Abstract
Toxicity in natural ecosystems is usually not due to exposure to a single substance, but is rather the result of exposure to mixtures of toxic substances. Knowing the effects of contaminants as a mixture compared to their effects in isolated form is therefore important. This study aimed to evaluate the oxidative stress induced by binary mixtures of diclofenac with paracetamol, ibuprofen, naproxen, and acetylsalicylic acid and by these nonsteroidal anti-inflammatory drugs (NSAIDs) in isolated form, using Hyalella azteca as a bioindicator. The median lethal concentration (LC50) and the lowest observed adverse effect level (LOAEL) of each NSAID were obtained. Amphipods were exposed for 72 h to the latter value in isolated form and as binary mixtures. The following biomarkers were evaluated: lipid peroxidation (LPX), protein carbonyl content (PCC), and activity of the antioxidant enzymes: superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). Significant increases in LPX and PCC with respect to the control group (p ≤ 0.05) were induced by NSAIDs both in isolated form and as binary mixtures. Changes in SOD, CAT, and GPx activity likewise occurred with NSAIDs in isolated form and as binary mixtures. In conclusion, NSAIDs used in this study induce oxidative stress on H. azteca both in isolated form and as binary mixtures, and the interactions occurring between these pharmaceuticals are probably antagonistic in type.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pharmaceuticals for human and veterinary use have become an important source of contamination and are now termed emerging chemicals of concern. Diverse studies have shown a remarkable increase worldwide in the levels of these contaminants in surface and groundwater, eliciting toxicity problems in diverse hydrobionts (Cleuvers 2004; Montforts et al. 2007; Spindler et al. 2007).
Studies regarding the occurrence of pharmaceutical products in aquatic systems have been conducted mainly in areas of high population density in countries such as the USA, Germany, Switzerland, Denmark, the Netherlands, and France, but these agents have also been detected in the North Sea and Antarctica (Fent et al. 2006). These studies have found that the typical pharmaceuticals detected such as antibiotics, hormones, antihypertensive agents, and nonsteroidal anti-inflammatory drugs (NSAIDs) (Araujo et al. 2011) span over several magnitudes of order from nanograms per liter to micrograms per liter.
NSAIDs are a heterogeneous group of pharmaceuticals with analgesic, anti-inflammatory, and antipyretic properties. The most commonly used NSAIDs at the world level, based on the number of prescriptions and sales, are acetylsalicylic acid (ASA), paracetamol (PCM), diclofenac (D), ibuprofen (IBP), and naproxen (NPX) (Katzung 2007).
NSAIDs studied in this work belong to the group of arylpropionic derivatives (IBP and NPX), salicylate derivatives (ASA), arylacetic derivatives (D), and p-aminophenol derivatives (PCM). These drugs are the most consumed NSAIDs in Mexico. In addition, several studies have shown that these drugs are highly toxic in aquatic species (Cleuvers 2004; Brun et al. 2006; Khetan and Collins 2007).
There are diverse antecedents of the identification and quantification of NSAIDs in different water bodies throughout the world, most notably the studies by Parolini et al. (2009), who identified IBP, NPX, and ASA in municipal effluent in Italy, and Ternes (1998), who noted the occurrence of D, IBP, NPX, and ASA at concentrations >1 μg/l in treatment plant effluent. Other scholars report concentrations of 0.1–2.5 μg D/l, 0.01–4 μg IBP/l, and 0.1–12.5 μg NPX/l in wastewater treatment plant effluents (Metcalfe et al. 2003; Andreozzi et al. 2004; Carballa et al. 2004; Quintana et al. 2005; Tauxe-Wuersch et al. 2005).
In Mexico, there is little information on the occurrence of pharmaceutical products in water bodies. A single study by Siemens et al. (2008) reports concentrations of 0.22–0.38 μg IBP/l, 2.84–6.74 μg NPX/l, and 0.25–0.50 μg D/l in the Mezquital Valley irrigation system. NSAID-induced toxicity in aquatic organisms has been shown to elicit alterations to reproduction and growth in invertebrates as well as nephrotoxicity in vertebrates (Cleuvers 2004; Brun et al. 2006; Khetan and Collins 2007).
Our study team has shown that PCM, D, NPX, and IBP induce oxidative stress on microcrustaceans such as Daphnia magna, amphipods such as Hyalella azteca, and commercially valuable freshwater fish such as Cyprinus carpio, negatively affecting biomolecules such as proteins, membrane lipids, and DNA (Oviedo-Gómez et al. 2010; Gómez-Oliván et al. 2012; Islas-Flores et al. 2013; Gómez-Oliván et al. 2014; Islas-Flores et al. 2014).
Oxidative stress, considered one of the major mechanisms of action of toxicants, has become one of the most commonly used biomarkers because of its capacity to evaluate general damage to biomolecules such as lipids, proteins, and DNA. Oxidative stress is defined as an imbalance between reactive oxygen species (ROS)—including the superoxide anion radical (O2 −•), hydrogen peroxide (H2O2), and hydroxyl radical (OH•)—and antioxidant systems of the body. Normally, ROS are produced in cells as a result of metabolic processes (Vlahogianni et al. 2007). To minimize oxidative damage to cellular components, organisms have developed antioxidant defenses. The most important antioxidant enzymes are superoxide dismutase (SOD, which converts O2 − to H2O2), catalase (CAT, which reduces H2O2 to water), and glutathione peroxidase (GPx, which detoxifies H2O2 and organic hydroxy-peroxides formed, for example, by lipid peroxidation) (Barata et al. 2005).
H. azteca is a sentinel organism that is widely used to evaluate toxicity in water bodies because of the many advantages it offers, such as being a bioindicator of water and sediment quality and being easy to reproduce and maintain under laboratory conditions and also highly sensitive to diverse xenobiotics. Toxicity in natural ecosystems is usually not due to exposure to a single substance, but is the result of exposure to mixtures of toxic substances (Backhaus et al. 2003; Silva et al. 2002). Thus, the present study aimed to evaluate oxidative stress induced by binary mixtures of D with PCM, IBP, NPX, and ASA and by these NSAIDs in isolated form, at sublethal concentrations on H. azteca.
Materials and methods
Procurement, culturing, and maintenance of specimens
H. azteca was collected from its natural habitat in Lake San Miguel de Almaya, municipality of Capulhuac (State of Mexico), and transported to the laboratory in plastic bags with constant aeration. During culture, specimens were maintained in reconstituted water (NaHCO3 = 174 mg/l, MgSO4 = 120 mg/l, KCl = 8 mg/l, and CaSO4⋅2H2O = 120 mg/l; all reagents were obtained from Sigma-Aldrich, St. Louis, MO) pH 7.5–8.5 at room temperature with constant oxygen (6.4–6.6 mg/l, O2) and a 12-h/12-h light/dark photoperiod and were fed ground lettuce ad libitum. Specimens used in toxicity assays were third-generation neonates obtained by sexual reproduction from a 4-month-old culture.
Artificial sediment
The artificial sediment used was 70 % sand (0.2 mm), 20 % kaolinite (<0.002 mm), and 10 % organic matter (0.2 mm). The organic matter source was lamb compost inactivated by dry heating at 55–60 °C for 3 days. The sediment was sterilized with three 15-min autoclave cycles at 121 °C and 15-lb pressure, separated by 1-h intervals.
Determination of the median lethal concentration (LC50) and equieffective concentration
Test systems
Test systems were set up by adding reconstituted water and artificial sediment in a 3:1 ratio to 150-ml polyethylene containers equipped with constant oxygenation and maintained under a 12-h/12-h photoperiod at room temperature. Static systems were used, the medium was not replaced, and no food was provided to the specimens during exposure.
Determination of the LC50
To establish the concentration to be used to evaluate oxidative stress, the LC50 of D, PCM, IBP, NPX, and ASA in H. azteca was determined. To this end, five exposure systems were set up for each NSAID, except IBP for which six systems were used. The following concentrations were added to these systems: D (4.2, 6.6, 10.5, 16.6, and 27 mg/l), PCM (2.4, 3.8, 6.0, 9.6, and 15.1 mg/l), IBP (1.2, 1.5, 1.8, 2.1, 2.4, and 2.7 mg/l), NPX (2.5, 3.9, 4.9, 6.2, and 9.8 mg/l), and ASA (2.5, 2.7, 2.8, 3.0, and 3.2 mg/l). A pharmaceutical-free control system was set up and 10 specimens were placed in each test system. The exposure period was 72 h, after which the number of dead (immobile) specimens was counted. LC50 values were obtained using the Probit analysis v1.5 software of the US Environmental Protection Agency (USEPA).
Graph plots of these LC50 estimates were used to derive the lowest observed adverse effect level (LOAEL) values, these being the lowest concentration or amount of a substance, found by experiment or observation, which elicits an adverse alteration in morphology, functional capacity, growth, development, or life span of a target organism distinguishable from normal (control) organisms of the same species and strain under defined conditions of exposure. These values were considered equieffective concentrations (the minimum concentration at which an effect was observed) for each NSAID evaluated in the oxidative stress experiment both in isolated form and as binary mixtures.
It was decided to use D as the fixed component of all binary mixtures since previous studies in our laboratory have shown its capacity to induce oxidative stress (Oviedo-Gómez et al. 2010; Islas-Flores et al. 2013). Pharmaceuticals evaluated in the present study were PCM, IBP, NPX, and ASA.
Evaluation of oxidative stress biomarker
Determination of oxidative stress induced by NSAIDs in isolated form
A sublethal toxicity assay was performed to determine the oxidative stress induced on H. azteca by each NSAID in isolated form. To each exposure system, 150 mg (wet weight) of the amphipod and a concentration equal to the LOAEL of the corresponding NSAID were added. After 72 h of exposure, specimens were removed and homogenized in 1 ml Tris buffer solution pH 7. The supernatant was centrifuged at 12,500 × g for 15 min at −4 °C. The following oxidative stress biomarkers were evaluated: lipid peroxidation (LPX); protein carbonyl content (PCC) in order to assess oxidized protein levels; and activity of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). Total protein content (Bradford 1976) was determined and was used to express the results of the biomarkers evaluated. All biochemical assays were done on the supernatant, except for LPX assessment in which the bud was used.
Determination of oxidative stress induced by binary mixtures
A sublethal toxicity assay was performed to evaluate the oxidative stress induced on H. azteca by binary mixtures of the NSAIDs. To each exposure system, 150 mg (wet weight) of the amphipod and a concentration equal to the LOAEL of each NSAID in the corresponding binary mixture (D-PCM, D-IBP, D-NPX, and D-ASA) were added. After exposure for 72 h, the same procedure as discussed previously was used to evaluate oxidative stress.
Cellular oxidation indicators
Determination of LPX
LPX was determined using the thiobarbituric acid-reactive substances method (Büege and Aust 1978). One hundred microliters of the supernatant was added to Tris-HCl buffer solution pH 7.4 (Sigma-Aldrich, St. Louis) to complete a volume of 1 ml. Samples were incubated at 37 °C for 30 min; 2 ml TBA-TCA reagent [0.375 % thiobarbituric acid (Fluka-Sigma-Aldrich, Toluca, Mexico) in 15 % trichloroacetic acid (Sigma-Aldrich, St. Louis)] was added, and the samples were shaken in a vortex. They were then heated to boiling for 45 min and allowed to cool, and the precipitate was removed by centrifugation at 3,000 × g for 10 min. Absorbance was read at 535 nm against a reaction blank. MDA content was calculated using the molar extinction coefficient (MEC) of malondialdehyde (MDA) (1.56 × 105 M/cm). Results were expressed as millimolars of MDA per milligram protein.
Determination of PCC
PCC was determined using the method of Levine et al. (1994) as modified by Parvez and Raisuddin (2005) and Burcham (2007). One hundred fifty microliters of 10 mM in 2 M HCl was added to 100 μl of the supernatant, and the resulting solution was incubated at room temperature for 1 h in the dark. Next, 500 μl of 20 % trichloroacetic acid was added and the solution was allowed to rest for 15 min at 4 °C. The precipitate was centrifuged at 11,000 × g for 5 min. The bud was washed several times with 1:1 ethanol/ethyl acetate, then dissolved in 1 ml of 6 M guanidine solution (pH 2.3), and incubated at 37 °C for 30 min. All reagents were obtained from Sigma-Aldrich, St. Louis. Absorbance was read at 366 nm. Results were expressed as nanomolars of reactive carbonyls formed (C = O) per milligram protein, using the MEC of 21,000 M/cm.
Antioxidant activity
Determination of SOD activity
SOD activity was determined by the method of Misra and Fridovich (1972). Two hundred sixty microliters of carbonate buffer solution (50 mM sodium carbonate and 0.1 mM EDTA) pH 10.2, plus 200 μl adrenaline (30 mM), was added to 40 μl of the supernatant in a 1-cm cuvette; all reagents were from Sigma-Aldrich, St. Louis. Absorbance was read at 480 nm after 30 s and 5 min. Enzyme activity was determined using the MEC of SOD (21 M/cm). Results were expressed as international units of SOD per milligram protein.
Determination of CAT activity
CAT activity was determined by the method of Radi et al. (1991). To 20 ml of the supernatant, 1 ml isolation buffer solution was added [0.3 M saccharose (Vetec-Sigma-Aldrich, St. Louis), 1 ml EDTA (Sigma-Aldrich, St. Louis), 5 mM HEPES (Sigma-Aldrich, St. Louis) and 5 mM KH2PO4 (Vetec-Sigma-Aldrich, St. Louis)], plus 0.2 ml of a hydrogen peroxide solution (20 mM, Vetec-Sigma-Aldrich, St. Louis). Absorbance was read at 240 nm after 0 and 60 s. Results were derived by substituting the absorbance value obtained for each of these times using the formula: CAT concentration = (A 0 − A 60) / MEC), where the MEC of H2O2 is 0.043 mM/cm and expressed as micromolars of H2O2 per milligram protein.
Determination of GPx activity
GPx activity was determined by the method of Gunzler and Flohe-Clairborne (1985) as modified by Stephensen et al. (2000). To 100 μl of the supernatant, 10 μl glutathione reductase was added (2 U glutathione reductase, Sigma-Aldrich, St. Louis), plus 290 μl reaction buffer [50 mM K2HPO4 (Vetec, St. Louis), 50 mM KH2PO4 (Vetec, St. Louis) pH 7.0, 3.5 mM reduced glutathione (Sigma-Aldrich, St. Louis), 1 mM sodium azide (Sigma-Aldrich, St. Louis), and 0.12 mM NADPH (Sigma-Aldrich, St. Louis)] and 100 μl H2O2 (0.8 mM, Vetec, St. Louis). Absorbance was read at 340 nm at 0 and 60 s. Enzyme activity was estimated using the equation: GPx concentration = (A 0 − A 60) / MEC), where the MEC of NADPH = 6.2 mM/cm. Results were expressed as millimolars of NADPH per milligram protein.
Determination of protein content
To 25 μl of the supernatant, 75 μl deionized water and 2.5 ml Bradford’s reagent were added (0.05 g Coomassie Blue dye, 25 ml of 96 % ethanol, and 50 ml H3PO4, in 500 ml deionized water). The test tubes were shaken and allowed to rest for 5 min prior to reading of absorbance at 595 nm and interpolation on a bovine albumin curve (Bradford 1976).
Statistical analysis
In the acute toxicity assay (72-h LC50 of D, ASA, IBP, NPX, and PCM), Probit analysis was performed and significance assessed by the degree of 95 % LC50 overlap (EPA Analysis Program v1.5). The χ 2 linear adjustment test was not significant at p < 0.05.
In the sublethal toxicity assays, statistical evaluation of the results was done with one-way analysis of variance (ANOVA), and differences between means were compared using the Tukey-Kramer multiple comparisons test, with p set at <0.05. Statistical determinations were made with the SPSS v10 software package (SPSS, Chicago, IL).
Results
LC50 of NSAIDs
Table 1 shows the LC50 of the NSAIDs as well as their 95 % confidence intervals. Values vary considerably, the lowest being 1.7 mg/l for IBP and the highest 7.7 mg/l for PCM. The χ 2 linear adjustment test was not significant at p ≤ 0.05.
In order to evaluate the lowest concentration of NSAIDs used in this study that causes an alteration, the LOAEL was calculated using the concentration–response curve and Probit analysis obtained from the acute assay. The results obtained are shown in Table 1, where the lowest value of LOAEL was for IBP and greater for PCM.
Evaluation of oxidative stress biomarkers
Cellular oxidation indicators
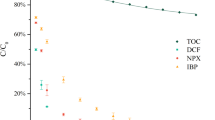
The amount of MDA induced by NSAIDs in isolated form and as binary mixtures is shown in Fig. 1. Significant increases with respect to the control group (p ≤ 0.05) were induced by D, PCM, NPX, and ASA in isolated form as well as by binary mixtures of D with PCM, NPX, and ASA. When the NSAIDs evaluated were compared, significant differences were observed in the mixtures D-PCM, D-IBP, D-NPX, and D-ASA by comparison to D (p ≤ 0.05).
Lipid peroxidation (LPX) at 72 h in H. azteca exposed to NSAIDs in isolated form and as binary mixtures. Values are the mean ± SE. *p < 0.05, significantly different from the control group, ANOVA and Tukey-Kramer. a p < 0.05 (significantly different from D); b p < 0.05 (significantly different from the other NSAID present in the mixture)
Protein oxidation in specimens exposed to NSAIDs in isolated form or as binary mixtures is shown in Fig. 2. In general, most NSAIDs both in isolated form and as binary mixtures induced significant increases with respect to the control group (p ≤ 0.05). However, in the case of D in isolated form and its binary mixtures with PCM and IBP, no significant differences were found, although a tendency toward increased PCC was observed. Comparisons among the evaluated NSAIDs show that PCM, IBP, NPX, and ASA in isolated form and binary mixtures of D with NPX and ASA induced significant increases by comparison to D (p ≤ 0.05). The most pronounced change in this biomarker occurred with ASA in isolated form.
Protein carbonyl content (PCC) at 72 h in H. azteca exposed to NSAIDs in isolated form and as binary mixtures. Values are the mean ± SE. *p < 0.05, significantly different from the control group, ANOVA and Tukey-Kramer. a p < 0.05 (significantly different from D); b p < 0.05 (significantly different from the other NSAID present in the mixture)
Antioxidant activity
Figure 3 shows SOD activity results. In general, all NSAIDs in isolated form induced significant decreases with respect to the control group (p ≤ 0.05). A similar behavior was observed with binary mixtures of D with PCM and NPX. D-ASA induced a significant increase in SOD activity with respect to the control group. Finally, significant differences with respect to D were observed in the mixtures D-IBP and D-ASA (p ≤ 0.05). In the mixture D-NPX, a significant decrease only with NPX was found (p ≤ 0.05).
Superoxide dismutase (SOD) activity at 72 h in H. azteca exposed to NSAIDs in isolated form and as binary mixtures. Values are the mean ± SE. *p < 0.05, significantly different from the control group, ANOVA and Tukey-Kramer. a p < 0.05 (significantly different from D); b p < 0.05 (significantly different from the other NSAID present in the mixture)
CAT activity, expressed as millimolars of H2O2 per milligram protein, is shown in Fig. 4. D, IBP, and NPX in isolated form induced a significant increase with respect to the control group (p ≤ 0.05), while ASA induced a significant decrease (p ≤ 0.05). Significant increases occurred when binary mixtures of D with PCM, IBP, and NPX were used. A significant difference (p ≤ 0.05) was observed with D-IBP and D-NPX by comparison to D. The mixture D-PCM only induced a significant increase with respect to PCM (p ≤ 0.05).
Catalase (CAT) activity at 72 h in H. azteca exposed to NSAIDs in isolated form and as binary mixtures. Values are the mean ± SE. *p < 0.05, significantly different from the control group, ANOVA and Tukey-Kramer. a p < 0.05 (significantly different from D); b p < 0.05 (significantly different from the other NSAID present in the mixture)
Figure 5 shows GPx activity results. As regards NSAIDs in isolated form, only in the case of NPX was a significant increase observed with respect to the control group (p ≤ 0.05) and with ASA was a significant decrease observed. Among the mixtures, only DCF-ASA obtained a significant decrease with respect to the control group. A similar behavior was observed in comparing these two mixtures with D, NPX, and ASA in isolated form.
Glutathione peroxidase (GPx) activity at 72 h in H. azteca exposed to NSAIDs in isolated form and as binary mixtures. Values are the mean ± SE. *p < 0.05, significantly different from the control group, ANOVA and Tukey-Kramer. a p < 0.05 (significantly different from D); b p < 0.05 (significantly different from the other NSAID present in the mixture)
Binary mixtures
Table 2 shows the values of oxidative stress biomarkers evaluated in isolated systems, in mixtures, and calculated additivity. As can be observed, the changes induced by the mixtures did not exceed the algebraic sum of the changes induced by each of the NSAIDs in the mixture.
Discussion
LC50 of NSAIDs
Based on the results obtained in the sublethal toxicity assay (Table 1), IBP is the most toxic and PCM the least toxic of the NSAIDs evaluated herein. Directive 93/67/EEC of the Commission of the European Communities (1996) classifies pharmaceutical products according to their LC50 value: <1 mg/l is extremely toxic, 1–10 mg/l is toxic, and 10–100 mg/l is hazardous to aquatic organisms. Based on this classification, all NSAIDs in the present study are toxic to H. azteca.
The LC50 values of D, IBP, NPX, PCM, and ASA found in H. azteca in the present study may be explained by the fact that these NSAIDs act by blocking the enzyme cyclooxygenase. The latter is responsible for catalyzing arachidonic acid degradation in prostaglandin production (Cha et al. 2006; Fortier et al. 2008). These eicosanoids act as autocrine and paracrine messengers and, in invertebrate species such as H. azteca, as important mediators during reproduction and in the immune system (Deridovich and Reunova 1993; Stanley 2000; Fortier et al. 2008). Prostaglandins are also involved in neurotransmission and the transport of ions across cell membranes (Arkhipova et al. 2005). The final result of these actions in the present study might have been inhibition of neurotransmission evidenced by H. azteca immobilization.
Evaluation of oxidative stress biomarkers
Cellular oxidation indicators
Diverse studies have shown that NSAIDs are unstable and are photodegradable to metabolites more toxic to aquatic organisms than the unaltered pharmaceuticals (Borgmann et al. 2007; Araujo et al. 2011). The main metabolites present in water bodies as a result of bacterial degradation and abiotic characteristics (pH and light) are as follows: for D, 5,4′-dihydroxy-diclofenac, 3′-hydroxy-diclofenac, 4′-hydroxymethyl diclofenac, 3′-hydroxy-4′-hydroxymethyl diclofenac, 4′-hydroxy- and 5′-hydroxy-diclofenac (Deng et al. 2003); for PCM, p-hydroxyacetanilide, p-hydroxyacetanilide glucuronide, and N-acetyl benzoquinoneimine (Mycek et al. 2004); for IBP, 1-hydroxy and 2-hydroxy ibuprofen and arylcarboxyl ibuprofen (Carballa et al. 2004); for NPX, naproxen-β-1-O-acyl glucuronide and 6-O-desmethyl naproxen (Huq 2006); and for ASA, acetylsalicylic acid, gentisic acid (2,5-dihydrobenzoic acid), o-hydroxyhippuric acid, salicyluric acid, and catechol (Ternes et al. 1999; Metcalfe et al. 2003; Carballa et al. 2004).
The biotransformation of NSAIDs once they enter the body must also be considered. The major pathway of biotransformation of carboxylate NSAIDs (ASA, D, NPX, and IBP) is glucuronic acid conjugation catalyzed by the uridine diphosphoglucuronosyl transferase superfamily of enzymes, which results in acyl glucuronides (Pritchard 1993). These compounds are reactive intermediates that can undergo acyl migration and hydrolysis. They can also form adducts with nucleophilic amino acid residues. Many NSAID-derived acyl glucuronides, including those obtained from D and IBP, have been shown to form covalent bonds with intra- and extracellular proteins, with toxicological consequences (Boelsterli 2007).
In the present study, most NSAIDs both in isolated form and as binary mixtures induced increases in LPX (Fig. 1). Such increases may be explained by the oxidative metabolism of NSAIDs. During this process, specifically in phase I, cytochrome P450 produces an oxygenated intermediate called the oxycytochrome P450 complex [P450 (Fe3+) 02 −] which is highly unstable and is decoupled during NSAID biotransformation, enabling the release of the superoxide anion radical (Doi et al. 2002), a highly oxidant species with the capacity to peroxidize membrane lipids.
In the case of binary mixtures of D with PCM and IBP, significant decreases were observed by comparison to D in isolated form, while binary mixtures of D with NPX and ASA induced significant increases also by comparison to D in isolated form, although these increases never exceeded the algebraic sum of the individual changes found with the two NSAIDs involved in the interaction. Based on this result, it is possible to infer that antagonistic interactions take place between D and the NSAIDs evaluated.
Protein oxidation results in the loss of sulfhydryl groups and rearrangement of the resonance structure of amino acids, affecting protein function and consequently the integrity of the body (Parvez and Raisuddin 2005). Our results show that significant increases in PCC were induced by NSAIDs both singly (PCM, IBP, NPX, and ASA) and as binary mixtures of D with NPX and ASA. The oxidant agent peroxynitrite may have been responsible for such increases since some NSAIDs, after ingestion, are known to come in contact with the vasculature where they acetylate the COX2 enzyme. The latter is present in the endothelium or in circulating leukocytes and produces 15-epi-lipoxin A4, which favors nitric oxide (NO) synthesis mediated by constitutive (eNOS) and inducible (iNOS) nitric oxide synthase (Paul-Clark et al. 2004). When the superoxide anion and NO combine, they form a reactive nitrogen species (RNS), the peroxynitrite radical, via a diffusion-limited reaction (Hule and Padmaja 1993). Peroxynitrite is known to induce protein oxidation and nitration in the absence of GSH, eliciting mitochondrial dysfunction and eventually leading to irreversible damage and severe loss of cellular ATP (Jaeschke et al. 2003).
Protein oxidation differs from the process of oxidation in other macromolecules and may affect both the straight chains and side chains of amino acids. When less reactive species of ROS are involved, this reaction produces specific sulfurate amino acid residues (-SH, -S-CH3) accompanied by changes in protein structure, function, and activity, this oxidation being reversible. On the other hand, more reactive species of ROS form nonreversible products (-CO•) and are usually intracellularly degraded although they sometimes accumulate in cells and tissues (Almroth et al. 2005; Alves de Almeida et al. 2007).
There is little information on NSAID-induced protein damage in aquatic organisms, but exposure of H. azteca for 72 h to PCM and D induced increases in this biomarker (Oviedo-Gómez et al. 2010; Gómez-Oliván et al. 2012). Asensio et al. (2007) note that inhibition of NSAID-induced G6PD elicits damage through protein thiol oxidation.
Our results show that binary mixtures of D with NPX and ASA induced significant increases by comparison to D in isolated form, although in this case as with LPX, the changes induced by the mixtures did not exceed the algebraic sum of the changes induced by each of the NSAIDs in the mixture. Thus, based on these results, an antagonistic interaction may be inferred to have occurred.
Antioxidant activity
Antioxidant defenses can be induced by environmental contaminants under prooxidant conditions. Antioxidant levels initially increase in order to offset oxidative stress, but prolonged exposure leads to their depletion (Bebianno et al. 2005). SOD catalyzes the transformation of superoxide anion (O2⋅) to hydrogen peroxide (H2O2) which is metabolized to O2 and water by CAT and GPx (van der Oost et al. 2003).
The superoxide anion radical is a minor product of mitochondrial respiration (van der Oost et al. 2003) and, in the case of NSAIDs, of the oxidative metabolism of mitochondria. Thus, exposure of H. azteca to NSAIDs in isolated form or as binary mixtures should result in increased SOD activity. Paradoxically, in our study, NSAIDs in isolated form and as binary mixtures induced a significant diminution in this activity in H. azteca with respect to the control group. This decrease may be due to potential saturation of this antioxidant system as a result of the amount of ROS and RNS produced by the oxidative metabolism of H. azteca. Jira et al. (1997) suggest that SOD activity may be inhibited if there is an increase in superoxide anion, H2O2, and peroxynitrite.
In the case of the mixture D-ASA, a significant increase was induced with respect to the control group. Since NSAIDs—including D and ASA—affect the mitochondrion and consequently also oxidative phosphorylation, increased ROS production particularly of O• 2 − may occur, resulting in increased SOD activity and higher levels of hydrogen peroxide (Asensio et al. 2007), as evident in the present study with exposure of H. azteca to these compounds.
A significant increase was observed with binary mixtures of D with IBP and ASA by comparison to D in isolated form. This increase was higher than the algebraic sum of the individual changes induced by each NSAID in the mixture, and it is therefore inferred that a synergistic interaction occurred.
The metalloenzyme CAT is responsible for transforming H2O2 to water and O2 (Chandran et al. 2005; Peraza 2008). In our study, a significant increase in CAT activity was observed with most NSAIDs in isolated form (D, IBP, and NPX) and as binary mixtures of D with PCM, IBP, and NPX. This may be due to an increase in hydrogen peroxide production acting as signal to activate CAT in order to transform this highly toxic radical to less toxic compounds. A similar response has been obtained in other aquatic organisms exposed to other contaminants such as metals and pesticides (Huang et al. 2007). However, it is worth noting that excess production of peroxide not only activates CAT, but also favors NSAID oxidation and production of reactive metabolites, potentially affecting other biomolecules (Galati et al. 2002; Huq 2006).
Binary mixtures of D with IBP and NPX induced significant increases by comparison to D in isolated form. However, these increases never exceeded the sum of the individual changes induced by each of the NSAIDs in the mixture. In the case of D-ASA, CAT activity decreased significantly by comparison to D. In both cases, an antagonistic interaction may have occurred.
Another major enzyme involved in detoxification of peroxides is GPx, which converts H2O2 to water, and lipid peroxides to hydroxides of nonreactive fatty acids (Cárdenas et al. 2008). In our study, GPx activity increased significantly with NPX in isolated form and with the mixture D-NPX. In ASA in isolated form and in the mixture D-ASA, GPx activity decreased. However, no significant changes were observed with any other NSAID or binary mixture. GPx is the most important enzyme for inactivation of extraperoxisomal H2O2. This reaction involves GSH, which is oxidized to GSSG and again reduced by glutathione reductase (GR) in order to be reused, using NADPH as a cofactor (Boelsterli 2007).
GSH, along with some coupled enzyme systems, is one of the major lines of antioxidant defense. This molecule is a tripeptide composed of glutamate, cysteine, and glycine, which is present in the cytosol, mitochondria, and nucleus of cells (except for erythrocytes). Its antioxidant role is associated with three characteristics: it is a radical sequestration agent (nonenzymatically), it favors oxidized protein regeneration, and it is a cosubstrate of GPx and therefore involved in H2O2 degradation (Boelsterli 2007).
GSH is regulated according to body needs and its synthesis is increased in conditions of oxidative stress. However, it can also be decreased: GSH levels are reduced when the molecule is continuously conjugated to reactive electrophiles by exposure to xenobiotics or prooxidant agents that increase oxidative stress (Boelsterli 2007).
Huq (2006) notes that in the biotransformation of NSAIDs such as NPX, both ROS and electrophilic metabolites are produced which can diminish intracellular glutathione levels and, consequently, compromise cell antioxidant status. On the other hand, Galati et al. (2002) found that NSAIDs are also biotransformed by peroxidases. These enzymes use intracellular H2O2 or hydroperoxides to oxidize and produce radicals from NSAIDs. Such radicals may have been responsible for the behavior of this biomarker in our study.
The binary mixture of D-NPX induced significant increases of GPx by comparison to D in isolated form. However, these increases did not exceed the sum of the changes occurring with each of the NSAIDs in the interaction, and therefore, an antagonistic interaction is assumed to have occurred.
Conclusions
The NSAIDs IBP, D, PAR, NPX, and ASA induce oxidative stress in isolated form, while the mixtures D-IBP, D-PAR, D-NPX, and D-ASA induced changes in oxidative stress biomarkers that allow us to assume that interactions between them are fundamentally antagonistic in type. Evaluating the toxicity of this group of pharmaceuticals and their mixtures on aquatic organisms may help predict their potential effects on other organisms in the food chain which are also exposed to these agents.
References
Almroth, B. C., Sturve, J., Berglund, A., & Förlin, L. (2005). Oxidative damage in eelpout (Zoarces viviparous) measured as protein carbonyls and TBARS, as biomarkers. Aquatic Toxicology, 73, 171–180.
Alves de Almeida, E., Dias, B. A., de Melo, L. A., Regina, M. G., Miyamoto, S., Onuki, J., Fujita, B. L., Machado, G. C., Manso, P. F., Ronsein, G., Sigolo, C., Barbosa, B. C., Gracioso, M. A., Gennari de Medeiros, M., & Di Mascio, P. (2007). Oxidative stress in Perna perna and other bivalves as indicators of environmental stress in the Brazilian marine environment: antioxidants, lipid peroxidation and DNA damage. Comparative Biochemistry and Physiology, Part A, 146, 588–600.
Andreozzi, R., Caprio, V., Ciniglia, C., De Champdoré, M., Lo Giudice, R., Marotta, F., & Zuccato, E. (2004). Antibiotics in the environment: occurrence in Italian STPs, fate and preliminary assessment on algal toxicity of amoxicillin. Environmental Toxicology and Chemistry, 38, 6832–6837.
Araujo, L., Villa, N., Camargo, N., Bustos, M., García, T., & Prieto, A. (2011). Persistence of gemfibrozil, naproxen and mefenamic acid in natural waters. Environmental Chemistry Letters, 9, 13–18.
Arkhipova, O.V., Grishin, S.N., Sitdikova, G.F., & Zefirov, A.L. (2005). Presynaptic effects of arachidonic acid and prostaglandin E2 in the frog neuromuscular synapse. Rossiiskii Fiziologicheskii Zhurnal Imeni I.M. Sechenova, 91, 268–276.
Asensio, C., Levoin, N., Guillaume, C., Guerquin, M., Rouguieg, K., Chrétien, F., Chapleur, Y., Netter, P., Minn, A., & Lapicque, F. (2007). Irreversible inhibition of glucose-6-phosphate dehydrogenase by the coenzyme A conjugate of ketoprofen: a key to oxidative stress induced by non-steroidal anti-inflammatory drugs? Biochemical Pharmacology, 73, 405–416.
Backhaus, T., Altenburger, R., Arrhenius, A., Blanck, H., Faust, M., Finizio, A., Gramatica, P., Grote, M., Junghans, M., Meyer, W., Pavan, M., Porsbring, T., Scholze, M., Todeschini, R., Vighi, M., Walter, H., & Grimme, L. H. (2003). The BEAM-project: prediction and assessment of mixture toxicities in the aquatic environment. Continental Shelf Research, 23, 1757–1769.
Barata, C., Varo, I., Navarro, J.C., Arun, S., & Porte, C. (2005). Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds. Comparative Biochemistry and Physiology, 140(C), 175–186.
Bebianno, M. J., Company, R., Serafim, A., Cosson, R. P., & Fiala-Medoni, A. (2005). Antioxidant systems and lipid peroxidation in Bathymodiolusazoricus from Mid-Atlantic Ridge hydrothermal vent fields. Aquatic Toxicology, 75, 354–373.
Boelsterli, U. A. (2007). Mechanistic toxicology. The molecular basis of how chemicals disrupt biological targets (2nd ed.). Boca Ratón: CRC Press.
Borgmann, U., Bennie, D. T., Ball, A. L., & Palabrica, V. (2007). Effect of a mixture of seven pharmaceuticals on Hyalella azteca over multiple generations. Chemosphere, 66, 1278–1283.
Bradford, M. (1976). A rapid and sensitive method for the quantitation of microorganism quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry, 72, 248–254.
Brun, L., Bernier, M., Losier, R., Doe, K., Jackman, P., & Lee, H. (2006). Pharmaceutically active compounds in Atlantic Canadian sewage treatment plant effluents and receiving waters and potential for environmental effects as measured by acute and chronic aquatic toxicity. Environmental Toxicology and Chemistry, 26(Suppl 8), 2163–2176.
Büege, J. A., & Aust, S. D. (1978). Microsomal lipid peroxidation. Methods in Enzymology, 52, 302–310.
Burcham, P. C. (2007). Modified protein carbonyl assay detects oxidised membrane proteins: a new tool for assessing drug- and chemically-induced oxidative cell injury. Journal of Pharmacological and Toxicological Methods, 56, 18–22.
Carballa, M., Omil, F., Lema, J. M., Llompart, M., García-Jares, C., Rodríguez, I., Gómez, M., & Ternes, T. (2004). Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Research, 38, 2918–2926.
Cárdenas, R.N., Medina, C.O., & Pedraza C.J. (2008). Glutation peroxidasas: una familia de enzimas. In F. Konigdberg-Fainstein (Ed.), Radicales libres y estrés oxidativo. Aplicaciones médicas (Capítulo 13: pp. 201–217). Mexico, DF: Manual Moderno.
Cha, Y. I., Solnica-Krezel, L., & DuBois, R. N. (2006). Fishing for prostanoids: deciphering the developmental functions of cyclooxygenase-derived prostaglandins. Developmental Biology, 289, 263–272.
Chandran, R., Sivakumar, A. A., Mohandass, S., & Aruchami, M. (2005). Effect of cadmium and zinc on antioxidant enzyme activity in the gastropod, Achatina fulica. Comparative Biochemistry and Physiology, 140, 422–426.
Cleuvers, M. (2004). Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen and acetylsalicylic acid. Ecotoxicology and Environmental Safety, 59, 309–315.
Commission of the European Communities (1996). Technical guidance document in support of Commission Directive 93/67/EEC on risk assessment for new notified substances and Commission Regulation (EC) No. 1488/94 on risk assessment. Luxembourg: Office for Official Publications of the European Communities.
Deng, A., Himmelsbach, M., Zhu, Q. Z., Frey, S., Sengl, M., Buchberger, W., Niessner, R., & Knopp, D. (2003). Residue analysis of the pharmaceutical diclofenac in different water types using ELISA and GC-MS. Environmental Science & Technology, 37, 3422–3429.
Deridovich, I. I., & Reunova, O. V. (1993). Prostaglandins: reproduction control in bivalve mollusks. Comparative Biochemistry and Physiology, 104, 23–27.
Doi, H., Iwasaki, H., Masubuchi, Y., Nishigaki, R., & Horie, T. (2002). Chemiluminescence associated with the oxidative metabolism of salicylic acid in rat liver microsomes. Chemico-Biological Interactions, 140, 109–119.
Fent, K., Weston, A. A., & Caminada, D. (2006). Ecotoxicology of human pharmaceuticals. Aquatic Toxicology, 76, 122–159.
Fortier, M. A., Krishnaswamy, K., Danyod, G., Boucher-Kovalik, S., & Chapdalaine, P. (2008). A postgenomic integrated view of prostaglandins in reproduction: implications for other body systems. Journal of Physiology and Pharmacology, 59, 65–89.
Galati, G., Tafazoli, S., Sabzevari, O., Chan, T. S., & O’Brien, P. J. (2002). Idiosyncratic NSAID drug induced oxidative stress. Chemico-Biological Interactions, 142, 25–41.
Gómez-Oliván, L. M., Neri-Cruz, N., Galar-Martínez, M., Vieyra-Reyes, P., García-Medina, S., Razo-Estrada, C., Dublán-García, O., & Corral-Avitia, A. Y. (2012). Assessing the oxidative stress induced by paracetamol spiked in artificial sediment on Hyalella azteca. Water, Air, & Soil Pollution, 223(1), 5097–5104.
Gómez-Oliván, L. M., Galar-Martínez, M., García-Medina, S., Valdés-Alanís, A., Islas-Flores, H., & Neri-Cruz, N. (2014). Genotoxic response and oxidative stress induced by diclofenac, ibuprofen and naproxen in Daphnia magna. Drug and Chemical Toxicology. doi:10.3109/01480545.2013.870191. Published on line.
Gunzler, W., & Flohe-Clairborne, A. (1985). Glutathione peroxidase. In R. A. Green-Wald (Ed.), Handbook of methods for oxygen radical research (pp. 285–290). Boca Ratón: CRC Press.
Huang, D. J., Zhang, Y. M., Song, G., Long, J., Liu, J. H., & Ji, W. H. (2007). Contaminants-induced oxidative damage on the carp Cyprinus carpio collected from the Upper Yellow River, China. Environmental Monitoring and Assessment, 128, 483–488.
Hule, R. E., & Padmaja, S. (1993). The reaction rate of nitric oxide with superoxide. Free Radical Research Communications, 18, 195–199.
Huq, F. (2006). Molecular modeling analysis of the metabolism of naproxen. Journal of Pharmacology and Toxicology, 1, 346–353.
Islas-Flores, H., Gómez-Oliván, L. M., Galar-Martínez, M., Colín-Cruz, A., Neri-Cruz, N., & García-Medina, S. (2013). Diclofenac-induced oxidative stress in brain, liver, gill and blood of common carp (Cyprinus carpio). Ecotoxicology and Environmental Safety, 9, 32–38.
Islas-Flores, H., Gómez-Oliván, L. M., Galar-Martínez, M., García-Medina, S., Neri-Cruz, N., & Dublán-García, O. (2014). Effect of ibuprofen exposure on blood, gill, liver, and brain on common carp (Cyprinus carpio) using oxidative stress biomarkers. Environmental Science & Pollution Research. doi:10.1007/s11356-013-2477-0. Published on line.
Jaeschke, H., Knight, T. R., & Bajt, M. L. (2003). The role of oxidant stress and reactive nitrogen species in acetaminophen hepatotoxicity. Toxicology Letters, 144, 279–288.
Jira, W., Spiteller, G., & Richter, A. (1997). Increased levels of lipid oxidation products in low density lipoproteins of patients suffering from rheumatoid arthritis. Chemistry and Physics of Lipids, 87(1), 81–89.
Katzung, B. (2007). Farmacología Básica y Clínica (9th ed.). Mexico: Manual Moderno.
Khetan, S., & Collins, T. J. (2007). Human pharmaceuticals in the aquatic environment: a challenge to green chemistry. Chemical Reviews, 107, 2319–2364.
Levine, R. L., Williams, J. A., Stadtman, E. R., & Shacter, E. (1994). Carbonyl assays for determination of oxidatively modified proteins. Methods in Enzymology, 233, 346–357.
Metcalfe, C. D., Koenig, B. G., Bennie, D. T., Servos, M., Ternes, T. A., & Hirsch, R. (2003). Occurrence of neutral and acidic drugs in the effluents of Canadian sewage treatment plants. Environmental Toxicology & Chemistry, 22(12), 2872–2880.
Misra, H. P., & Fridovich, I. (1972). The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Journal of Biological Chemistry, 247, 3170–3175.
Montforts, M., Brandt, I., & Hutchinson, T. (2007). Summary of workshop on environmental assessment of human medicines: development and use of aquatic toxicity data. Drug Information Journal, 41, 200–203.
Mycek, M. J., Harvey, R. A., & Champe, P. C. (2004). Farmacología (pp. 499–518). Mexico: McGraw-Hill.
Oviedo-Gómez, D., Galar-Martínez, M., García-Medina, S., Razo-Estrada, C., & Gómez-Oliván, L. (2010). Diclofenac-enriched artificial sediment induces oxidative stress in Hyalella azteca. Environmental Toxicology and Pharmacology, 29, 39–43.
Parolini, M., Binelli, A., Cogni, D., Riva, C., & Provini, A. (2009). An in vitro biomarker approach for the evaluation of the ecotoxicity of non-steroidal anti-inflammatory drugs (NSAIDs). Toxicology In Vitro, 23(5), 935–942.
Parvez, S., & Raisuddin, S. (2005). Protein carbonyls: novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish Channa punctata (Bloch). Environmental Toxicology and Pharmacology, 20, 112–117.
Paul-Clark, M. J., van Cao, T., Moradi-Bidhendi, N., Cooper, D., & Gilroy, D. W. (2004). 15-Epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. Journal of Experimental Medicine, 200, 69–78.
Peraza, R. L. (2008). Catalasa. In F. Konigdberg (Ed.), Radicales libres y estrés oxidativo. Aplicaciones médicas (pp. 183–200). Mexico: Manual Moderno.
Pritchard, J. B. (1993). Aquatic toxicology—past, present, and prospects. Environmental Health Perspectives, 100, 249–257.
Quintana, J. B., Weiss, S., & Reemtsma, T. (2005). Pathways and metabolites of microbial degradation of selected acidic pharmaceuticals and their occurrence in municipal wastewater treated by a membrane bioreactor. Water Research, 39, 2654–2664.
Radi, R., Turrens, J. F., Chang, L. Y., Bush, K. M., Carpo, J. D., & Freeman, B. A. (1991). Detection of catalase in rat heart mitochondria. Journal of Biological Chemistry, 266, 22028–22034.
Siemens, J., Huscheka, G., Siebeb, C., & Kaupenjohann, M. (2008). Concentrations and mobility of human pharmaceuticals in the world’s largest wastewater irrigation system, Mexico City–Mezquital Valley. Water Research, 42, 2124–2134.
Silva, E., Rajapakse, N., & Kortenkamp, A. (2002). Something from “nothing”—eight weak chemicals combined at concentrations below NOEC’s produce significant mixture effects. Environmental Science and Technology, 36(8), 1751–1756.
Spindler, P., Montforts, M., Olejniczak, K., & Koschorreck, J. (2007). Environmental assessment for human medicines in the European Union. Drug Information, 41, 149.
Stanley, D. W. (2000). Eicosanoids in invertebrate signal transduction systems (pp. 231–234). Princeton: Princeton University Press.
Stephensen, E., Svavarsson, J., Sturve, J., Ericson, G., Adolfsson-Erici, M., & Förlin, L. (2000). Biochemical indicators of pollution exposure in shorthorn sculpin (Myoxocephalus scorpius), caught in four harbours on the southwest coast of Iceland. Aquatic Toxicology, 48(4), 431–442.
Tauxe-Wuersch, A., De Alencastro, L. F., Grandjean, D., & Tarradellas, J. (2005). Occurrence of several acidic drugs in sewage treatment plants in Switzerland and risk assessment. Water Research, 39, 1761–1772.
Ternes, T. A. (1998). Occurrence of drugs in German sewage treatment plants and rivers. Water Research, 32, 3245–3260.
Ternes, T. A., Stumpf, M., Muller, J., Haberer, K., Wilken, R. D., & Servos, M. (1999). Behavior and occurrence of estrogens in municipal sewage treatment plants I. Investigations in Germany, Canada and Brazil. Science of the Total Environment, 225, 81–90.
Van der Oost, R., Beyer, J., & Vermeulen, N. P. E. (2003). Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology, 13, 57–149.
Vlahogianni, T., Dassenakis, M., Scoullos, M. J., & Valavanidis, A. (2007). Integrated use of biomarkers (superoxide dismutase, catalase and lipid peroxidation) in mussels Mytilus galloprovincialis for assessing heavy metals’ pollution in coastal areas from the Saronikos Gulf of Greece. Marine Pollution Bulletin, 54, 1361–1371.
Acknowledgments
This study was made possible by support from the Consejo Nacional de Ciencia y Tecnología (CONACyT-Mexico, Project 151665).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez-Oliván, L.M., Neri-Cruz, N., Galar-Martínez, M. et al. Binary mixtures of diclofenac with paracetamol, ibuprofen, naproxen, and acetylsalicylic acid and these pharmaceuticals in isolated form induce oxidative stress on Hyalella azteca . Environ Monit Assess 186, 7259–7271 (2014). https://doi.org/10.1007/s10661-014-3925-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3925-0