Abstract
Traditional sampling methods for dissolved organic matter (DOM) in streams limit opportunities for long-term studies due to time and cost constraints. Passive DOM samplers were constructed following a design proposed previously which utilizes diethylaminoethyl (DEAE) cellulose as a sampling medium, and they were deployed throughout a temperate stream network in Indiana. Two deployments of the passive samplers were conducted, during which grab samples were frequently collected for comparison. Differences in DOM quality between sites and sampling methods were assessed using several common optical analyses. The analyses revealed significant differences in optical properties between sampling methods, with the passive samplers preferentially collecting terrestrial, humic-like DOM. We assert that the differences in DOM composition from each sampling method were caused by preferential binding of complex humic compounds to the DEAE cellulose in the passive samplers. Nonetheless, the passive samplers may provide a cost-effective, integrated sample of DOM in situations where the bulk DOM pool is composed mainly of terrestrial, humic-like compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dissolved organic matter (DOM) refers to a class of filterable organic compounds common to soil, marine, and freshwater habitats. DOM is an essential component to carbon cycling in aquatic ecosystems but is generally difficult to collect and characterize (Lam and Simpson 2006). In streams, DOM can be broadly classified as allochthonous or autochthonous: the former being material originating from the terrestrial environment and the latter being produced within the stream through algal and microbial activity. Typical compounds released by stream microorganisms include amino acids, carboxylic acids, and sugars, whereas terrestrial DOM is composed of complex carbohydrates, organic acids, and humic compounds produced from the decay of plant matter (Kaplan and Newbold 2003). Stream DOM fractions may provide carbon and nitrogen to stream microorganisms, adsorb to metal oxides and mineral surfaces, donate electrons for oxidation-reduction reactions, or pass unchanged through a stream network (Leenheer and Croué 2003; Jaffé et al. 2008). Thus, spatial variation in DOM throughout a stream system reflects the biogeochemical processes and changing sources present within its watershed (Fellman et al. 2009).
Certain properties of a DOM sample may be identified using fluorescence analysis. A variety of common DOM compound classes (humic and fulvic acids, proteins, and some microbial products) possess specific, measurable fluorescent properties. Each of these component types can be described as microbially or terrestrially derived (Cory and McKnight 2005; Jaffé et al. 2008). A fluorescence index (FI) can be used to indicate a relative abundance of terrestrial and microbial DOM fractions. The FI is calculated as the ratio of emission intensities of a sample at 520 and 470 nm at an excitation wavelength of 370 nm (Cory and McKnight 2005). FI values typically range from 1 to 2 but can exceed 2 in samples with extremely high microbial production. Higher FI values (>1.5) indicate a predominantly microbial source of DOM, whereas lower values indicate DOM of terrestrial origin (McKnight et al. 2001). The slope ratio (S R) compares the slopes of absorbance between short (275–295 nm) and long (350–400 nm) UV wavelengths and is inversely related to the molecular weight of DOM in a sample (Helms et al. 2008). Another common optical metric is specific UV absorbance at 254 nm (SUVA254), which is used to describe the aromaticity of DOM (Weishaar et al. 2003). As the aromaticity of the DOM increases (increasing SUVA254 values), the DOM is presumed to be less bioavailable.
Traditional DOM sampling requires samples to be concentrated by extraction through columns packed with Amberlite XAD ion exchange resins (XAD-8 and XAD-7HP) composed of polymethyl methacrylate. This provides a rich sampling of all component groups, but the process requires large volumes of sample water, which must be acidified to within the functional range (pH ~2) of the resin. These requirements make long-term monitoring studies costly, especially in sites with limited access (Lam and Simpson 2006). In a few studies, the DOM samples isolated with XAD resins have been compared to the samples isolated with diethylaminoethyl (DEAE) cellulose, which functions best at circumneutral pH. The studies found that DOM isolated with DEAE cellulose was generally more humic and less bioavailable in character than DOM isolated with XAD resins (Hejzlar et al. 1994; Peuravuori et al. 2002). Lam and Simpson (2006) proposed a design for a passive DOM sampler utilizing a DEAE cellulose core. The design allows for a sampler to be deployed in a stream for up to a month, providing an integrated sample of DOM compounds present over an extended period of time with relatively little cost.
The alternative to passive samplers is the frequent collection of grab samples, which is often not feasible. Reliable passive samplers would have many applications in stream monitoring as well as in long-term studies of stream biogeochemistry. In this study, the passive samplers were built based on the design of Lam and Simpson (2006) and the efficacy of the samplers was evaluated. The optical properties of DOM extracted from passive samplers were compared with those from grab samples collected over two 4-week periods during the spring and summer of 2011. In addition to the field study, a laboratory study was conducted to determine possible effects of the exterior sampler membrane and adsorptive properties of DEAE cellulose.
Methods
Field study
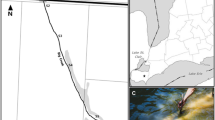
The passive samplers were constructed following the methods detailed in Lam and Simpson (2006). Each sampler consisted of a 7 cm × 2.5 cm packet of a 0.45-μm pore size polysulfone membrane packed with 0.25 g of DEAE cellulose. Membranes were initially cleaned by soaking in 0.1 M HCl and ultrapure water. DEAE cellulose resin was thoroughly cleaned through ten rinse cycles of 0.1 M HCl and ultrapure water and then 0.1 M NaOH and ultrapure water. The resin was then repeatedly washed in ultrapure water to remove any unwanted residual organic carbon and then freeze-dried until sampler construction. Membrane packets were formed using a heat sealer and soaked in 0.1 % sodium azide solution to inhibit any biofilm formation during deployment. Next, 0.25 g of resin was packed into each open membrane packet and the packets were sealed shut. Sampling packets were enclosed in protective housings of a 2.5-cm-internal diameter PVC pipe with caps at each end. Seven 6-mm holes were drilled through each cap to allow water flow past the sampler membranes. Housings were strapped to bricks with cable ties prior to deployment and were oriented parallel to the stream flow (Fig. 1a).
A total of 30 passive samplers were deployed over two 28-day periods in early May and late July 2011. For each sampling period, three samplers were deployed at five sites on Jack’s Defeat Creek near Ellettsville, Indiana, USA. The watershed of Jack’s Defeat Creek is predominantly forest and low-intensity agriculture (Gardner and Royer 2010). Site 1 was an open, sunny reach that flowed through a small pasture. Site 2 was heavily shaded for most of the day and located approximately 1.5 km downstream of site 1. Sites 3 and 4 were located just upstream and downstream, respectively, of the outfall from a small wastewater treatment plant. Site 5 was located on a small tributary to Jack’s Defeat Creek near the location of site 1.
Grab samples for dissolved organic carbon (DOC) concentration and fluorescence analysis were collected from each site twice a week during each 28-day deployment period. Samples were filtered with Whatman GF/F filters (0.7 μm nominal pore size), and the samples for DOC concentration were acidified at the time of collection. A mix of the grab samples from site 1 was created for each sampling period by combining 5 mL of each grab sample into a single composite sample. The mix sample was used for excitation-emission analysis as described below. Water chemistry and nutrient concentrations were monitored throughout the study to characterize each study reach. Samples for nitrate (NO3 −), ammonium (NH4 +), and soluble reactive phosphorus (SRP) were filtered on the site with membrane filters (0.45 μm pore size).
Resin-bound DOM extraction
After the passive samplers were retrieved from the stream (Fig. 1b), the membrane packets were cleaned with ultrapure water to remove any debris or biofilm on the exteriors. Packets were then carefully cut open (Fig. 1c), and all DEAE cellulose was scraped into centrifuge tubes using a squirt bottle of ultrapure water. Forty milliliters of 0.1 M sodium hydroxide was added to each centrifuge tube and agitated to suspend the cellulose. All tubes were then centrifuged at 2000g for 30 min until the cellulose formed a small pellet. The extraction process was repeated three more times, and the supernatant of each extraction was combined, yielding approximately 160 mL of the concentrated sample from each passive sampler (Fig. 1d).
To avoid modifying the pH-dependent fluorescent properties, each sample was agitated with 5 g of Amberjet 1200H Plus resin for 30 min. Further additions of concentrated NaOH or HCl were added if necessary to achieve a final pH of 6–8. Samples were then divided into separate dark HDPE bottles for DOC analysis and amber glass bottles for fluorescence analysis. Fluorescence samples were diluted prior to analysis to reduce potential inner filter effects. All the samples were stored frozen until analysis.
Sample analysis
DOC concentration was determined via high-temperature oxidation using a TOC-CPN analyzer (Shimadzu, Inc., Columbia, MD, USA) with a detection limit of 0.2 mg L−1. A Lachat QuikChem 8500 Flow Injection Analysis System (Hach Inc., Loveland, CO, USA) was used to determine concentrations of SRP, NO3 −-N, and NH4 +-N. Detection limits were 5 μg L−1 for SRP, 3 μg L−1 for NO3 −-N, and 10 μg L−1 for NH4 +-N. For the DOC and nutrient analyses, blanks, duplicates, and commercial standards were measured to ensure the accuracy of the measured concentrations. Absorption spectra were measured over a range of 200 to 600 nm at 1-nm intervals using a spectrophotometer (model UV-2101PC; Shimadzu, Inc.) and a 1-cm glass cuvette, while excitation and emission data were collected using a luminescence spectrophotometer (model LS-50B; Perkin-Elmer, Inc., Waltham, MA, USA) and a 1-cm quartz cuvette. Emission was measured from 300 to 550 nm (2-nm intervals) over excitation wavelengths of 240 to 450 nm (10-nm intervals) using a 5-nm slit width.
Fluorescence indices and slope ratio were measured for all grab samples and extracted samples in the field and laboratory studies. FI was calculated as the ratio of emission intensities at 520 and 470 nm and excitation at 370 nm, after data were corrected from a blank Raman scan and absorbance data (Cory and McKnight 2005). Linear regressions were performed on log-transformed absorption data over the ranges 275–295 and 350–400 nm. S R was calculated as \( {S}_{\mathrm{R}}=\frac{S_{275-295}}{S_{350-400}} \), where S is the slope of the regression equation for the respective range of wavelengths (Helms et al. 2008). SUVA254 was determined by dividing the blank-corrected absorbance of a sample at 254 nm by the DOC concentration in that sample (Weishaar et al. 2003).
Membrane effects
In addition to field deployment of passive sampler units, a laboratory study was conducted to assess potential differences in DOM sorption due to the polysulfone membrane. A large grab sample was collected from site 1 in June 2012. The grab sample was returned to the laboratory on ice within 15 min and filtered as described above for fluorescence samples. The sample was treated with sodium azide to a 0.005 % solution to prevent any potential microbial DOM consumption during the experiment.
The sample was then divided into nine 400-mL amber glass bottles. In addition, the initial DOC and fluorescence samples were removed and frozen. Three of the amber bottles were treated with a membrane-bound DEAE cellulose packet identical to those used in the field study, three were treated with 0.25 g of free-floating DEAE cellulose, and three were used as control samples.
The samples were incubated for 21 days on a shaker table at a moderate speed. Solar exposure was limited by storage in the amber bottles out of direct sunlight. After the test period, the samples for DOC and fluorescence analysis were drawn off from the control container and residual water from DEAE cellulose test containers. Free-floating resin was removed via filtration, and resin-bound DOM was extracted for DOC and fluorescence analysis as described above.
Statistical analyses
Because normality could not be confirmed, the Kruskal-Wallis test, with α = 0.05, was used to examine the effects of site and sample type (grab vs. extract) on slope ratio, fluorescence index, and SUVA254. A one-way ANOVA was used to examine differences among treatments in the membrane-effects experiment, for which the normality of the data was confirmed using the Shapiro-Wilk test. All statistical analyses were performed in IBM SPSS Statistics (v. 20; IBM Corp., Armonk, NY, USA).
Results
Stream chemistry
Total rainfall during the May deployment was 8.6 cm compared to 2.2 cm during the July period. Water temperature averaged about 18 °C in May and increased to about 25 °C in July. All of the sites had circumneutral pH and specific conductivity of 400–500 μS cm−1 (Table 1). Mean DOC concentrations were typically around 3 mg L−1, although the samples with >5 mg L−1 of DOC were occasionally collected. NH4 +-N was below the detection limit at all sites, whereas NO3 −-N concentrations were >1 mg L−1 in May. SRP was low at all sites except site 4, which was located downstream of the WWTP outfall. Site 4 had the highest concentrations of NO3 −-N, SRP, and DOC throughout the study (Table 1).
Field study optical analyses
There were significant differences in FI between the grab and extract samples in both May (p = 0.002) and July (p < 0.0005) deployments (Fig. 2). No statistically significant differences were observed between sites within the extract samples, although the site 4 grab sample FI values were significantly higher than all other sites during the July deployment and all but site 5 during the May deployment. There were differences in S R between sampling methods (p = 0.048) and sites within the grab samples (p = 0.003) during the July deployment. SUVA254 was significantly higher in the extract samples from all sites during both deployments (p < 0.0005).
Overall, the grab samples had higher mean FI values than did the extract samples, whereas the extract sample had much higher mean SUVA254 than did the grab samples (Fig. 3). The mean S R values were often similar between the grab and extract samples. The samples from site 4 consistently had the highest values for FI and, within a sampling period, deviated the most from the 1:1 line for S R (Fig. 3). To illustrate differences in optical properties between the sampling methods, Fig. 4 shows comparisons of excitation and emission matrices from the site 1 grab sample mixtures and extract samples.
Membrane effects
The initial sample FI averaged 1.4, and following incubation, there were no differences in FI values among the three treatments (one-way ANOVA, p > 0.05), with each having a mean FI value of 1.5. For SUVA254, both of the DEAE cellulose treatments (packet and free-floating resin) were different from the control (one-way ANOVA, p < 0.001; Tukey’s post hoc test, p < 0.05), but not different from each other. The mean (±1 standard error) SUVA254 was 0.059 ± 2 × 10−5, 0.012 ± 0.002, and 0.011 ± 0.002 for control, packet, and free-floating resin samples, respectively. A similar result was found for S R, in which the DEAE cellulose treatments both differed from the control, but not from each other based on Tukey’s post hoc comparisons (one-way ANOVA, p < 0.024 for main effect). The mean (±1 standard error) S R was 0.71 ± 0.18, 1.06 ± 0.07, and 1.04 ± 0.10 for control, packet, and free-floating resin samples, respectively.
Discussion
Polysulfone membrane effects
While S R and SUVA254 were different between the control and extract samples in the laboratory study, neither of these were statistically different between membrane-bound and free-floating resin extracts. There were no significant differences between FI values in the laboratory study. Given the close similarities in optical properties between the extract treatments, it is unlikely that the membrane effectively excludes any dissolved compounds that the resin is capable of adsorbing.
Differences in optical properties between sampling techniques
No extract sample had an FI value exceeding 1.7. This held true even at site 4 during the summer sampling, where the grab sample FI values often exceeded 2. Compared to the other sites, site 4 received more microbial DOM due to the input of effluent from the wastewater treatment plant and was the only site for which FI differed significantly between the two sampling methods. The extract samples never indicated dominance of DOM of microbial origin. However, based on the grab sample FI values, the DOM at site 4 was predominantly microbial in origin. Lam and Simpson (2006) found low molecular weight sugars to be slightly lower in extract samples than grab samples using NMR analysis. Their explanation was that such sugars are relatively soluble and may not remain adsorbed to the DEAE cellulose for the entire sampling period. Though sugars do not fluoresce, if similar low molecular weight, fluorescent microbial DOM compounds do not remain tightly bound to adsorption sites over the study duration, FI values from the extract samples may underrepresent the microbial contribution to the DOM pool. This could explain the discrepancy in FI values between the grab and extract samples at site 4.
S R was significantly higher in the site 4 grab samples than in corresponding extracts and other sites. As S R is inversely related to the average molecular weight of DOM, it follows that DOM extracted from the samplers was generally larger than the average for bulk DOM in the stream (Helms et al. 2008). Treated wastewater DOM is composed of relatively small microbial products (Imai et al. 2002), which is reflected in S R data from the site 4 grab samples, but not extracts. In both study periods, SUVA254 was greater in the extract samples than in the grab samples at all sites. This suggests that aromatic DOM was preferentially collected by the DEAE cellulose, while less aromatic compounds were more likely to be missed by the samplers. Higher SUVA254 values are generally associated with humic-like DOM of terrestrial origin (Jaffé et al. 2008), which supports the findings from FI and S R analyses, suggesting that the extracts contained a greater proportion of large molecular weight, terrestrial DOM (Fig. 2).
Figure 4 illustrates the differences and similarities in excitation-emission matrices (EEMs) collected using the two sampling methods. In all samples, fluorescence was prominent in a region associated with terrestrial humic-like fluorescence [excitation (ex.) 260 nm, emission (em.) 400–460 nm]. Grab sample mixtures also featured fluorescence in a region (ex. 275 nm, em. 305–340 nm) typically associated with protein-like autochthonous products. This region of fluorescence was present in the extract samples, but at lower relative intensities. This difference was particularly evident in the May sampling period and suggests that these compounds were not effectively collected by the resin. Extract EEMs had relatively greater intensity in a region characterized by microbial humic-like fluorescence (ex. 240 nm and 290–310 nm, em. 350–400 nm and 370–420 nm), indicating another difference in the quality of DOM collected between the two techniques (Coble 1996; Parlanti et al. 2000).
Traditional sampling techniques
Studies on the sampling capabilities of DEAE cellulose have shown the resin to be quite effective at adsorbing humic acids due to their many functional groups (Miles et al. 1983). DOM sampling from forested temperate lakes showed that 30–80 % of DOM adsorbed to DEAE cellulose was composed of humic acids, compared to 21–57 % from the same lakes using the traditional column extraction method (Peuravuori et al. 2002). DEAE cellulose relies on adsorption by charged functional groups, but the traditional method using polymethyl methacrylate resins employs a fundamentally different mechanism. Acidification is required for samples extracted onto polymethyl methacrylate resins to reduce the ionic character of the DOM, which then relies on Van der Waals forces to keep the nonionic surfaces adsorbed. During the passive sampler deployment, weakly adsorbed DOM (e.g., low molecular weight compounds) may be gradually replaced with terrestrial humic compounds due to their greater abundance of functional groups. As large humic substances make up a greater fraction of terrestrial DOM than of microbial DOM (Kaplan and Newbold 2003), this preferential adsorption may also explain the lower FI values seen in site 4 extracts, a site that is clearly dominated by microbial DOM from the treated wastewater effluent.
Conclusions
Optical analyses revealed statistically significant differences in DOM quality between sampling techniques and suggested that the passive samplers indicate higher abundances of high molecular weight, terrestrial DOM than might actually occur in a given stream. The extent to which this matters for a study depends on the type and scale of questions being addressed. In the present study, differences in two of the optical metrics between sampling methods were significant only at a site receiving treated wastewater effluent. Although the passive samplers appeared to neglect some microbial DOM, they likely are useful for studies in forested headwaters or other systems with predominantly terrestrial DOM inputs. Additionally, the passive samplers provide an inexpensive, time-integrated sample of DOM that can be readily isolated and analyzed. Caution is warranted, however, in relating DOM collected with passive samplers to microbial community structure or biogeochemical processes, both of which might be dependent on labile, low molecular weight DOM rather than the terrestrial, humic DOM captured by the DEAE cellulose.
References
Coble, P. G. (1996). Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Marine Chemistry, 51(4), 325–346.
Cory, R. M., & McKnight, D. M. (2005). Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environmental Science and Technology, 39(21), 8142–8149.
Fellman, J. B., Hood, E., D’Amore, D. V., Edwards, R. T., & White, D. (2009). Seasonal changes in the chemical quality and biodegradability of dissolved organic matter exported from soils to streams in coastal temperate rainforest watersheds. Biogeochemistry, 95(2/3), 277–293.
Gardner, K. M., & Royer, T. V. (2010). Effect of road salt application on seasonal chloride concentrations and toxicity in south-central Indiana streams. Journal of Environmental Quality, 39(3), 1036–1042.
Hejzlar, J., Szpakowska, B., & Wershaw, R. L. (1994). Comparison of humic substances isolated from peatbog water by sorption on DEAE-cellulose and Amberlite XAD-2. Water Resources, 28(9), 1961–1970.
Helms, J. R., Stubbins, A., Ritchie, J. D., Minor, E. C., Keiber, D. J., & Mopper, K. (2008). Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnology and Oceanography, 53(3), 955–969.
Imai, A., Fukushima, T., Matsushige, K., Yong-Hwan, K., & Choi, K. (2002). Characterization of dissolved organic matter in effluents from wastewater treatment plants. Water Research, 36(4), 859–870.
Jaffé, R., McKnight, D., Maie, N., Cory, R., McDowell, W. H., & Campbell, J. L. (2008). Spatial and temporal variations in DOM composition in ecosystems: the importance of long-term monitoring of optical properties. Journal of Geophysical Research, 113, G04032. doi:10.1029/2008JG000683.
Kaplan, L. A., & Newbold, J. D. (2003). The role of monomers in stream ecosystem metabolism. In S. E. G. Findlay & R. L. Sinsabaugh (Eds.), Aquatic ecosystems: interactivity of dissolved organic matter (pp. 97–119). London: Elsevier Science.
Lam, B., & Simpson, A. J. (2006). Passive sampler for dissolved organic matter in freshwater environments. Analytical Chemistry, 78(24), 8194–8199.
Leenheer, J., & Croué, J. (2003). Characterizing dissolved organic matter. Environmental Science and Technology, 37(1), 18–26.
McKnight, D. M., Boyer, E. W., Westerhoff, P. K., Doran, P. T., Kulbe, T., & Andersen, D. T. (2001). Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnology and Oceanography, 46(1), 38–48.
Miles, C. J., Tuschall, J. R., Jr., & Brezonik, P. J. (1983). Isolation of aquatic humus with diethylaminoethylcellulose. Analytical Chemistry, 55(2), 410–411.
Parlanti, E., Wörz, K., Geoffroy, L., & Lamotte, M. (2000). Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Organic Geochemistry, 31(12), 1765–1781.
Peuravuori, J., Koivikko, R., & Pihlaj, K. (2002). Characterization, differentiation and classification of aquatic humic matter separated with different sorbents: synchronous scanning fluorescence spectroscopy. Water Research, 36(18), 4552–4562.
Weishaar, J. L., Aiken, G. R., Bergamaschi, B. A., Fram, M. S., Fujii, R., & Mopper, K. (2003). Evaluation of specific ultra-violet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environmental Science and Technology, 37(20), 4702–4708.
Acknowledgments
We thank Laura T. Johnson and Annie Bowling for their guidance and help in the field and laboratory. Financial support was provided by Indiana University and the National Science Foundation (grant DEB-0743396).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Warner, D.L., Oviedo-Vargas, D. & Royer, T.V. Evaluation of passive samplers for the collection of dissolved organic matter in streams. Environ Monit Assess 187, 4208 (2015). https://doi.org/10.1007/s10661-014-4208-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4208-5