the ESRI international user conference (pp. 12–16).
de Vries, W., Romkens, P. F., & Schutze, G. (2007). Critical soil concentrations of cadmium, lead, and mercury in view of health effects on humans and animals. Reviews of Environmental Contamination and Toxicology, 191, 91.
Feng, X., Li, P., Qiu, G., Wang, S., Li, G., Shang, L., Meng, B., Jiang, H., Bai, W., & Li, Z. (2008). Human exposure to methylmercury through rice intake in mercury mining areas. Environmental Science and Technology, 42, 326–332.
Gaudette, H. E., Flight, W. R., Toner, L., & Folger, D. W. (1974). An inexpensive titration method for the determination of organic carbon in recent sediments. Journal of Sedimentary Petrology, 44, 249–253.
Gisbert, C., Ros, R., de Haro, A., Walker, D. J., Pilar Bernal, M., Serrano, R., & Avino, J. N. (2003). A plant genetically modified that accumulates Pb is especially promising for phytoremediation. Biochemical and Biophysical Research Communications, 3,303(2), 440–445.
Guo, G., Wu, F., Xie, F., & Zhang, R. (2012). Spatial distribution and pollution assessment of heavy metals in urban soils from southwest China. Journal of Environmental Sciences, 24(3), 410–418.
Guven, D. E., & Akinci, G. (2011). Comparison of acid digestion techniques to determine heavy metals in sediment and soil samples. Gazi University Journal of Science, 24(1), 29–34.
Harter, T. (2003). Groundwater quality and groundwater pollution. UC Agriculture & Natural Resources Farm, https://doi.org/10.3733/ucanr.8084. Retrieved from https://escholarship.org/uc/item/0vw7400h.
He, Z. L., Yang, X. E., & Stoffella, P. J. (2005). Trace elements in agro ecosystems and impacts on the environment. Journal of Trace Elements in Medicine and Biology, 19(2–3), 125–140.
Holmes, P., James, K. A. F., & Levy, L. S. (2009). Is low-level environmental mercury exposure of concern to human health? Science of the Total Environment, 408, 171–182.
Jantschi, L., Loan Suciu, Cosma, C., Todica, M., & Bolboaca, S.D. (2008). Analysis of Soil Heavy Metal Pollution and Pattern in Central Transylvania. Int. J. Mol. Sci, 9, 434–453.
Jayakumar, R., Dhanakumar, S., Kalaiselvi, K., & Palanivel, M. (2015). Multivariate statistical analysis of heavy metals and other hydro chemical haracteristics in industrially polluted groundwater resources of Mettur, India. Chemical Science Transactions, 4(3), 728–735.
Kabir, E., Ray, S., Kim, K.-H., Yoon, H.-O., Jeon, E.-C., Kim, Y. S., Cho, Y.-S., Yun, S.-T., & Brown, R. J. C. (2012). Current status of trace metal pollution in soils affected by industrial activities. The Scientific World Journal, 916705. https://doi.org/10.1100/2012/916705.
Lee, C. S., Li, X., Shi, W., Cheung, S. C., & Thornton, I. (2006). Metal contamination in urban, suburban, and country park soils of Hong Kong: a study based on GIS and multivariate statistics. Science of the Total Environment, 356, 45–61.
Luo, C., Liu, C., Wang, Y., Liu, X., Li, F., Zhang, G., & Li, X. (2011). Heavy metal contamination in soils and vegetables near an e-waste processing site, South China. Journal of Hazardous Materials, 186, 481–490.
Mandal, A., & Sengupta, D. (2006). An assessment of soil contamination due to heavy metals around a coal-fired thermal power plant in India. Environmental Geology International Journal of Geosciences, 51(3), 409–420.
Mathew, M., Mohanraj, R., Azeez, P. A., & Pattabhi, S. (2003). Speciation of heavy metals in bed sediments of wetlands in urban Coimbatore, India. Bulletin of Environmental Contamination and Toxicology, 70, 800–808.
Mathiyazhagan, N., & Natarajan, D. (2011). Bioremediation on effluents from Magnesite and Bauxite mines using Thiobacillus Spp and Pseudomonas Spp. Journal of Bioremediation & Biodegradation, 2, 115.
Mathiyazhagan, N., & Natarajan, D. (2012). Physicochemical assessment of waste dumps of Magnesite and Bauxite Mine in summer and rainy season. International Journal of Environmental Sciences, 2(3), 2243–2252.
Meagher, R. B., & Heaton, A. C. P. (2005). Strategic for the engineered phytoremediation of toxic element pollution: mercury and arsenic. Journal of Industrial Microbiology & Biotechnology, 32, 502–513.
Muller, G. (1979). Heavy metals in the sediment of the Rhine – Changes seity. Umschau in Wissenschaft und Technik, 79, 778–783.
Muller, G. (1981). The heavy metal pollution of the sediments of Neckars and its tributary: stocktaking. Chemiker Zeitung, 105, 157–164.
Nazir, R., Khan, M., Masab, M., Rehman, H. U., Rauf, N. U., Shahab, S., Ameer, N., Sajed, M., Ullah, M., Rafeeq, M., & Shaheen, Z. (2015). Accumulation of heavy metals (Ni, Cu, Cd, Cr, Pb, Zn, Fe) in the soil, water and plants and analysis of physico-chemical parameters of soil and water collected from Tanda Dam kohat. Journal of Pharmaceutical Sciences and Research, 7(3), 89–97.
Nazzal, Y., Rosen, M. A., & Al-Rawabdeh, A. M. (2013). Assessment of metal pollution in urban road dusts from selected highways of the Greater Toronto Area in Canada. Environmental Monitoring and Assessment, 185(2), 1847–1858.
Nriagu, J. O. (1994). Arsenic in the Environment (pp. 439). Canada; John Wiley & Sons.
Nriagu, J. O., Jinabhai, C. C., Naidoo, R., & Coutsoudis, A. (1996). Atmospheric lead pollution in Kwazulu/Natal. Science of the Total Environment, 191, 69–76.
Ogeleka, D. F., Ugwueze, V. I., & Okieimen, F. E. (2016). Ecotoxicological assessment of cadmium and lead exposure to terrestrial sentinels - snails (Archachatina marginata). International Journal of Research in Chemistry and Environment, 6(4), 1–9.
Ojha, P. K., & Chaudhary, N. K. (2017). Soil quality assessment posed by industrial effluents in Bansbari industrial area of Morang District, Nepal. Elixir Pollution, 106, 45906–45908.
Olubunmi, F. E., & Olorunsola, O. E. (2010). Evaluation of the status of heavy metal pollution of sediment of Agbabu bitumen deposit area, Nigeria. European Journal of Scientific Research, 41(3), 373–382.
Pantelica, A., Cercasov, A., Steinnes, E., Bode, P., & Wolterbeek, B. (2008). Investigation by INAA, XRF, ICPMS and PIXE of Air Pollution Levels at Galati (Siderurgical Site). Book of abstracts, 4th Nat. Conf. of Applied Physics (NCAP4), Galati, Romania.
Premkumar, L. (2007). Unfolding Disaster: A Study of Chemplast Sanmar’s Toxic Contamination in Mettur – Tamilnadu. https://www.scribd.com/document/126704364.
Salomons, W., & Forstner, U. (1984). Metals in the Hydrocycle. Berlin – Heidelberg - New York - Tokyo. Springer Verlag, 349 (149), 13: 267–267.
Schutze, G., de Vries, W., & Romkens, P. F. (2007). Critical soil concentration of cadmium, lead and mercury in view of health effects on humans and animals. Reviews of Environmental Contamination and Toxicology, 191, 91–130.
Seaward, M. R. D., & Richardson, D. H. S. (1990). Atmospheric Sources of Metal Pollution and Effects on Vegetation. Heavy Metal Tolerance in Plants: Evolutionary Aspects (pp. 75–92). Florida: CRC Press.
Soriano, A., Pallares, S., Pardo, F., Vicente, A. B., Sanfeliu, T., & Bech, J. (2012). Deposition of heavy metals from particulate settleable matter in soils of an industrialised area. Journal of Geochemical Exploration, 113, 36–44.
Srinivas, C. H., Piska Ravi Shankar, Venkateshwar, C., Satyanarayana Rao, M. S., & Ravider Reddy, R. (2000). Studies on ground water quality of Hyderabad. Pollution Research, 19(2), 285–289.
Stihi, C., Bancuta, A., Popescu, I. V., Virgolici, M., Cimpoca, V., Gugiu, M., & Vlaicu, G. (2006). Air pollution studies using PIXE and ICP methods. Journal of Physics: Conference Series, 41, 565.
Suciu, I., Cosma, C., Todica, M., Bolboaca, S. D., & Jantschi, L. (2008). Analysis of soil heavy metal pollution and pattern in Central Transylvania. International Journal of Molecular Sciences, 9, 434–453.
Tsuji, L. J. S., & Karagatzides, J. D. (2001). Chronic Lead Exposure, Body Condition, and Testis Mass in Wild Mallard Ducks. Journal Bulletin of Environmental Contamination and Toxicology 67(4), 489–495.
Turekian, K. K., & Wedepohl, K. H. (1961). Distribution of the elements in some major units of the Earth’s crust. Geological Society of America, 72, 175–192.
United States Environmental Protection Agency. (2000). Electrokinetic and phytoremediation in situ treatment of metal-contaminated soil:state-of-thepractice. EPA/542. US Environmental Protection Agency, Office of Solid Waste and Emergency Response Technology Innovation Office, Washington, DC, USA.
Verma, C., Madan, S., & Hussain, A. (2016). Heavy metal contamination of groundwater due to fly ash disposal of coal-fired thermal power plant, Parichha, Jhansi. India Civil & Environmental Engineering, 3, 1179243.
Walsh, L. M., Sumner, M. E., & Keeney, R. (1977). Occurrence and distribution of arsenic in soils and plants. Environmental Health Perspectives, 19, 67–71.
Wu, G., Kang, H., Zhang, X., Shaob, H., Chu, L., & Ruan, C. (2009). A critical review on the bio-removal of hazardous heavy metals from contaminated soils: Issues, progress, eco-environmental concerns and opportunities. Journal of Hazardous Materials, 22.
Zhao, H., Xia, B., Fan, C., Zhao, P., & Shen, S. (2012). Human health risk from soil heavy metal contamination under different land uses near Dabaoshan Mine, Southern China. Science of the Total Environment, 417–418, 45–54.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramesh kumar, K., Anbazhagan, V. Analysis and assessment of heavy metals in soils around the industrial areas in Mettur, Tamilnadu, India. Environ Monit Assess 190, 519 (2018). https://doi.org/10.1007/s10661-018-6899-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-018-6899-5