Abstract
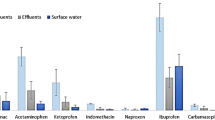
Nonsteroidal anti-inflammatory drugs (NSAIDs) are a group of molecules representing one of the most relevant therapeutic class found in the aquatic ecosystems worldwide. NSAIDs are commonly and extensively used for their analgesic, antipyretic and anti-inflammatory properties to cure pain and inflammation in both human and veterinary therapy. Because of the huge, continuative and increasing use, as well as their specific pharmacokinetic properties, after medical use they are excreted in their native form or as metabolites and enter the aquatic ecosystems. A number of monitoring surveys has reported levels of NSAIDs exceeding 1 μg/L in influent and effluents of Wastewater Treatment Plants (WWTPs), while lower concentrations have been found in surface waters, ranging in the ng/L – μg/L range. Among NSAIDs, paracetamol, diclofenac, and ibuprofen are the most detected therapeutics found in aquatic ecosystems. Although the concentrations of these molecules in surface waters are quite low, their high biological activity might confer them a potential toxicity towards non-target aquatic organisms. The present chapter aims at reviewing the adverse effects induced by paracetamol, diclofenac, and ibuprofen towards different freshwater invertebrates belonging to different taxa. Although acute toxicity of paracetamol, diclofenac, and ibuprofen occur only at high, unrealistic concentrations, sublethal effects were caused by low, environmentally relevant concentrations of these drugs. For these reasons, further studies represent a priority in order to enlarge the knowledge on NSAID toxicity towards aquatic organisms and to shed light on their real ecological hazard towards aquatic communities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 NSAIDs in Freshwater Ecosystems
In the last two decades, pharmaceuticals have been identified as emerging contaminants for aquatic ecosystems. Emerging contaminants are synthetic or natural compounds that has recently been found in natural ecosystems and for which environmental or public health risks are limited or yet to be established. These molecules are not routinely monitored, and, even if their environmental concentrations are low, they are suspected to cause adverse effects towards ecosystems [1, 2]. The presence of pharmaceutical compounds in aquatic ecosystems represents one of the main concerns that ecotoxicology has to face in recent years [3,4,5]. Pharmaceuticals are extensively and increasingly being used both in human and veterinary medicine, as well as in agriculture and aquaculture [5]. After the use, these molecules are excreted in their native form or as active metabolites entering the sewage, which has been individuated as the main spreading pharmaceuticals after therapeutic use or improper disposal of unused medicines to the environment. As traditional wastewater treatment plants (WWTPs) have a limited efficiency for the removal of several therapeutic drugs, these molecules are discharged in WWTP effluents in unneglectable concentrations, resulting in contamination of surface waters and, rarely, groundwater and drinking water [4]. Moreover, sewage sludge originated from WWTPs and manure from zootechnical breeding farms have been identified as a secondary source of pharmaceuticals, contributing to aquatic contamination as a consequence of their use in agriculture and the subsequent runoff. Pharmaceuticals have been designed to have a specific mode of action, targeting specific organs, metabolic pathways, or receptors in order to modulate physiological functions of the orgnism, to treat a desease and to restore the health of the organism. Thus, because of their usefulness, pharmaceuticals play a pivotal role in our society and are commonly used, and often abused, worldwide. For instance, in the European Union (EU) alone, it has been estimated that about 3,000 different substances are commonly used in human therapy such as anti-inflammatory drugs, contraceptives, antibiotics, β-blockers, lipid regulators, neuroactive compounds, and many others [3]. Similarly, a large number of these molecules are used also in veterinary applications. Following the trend of production and use, several therapeutics commonly used in human and veterinary therapy as contraceptives, β-blockers, antiepileptic, anti-inflammatory, antidepressants, or antibiotics have been found at concentrations ranging from a few ng/L to few mg/L in wastewater, surface water, and groundwater worldwide [4, 6]. Although the environmental concentrations measured in aquatic ecosystems are often quite low, pharmaceuticals are designed to be biologically active at low concentrations; for this reason pharmaceuticals revealed in environment might represent a potential risk for chronically exposed, non-target organisms [3, 5]. Considering the potential hazard of pharmaceuticals towards ecosystems some international actions have been planned. For instance, the European Union has included 17α-ethinylestradiol, 17β-estradiol, and diclofenac to the list of the Water Framework Directive (2013/39/EU) as priority molecules to be monitored in aquatic ecosystems.
Nonsteroidal anti-inflammatory drugs (NSAIDs) represent one of the most relevant therapeutic class found in the aquatic ecosystems. NSAIDs are largely used for their analgesic, antipyretic, and anti-inflammatory properties to cure pain and inflammation. They inhibit the synthesis and the release of prostaglandins from arachidonic acid, acting as non-selective inhibitors of cyclooxygenase enzymes, including both the cyclooxygenase-1 (COX-1) and the cyclooxygenase-2 (COX-2) isoforms [7]. Different NSAIDs have been prescribed extensively throughout the world. For instance, more than 70 million prescriptions are written each year in the United States, while considering the over-the-counter use, more than 30 billion NSAID doses are consumed annually in the United States alone [8]. Because of the huge, continuative and increasing use, as well as their specific pharmacokinetic properties, NSAIDs can reach detectable concentrations both in sewage and in surface water [9], accounting for 15% of pharmaceuticals measured in aquatic ecosystems worldwide [4]. Diverse monitoring surveys have reported levels of NSAIDs exceeding 1 μg/L in influent and effluents of WWTPs, while lower concentrations have been found in surface waters [4, 10]. Among NSAIDs, paracetamol, diclofenac, and ibuprofen are the most detected therapeutics found in aquatic ecosystems [4].
Paracetamol (PCM; N-(4-hydroxyphenyl)acetamide) is an analgesic and antipyretic agent. Although PCM does not own a proper anti-inflammatory action, it is usually considered in the NSAID group by a toxicological point of view because of its mode of action, similar to that of NSAIDs [11]. As PCM is considered a safe drug at therapeutic doses, it can be purchased as an over-the-counter drug in most countries. According to its extensive use, PCM is one of the most frequently detected pharmaceuticals in surface waters, wastewaters, and drinking water. For instance, Kolpin et al. [12] detected PCM in 24% of samples during a survey performed in 139 US streams, at a median concentration of 0.11 μg/L, with concentrations up to 10 μg/L. The median concentration of PCM measured in surface waters worldwide was 0.055 ± 0.051 μg/L [13, 14], while in wastewaters PCM was detected at a median concentration of 48 ± 75 μg/L [14, 15].
Diclofenac (DCF; 2-[(2,6-dichlorophenyl)amino] phenylacetic acid) is a phenylacetic acid NSAID used to reduce inflammation and pain associated with arthritis, osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis [16]. According to its huge over-the-counter sale, coupled with the great number of medical prescriptions, DCF has been identified as one of the main pharmaceuticals contaminating the aquatic ecosystems. Dermal application results the main source of DCF in water [17]; in fact, because of the relative small absorption on skin (5–10%), the most of the pharmaceutical is released to water by washing [18]. Moreover, as traditional WWTPs have a limited efficiency of DCF removal, this drug was commonly detected at low μg/L range in WWTP effluents of Europe and North and South America [19, 20]. Accordingly, DCF was commonly detected also in surface waters, in concentrations ranging between low ng/L up to low μg/L [10,11,12,13,14]. Despite these findings, the information of the environmental fate and the adverse effects of DCF towards non-target aquatic organisms is still limited.
Ibuprofen (IBU; ((+/−)-2-(p-isobutylphenyl) propionic acid with R and S isomers) is used to relieve the symptoms of arthritis, rheumatic disorders, pain, and fever [21]. IBU represents one of the core pharmaceuticals included in the “Essential Drug List” of the World Health Organization (WHO), and it is therefore produced in large amounts worldwide [22]. Because of its huge over-the-counter sale, large prescription volume, and high excretion rate (estimated as 70–80% of the ingested therapeutic dose), IBU has been identified as one of the main pharmaceuticals in aquatic ecosystems. Moreover, IBU has relatively high mobility into aquatic environments but a lower persistence in comparison with other pharmaceuticals [23]. IBU has been detected in moderate concentrations (up to tens of μg/L) both in the effluents of WWTPs and in surface waters during surveys carried out in both Europe and North America [10, 12, 24].
Although the occurrence of low to moderate concentrations of paracetamol, diclofenac, and ibuprofen has been demonstrated in aquatic ecosystems worldwide, the information concerning their potential toxicity towards non-target aquatic organisms is still limited. For this reason, the aim of the present study is to review the adverse effects induced by the exposure to paracetamol, diclofenac, and ibuprofen towards freshwater invertebrates that was performed in order to shed light on the potential hazard of these pharmaceutical compounds towards non-target organisms and to lay the foundations for further ecotoxicological investigations.
2 Adverse Effects of NSAIDs of Freshwater Organisms
The studies of the adverse effects induced by the exposure to paracetamol, diclofenac, and ibuprofen towards freshwater invertebrates have been performed on different model species belonging to different taxa, from algae to mussels. Thus, the effects induced to different organisms by the exposure to each single molecule are discussed in the paragraphs below.
2.1 Effects Induced by Paracetamol Exposure
The toxicity of paracetamol (PCM) towards non-target, freshwater invertebrates has been investigated on algae (Pseudokirchneriella subcapitata), cyanobacteria (Cylindrospermopsis raciborskii), cnidarian (Hydra vulgaris), rotifers (Plationus patulus), crustaceans (Daphnia magna, Daphnia longispina, and Moina macrocopa), bivalves (Dreissena polymorpha and Corbicula fluminea), as well as plants (Lemna minor and Lemna gibba) (Table 1).
Acute toxicity of PCM to D. magna was calculated as 5.32 ± 0.73 mg/L [25]. A study performed by Nunes and coauthors [26] investigated the toxicity of paracetamol towards different freshwater species, from algae to plants. This study assessed the growth inhibition of the microalga P. subcapitata after the exposure for 72 h to seven PCM concentrations, ranging from 87.8 and 1,000 mg/L and the growth inhibition of the cyanobacterium C. raciborskii exposed to eight paracetamol concentrations, ranging from 48.4 to 510.2 mg/L. Moreover, acute and chronic toxicity of PCM was assessed in the crustaceans D. magna and D. longispina. Acute toxicity of PCM towards D. magna and D. longispina was assessed through static exposures to five (range 48.6–85 mg/L) and eight (range 4.0–8.9 mg/L) PCM concentrations, respectively. Chronic toxicity was assessed by a reproduction test exposing D. longispina 7.9, 11.8, 17.8, 26.7, 40.0, and 60.0 mg/L, while D. magna to 0.53, 0.79, 1.2, 1.7, 2.7, and 4.0 mg/L of PCM. Lastly, acute toxicity of increasing PCM concentrations (five concentrations ranging between 62.5 and 1,000 mg/L) towards L. minor and L. gibba was investigated. Paracetamol toxicity was widely variable among species, even among phylogenetically related ones. Paracetamol was toxic to all test organisms in the tested concentration range, with the exception of L. gibba, whereby no acute effects occurred also at concentrations up to 1,000 mg/L. Considering acute toxicity in terms of EC50, the scale of toxicity, from the most sensitive to the most tolerant model organism, was the following: D. magna < D. longispina < C. raciborskii < P. subcapitata < L. minor < L. gibba. PCM caused mortality in the reproduction test with D. magna at the highest tested concentrations (between 1.2 and 1.7 mg/L), so that no organisms survived over the whole duration of the experiment, although they generated offspring. Differently, D. longispina showed a significant delay in the first reproductive event and a reduction in the fecundity. A study by Sarma and coauthors [27] exposed the rotifer Plationus patulus and the cladoceran Moina macrocopa to increasing concentrations of PCM (2, 4, 8, 16, and 32 mg/L) in order to assess changes in population growth. Population growth curves of both the species were affected by the exposure to increasing concentrations of PCM, showing a decrease in organism density with increasing levels of drug. Moreover, the daily rate of population increase was negatively and significantly affected by PCM exposure in both the zooplanktonic species. A 7-day exposure to 10, 100 μg/L, 1.0 and 10 mg/L of PCM did not affect the survival of Hydra vulgaris specimens at concentrations up to 1.0 mg/L, while after 17 days neither feeding nor bud formation was adversely affected. Moreover, the ability of dissected polyps to regenerate a hypostome, tentacles, and foot was not inhibited [28]. Biochemical effects of PCM exposure were investigated in the freshwater clam Corbicula fluminea following short- (96 h) and long-term (28 days) exposures to 0.05, 0.48, 4.82, and 532.78 mg/L of PCM and 3.88, 7.74, 15.49, 30.98, and 61.95 μg/L of PCM, respectively [29]. Effects of PCM exposure on some oxidative stress endpoints, namely, catalase (CAT), glutathione S-transferases (GSTs), glutathione reductase (GRed), and lipid peroxidation were investigated. No mortality was observed in clams over short- or long-term exposures. PCM did not modulate CAT activity but induced a significant decrease of GSTs activity following both short- and long-term exposure (LOEC values of 532.78 mg/L and 30.98 μg/L, respectively). Moreover, PCM treatment induced a significant dose-dependent decrease of GRed activity in both short- and long-term exposures. A significant increase of lipid peroxidation was noted at the end of short- and long-term exposure to the highest PCM tested concentrations. These results indicated that the exposure to increasing PCM concentration caused notable changes in the cellular redox status of C. fluminea. The cytogenotoxicity of PCM was investigated through an in vitro approach by exposing the hemocytes collected from the zebra mussel D. polymorpha for 1 h to 30, 150, and 450 μg/L [30]. Cytotoxicity was evaluated by the neutral red retention assay (NRRA) while genotoxicity by SCGE (single cell gel electrophoresis) and DNA diffusion assay. Significant cytotoxic and genotoxic effects were after the exposures to all the tested concentrations according to a dose-dependent relationship. PCM exposure induced significant alterations of the oxidative status of the zebra mussel D. polymorpha [31]. Zebra mussels were exposed for 96 h to three PCM concentrations (0.154, 0.75, and 1.51 μg/L), and cytogenotoxicity was assessed in mussel hemocytes through the application of a suite of eight different biomarkers, namely, the lysosomal membrane stability (neutral red retention assay), the single cell gel electrophoresis (SCGE) assay, the micronucleus test (MN test), and assessments of the apoptotic frequency (DNA diffusion assay). The alteration of mussel oxidative status was assessed by measuring the activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and the detoxifying enzyme glutathione S-transferase (GST). No mortality of zebra mussel over the test or changes in hemocyte viability were induced by PCM exposure. Although PCM did not induce primary genetic damage in zebra mussel hemocytes at all the tested concentration, a significant increase of fixed genetic damage, in terms of both micronuclei and apoptotic frequency, was noted at the end of the exposure to the highest tested concentrations. Moreover, a significant destabilization of lysosomal membranes and significant modulation of CAT, GPx, and GST activity was induced by the exposure to high PCM concentrations. All these data suggested that the exposure to environmental concentrations of PCM might modulate the oxidative status of freshwater invertebrates, leading to oxidative stress situation and genetic damage.
2.2 Effects Induced by Diclofenac Exposure
Acute and chronic toxicity of diclofenac (DCF) towards non-target, freshwater invertebrates has been investigated on rotifers (Plationus patulus), crustaceans (Daphnia magna and Moina macrocopa), diptera (Chironomus riparius), bivalves (Dreissena polymorpha), and gastropods (Lymnaea stagnalis) (Table 2).
Complete mortality of D. magna specimens was caused after only 24 h exposure to high levels of DCF (486 mg/L). DFC exposure caused 50% mortality in D. magna after 21 days exposure to 2.00 ± 0.30 mg/L and a significant reduction of egg production at the lowest exposure concentrations of 0.50 mg/L [25]. A study by de Oliveira and coauthors [32] calculated a diclofenac (EC50 = 123.3 mg/L) in D. magna, but no effect on population increase was noted after the exposure to increasing DCF concentrations (range 29.5–75 mg/L). Toxic effects of 21 days exposure to DCF (5, 50, 500, and 5,000 μg/L) on survival, growth rate, and reproduction, as well effects on the expression of the genes related to the detoxification metabolism, growth, development, and reproduction, such as HR96, P-gp, CYP360A8, CYP314, GST, EcR, and Vtg after 96 h exposure, were investigated in D. magna specimens [33]. Significant toxic effects of DCF to D. magna were observed at 50 μg/L, whereby the expression of the selected genes was inhibited after 24 h of exposure, while they were increased after 48 h. Despite modulation of gene expression, no significant effects were observed in molting frequency, number of eggs produced in the first brood, total number of eggs per individual, total number of broods per individual, body length, and growth rate. In contrast, the exposure to increasing concentrations of DCF (2, 4, 8, 16, and 32 mg/L) affected the population growth curves of the rotifer Plationus patulus and the cladoceran Moina macrocopa, leading to a decrease in organism density with increasing levels of drug, as well as a negative effect on the daily rate of population increase [27]. Another research investigated the toxicity of DCF at biochemical level in D. magna by assessing the modulation of hsp70 level as a biomarker for proteotoxicity [34]. Hsp70 induction occurred at high levels of DCF, as the LOEC was calculated at 40 mg/L. The cytogenotoxicity of DCF was investigated through an in vitro approach by exposing hemocytes from the zebra mussel D. polymorpha for 1 h to 60, 126, and 250 μg/L [30]. Cytotoxicity was evaluated by the neutral red retention assay (NRRA) while genotoxicity by SCGE (single cell gel electrophoresis) and DNA diffusion assay. A significant cytotoxic effect was noted only after the exposure to 250 mg/L of DCF, while genotoxicity occurred after the exposures to all the tested concentrations. A further in vitro experiment [35] investigated the toxicity of increasing DCF concentrations (0.001, 0.01, 0.1, 1, and 10 mg/L) on three different cell typologies from the zebra mussel (Dreissena polymorpha), namely, hemocytes, gill, and digestive gland cells. At the end of the exposure (i.e., 96 h), viability of DCF treated gill cells was significantly reduced already at the lowest concentration with respect to baseline levels. Viability of DCF-treated digestive gland cells was significantly reduced already after 48 h exposure to 0.01 mg/L, while hemocyte viability was affected already at the lowest concentration (0.001 mg/L). Zebra mussels specimens were exposed for 96 h to increasing concentrations (95, 318, and 637 ng/L) of DCF through an in vivo approach [36]. Cytogenotoxicity was assessed by means of the single cell gel electrophoresis assay, the apoptotic frequency, the micronucleus test (MN test), and the lysosomal membrane stability (neutral red retention assay) in mussel hemocytes. Moreover, the activity of catalase, superoxide dismutase, glutathione peroxidase, and the phase II detoxifying enzyme glutathione S-transferase was measured as oxidative stress biomarkers. Negligible cyto- and genotoxicity of DCF was noted towards the zebra mussel hemocytes; in fact only a slight decrease of lysosomal membrane stability was observed at the end of exposure to the highest tested concentration (637 ng/L). DCF toxicity of gastropods was assessed by exposing Lymnaea stagnalis specimens for 3 days to environmental realistic (1–10 μg/L) and therapeutic concentrations (100–1,000 μg/L) of DCF [37]. Effects on immune parameters of individual snails were measured, namely, hemocyte density and viability, hemocyte phagocytosis capacity, and hemocyte-related oxidative activities (basal and NADPH-oxidase). Diclofenac induced immune responses, while no immunosuppression was observed. DCF significantly affected the immunocapacity and the immunoefficiency of the snails’ hemocytes. This effect is typical of an inflammatory response, confirmed by the increase of the NADPH-oxidase activity, mainly at 1,000 μg/L. The effects of exposure to DCF towards the Chironomus riparius was assessed through an experiment using spiked sediment [38]. A 10-day chronic toxicity test with C. riparius was performed to assess effects on survival, growth, and developmental stage, in terms of biomass, as well as emergence rates and sex ratio after 21 days of exposure. No effects on survival and no change in the sex ratio was induced by DCF exposure. In contrast, DCF decreased the emergence ratio in organisms exposed at concentrations of 34.0 μg/g of DCF.
2.3 Effects Induced by Ibuprofen Exposure
Acute and chronic toxicity of ibuprofen (IBU) towards non-target, freshwater invertebrates has been investigated on crustaceans (Daphnia magna), cnidarian (Hydra vulgaris), bivalves (Dreissena polymorpha and Corbicula fluminea), and gastropods (Planorbis carinatus) (Table 3).
Acute toxicity on D. magna occurred at lower concentrations compared to DCF. In fact, complete mortality of D. magna specimens was caused after only 24-h exposure to high levels of IBU (200 mg/L), while EC50 was calculated as 3.97 ± 0.43 mg/L [25]. A 14-day exposure of D. magna to IBU (concentration range 20, 40, and 80 mg/L) measuring chronic effects on life history traits and population performance was performed by Heckmann and coauthors [22]. Population growth rate was significantly reduced at all the IBU tested concentrations, while D. magna survival was affected only by the exposure to 80 mg/L of IBU. Reproduction was influenced by the exposure to low concentrations of IBU, whereby the 14-day EC50 was calculated as 13.4 mg/L but was utterly inhibited at 80 mg/L. Similar results were obtained by Hayashi and coauthors [21], who exposed D. magna (5-days old) to the same range of IBU concentrations than [22] (i.e., 20, 40 and 80 mg/L) for 10 days. Individuals exposed to higher concentrations produced significantly fewer offspring than controls, while no reproduction occurred at 80 mg/L. Moreover, at first reproduction was delayed at all the tested IBU concentrations. D. magna survival was affected after the exposure to 80 mg/L during the 10-day exposure, while the population growth rates were >1 after the exposure to control, 20 and 40 mg/L of IBU, suggesting and increasing population, <1 at 80 mg/L of IBU, suggesting a decreasing population trend [39]. A recent study by Wang and coauthors [39] investigated the modulation of the expression of CYP360A, CYP314, and GST genes involved in the detoxification process and the responses of their associated enzymes activity, as well as in some physiological parameters (e.g., growth and reproduction) in D. magna exposed to environmentally relevant concentrations of IBU (0.5, 5, and 50 μg/L). IBU did not affect the total amount of eggs produced per female, total number of brood per female, and body length of D. magna specimens. By a molecular and biochemical point of view, IBU treatment inhibited the expression of CYP360A gene at 0.5 μg/L while induced its expression at 50 μg/L. Similar trend was also noted for GST gene, while the gene CYP314 showed an inhibition after short time exposure (6 h). Conversely, the gene CYP314 showed an overexpression after prolonged exposure time (48 h at 0.5 μg/L). Erythromycin N-demethylase (ERND) and aminopyrine N-demethylase were both inhibited after short time exposure (6 h). However, they were both overexpressed after prolonged exposure time (48 h) at 0.5 μg/L. Moreover, an induction of glutathione S-transferase (GST), superoxide dismutase (SOD), and catalase (CAT) activity was observed in short-term exposure to IBU, while EROD and methane dicarboxylic aldehyde (MDA) content increased in a dose-dependent manner [41]. A 7-day exposure to 10, 100 μg/L, 1.0, and 10 mg/L of IBU did not influence the survival of H. vulgaris at concentrations up to 1.0 mg/L, while after 17 days neither feeding nor bud formation nor the ability of dissected polyps to regenerate a hypostome, tentacles, and foot was affected [28]. However, a further study showed that regeneration was significantly inhibited at 5 mg/L of IBU, while the 96-h IC50 (i.e., the concentration that inhibits 50% of the embryos to develop) was calculated as 3.84 mg/L (confidence interval 2.36–6.26 mg/L) [44]. IBU exposure also induced sublethal effects towards mollusks. The cytogenotoxicity of IBU was investigated through an in vitro approach by exposing zebra mussel hemocytes for 1 h to 45, 450, and 909 μg/L [34]. A significant decrease in the stability of lysosomal membranes was noted after the exposure to 450 and 909 μg/L of IBU, while genotoxicity occurred after the exposures to all the tested concentrations. A further in vivo exposure of the zebra mussel showed that the 96 h treatment with 0.2, 2, and 8 mg/L of IBU induced a slight cytogenotoxicity (i.e., NRRA, SCGE assay, apoptosis, and MN test) on hemocytes at the IBU concentration of 0.2 mg/L, while higher IBU concentrations (2 and 8 mg/L) cause a significant increase of both cellular and primary and fixed genetic damage. In addition, IBU significantly altered the activity of antioxidant and detoxifying enzymes at all the tested concentrations, suggesting the imbalance of oxidative status and a possible onset of oxidative stress [41]. A study performed on the zebra mussel exposed for 7 days to increasing IBU concentrations (0.206, 2.06, 20.6, and 206.3 μg/L) investigated the effects of this NSAIDs at molecular level, assessing the mRNA changes of enzymes and other proteins involved in the prevention of protein damage (heat shock protein 70) and oxidative stress (superoxide dismutase, catalase, metallothionein), biotransformation (glutathione S-transferase, aryl hydrocarbon receptor), elimination (P-glycoprotein), and reversible protein posttranslational modification (protein phosphatase 2A). Mussels exposed to the lowest tested concentrations of IBU experienced an oxidative stress situation as showed by induced mRNA levels in the digestive gland of mussels recorded for catalase and metallothionein, as well as superoxide dismutase, after 1 and 4 days of exposure, respectively. At higher concentrations, an increase in transcript levels of glutathione S-transferase occurred, suggesting the activation of biotransformation processes of IBU or by-products deriving from oxidative stress [40]. Moreover, responses induced by 21-days exposure to increasing IBU concentrations (0.1, 1.5, 10, 15, 50 μg/L), in terms of general stress (lysosomal membrane stability), biomarkers of phase I and II (ethoxyresorufin-O-deethylase, dibenzylfluorescein dealkylase, glutathione S-transferase), oxidative stress (glutathione reductase, glutathione peroxidase, lipid peroxidation), and DNA damage were investigated in the clam Corbicula fluminea. IBU induced a destabilization of lysosomal membrane at all the tested concentrations. Moreover, IBU activated both phase I and II enzymes, including glutathione reductase and glutathione peroxidase, at the highest tested concentration (50 μg/L). Moreover, an increase of lipid peroxidation, but not of DNA damage, was observed at the end of the exposure to 50 μg/L [42]. Individuals of the freshwater Keeled rams horn snails (Planorbis carinatus) were exposed for 72 h to 0.1, 1.0, 10, and 100 mg/L of IBU and to 0.32, 1.0, 3.2, and 10 mg/L of IBU for 21 days. The 48 and 72 h LC50 values were both 17.1 mg/L (95% confidence intervals 5.9–72.3 mg/L), while the 21 days LOEC and NOEC based on individual survival were calculated as 45.36 and 5.36 mg/L, respectively. The 21-day LOEC and NOEC calculated for snail reproduction (i.e., hatching success) were 5.36 and 2.43 mg/L, respectively, while the LOEC and NOEC calculated for growth were 2.43 and 1.02 mg/L, respectively [43].
3 Conclusions
The results reported in the present review show that three of the most common NSAIDs found in the aquatic ecosystems worldwide might represent a serious hazard towards non-target, freshwater invertebrates. In fact, although acute toxicity of PCM, DCF, and IBU occurs only at high concentrations, much higher than those measured in freshwaters, sublethal effects due to chronic exposures cannot be neglected. In fact, studies performed on different model species belonging to different taxa showed that the exposure to low, environmentally relevant concentrations of PCM, DCF, and IBU can induce notable adverse effects at molecular, biochemical, and cellular level, while effects at individual level (e.g., growth, survival, reproduction) seem to be improbable. The sublethal effects pointed out by short- and mid-term exposures might be also more worrisome considering that freshwater invertebrates are exposed to NSAID concentrations for their whole lifespan. In addition, considering the increasing production and use of NSAIDs might lead to a notable increase in freshwater environmental levels and, consequently, to an enhancement of the hazard of these pharmaceuticals towards non-target, freshwater invertebrates. For these reasons, further studies should be needed to enlarge the knowledge on NSAID toxicity towards aquatic organisms, considering long-term exposures and the use of alternative and innovative assays to shed light on the mechanisms of action of these pharmaceuticals. Lastly, considering that NSAIDs occur in aquatic ecosystems in complex “cocktails,” studies of toxicity of NSAID mixture toxicity should be a priority in environmental risk assessment for this molecules in order to explore their real ecological hazard towards aquatic communities.
References
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107:907–938
Ellis JB (2006) Pharmaceutical and personal care products (PPCPs) in urban receiving waters. Environ Pollut 144(1):184–189
Fent K, Weston AA, Caminada D (2006) Ecotoxicology of human pharmaceuticals. Aquat Toxicol 76(2):122–159
Santos LH, Araújo AN, Fachini A, Pena A, Delerue-Matos C, Montenegro MCBSM (2010) Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J Hazard Mater 175(1-3):45–95
Boxall AB, Rudd MA, Brooks BW, Caldwell DJ, Choi K, Hickmann S, Innes E, Ostapyk K, Staveley JP, Verslycke T, Ankley GT (2012) Pharmaceuticals and personal care products in the environment: what are the big questions? Environ Health Perspect 120(9):1221–1229
Al Aukidy M, Verlicchi P, Voulvoulis N (2014) A framework for the assessment of the environmental risk posed by pharmaceuticals originating from hospital effluents. Sci Total Environ 493:54–64
Gierse JK, Hauser SD, Creely DP, Koboldt C, Rangwala SH, Isakson PC, Seibert K (1995) Expression and selective inhibition of the constitutive and inducible forms of human cyclo-oxygenase. Biochem J 305(2):479–484
Wiegand TJ, Vernetti CM (2017) Nonsteroidal anti-inflammatory drug (NSAID) toxicity. https://emedicine.medscape.com/article/816117-overview. Accessed 14 Nov 2019
Cleuvers M (2004) Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol Environ Saf 59(3):309–315
Metcalfe C, Miao XS, Hua W, Letcher R, Servos M (2004) Pharmaceuticals in the Canadian environment. In: Pharmaceuticals in the environment. Springer, Berlin, pp 67–90
Misra R, Pandey H, Chandra M, Agarwal PK, Pandeya SN (1990) Effects of commonly used nonsteroidal anti-inflammatory drugs on gastric mucosa. A clinical, endoscopic and histopathological study. J Assoc Physicians India 38(9):636–638
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999− 2000: A national reconnaissance. Environ Sci Technol 36(6):1202–1211
Bound JP, Voulvoulis N (2006) Predicted and measured concentrations for selected pharmaceuticals in UK rivers: implications for risk assessment. Water Res 40(15):2885–2892
Gros M, Petrović M, Barceló D (2006) Multi-residue analytical methods using LC-tandem MS for the determination of pharmaceuticals in environmental and wastewater samples: a review. Anal Bioanal Chem 386(4):941–952
Han GH, Hur HG, Kim SD (2006) Ecotoxicological risk of pharmaceuticals from wastewater treatment plants in Korea: occurrence and toxicity to Daphnia magna. Environ Toxicol Chem Int J 25(1):265–271
Todd PA, Sorkin EM (1988) Diclofenac sodium. Drugs 35(3):244–285
Heberer T, Feldmann D (2005) Contribution of effluents from hospitals and private households to the total loads of diclofenac and carbamazepine in municipal sewage effluents – modeling versus measurements. J Hazard Mater 122(3):211–218
Letzel M, Metzner G, Letzel T (2009) Exposure assessment of the pharmaceutical diclofenac based on long-term measurements of the aquatic input. Environ Int 35(2):363–368
Roberts PH, Thomas KV (2006) The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci Total Environ 356(1-3):143–153
Gómez MJ, Bueno MM, Lacorte S, Fernández-Alba AR, Agüera A (2007) Pilot survey monitoring pharmaceuticals and related compounds in a sewage treatment plant located on the Mediterranean coast. Chemosphere 66(6):993–1002
Hayashi Y, Heckmann LH, Callaghan A, Sibly RM (2008) Reproduction recovery of the crustacean Daphnia magna after chronic exposure to ibuprofen. Ecotoxicology 17(4):246–251
Heckmann LH, Callaghan A, Hooper HL, Connon R, Hutchinson TH, Maund SJ, Sibly RM (2007) Chronic toxicity of ibuprofen to Daphnia magna: effects on life history traits and population dynamics. Toxicol Lett 172(3):137–145
Buser HR, Poiger T, Müller MD (1999) Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater. Environ Sci Technol 33(15):2529–2535
Ashton D, Hilton M, Thomas KV (2004) Investigating the environmental transport of human pharmaceuticals to streams in the United Kingdom. Sci Total Environ 333(1-3):167–184
Du J, Mei CF, Ying GG, Xu MY (2016) Toxicity thresholds for diclofenac, acetaminophen and ibuprofen in the water flea Daphnia magna. Bull Environ Contam Toxicol 97(1):84–90
Nunes B, Antunes SC, Santos J, Martins L, Castro BB (2014) Toxic potential of paracetamol to freshwater organisms: a headache to environmental regulators? Ecotoxicol Environ Saf 107:178–185
Sarma SSS, González-Pérez BK, Moreno-Gutiérrez RM, Nandini S (2014) Effect of paracetamol and diclofenac on population growth of Plationus patulus and Moina macrocopa. J Environ Biol 35(1):119
Pascoe D, Karntanut W, Müller CT (2003) Do pharmaceuticals affect freshwater invertebrates? A study with the cnidarian Hydra vulgaris. Chemosphere 51(6):521–528
Brandão FP, Pereira JL, Gonçalves F, Nunes B (2014) The impact of paracetamol on selected biomarkers of the mollusc species Corbicula fluminea. Environ Toxicol 29(1):74–83
Parolini M, Binelli A, Cogni D, Riva C, Provini A (2009) An in vitro biomarker approach for the evaluation of the ecotoxicity of non-steroidal anti-inflammatory drugs (NSAIDs). Toxicol In Vitro 23(5):935–942
Parolini M, Binelli A, Cogni D, Provini A (2010) Multi-biomarker approach for the evaluation of the cyto-genotoxicity of paracetamol on the zebra mussel (Dreissena polymorpha). Chemosphere 79(5):489–498
de Oliveira LLD, Antunes SC, Gonçalves F, Rocha O, Nunes B (2016) Acute and chronic ecotoxicological effects of four pharmaceuticals drugs on cladoceran Daphnia magna. Drug Chem Toxicol 39(1):13–21
Liu Y, Wang L, Pan B, Wang C, Bao S, Nie X (2017) Toxic effects of diclofenac on life history parameters and the expression of detoxification-related genes in Daphnia magna. Aquat Toxicol 183:104–113
Haap T, Triebskorn R, Köhler HR (2008) Acute effects of diclofenac and DMSO to Daphnia magna: immobilisation and hsp70-induction. Chemosphere 73(3):353–359
Parolini M, Quinn B, Binelli A, Provini A (2011) Cytotoxicity assessment of four pharmaceutical compounds on the zebra mussel (Dreissena polymorpha) haemocytes, gill and digestive gland primary cell cultures. Chemosphere 84(1):91–100
Parolini M, Binelli A, Provini A (2011) Assessment of the potential cyto–genotoxicity of the nonsteroidal anti-inflammatory drug (NSAID) diclofenac on the zebra mussel (Dreissena polymorpha). Water Air Soil Pollut 217(1-4):589–601
Boisseaux P, Noury P, Thomas H, Garric J (2017) Immune responses in the aquatic gastropod Lymnaea stagnalis under short-term exposure to pharmaceuticals of concern for immune systems: Diclofenac, cyclophosphamide and cyclosporine A. Ecotoxicol Environ Saf 139:358–366
Nieto E, Corada-Fernández C, Hampel M, Lara-Martín PA, Sánchez-Argüello P, Blasco J (2017) Effects of exposure to pharmaceuticals (diclofenac and carbamazepine) spiked sediments in the midge, Chironomus riparius (Diptera, Chironomidae). Sci Total Environ 609:715–723
Wang L, Peng Y, Nie X, Pan B, Ku P, Bao S (2016) Gene response of CYP360A, CYP314, and GST and whole-organism changes in Daphnia magna exposed to ibuprofen. Comp Biochem Physiol C Toxicol Pharmacol 179:49–56
Contardo-Jara V, Lorenz C, Pflugmacher S, Nützmann G, Kloas W, Wiegand C (2011) Exposure to human pharmaceuticals Carbamazepine, Ibuprofen and Bezafibrate causes molecular effects in Dreissena polymorpha. Aquat Toxicol 105(3-4):428–437
Parolini M, Binelli A, Provini A (2011) Chronic effects induced by ibuprofen on the freshwater bivalve Dreissena polymorpha. Ecotoxicol Environ Saf 74(6):1586–1594
Aguirre-Martínez GV, Del Valls AT, Martín-Díaz ML (2015) Yes, caffeine, ibuprofen, carbamazepine, novobiocin and tamoxifen have an effect on Corbicula fluminea (Müller, 1774). Ecotoxicol Environ Saf 120:142–154
Pounds N, Maclean S, Webley M, Pascoe D, Hutchinson T (2008) Acute and chronic effects of ibuprofen in the mollusc Planorbis carinatus (Gastropoda: Planorbidae). Ecotoxicol Environ Saf 70(1):47–52
Quinn B, Gagné F, Blaise C (2009) Evaluation of the acute, chronic and teratogenic effects of a mixture of eleven pharmaceuticals on the cnidarian, Hydra attenuata. Sci Total Environ 407(3):1072–1079
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Parolini, M. (2020). Adverse Effects Induced by Nonsteroidal Anti-inflammatory Drugs on Freshwater Invertebrates. In: Gómez-Oliván, L.M. (eds) Non-Steroidal Anti-Inflammatory Drugs in Water. The Handbook of Environmental Chemistry, vol 96. Springer, Cham. https://doi.org/10.1007/698_2020_547
Download citation
DOI: https://doi.org/10.1007/698_2020_547
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-56293-9
Online ISBN: 978-3-030-56294-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)