Abstract
The Hara Mangrove Forest of the Persian Gulf is undergoing increasing pollution from industrial, municipal, and petroleum sources; however, little research in ecotoxicology has been carried out in this ecosystem. In the present study, mercury distribution and accumulation were investigated in muscle, liver, kidney, and feather of the resident Western reef heron (n = 15) and the migratory Siberian gull (n = 15). We also evaluated the relation between Hg concentrations, sex, and age (juvenile vs. adult). Results showed that the highest concentrations of Hg were recorded in the feather (35 ± 0.14–3.0 ± 0.27 mg kg−1 dw) and at 3.7-, 1.6-, and 1.3-fold in muscle, kidney, and liver, respectively. Concentrations of mercury in tissues of migratory birds were two times higher than in resident birds; geographical differences and feeding habits were used to explain these variations. We found a weak relationship between Hg concentrations in feathers and internal tissues (r ≤ 0.50); conversely, liver presented strong positive correlations with other soft tissues, especially kidney (p > 0.05; r = 0.82). Results showed that sex and age have no significant effects on T-Hg accumulation in these birds (p > 0.05; r < −0.01). Based on these findings, Hg concentrations were low in both species. Therefore, Hg contamination of this aquatic ecosystem is not a threat. Accordingly, we recommend the use of the Western reef heron as a bioindicator of mercury pollution in this region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental mercury (Hg) pollution threatens humans and wildlife (Seewagen 2010). In aquatic ecosystems, Hg is converted to methylmercury (MeHg) and bioaccumulates and biomagnifies through all levels of the food chain (Lindqvist et al. 1991). Seabirds have been employed as credible indicators of environmental heavy metal pollution because they are often at the top of food chains and are common and widespread, abundant, long-lived, and of interest to the public (Burger and Gochfeld 2004; Monteiro and Furness 1995; Hollamby et al. 2006). The reported variations of mercury concentrations in specific targeted tissues within bird species potentially reflect the effects of factors such as molt, age, sex, season, and laying sequence (Monteiro and Furness 1995). Variations in mercury concentrations between bird species potentially reflect many factors, including feeding and migratory habits, body size, life span, molt strategy, and taxonomic influences on physiology (Walsh 1990). Migratory birds can be used to assess exposure in distant regions, while resident species can indicate local levels of pollutants (Rothschild and Duffy 2005). A comparative study of mercury levels between species is essential because it will allow us to identify the more sensitive species by representative mercury levels versus reported behavioral or reproductive abnormalities (Zamani-Ahmadmahmoodi et al. 2009). Many papers have reported on mercury levels in both tissue and feather of birds in order to assess mercury contamination in migratory and resident birds (Lewis et al. 1993; Lavoie et al. 2010). Lucia et al. (2010) studied Hg concentrations in liver, kidney, muscle, and feather of migratory and resident aquatic birds on the southwest Atlantic coast of France. These researchers found that Hg accumulation was less in muscle and higher in liver, kidney, and feathers, respectively. The northeast Siberian seabirds exhibited higher Hg levels in resident organisms (Ruelas-Inzunza et al. 2007). In tissues of seabirds from the Persian Gulf, concentrations of mercury in tissues of migratory birds were considerably higher than in resident birds (Zamani-Ahmadmahmoodi et al. 2009). Gulls had significantly higher levels of mercury than the eiders in the Bering Sea, and interspecific differences were detected due to differences in diet (Burger and Gochfeld 2007). Siberian gulls had greater metal concentrations in kidney, liver, pectoral muscle, and feather than Western reef heron from southern Iran (Mansouri et al. 2012a, b).
The present study documents, for the first time, mercury distribution in tissues of two species of waterbirds, Western reef heron (Egretta gularis) and Siberian gull (Larus heuglini), obtained from Hara International Wetland, located in the northern Persian Gulf. Resident Western reef herons are abundant and native breeders of this wetland. The Siberian gull is a migratory species, journeying every autumn from the tundra of northern Russia to these wetlands and remaining throughout the winter.
The aim of this study was to analyze total mercury concentrations in four tissues (liver, kidney, muscle, and breast feather) of two waterbird species with their differing life strategies, resident versus migratory, which leads to exposure to pollution in different environments. In addition, our objective was to determine whether age and gender can affect the levels of mercury in these species. Furthermore, relationships between Hg levels in feathers and internal tissues were determined to evaluate the utility of feathers for Hg monitoring of birds with the hypothesis that breast feathers are the best indicator of whole-body burdens, particularly for mercury.
Materials and methods
Study area
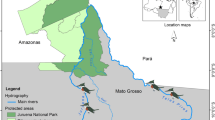
Hara International Wetland is located between 26°40′–26°59′ N, 55°21′–55°52′ E on the northern shores of the Persian Gulf. It comprises 856.86 km2 and contains 86 % of the mangrove forests of Iran. It is part of the narrow Khouran Strait, situated between Qeshm Island and Iran’s mainland, consisting of marine and coastal mangrove communities and a system of branches of lagoon (Fig. 1) (Zehzad and Majnoonian 1998). The Hara Mangrove Forest is a UNESCO Biosphere Reserve (BR) in Iran and hosts one of the largest breeding colonies of herons and egrets in Iran and the Middle East (Evans 1994).
Sample preparation
The birds examined in this study (n = 30) were hunted and collected in October and December 2011 from a region under license of the Environmental Protection Agency of Hormozgan Province. Samples were immediately transported to the laboratory on ice; the birds’ weight, length, and wing length were recorded. The birds were dissected immediately, and muscle, liver, kidney, and feather were removed from the specimens. Pectoral muscle and breast feathers were used for the analysis. Sex was determined by identifying the sex organ during dissection. Plumage characteristics (plumage coloration, and feather wear and shape) and size used to identify juvenile and adult. Relevant biological data are provided in Table 1. Remains were kept in clean plastic bags at −20 °C. Feathers were washed in deionized water and acetone and then dried in an oven at 40 °C for 24 h. All other tissues were placed in a freeze dryer for 48 h, and then powdered samples were homogenized by Polytron Homogenizer (Kinematica, India). The moisture content (average ± standard error) for each tissue (muscle, 63.28 ± 0.09 %; liver, 59.74 ± 0.07 %; kidney, 57.76 ± 0.05 %) was determined.
Analytical procedure
Mercury concentration of samples was measured using the Advanced Mercury Analyzer (LECO AMA 254) according to ASTM Standard No. D6722. Relative standard deviation (RSD) of the method was in the range of 1.5–7.0 %. The AMA 254 had a detection limit of 0.01 ng of Hg. All samples analyzed were within the calibration range. Accuracy of the method for determining total mercury analysis was checked by running three samples based on Standard Reference Materials (SRM), Institute of Standards and Technology, SRM 1633b, SRM 2709, and SRM 2711 in seven replicates. It was found to give results in good agreement with the certified values in seven replicates, and a recovery of 98.4 ± 2.8 % was obtained. In this paper, concentrations are expressed in milligrams per kilogram in dry weight (mg kg−1 dw).
Statistical analysis
Statistical analysis was performed using SPSS software v. 16. Normality of data was assessed by a Kolmogorov-Smirnov test. Values given are the mean ± standard errors, and we considered a p value <0.05 to be statistically significant. Mercury concentrations in feather, liver, kidney, and muscle were tested with the Dunnett T3 post hoc test of one-way analysis of variance (ANOVA), at α = 0.05 probability. An independent t test was utilized to determine the significant difference between two groups of adults and juveniles in terms of mercury accumulation in different tissues and also between sexes in both species. Spearman’s correlation coefficients were calculated to check the relationship between Hg concentrations among different tissues.
Results and discussion
Intraspecific differences
Mercury concentrations were detected in four types of bird tissue: muscle, liver, kidney, and breast feather. The highest concentrations of Hg were recorded in the feather and the smallest in the muscle (0.57–5.33 and 0.27–0.99 mg kg−1 dw, respectively), The sequence of average mercury concentrations in migratory birds was feather > kidney > liver > muscle, and for resident birds, the order was feather > liver > kidney > muscle (Table 1). This high variation in ratios among tissues of species has been noted in other studies: highest mercury concentrations were reported in avian feather, followed by liver, kidney, heart, and muscle tissues (Burger 1993). In addition, Eagles-Smith et al. (2008) reported highest total Hg concentrations in feathers, followed by kidney and liver, and then muscle. The highest levels of mercury were detected in the livers of migratory birds, whereas in resident birds, mercury levels of kidney were highest (Zamani-Ahmadmahmoodi et al. 2009). Ruelas-Inzunza et al. (2007) reported highest Hg levels in liver and feathers for resident and migratory Siberian seabird, respectively. However, Hg poisoning can indicate that kidney concentrations may be elevated to near the liver concentration. It should be noted that birds differ from mammals in having a renal entrance system; venous blood from the terminal portion of the digestive tract flows to the kidney rather than the liver, as in mammals (Wolfe et al.1998).
In most research, liver and breast feathers are most commonly used in monitoring and toxicological assessments of Hg exposure in birds. Although sensitivity to methylmercury toxicity varies among different species, concentrations of Hg of 5–15 mg kg−1 in feather have been related to adverse effects on growth and reproduction of birds (Wiener et al. 2003; La Sala et al. 2011). Zillioux et al. (1993) concluded that a threshold for major toxic effects in waterbirds is 5 mg kg−1 in liver. In our study, the Hg levels of feathers and other tissues were below the Hg toxicity threshold that may affect birds. Scheuhammer (1991) suggests that feather Hg concentrations >20 mg kg−1 can result from diets containing Hg concentrations >1 mg kg−1, and these concentrations are indicative of a wetland that exhibits an Hg risk for birds. Non-migratory and non-nomadic species are an indication of local environmental contamination (Hollamby et al. 2006). We conclude that mercury concentrations in the Western reef heron closely reflect mercury contamination in Hara International Wetland. On the other hand, the mercury burden of the Siberian gull is a reflection of diversity in food items from its wider geographical locations, with perhaps much higher mercury pollution encountered in the environmental conditions in their sites of origin outside Iran. Furthermore, mercury concentration in tissues of the adult Western reef heron from the Hara International Wetland was lower than those reported for birds of the same species from Iran (Zamani-Ahmadmahmoodi et al. 2010; 2009), but higher than birds from the Gulf of California (Table 2) (Ruelas-Inzunza et al. 2007). Mercury in the Siberian gull in this study was lower than or equal to values reported for birds of the same species from Iran and Siberia (Russia) (Kim et al. 1996), the UK (Hutton 1981), and Japan (Agusa et al. 2005) (Table 2).
Interspecific differences
The comparison of mean Hg concentration in the four tissues of each bird showed that there were significant differences among tissues in two birds (Dunnett T3 post hoc test, p < 0.05), except between liver and kidney of the Siberian gull (F = 0.127, p > 0.731) and feather and liver in Western reef heron (F = 0.035, p > 0.762). The results showed that there was significant difference among tissues of interspecies mercury concentration (p < 0.05) (Fig. 2). Mercury concentration in tissues of migratory birds was higher than those detected in tissues of resident species. For example, mercury was 2.22 times higher in feather, 1.61 times higher in liver, 2.41 times higher in kidney, and 1.95 times higher in muscle of Siberian gull when compared to Western reef heron (Fig. 2). Contaminant exposure among species varies with diet, foraging behavior, and migration patterns (Braune and Noble 2009). Contamination from higher latitudes and warmer southern regions has been noted through atmospheric deposition. Species (like Siberian gull) that spend the most time in the higher latitudes may show higher mercury concentrations due to mercury deposition in these latitudes (Lavoie et al. 2010; Mallory et al. 2006; Braune et al. 2005). On the other hand, food consumption is known to be a very important route of exposure for contaminants’ uptake in animals. High levels can be reached in food chains by upper trophic level consumers such as seabirds and can potentially cause adverse effects (AMAP 1998; Wolfe et al. 1998). In this study, Western reef heron has less mercury accumulation in the body because this bird eats mainly smaller fish and also feeds on crustaceans, amphibians, and shells. However, the gulls had mainly larger fish, mollusks, and a few sea urchins in their stomachs (Irons et al. 1986). Top predator birds that eat larger fish have higher mercury concentrations (Houserova et al. 2007); therefore, the scavenging habits of gulls may also have exposed them to high Hg concentrations (Burger and Gochfeld 2001). Generally, species that typically live longer and can eat larger food items, as do Siberian gulls, are expected to have higher levels of pollutants in their bodies.
Sex differences
There was no significant difference between the sexes in Hg concentration in tissues of the two species (p > 0.05). There is little evidence for differentiating the mercury levels of the two sexes in birds (Ackerman et al. 2008). Although the female can eliminate mercury into eggs, the amount shed this way is usually very small compared with the amount put into feathers during molt (Agusa et al. 2005; Furness and Camphuysen 1997). In the present study, because males and females are relatively similar in size, prey size is not different; furthermore, foraging niches are similar, hence there is little difference in mercury burden between the sexes (Evers et al. 2005). In addition, because our study was conducted in non-breeding season, mercury concentrations did not differ between the sexes. As Ackerman et al. (2008) state, when tissues were analyzed in non-breeding season, mercury concentrations were no different between the sexes.
Age differences
According to the results of an independent t test, there was no significant difference between two groups of adults and juveniles in terms of mercury accumulation in different tissues of Siberian gull (p > 0.05). Similar results were reported for Hg in tissues of known-aged birds (Agusa et al. 2005; Hutton and Goodman 1980). This might result from a special mercury dynamic among birds that mainly is based on the balance between Hg dietary intake and the elimination of plumage during the annual molt (Monteiro and Furness 1995). Adult migratory birds can eliminate accumulated contamination in their tissues via the molting process in their overwintering areas (Furness and Camphuysen 1997; La Sala et al. 2011). However, Burger and Gochfeld (2002) (stated that age can also be a factor in the accumulation of contaminant residues, although age-related differences depend on the contaminant and the species being studied.
Relationships of Hg levels among tissues
The relationship between mercury concentrations in internal tissues showed that there was significant correlation among those tissues in both birds (Fig. 3) (Spearman’s correlation, r = 0.53–0.82). A positive correlation was observed between liver and other soft tissues, especially kidney. Conversely, the relationships between T-Hg in breast feathers and internal tissues were substantially weaker (p > 0.05). This suggests that feathers are relatively poor indicators of Hg concentrations in internal tissues. However, the results of our study concur with other recent studies (Eagles-Smith et al. 2008; Evers et al. 2005; Kenow et al. 2003). One goal of the multi-tissue studies is to determine which single tissue would provide the best estimate of levels in other tissues (Muirhead and Furness 1988). Liver has become a standard tissue for contaminant testing (Burger and Gochfeld 2004). Feathers represent a discrete depuration and sequestration repository for Hg only during the period of feather growth and do not reflect changes in tissue concentrations (Bearhop et al. 2000; Braune and Gaskin 1987). Consequently, depending on when feather growth occurred relative to the time of tissue sampling, the relationship will change.
Conclusion
Total mercury was determined in four tissues (muscle, liver, kidney, and breast feather) of Western reef heron (resident) and Siberian gull (migratory). Results explain the accumulatory behavior of Hg in selected tissues of these birds. Concentrations of mercury in tissues of migratory birds were considerably higher than in resident species. Our data demonstrate that breast feathers were weaker indicators of Hg concentrations in internal tissues. No differences of Hg levels were observed in connection with age or sex. Hg levels in tissues of both birds were lower than or similar to the same species from Iran and other places. Mercury levels of tissues were below the threshold of 5–15 mg kg−1 that may affect birds. Piscivorous birds have particular importance for pollution monitoring as a bioindicator of aquatic ecosystems (Hollamby et al. 2006; Burger and Gochfeld 2004); therefore, Western reef heron as a resident species, with its high population frequency and breeding capability, can be introduced as a bioindicator of mercury pollution in the Hara International Wetland of the Persian Gulf.
References
Ackerman, J. T., Eagles-Smith, C. A., Takekawa, J. Y., Adelsbach, T. L., & Bluso, J. D. (2008). Mercury concentration in blood and feathers of prebreeding Forster’s terns in relation to space use of San Francisco Bay, California, USA, habitats. Environmental Toxicology, 27(4), 897–908.
Agusa, T., Matsumoto, T., Ikemoto, T., Anan, Y., Kubota, R., Yasunaga, G., & Shibata, Y. (2005). Body distribution of trace elements in black-tailed gulls from Rishiri Island, Japan: age-dependent accumulation and transfer to feathers and eggs. Environmental Toxicology and Chemistry, 24(9), 2107–2120.
AMAP. (1998). Assessment report: arctic pollution issues (Vol. 12, p. 859). Arctic Monitoring and Assessment Programme (AMAP): Oslo.
Bearhop, S., Ruxton, G. D., & Furness, R. W. (2000). Dynamics of mercury in blood and feathers of great skuas. Environmental Toxicology and Chemistry, 19, 1638–1643.
Braune, B. M., & Gaskin, D. E. (1987). Mercury levels in Bonaparte’s gulls (Larus philadelphia) during autumn molt in the Quoddy Region, New Brunswick, Canada. Archives of Environmental Contamination and Toxicology, 16(5), 539–549.
Braune, B. M., & Noble, D. G. (2009). Environmental contaminants in Canadian shorebirds. Environmental Monitoring and Assessment, 148(1–4), 185–204.
Braune, B. M., Outridge, P. M., Fisk, A. T., Muir, D. C. G., Helm, P. A., Hobbs, K., & Stirling, I. (2005). Persistent organic pollutants and mercury in marine biota of the Canadian Arctic: an overview of spatial and temporal trends. Science of the Total Environment, 351, 4–56.
Burger, J. (1993). Metals in avian feathers: bioindicators of environmental pollution. Reviews of Environmental Contamination and Toxicology, 5, 203–311.
Burger, J., & Gochfeld, M. (2001). Metal levels in feathers of cormorants, flamingos and gulls from the coast of Namibia in Southern Africa. Environmental Monitoring and Assessment, 69(2), 195–203.
Burger, J., & Gochfeld, M. (2002). Effects of chemicals and pollution on seabirds. In E. A. Schreiber & J. Burger (Eds.), Biology of marine birds (pp. 485–525). Boca Raton: CRC Press.
Burger, J., & Gochfeld, M. (2004). Marine birds as sentinels of environmental pollution. EcoHealth, 1(3), 263–274.
Burger, J., & Gochfeld, M. (2007). Metals and radionuclides in birds and eggs from Amchitka and Kiska Islands in the Bering Sea/Pacific Ocean ecosystem. Environmental Monitoring and Assessment, 127, 105–117.
Eagles‐Smith, C. A., Ackerman, J. T., Adelsbach, T. L., Takekawa, J. Y., Miles, A. K., & Keister, R. A. (2008). Mercury correlations among six tissues for four waterbird species breeding in San Francisco Bay, California, USA. Environmental Toxicology and Chemistry, 27(10), 2136–2153.
Evans, M. I. (1994). Important bird areas in the Middle East. Cambridge: UK. Bird Life International.
Evers, D. C., Burgess, N. M., Champoux, L., Hoskins, B., Major, A., Goodale, W. M., & Daigle, T. (2005). Patterns and interpretation of mercury exposure in freshwater avian communities in northeastern North America. Ecotoxicology, 14(1–2), 193–221.
Furness, R. W., & Camphuysen, K. C. (1997). Seabirds as monitors of the marine environment. ICES Journal of Marine Science Journal du Conseil, 54(4), 726–737.
Hollamby, S., Afema-Azikuru, J., Waigo, S., Cameron, K., Gandolf, A. R., Norris, A., & Sikarskie, J. G. (2006). Suggested guidelines for use of avian species as biomonitors. Environmental Monitoring and Assessment, 118(1–3), 13–20.
Houserova, P., Kubáň, V., Kráčmar, S., & Sitko, J. (2007). Total mercury and mercury species in birds and fish in an aquatic ecosystem in the Czech Republic. Environmental Pollution, 145(1), 185–194.
Hutton, M. (1981). Accumulation of heavy metals and selenium in three seabird species from the United Kingdom. Environmental Pollution Series A, Ecological and Biological, 26(2), 129–145.
Hutton, M., & Goodman, G. T. (1980). Metal contamination of feral pigeons Columba livia from the London area: part 1—tissue accumulation of lead, cadmium and zinc. Environmental Pollution Series A, Ecological and Biological, 22(3), 207–217.
Irons, D. B., Anthony, R. G., & Estes, J. A. (1986). Foraging strategies of glaucous-winged gulls in a rocky intertidal community. Ecology, 67, 1460–1474.
Kenow, K. P., Gutreuter, S., Hines, R. K., Meyer, M. W., Fournier, F., & Karasov, W. H. (2003). Effects of methyl mercury exposure on the growth of juvenile common loons. Ecotoxicology, 12(1–4), 171–181.
Kim, E. Y., Ichihashi, H., Saeki, K., Atrashkevich, G., Tanabe, S., & Tatsukawa, R. (1996). Metal accumulation in tissues of seabirds from Chaun, northeast Siberia, Russia. Environmental Pollution, 92(3), 247–252.
La Sala, L. F., Petracci, P. F., Smits, J. E., Botté, S., & Furness, R. W. (2011). Mercury levels and health parameters in the threatened Olrog’s gull (Larus atlanticus) from Argentina. Environmental Monitoring and Assessment, 181(1–4), 1–11.
Lavoie, R. A., Champoux, L., Rail, J. F., & Lean, D. R. (2010). Organochlorines, brominated flame retardants and mercury levels in six seabird species from the Gulf of St. Lawrence (Canada): relationships with feeding ecology, migration and molt. Environmental Pollution, 158(6), 2189–2199.
Lewis, S. A., Becker, P. H., & Furness, R. W. (1993). Mercury levels in eggs, tissues, and feathers of herring gulls Larus argentatus from the German Wadden Sea Coast. Environmental Pollution, 80(3), 293–299.
Lindqvist, O., Johansson, K., Bringmark, L., Timm, B., Aastrup, M., Andersson, A., & Meili, M. (1991). Mercury in the Swedish environment—recent research on causes, consequences and corrective methods. Water, Air, and Soil Pollution, 55(1–2), xi–261.
Lucia, M., André, J. M., Gontier, K., Diot, N., Veiga, J., & Davail, S. (2010). Trace element concentrations (mercury, cadmium, copper, zinc, lead, aluminum, nickel, arsenic, and selenium) in some aquatic birds of the southwest Atlantic Coast of France. Archives of Environmental Contamination and Toxicology, 58(3), 844–53.
Mallory, M. L., Gilchrist, H. G., Braune, B. M., & Gaston, A. J. (2006). Marine birds as indicators of Arctic marine ecosystem health: linking the northern ecosystem initiative to long-term studies. Environmental Monitoring and Assessment, 113(1), 31–48.
Mansouri, B., Babaei, H., & Hoshyari, E. (2012a). Heavy metal contamination in feathers of Western reef heron (Egretta gularis) and Siberian gull (Larus heuglini) from Hara Biosphere Reserve of southern Iran. Environmental Monitoring and Assessment, 184(10), 6139–6145.
Mansouri, B., Babaei, H., Hoshyari, E., Khodaparast, S. H., & Mirzajani, A. (2012b). Assessment of trace- metal concentrations in Western reef heron (Egretta gularis) and Siberian gull (Larus heuglini) from southern Iran. Archives of Environmental Contamination and Toxicology, 63(2), 280–287.
Monteiro, L. R., & Furness, R. W. (1995). Seabirds as monitors of mercury in the marine environment. Water, Air, and Soil Pollution, 80(1–4), 851–870.
Muirhead, S. J., & Furness, R. W. (1988). Heavy metal concentrations in the tissues of seabirds from Gough Island, south Atlantic Ocean. Marine Pollution Bulletin, 19(6), 278–283.
Rothschild, R. F., & Duffy, L. K. (2005). Mercury concentrations in muscle, brain and bone of Western Alaskan waterfowl. Science of the Total Environment, 349(1), 277–283.
Ruelas-Inzunza, J., Páez-Osuna, F., & Arvizu-Merin, M. (2007). Mercury distribution in selected tissues of migratory and resident avifauna from Altata-Ensenada del Pabellón Lagoon, southeast Gulf of California. Bulletin of Environmental Contamination and Toxicology, 78(1), 39–43.
Scheuhammer, A. M. (1991). Effects of acidification on the availability of toxic metals and calcium to wild birds and mammals. Environmental Pollution, 71(2), 329–375.
Seewagen, C. L. (2010). Threats of environmental mercury to birds: knowledge gaps and priorities for future research. Bird Conservation International, 20(02), 112–123.
Walsh, P. (1990). The use of seabirds as monitors of heavy metals in the marine environment (pp. 183–204). Boca Raton: CRC Press.
Wiener, J. G., Krabbenhoft, D. P., Heinz, G. H., & Scheuhammer, A. M. (2003). Ecotoxicology of mercury. In D. J. Hoffman, B. A. Rattner, G. A. Burton, & J. Cairns (Eds.), Handbook of ecotoxicology (2nd ed., pp. 409–463). Boca Raton: CRC Press.
Wolfe, M. F., Schwarzbach, S., & Sulaiman, R. A. (1998). Effects of mercury on wildlife: a comprehensive review. Environmental Toxicology and Chemistry, 17(2), 146–160.
Zamani-Ahmadmahmoodi, R., Esmaili-Sari, A., Ghasempouri, S. M., & Savabieasfahani, M. (2009). Mercury in wetland birds of Iran and Iraq: contrasting resident Moorhen, Gallinula chloropus, and migratory common teal, Anas crecca, life strategies. Bulletin of Environmental Contamination and Toxicology, 82(4), 450–453.
Zamani-Ahmadmahmoodi, R., Esmaili-Sari, A., Savabieasfahani, M., & Bahramifar, N. (2010). Cattle egret (Bubulcus ibis) and little egret (Egretta garzetta) as monitors of mercury contamination in Shadegan Wetlands of south-western Iran. Environmental Monitoring and Assessment, 166(1–4), 371–7.
Zehzad, B. & Majnoonian, H. (1998). Hara Protected Area (Biosphere Reserve). Department of the Environment & Shahid Beheshti University Research Bureau. 70 pp.
Zillioux, E. J., Porcella, D. B., & Benoit, J. M. (1993). Mercury cycling and effects in freshwater wetland ecosystems. Environmental Toxicology and Chemistry, 12(12), 2245–2264.
Acknowledgments
This research was funded by Tarbiat Modares University (TMU) of Iran. We thank Amir Majidi and Ali H. Nejad for the field assistance. We also thank Ali Kazemi and Jabber Azami for laboratory support and statistical advice. We are indebted to the anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Majidi, Y., Bahramifar, N. & Ghasempouri, S.M. Pattern of mercury accumulation in different tissues of migratory and resident birds: Western reef heron (Egretta gularis) and Siberian gull (Larus heuglini) in Hara International Wetland—Persian Gulf. Environ Monit Assess 187, 4082 (2015). https://doi.org/10.1007/s10661-014-4082-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4082-1